Abstract
Prevalence and predictors of Transmitted Drug Resistance (TDR), defined as the presence of at least one WHO surveillance drug resistance mutation (SDRM), were investigated in antiretroviral-naïve HIV-1infected patients, with a genotypic resistance test (GRT) performed ≤6 months before starting cART between 2000 and 2010.
3163 HIV-1 sequences were selected (69% subtype B). Overall, the prevalence of TDR was 12% (13.2% subtype B, 9% non-B). TDR significantly declined overall and for the single drug classes. Older age independently predicted an increased odd whereas a more recent GRT, a higher HIV-RNA and C- versus B-subtype lower odds of TDR.
Keywords: Resistance epidemiology, Antiretroviral therapy, transmitted resistance, chronic HIV infection, recent HIV infection
Introduction
The introduction of combination Antiretroviral Therapy (cART) has allowed to obtain high rates[1-5] of success in controlling the replication of HIV. In Italy, the prevalence of TDR ranged from 5.9% to 15.1% in two studies between 1992 and 2007[6, 7]. We had analyzed the temporal trends and predictors of TDR in Italy in a previous study between 1996 and 2007[8].
The present study aimed to extend the follow-up of our previous work[8] and to evaluate possible predictors and trends of TDR prevalence in the Italian population from 2000 to 2010.
Materials and Methods
Sequences from HIV-1 infected, treatment naïve patients with a GRT performed ≤6 months before starting cART from 2000 to 2010 were retrieved from the ARCA databasewww.hivarca.net,8 and from separate databases at the Catholic University of Sacred Heart (CUSH) and at the University Hospital “Spedali Civili” of Brescia, Italy. For each patient, only the first genotype was considered. We defined an “acute/recent” infection if the first HIV positive test was obtained within 1-18 months since the last available negative test or if a primary HIV infection (PHI) could be demonstrated and a “non acute/non recent” infection when the time between an HIV negative and a subsequent positive test was longer than previously stated or when no negative test was available. TDR was defined as the detection of at least one mutation among those indicated in the WHO-recommended SDRM list updated in 2009 (available at: http://hivdb.stanford.edu/pages/WHOResistanceList.html)[9].
HIV-1 subtyping was automatically performed upon sequence upload by BLAST[8].
The changes in the prevalence of TDR per calendar year were evaluated with χ2 test for trend; logistic regression analysis was used in order to evaluate the predictors of TDR.
Results
A total of 3,163 (65% from ARCA, 14% from CUSH and 21% from Brescia) protease and reverse transcriptase sequences obtained between 2000 and 2010 from treatment-naive patients were analyzed. Two hundred twenty-six (7%) of 3,163 patients were diagnosed with an acute or recent HIV infection.
The evolution of patients characteristics over time is shown in table 1.
Table 1.
