Abstract
BACKGROUND
The prevention of bleeding with adequately sustained levels of clotting factor, after a single therapeutic intervention and without the need for further medical intervention, represents an important goal in the treatment of hemophilia.
METHODS
We infused a single-stranded adeno-associated viral (AAV) vector consisting of a bioengineered capsid, liver-specific promoter and factor IX Padua (factor IX–R338L) transgene at a dose of 5×1011 vector genomes per kilogram of body weight in 10 men with hemophilia B who had factor IX coagulant activity of 2% or less of the normal value. Laboratory values, bleeding frequency, and consumption of factor IX concentrate were prospectively evaluated after vector infusion and were compared with baseline values.
RESULTS
No serious adverse events occurred during or after vector infusion. Vector-derived factor IX coagulant activity was sustained in all the participants, with a mean (±SD) steady-state factor IX coagulant activity of 33.7±18.5% (range, 14 to 81). On cumulative follow-up of 492 weeks among all the participants (range of follow-up in individual participants, 28 to 78 weeks), the annualized bleeding rate was significantly reduced (mean rate, 11.1 events per year [range, 0 to 48] before vector administration vs. 0.4 events per year [range, 0 to 4] after administration; P = 0.02), as was factor use (mean dose, 2908 IU per kilogram [range, 0 to 8090] before vector administration vs. 49.3 IU per kilogram [range, 0 to 376] after administration; P = 0.004). A total of 8 of 10 participants did not use factor, and 9 of 10 did not have bleeds after vector administration. An asymptomatic increase in liver-enzyme levels developed in 2 participants and resolved with short-term prednisone treatment. One participant, who had substantial, advanced arthropathy at baseline, administered factor for bleeding but overall used 91% less factor than before vector infusion.
CONCLUSIONS
We found sustained therapeutic expression of factor IX coagulant activity after gene transfer in 10 participants with hemophilia who received the same vector dose. Transgene-derived factor IX coagulant activity enabled the termination of baseline prophylaxis and the near elimination of bleeding and factor use. (Funded by Spark Therapeutics and Pfizer; ClinicalTrials.gov number, NCT02484092.)
Hemophilia B is an X-linked bleeding disorder that results from a deficiency or dysfunction of coagulation factor IX. The bleeding manifestations of hemophilia are predictable on the basis of the plasma factor IX coagulant activity. The hallmark of hemophilia B, recurrent spontaneous hemarthrosis, occurs in severe cases (factor IX coagulant activity, <1% of the normal value) or moderate cases (factor IX coagulant activity, 1 to <5% of the normal value) and rarely in mild cases (factor IX coagulant activity, 5 to <40% of the normal value).1 The current standard of care for patients with hemophilia is exogenous factor that is infused intravenously 1 to 3 times per week to prevent bleeding or that is administered on demand in response to bleeding.2 Unfortunately, exogenous factor replacement requires regular vascular access, results in peaks and troughs of activity that require considerable planning of daily activities, and does not always eliminate bleeding.1,2 The level of factor IX coagulant activity that is effective for the elimination of spontaneous bleeding events is unknown. In patients with hemophilia A, a phenotypically similar bleeding disorder that is characterized by factor VIII deficiency, natural history data indicate that endogenous factor VIII activity of 12% or more of the normal value eliminates spontaneous hemarthrosis.3
Endogenous factor IX expression after gene therapy could address many of the limitations of current therapies with the use of a single vector infusion to maintain factor IX coagulant activity above the threshold that is effective in preventing spontaneous bleeding and preserving joint function. After early clinical trials that began in the late 1990s did not show sustained factor expression,4–7 clinical investigation of gene therapy for hemophilia coalesced around in vivo liver-directed gene transfer with recombinant adeno-associated viral (AAV) vectors.8,9 However, previous trials of AAV-mediated hemophilia B gene transfer resulted in either therapeutic but short-lived circulation of factor IX7,10 or durable but suboptimal expression levels.11 A consistent observation in all these studies was that the risk of an immune response against AAV-transduced hepatocytes was dependent on the vector dose.7,10,11 This observation highlights a basic therapeutic principle — the need to use the lowest dose that results in a clinical benefit while minimizing the risk of adverse events.