Descriptive characteristics of general population and of patients among different quartiles
| Total population N=3163 |
2000-2003 N=398 |
2004-2007 N=1793 |
2008-2010 N=972 |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Median Age (IQR) (years) | 37 (30-43) | 35 (30-41) | 37 (30-43) | 38 (31-46) | 0.001 |
|
| |||||
| Male sex (%) | 74 | 71 | 73 | 76 | 0.07 |
|
| |||||
| Median calendar year at genotyping (IQR) | 2006 (2005-2008) | ||||
|
| |||||
| Risk factors | |||||
| Heterosexual contacts (%) | 40 | 38 | 27 | 40 | 0.80 |
| Homosexual contacts (%) | 26 | 25 | 27 | 26 | 0.93 |
| Injecting drug use (%) | 11 | 14 | 12 | 9 | 0.002 |
| Other or unknown (%) | 23 | 23 | 20 | 25 | 0.05 |
|
| |||||
| Country of birth | |||||
| Italy (%) | 81 | 61 | 70 | 78 | <0.001 |
| Sub-Saharan Africa (%) | 8 | 4 | 8 | 8 | 0.007 |
| Latin America and Caribbean (%) | 5 | 5 | 4 | 3 | 0.30 |
| Eastern Europe (%) | 3 | 4 | 2 | 2 | 0.72 |
| Other (%) | 3 | 5 | 4 | 3 | 0.58 |
|
| |||||
| Patients followed at sites | |||||
| Northern Italy (%) | 46 | 45 | 54 | 41 | <0.001 |
| Central Italy (%) | 50 | 50 | 37 | 36 | <0.001 |
| Southern Italy and islands (%) | 4 | 5 | 9 | 23 | <0.001 |
|
| |||||
| Subtype, n (%) | |||||
| B | 69 | 77 | 72 | 61 | 0.002 |
| Non B | 22 | 18 | 23 | 24 | |
| Unknown | 9 | 6 | 6 | 16 | |
|
| |||||
| Distribution of NonB Subtypes, % | |||||
| F1 | 17.3 | 23.7 | 18.9 | 13.6 | |
| 02_AG | 15.8 | 15.1 | 19.5 | 11 | |
| C | 14 | 14 | 13.6 | 14.4 | |
| G | 6.2 | 5.4 | 8.3 | 13.7 | |
| Other (<5% each) | 18.5 | 17.1 | 19.6 | 7.4 | |
| Unknown | 28.2 | 24.7 | 20.1 | 39.9 | |
|
| |||||
| VL median (IQR) (log copies/ml) | 4.58 (4.04-5.15) | 4.42 (3.89-5.12) | 4.61 (4.06-5.16) | 4.58 (4.04-5.16) | 0.006 |
|
| |||||
| CD4 median (IQR) (cells/mm3) | 357 (201-518) | 361 (185-557) | 360 (226-527) | 342 (161-503) | 0.02 |
|
b: Predictors of TDR in all samples
| ||||
| Variable | Univariate analysis | Multivariate analysis | ||
|
| ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
|
| ||||
| Sex (male versus female) | 0.95 (0.74-1.22) | 0.71 | 0.84 (0.58-1.21) | 0.35 |