We hypothesized that an AAV vector expressing a high-specific-activity factor IX variant could be administered at low doses to drive therapeutic factor IX expression and minimize the risk of an AAV capsid immune response. Factor IX–R338L is a naturally occurring gain-of-function mutation in the factor IX catalytic domain that results in specific activity that is 8 to 12 times as high as that of nonmutant factor IX.12,13 Although the long-term expression of factor IX–R338L in canine models of hemophilia and preliminary data regarding short-term expression in a previous clinical trial did not show inhibitor formation or evidence of thrombosis, data have been limited regarding the safety and hemostatic efficacy of sustained expression of factor IX–R338L after gene transfer.10,13 Here, we report data on safety and sustained factor IX activity and preliminary data on efficacy in 10 participants who received the same low dose of therapeutic vector.
METHODS
STUDY DESIGN AND OVERSIGHT
This open-label, nonrandomized, multicenter, phase 1–2a study involving men with hemophilia B investigated the safety and kinetics of a single-stranded recombinant-AAV vector, SPK-9001. The institutional review board at each study site approved the protocol (available with the full text of this article at NEJM.org). Written informed consent was obtained from all the participants at enrollment. Immediately before vector infusion, participants received a 100% correction dose of their usual factor IX product to aid vector transduction.14 SPK-9001 was infused intravenously in an outpatient setting at a dose of 5×1011 vector genomes (vg) per kilogram of body weight over a period of 1 hour. Participants were followed for 52 weeks and thereafter were invited to enroll in a long-term follow-up protocol.
The study was funded by Spark Therapeutics and Pfizer; Pfizer had no other role in the study. The study was designed by the first author and by three authors who are employees of Spark Therapeutics (the sponsor), and the data were collected by site investigators (listed as authors) who vouch for the fidelity of the trial to the protocol. The clinical investigators (see the Supplementary Appendix, available at NEJM.org) and employees of the sponsor (all of whom are listed as authors of this article) analyzed the data. The first author prepared the first draft of the manuscript and, with two authors who are employees of the sponsor, wrote the manuscript with critical input from all the coauthors and without editorial assistance. The authors assume responsibility for the completeness and integrity of the data and analyses.
PARTICIPANTS
We enrolled men 18 years of age or older who had hemophilia B and a factor IX coagulant activity of 2% or less of the normal value with AAV-Spark100 neutralizing antibodies of 1:5 or less, as determined by an in vitro transduction assay,15 and who met screening criteria (see the Methods section in the Supplementary Appendix). At screening, annualized bleeding rates, factor consumption, and the number of infusions were retrospectively determined by means of review of participants’ records for the 52 weeks before enrollment and were compared with prospectively collected data after the infusion of vector.
VECTOR DESIGN AND PRODUCTION
This single-stranded recombinant-AAV vector, SPK-9001, consists of a bioengineered capsid (AAV-Spark100)16 and an expression cassette containing the apolipoprotein E gene hepatic-control region (APOE), a liver-specific human α1-antitrypsin (hAAT) promoter, and a codon-optimized F9–Padua minigene. The vector was manufactured by means of previously described methods.6,17,18 In brief, vector was produced by transient transfection of HEK293 cells, purified from clarified cell lysates by cation exchange chromatography and density gradient centrifugation, then diafiltrated into an isotonic formulation (see the Methods section in the Supplementary Appendix). The final product was tested in quality-control assays that were designed to ensure its purity, safety, and potency, including titer determination by means of quantitative polymerase chain reaction. In the final formulation, SPK-9001 was combined in defined ratios with empty capsids.
OBJECTIVES
The primary objective was to assess the safety of SPK-9001. Assessment after vector infusion included vital signs, physical examinations, reporting of adverse events, and laboratory testing that included factor IX coagulant activity, factor IX inhibitor, liver-function tests, and cellular and humoral immune response against the AAV capsid as assessed by means of the interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assay and the AAV-Spark100 neutralizing antibody assay, respectively. A cellular immune response against the FIX–R338L protein was analyzed by means of the interferon-γ ELISPOT assay, and a humoral response to nonmutant FIX protein was measured by means of the Nijmegen-modified Bethesda assay (lower limit of the normal range, <0.6 BU per milliliter). One-stage factor IX coagulant activity and liver-function studies (alanine aminotransferase and aspartate aminotransferase levels) were assayed on the day that samples were obtained in order to evaluate safety and evidence of an AAV capsid immune response and were run at individual study-site laboratories that were certified by the Clinical Laboratory Improvement Amendments (CLIA) program. The factor IX coagulant activity in each participant was cross-evaluated with the use of a STA-R Evolution instrument and TriniCLOT aPTT S reagent (Stago), which confirmed the results of the local laboratory at the individual study sites. The secondary study objective aimed to evaluate the pharmacokinetics of SPK-9001 — that is, the peak and steady-state vector-derived factor IX coagulant activity. Exploratory end points were vector shedding and efficacy studies, including changes from baseline in the annualized bleeding rate, consumption of exogenous factor IX concentrate, and infusions.
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize the prospectively observed longitudinal data. Prospective observations after the administration of vector were compared with retrospective data that were collected at screening regarding the 52-week period before study enrollment. Statistics were computed with the use of the two-tailed Wilcoxon matched-pairs signed-rank-test. Observed P values and W statistics are reported. (The W statistic is the sum of the signed ranks and provides information about the magnitude of the net difference between compared matched pairs. In a context in which the null hypothesis is true [i.e., no difference between matched pairs], the W statistic would approximate 0.19)
RESULTS
STUDY POPULATION
A total of 14 men with hemophilia B (factor IX level, ≤2% of the normal value), 18 to 53 years of age, were enrolled at four institutions. A total of 10 participants received an infusion of SPK-9001. Among the 4 participants who did not receive an infusion, 2 were found to be ineligible owing to hepatitis C virus (HCV)–related liver fibrosis, and 2 had the infusion deferred (see the Supplementary Results section in the Supplementary Appendix).
The 10 participants who received an infusion represented a heterogeneous population of patients with hemophilia, encompassing patients with HCV-related liver fibrosis of stage 1 or 2, varied degrees of hemophilic arthropathy, and varied genotypes, including 6 participants who were positive for factor IX cross-reacting material and 4 who were negative for factor IX cross-reacting material (Table 1). At baseline, 3 participants used factor IX concentrate on demand, and the remaining 7 participants used it as prophylaxis. Data represent a cumulative follow-up of 492 weeks among all the participants (mean follow-up among individual participants, 49.0±13.4 weeks; range, 28 to 78).
Table 1.
Characteristics of the Participants at Baseline and after Gene Transfer.*
| Characteristic | Participant Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| At baseline | ||||||||||