| Calendar year (per more recent) | 0.90 (0.86-0.94) | <0.001 | 0.93 (0.87-0.99) | 0.03 |
| Age (per 10 years more) | 1.01 (0.99-1.02) | 0.19 | 1.19 (1.04-1.36) | 0.01 |
| Risk category: | ||||
| Heterosexual contact (reference) | 1.00 | 1.00 | ||
| Homosexual contact | 1.25 (0.96-1.64) | 0.09 | 1.04 (0.73-1.47) | 0.84 |
| Injecting drug use | 1.08 (0.74-1.58) | 0.68 | 0.95 (0.62-1.46) | 0.83 |
| Other | 5.58 (0.92-33.6) | 0.06 | 2.87 (0.56-14.6) | 0.20 |
| Unknown | 0.69 (0.44-1.09) | 1.12 | 2.25 (0.22-22.6) | 0.49 |
| CD4 at GRT (per 100 cells/mm3 higher) | 1.06 (1.02-1.11) | 0.003 | 1.04 (0.99-1.09) | 0.17 |
| HIV-1 RNA at GRT (per 1 log10 copies/mL higher) | 0.76 (0.66-0.88) | <0.001 | 0.80 (0.67-0.95) | 0.01 |
| Clinical site: | ||||
| Northen Italy | 0.96 (0.77-1.21) | 0.74 | 0.92 (0.67-1.25) | 0.59 |
| Central Italy (reference) | 1.00 | 1.00 | ||
| Southern Italy | 0.65 (0.44-0.96) | 0.03 | 0.78 (0.52-0.72) | 0.30 |
| Origin: | ||||
| Italian (reference) | 1.00 | 1.00 | ||
| Sub-Saharan Africa | 0.45 (0..25-0.81) | 0.008 | 0.75 (0.32-1.72) | 0.49 |
| Latina America and Caribbean | 0.91 (0.53-1.59) | 0.76 | 0.90 (0.48-0.70) | 0.75 |
| Eastern Europe | 0.58 (0.23-1.46) | 0.25 | 0.69 (0.24-1.99) | 0.49 |
| Other | 1.87 (1.08-3.23) | 0.02 | 1.91 (1.02-3.59) | 0.04 |
| Subtype: | ||||
| B (reference) | 1.00 | 1.00 | ||
| C | 0.35 (0.16-0.76) | 0.008 | 0.31 (0.11-0.88) | 0.03 |
| F1 | 0.82 (0.50-1.35) | 0.44 | 0.95 (0.53-1.72) | 0.87 |
| G | 0.34 (0.10-1.09) | 0.07 | 0.21 (0.03-1.61) | 0.13 |
| CRF02_AG | 0.65 (0.37-1.42) | 0.13 | 0.56 (0.23-1.39) | 0.21 |
| Other | 0.70 (0.50-0.97) | 0.03 | 0.81 (0.53-1.22) | 0.31 |
| Non B-Subtype | 0.64 (0.48-0.86) | 0.003 | - | - |
| Duration of HIV infection: | ||||
| Acute/recent vs Non acute/non recent | 1.19 (0.80-1.77) | 0.38 | 1.14 (0.73-1.79) | 0.56 |