|
| ||||||||||
| Age — yr | 23 | 18 | 46 | 43 | 52 | 21 | 45 | 18 | 37 | 53 |
|
| ||||||||||
| Weight — kg | 81.8 | 55.6 | 97.9 | 101.3 | 87 | 70.9 | 87 | 68.2 | 82.8 | 68.1 |
|
| ||||||||||
| Factor IX activity — % of normal | <1.0 | <1.0 | <1.0 | 1.3 | 1 | <1.0 | 1.6 | <1.0 | <1.0 | 1.7 |
|
| ||||||||||
| Factor IX genotype | H221R | G309V | S136Cfs*6 | I382F | G396A | W194Stop | C19Y | W194Stop | S365I fs*15 | G367R |
|
| ||||||||||
| Cross-reacting material status | + | + | − | + | + | − | + | − | − | + |
|
| ||||||||||
| Factor IX treatment | ||||||||||
|
| ||||||||||
| Use | Prophylactic | Prophylactic | Prophylactic | On demand | On demand | Prophylactic | On demand | Prophylactic | Prophylactic | Prophylactic |
|
| ||||||||||
| Treatment type | EHL-FIX | rFIX | EHL-FIX | NA | NA | rFIX | NA | rFIX | pdFIX | EHL-FIX |
|
| ||||||||||
| Dosing regimen | 50 IU/kg weekly | 50 IU/kg three times per week | 100 IU/kg every 10 days | NA | NA | 30 IU/kg twice per week | NA | 30 IU/kg twice per week | 45 IU/kg weekly | 100 IU/kg every 10 days |
|
| ||||||||||
| No. of target joints | 0 | 4 | 4 | 2 | 1 | 0 | 2 | 0 | 5 | 0 |
|
| ||||||||||
| Infection history | ||||||||||
|
| ||||||||||
| HIV | No | No | Yes | No | No | No | No | No | No | No |
|
| ||||||||||
| Hepatitis C virus | No | No | Yes | No† | Yes | No | Yes | No | Yes | Yes |
|
| ||||||||||
| Hepatitis B virus | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
|
| ||||||||||
| Liver fibrosis (FibroScan result)‡ | NA | NA | F2 (8.3 kPa) | NA | F1 (6.4 kPa) | NA | F1 (7.1 kPa) | NA | F1 | F1 (7.1 kPa) |
|
| ||||||||||
| AAV neutralizing antibody | <1:1 | <1:1 | <1:1 | <1:1 | <1:1 | 1:1 | <1:1 | <1:1 | <1:1 | <1:1 |
|
| ||||||||||
| At vector administration | ||||||||||
|
| ||||||||||
| Ratio of full to empty capsids | 1:5 | 1:5 | 1:4 | 1:4 | 1:4 | 1:5 | 1:4 | 1:3 | 1:3 | 1:3 |
|
| ||||||||||
| After gene transfer | ||||||||||
|
| ||||||||||
| Follow-up — wk | 78 | 52 | 52 | 52 | 52 | 52 | 52 | 37 | 37 | 28 |
|
| ||||||||||
| Mean steady-state factor IX activity — % of normal | 30 | 36 | 25 | 41 | 36 | 18 | 14 | 27 | 81 | 29 |
|
| ||||||||||
| Factor IX inhibitor — BU | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
|
| ||||||||||
| Mean factor IX antigen — % of normal | NA | NA | 2.5 | NA | NA | 1.2 | NA | 2.2 | 7.0 | NA |
|
| ||||||||||
| AAV capsid immune response — wk after vector infusion | NA | NA | NA | NA | NA | NA | 6 | NA | 4 | NA |
|
| ||||||||||
| Thrombin-antithrombin at 52 wk§ | 2.6 | 3.6 | 4.4 | 2.8 | 5.9 | 2.2 | 3.6 | NA | NA | NA |
|
| ||||||||||
| Annualized bleeding rate | ||||||||||
|
| ||||||||||
| Before gene transfer — no. of events/yr | 4 | 3 | 12 | 0 | 10 | 2 | 32 | 0 | 48 | 0 |
|
| ||||||||||
| After gene transfer — no. of events/yr (% reduction) | 0 (100) | 0 (100) | 4 (67) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) |
|
| ||||||||||
| Annualized use of factor IX | ||||||||||
|
| ||||||||||
| At 52 wk before gene transfer | ||||||||||
|
| ||||||||||
| No. of infusions | 98 | 159 | 61 | 0 | 10 | 106 | 47 | 104 | 52 | 38 |
|
| ||||||||||
| Dose — IU/kg | 3009 | 8090 | 4024 | 0 | 911 | 2990 | 1761 | 3050 | 1884 | 3362 |
|
| ||||||||||
| After gene transfer | ||||||||||
|
| ||||||||||
| No. of infusions (% reduction) | 0 (100) | 0 (100) | 10 (84) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 2 (98) | 0 (100) | 0 (100) |
|
| ||||||||||
| Dose — IU/kg (% reduction) | 0 (100) | 0 (100) | 376 (91) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 117 (96) | 0 (100) | 0 (100) |
A plus sign indicates positive results, and a minus sign negative results. AAV denotes adeno-associated viral, BU Bethesda titer units, EHL-FIX extended half-life factor IX, HIV human immunodeficiency virus, NA not applicable, pdFIX plasma-derived factor IX, and rFIX recombinant factor IX.