The overall prevalence of any drug resistance mutation in patients with “non acute/non recent” infection was 11.7%; NRTI, NNRTI and major PI resistance mutations were detected in 7.4%, 5% and 2.9% of patients, respectively. The prevalence of any drug resistance mutations was significantly reduced in 2010 versus 2000 (8.2% vs 24.1%, p<0.001). The proportion of patients presenting any major IAS mutation associated to NRTI resistance, NNRTI resistance and PI resistance in this subgroup is shown in figure 1a.
Figure 1.
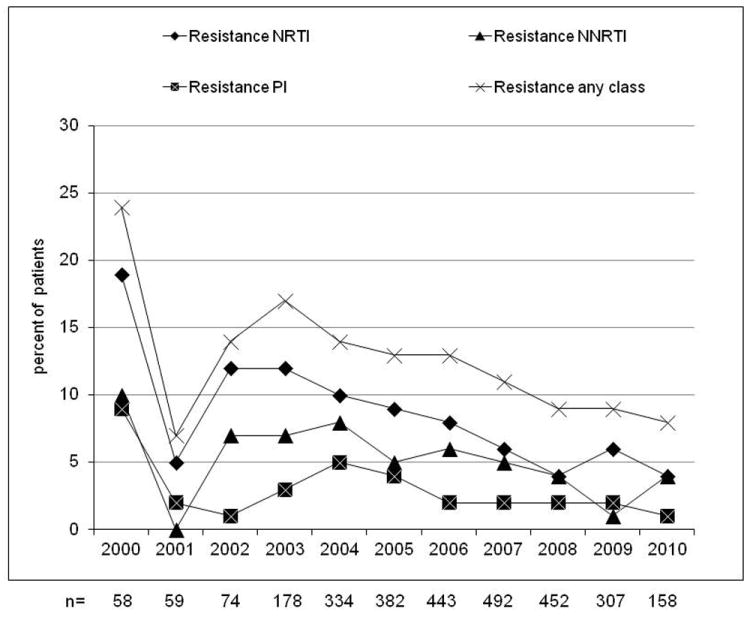
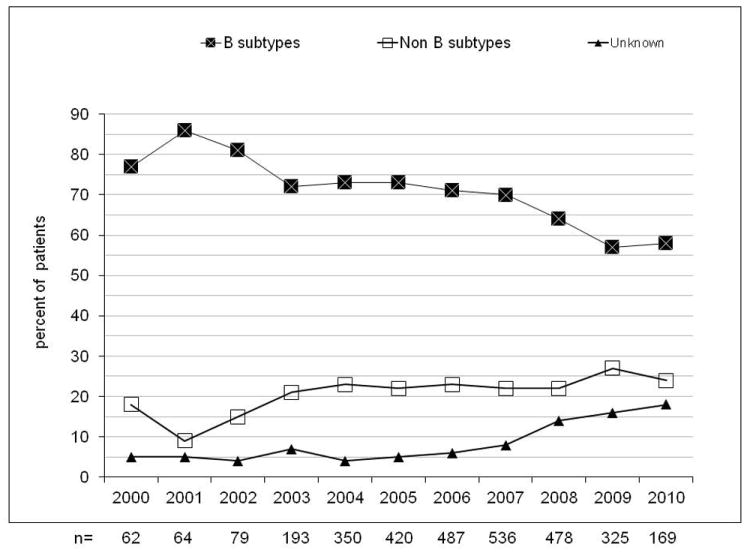
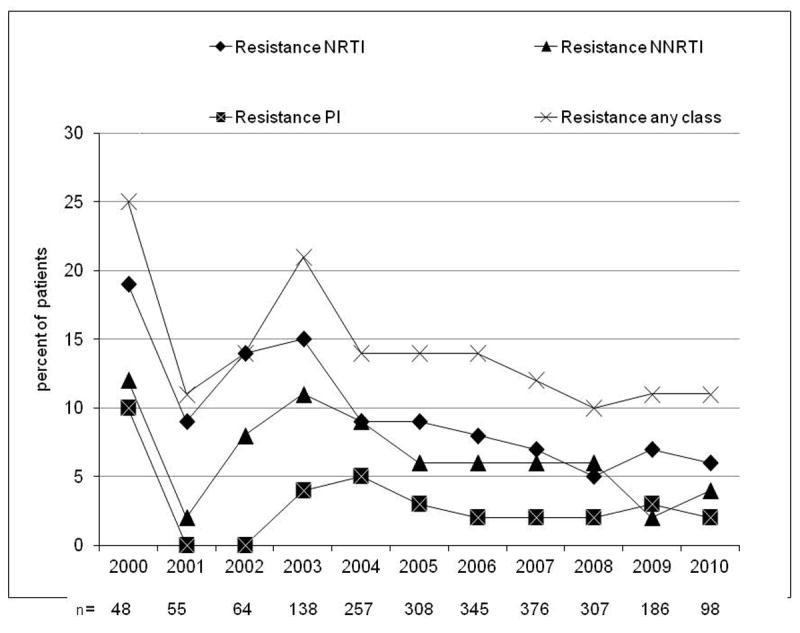
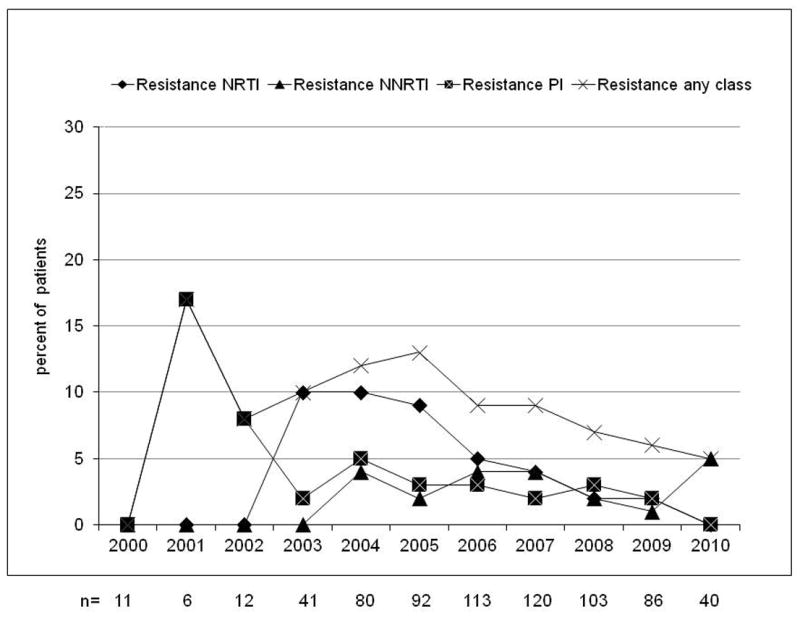
Evolution of resistance (at least one major IAS mutation) by calendar year from 2000 to 2010 in naive patients with non acute/non recent infection (A, n=2,937), evolution of the prevalence of B, non-B and unknown subtypes (B) and evolution of the prevalence of drug resistance in B subtype (C) and in non-B subtypes (D). NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The overall proportion of non-B strains increased from 18% in 2000 to 24% in 2010 (p<0.001); the prevalence of unknown subtypes was also increased from 5% to 18% (p<0.001) (see figure 1b). The CRF02_AG subtype showed an increase from 3/62 (5%) to 10/169 (6%, p=0.67) and F1 increased from 3/62 (5%) to 8/169 (5%, p=0.81). Among Italian natives, 310/2224 (13%) carried non-B viral strains; the prevalence of these subtypes increased over calendar years (11% in 2000–2003, 14% in 2004–07, 19% in 2008–10; overall p=0.01), peaking in 2009 (51/251, 25%).
The overall prevalence of subtype B HIV-1 strains was 69%. The prevalence of TDR was higher in B- than in non-B subtype (13% vs. 9%, p=0.002) carriers. The prevalence of TDR in subtype B and non-B was 8% and 5% for NRTIs (p=0.003), 6% and 3% for NNRTIs (p<0.001), and 3% each for PIs (p=0.94); the changes over calendar year in the trends for B and non-B subtypes is shown in figure 1c-d.
In patients diagnosed with an acute or recent HIV infection from 2000 to 2010 the overall prevalence of any drug resistance mutation was 14%; NRTI, NNRTI and major PI resistance mutations were detected in 7%, 7% and 3% of patients, respectively. Subtype B HIV-1 was detected in 185/226 (82%) samples; the most frequently observed non-B subtype was F1 (5%).
At multivariable logistic regression analysis, older age independently predicted higher odds of transmitted drug resistance mutations. More recent calendar year, higher plasma HIV-RNA and infection with non subtype B strains independently predicted reduced odds of TDR (see table 1b).
Discussion
This is an observational study aiming to analyze the prevalence of TDR in HIV-infected patients in three large Italian Cohorts. The declining trend in the prevalence of any TDR mutation that we had observed[8] was confirmed until 2010.
We observed a significantly increased proportion of treatment naïve patients carrying non-B subtype HIV-1 in the last 10 years. This confirms our previous observation reporting an increased prevalence of non subtype B strains over two decades (1983-2006) in Brescia[10].
At multivariate analysis, we observed a lower risk of TDR in patients tested for GRT in a more recent calendar year, probably as an effect of the improvement in the management of cART. The lower risk of TDR in patients with higher baseline viral load could be related to the higher fitness of wild-type HIV[11]. Finally, the lower prevalence of TDR in non-B subtype infected subjects is in line with the lower exposure to cART in countries where these subtypes are prevalent. We acknowledge that the surveillance list of resistance mutations for non-B subtypes is still being completely defined; anyway, we referred to the last available 2009 list[9] where a considerable amount of mutations were added to those previously recognized.
The analysis of TDR in patients with acute or recent HIV infection is of great interest but is limited by the low rates of documented seroconversion or diagnosed PHIs.
The high proportion of patients with unknown duration of HIV infection is an important confounder, but this is difficult to overcome as in clinical practice the proportion of patients with a recent negative test at the diagnosis of HIV infection always represents a minority. Finally, a relevant part of subtypes could not be determined, particularly in recent years.
In conclusion, the prevalence of TDR is declining in our multicentric cohort; this probably reflects the improvement in the management and prescription of antiretroviral drugs and the availability of new drugs.
Acknowledgments
MC wrote the first draft of the manuscript, also performing the appropriate literature search. CT contributed to the data interpretation and to the final version of the submitted draft. EMT, LA, AR, VM, NM, G Penco, BB, G Punzi, PC, G Parruti, PB, AG all contributed to the data collection. LM contributed to the data collection and to the editing of the final draft. RC edited the final draft. SDG conceived and designed the study, performed the statistical analyses and interpretations, and contributed to writing the first draft of the manuscript.
Funding.
This work was supported by DynaNets project (www.dynanets.org), a Future and Emerging Technologies (FET)-Open grant # 233847; Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN) project (http://ec.europa.eu/research/health/infectious-diseases/poverty-diseases/projects/185_en.htm), Seventh Framework Programme (FP7 2007–2013), grant # 223131; NIH, NINDS GRANTR01 NS063897-01A2 (Feb 2009–Jan 2014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
For the UCSC HIV resistance study group: Fabbiani M, Sidella L, Mondi A, Farina S, D’Avino A, Fanti I, Doino M, Prosperi M
For the Brescia HIV resistance study group: Gargiulo F, Benini A, Paraninfo G, Quiros-Roldan E, Castelnuovo F, Carosi G, Scalzini A.
The following centers currently contribute clinical and laboratory data to the ARCA database initiative.
Ancona—Clinica di Malattie Infettive, Andrea Giacometti; Ancona—Immunologia Clinica, Luca Butini; Ancona—Malattie Infettive, Romana del Gobbo; Ancona—Virologia, Stefano Menzo; Arezzo—Malattie Infettive, Danilo Tacconi; Ascoli Piceno—Malattie Infettive, Giovanni Corbelli; Aviano—Centro di Riferimento Oncologico, Stefania Zanussi; Bari—Clinica Malattie Infettive, Laura Monno; Bari—Virologia, Grazia Punzi; Bergamo—Malattie Infettive, Franco Maggiolo; Bergamo—Microbiologia e Virologia, Annapaola Callegaro; Bologna—Malattie Infettive S. Orsola, Leonardo Calza; Bologna—UO Microbiologia, Laboratorio Retrovirus, Maria Carla Re; Bolzano—Malattie Infettive, Raffaele Pristerà; Brescia—Fleming Labs, Paola Turconi; Cagliari—Centro S.I.D.A., Policlinico Universitario, Antonella Mandas; Città di Castello—Medicina Generale, Sauro Tini; Cremona—Malattie Infettive, Giuseppe Carnevale; Cremona—Servizio Immunoematologia e Medcina Trasfusionale, Elisabetta Paolini; Fermo—Malattie Infettive, Giorgio Amadio; Ferrara—Malattie Infettive, AOU S. Anna, Laura Sighinolfi; Firenze—Centro MTS, Giuliano Zuccati; Firenze—Ematologia Careggi, Massimo Morfini; Firenze—Immunoallergologia Careggi, Roberto Manetti; Firenze—Malattie Infettive Careggi, Paola Corsi; Firenze—Malattie Infettive Pediatria Meyer, Luisa Galli; Firenze—Malattie Infettive SM Annunziata, Massimo Di Pietro; Firenze—Malattie Infettive, Università, Filippo Bartalesi; Firenze—Virologia Careggi, Grazia Colao; Foligno—Malattie Infettive/SERT, Andrea Tosti; Genova— Clinica Malattie Infettive, AOU S. Martino, Antonio Di Biagio; Genova—Clinica Medica Immunologia, Maurizio Setti; Genova—Laboratorio di Igiene, Ospedale S. Martino, Bianca Bruzzone; Genova— Malattie Infettive, Ospedali Galliera, Giovanni Penco; Grosseto—Malattie Infettive, Michele Trezzi; Lecco—Malattie Infettive, Anna Orani; Livorno—Malattie Infettive, Riccardo Pardelli; Lucca—Malattie Infettive, Michele De Gennaro; Macerata—Malattie Infettive, Alessandro Chiodera; Mantova—Malattie Infettive, Ospedale ’C. Poma’, Alfredo Scalzini; Mantova—Virologia, Loredana Palvarini; Massa—Malattie Infettive, Paolo Almi; Messina—Malattie Infettive, Giovanni Todaro; Milano HSR—Studio MUSA, Nicola Gianotti; Milano—Clinica di Malattie Infettive, Ospedale S. Paolo, Paola Cicconi; Milano—Dipart. Scienze Cliniche, Sez. Malattie Infettive—Università degli Studi, Stefano Rusconi; Milano—Laboratorio Microbiologia, Ospedale L. Sacco (Dipart. Scienze Cliniche, Sez. Malattie Infettive), Maria Rita Gismondo; Milano—Laboratorio Microbiologia, Ospedale L. Sacco (Prima Divisione Malattie Infettive), Maria Rita Gismondo; Milano—Laboratorio Microbiologia, Ospedale L. Sacco (Seconda Divisione Malattie Infettive), Valeria Micheli; Milano—Laboratorio di diagnostica molecolare infettivologica, AO S. Paolo, Maria Luisa Biondi; Milano—Malattie Infettive, San Raffaele, Nicola Gianotti; Milano—Prima Divisione Malattie Infettive, Ospedale L. Sacco, Amedeo Capetti; Milano—Seconda Divisione Malattie Infettive, Ospedale L. Sacco, Paola Meraviglia; Milano—Virologia HSR, Enzo Boeri; Modena—Virologia, Monica Pecorari; Modena—Malattie Infettive, Cristina Mussini; Narni—SERT, Maurizio Santirocchi; Novara—Malattie Infettive, AO Maggiore, Diego Brustia; Novara—Virologia, Paolo Ravanini; Padova—Virologia, Federico Dal Bello; Palermo—Centro Riferimento AIDS, Università, Nino Romano; Palermo—Servizio Riferimento Regionale Diagnosi AIDS, Salvatrice Mancuso; Parma—Divisione Malattie Infettive ed Epatologia, Azienda Ospedaliera, Carlo Calzetti; Pavia—Ambulatorio Clinica Malattie Infettive, S. Matteo Renato Maserati; Pavia—Clinica Malattie Infettive e Tropicali, Gaetano Filice; Pavia—Virologia, S. Matteo, Fausto Baldanti; Perugia—Malattie Infettive, Daniela Francisci; Pescara—Malattie Infettive, Giustino Parruti; Pescara—Virologia, Ennio Polilli; Piacenza—Malattie Infettive, Daria Sacchini; Pisa—Malattie Infettive, Chiara Martinelli; Pisa—Pediatria I, Università, Rita Consolini; Pisa—Virologia, Linda Vatteroni; Pistoia—Malattie Infettive, Angela, Vivarelli; Prato—Malattie Infettive, Alessandro Nerli; Prato—Virologia, Lucia Lenzi; Reggio Emilia—Malattie Infettive, Giacomo Magnani; Rimini—Malattie Infettive, Patrizia Ortolani; Roma—Cattedra Malattie Infettive, Tor Vergata, Massimo Andreoni; Roma—IRCCS S. Gallicano, Guido Palamara; Roma—Immunologia Clinica, Umberto I, Caterina Fimiani; Roma—Istituto Superiore di Sanità, Lucia Palmisano; INMI Spallanzani, Andrea Antinori; Roma—Malattie Infettive e Tropicali, La Sapienza, Umberto I, Vincenzo Vullo; Roma—Medicina Sperimentale e Patologia, Sezione Virologia, La Sapienza, Ombretta Turriziani; Roma—Monitoraggio Terapie Antivirali e Antineoplastiche, INMI Spallanzani, Carlo Federico Perno; Roma—Virologia DMS, Tor Vergata, Carlo Federico Perno; Roma—Virologia Malattie Infettive, Tor Vergata, Marco Montano; Sanremo—Malattie Infettive, Giovanni Cenderello; Siena—Malattie Infettive, Angela Gonnelli; Siena—Virologia, Laura Romano; Terni—Malattie Infettive, Michele Palumbo; Torino—Laboratorio di Virologia, Ospedale Amedeo di Savoia, Valeria Ghisetti; Torino—Malattie Infettive, Amedeo di Savoia, Stefano Bonora; Trento—Malattie Infettive, Palma Delle Foglie; Treviso—Malattie Infettive, Cristina Rossi; Verbania—Malattie Infettive, Federica Poletti; Verbania—Virologia, Vincenzo Mondino; Verona—Centro di Medicina Preventiva, ULSS20, MarinaMalena;Verona—Malattie Infettive, Emanuela Lattuada.
Footnotes
Transparency Declaration
All Authors have nothing to declare in the period of research leading up to this publication and with specific reference to this paper.
MC, SDG, RC received funds for speaking, consultancy, advisory board membership, travel from MSD, J-C, Abbott, ViiV Healthcare, Gilead Science, BMS outside the present work.
MC was an employee of BMS from May 2010 to February 2011 and resigned before starting the present work.
References
- 1.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008 May 15;358(20):2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daar ES, Tierney C, Fischl MA, et al. Atazanavir Plus Ritonavir or Efavirenz as Part of a 3-Drug Regimen for Initial Treatment of HIV-1: A Randomized Trial. Ann Intern Med. 2011 Apr 5;154(7):445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. Epub 2011 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008 Aug 23;372(9639):646–55. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008 Jul 31;22(12):1389–97. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 5.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009 Sep 5;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. Epub 2009 Aug 3. Erratum in: Lancet. 2009 Dec 19-2010 Jan 1; 374(9707):2054. Lancet. 2009 Sep 5;374(9692):786. [DOI] [PubMed] [Google Scholar]
- 6.Riva C, Lai A, Caramma I. Transmitted HIV Type 1 drug resistance and Non-B subtypes prevalence among seroconverters and newly diagnosed patients from 1992 to 2005 in Italy. AIDS Res Hum Retroviruses. 2010 Jan;26(1):41–9. doi: 10.1089/aid.2009.0057. [DOI] [PubMed] [Google Scholar]
- 7.Alteri C, Svicher V, Gori C, et al. Characterization of the patterns of drug-resistance mutations in newly diagnosed HIV-1 infected patients naïve to the antiretroviral drugs. BMC Infectious Diseases. 2009;9:111. doi: 10.1186/1471-2334-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bracciale L, Colafigli M, Zazzi M, et al. Prevalence of transmitted HIV-1 drug resistance in HIV-1-infected patients in Italy: evolution over 12 years and predictors. JAC. 2009;64:607–615. doi: 10.1093/jac/dkp246. [DOI] [PubMed] [Google Scholar]
- 9.Bennet DE, Camacho R, Otelea D, et al. Drug Resistance Mutations for Surveillance of Transmitted HIV-1 Drug-Resistance: 2009 Update. PLoS ONE. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torti C, Lapadula G, Izzo I, et al. Heterogeneity and penetration of HIV-1 non-subtypes B virus in an Italian province: public health implications. Epidemiol Infect. 2010;138:1298–1307. doi: 10.1017/S0950268810000166. [DOI] [PubMed] [Google Scholar]
- 11.Harrison L, Castro H, Cane P, et al. The effect of transmitted HIV-1 drug resistance on pre-therapy viral load. AIDS. 2010 Jul 31;24(12):1917–22. doi: 10.1097/QAD.0b013e32833c1d93. [DOI] [PubMed] [Google Scholar]