Participant 4 was seropositive but did not have history of chronic hepatitis C infection.
Liver fibrosis is assessed on a scale of F0 to F4, with higher stages indicating a greater degree of fibrosis. FibroScan results are on a scale from 2.5 kPa to 75 kPa, with higher values indicating more severe fibrosis.
The normal range for thrombin–antithrombin values is 1.0 to 4.1 µg per liter.
SAFETY AND FACTOR IX–R338L EXPRESSION
A total of 40 adverse events occurred among the participants. There were no serious adverse events, including no deaths, no development of factor IX inhibitors, no thrombosis, and no laboratory evidence of excess activation of coagulation as assessed by thrombin–antithrombin values at the week 52 visit (Tables 1 and 2). The only adverse event that was deemed by the investigators to be related to the study vector occurred in Participant 9, who had a transient grade 1 elevation of the alanine aminotransferase level (to >2.5 times the upper limit of the normal range). Participant 7 had an increase in liver-enzyme levels above baseline that remained within the range of normal values and that did not meet the criteria regarding toxic effects. As has occurred in previous liver-directed AAV gene-transfer clinical trials, vector genomes were shed transiently in bodily fluids and cleared as outlined in Table S1 and Figure S1 in the Supplementary Appendix.7,20 Factor IX inhibitory antibodies did not develop in any participants, and no participants had a significant T-cell response to the factor IX–R338L transgene according to interferon-γ ELISPOT analysis (Fig. S2 in the Supplementary Appendix).
Table 2.
Summary of Adverse Events.*
| Event | No. of Events | Grade | Relation to Study Agent |
|---|---|---|---|
| Nervous system event | |||
| Headache | 3 | 1 | Unlikely |
| Peripheral neuropathy | 1 | 1 | Unlikely |
| Gastrointestinal event | |||
| Elevated level of aminotransferase | 1 | 1 | Likely |
| Gastrointestinal pain | 2 | 1–2 | Unlikely |
| Gastroesophageal reflux | 4 | 1–2 | Unlikely |
| Nausea | 2 | 1 | Unlikely |
| Diarrhea | 2 | 1 | Unlikely |
| Hematologic event | |||
| Normocytic anemia | 1 | 2 | Unlikely |
| Contusion | 1 | 1 | Unlikely |
| Infection | |||
| Upper respiratory tract infection | 6 | 1 | Unlikely |
| Sinusitis | 3 | 1–2 | Unlikely |
| Allergic rhinitis | 4 | 1 | Unlikely |
| Musculoskeletal event | |||
| Arthralgia | 1 | 1 | Unlikely |
| Arthritis | 4 | 1–2 | Unlikely |
| Muscle strain | 3 | 1–2 | Unlikely |
| Tendonitis | 1 | 1 | Unlikely |
| Hematuria | 1 | 1 | Unlikely |
The severity of adverse events was determined with the use of World Health Organization toxicity grading criteria. The relation of the event to the study agent was determined by the investigators.
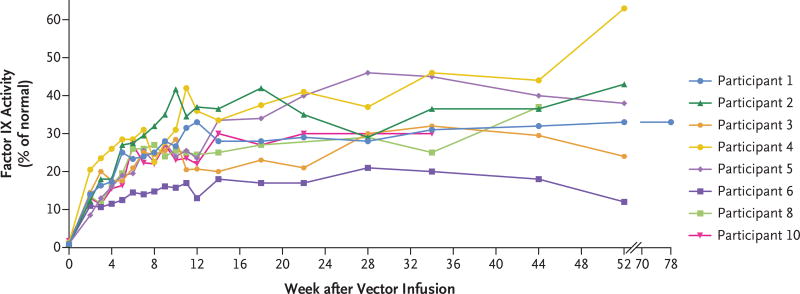
Vector-derived factor IX coagulant activity was observed within 1 week after vector infusion. Steady-state factor IX–R338L expression was reached within 14 weeks after the infusion of vector. Among all the participants, the mean (±SD) vector-derived factor IX coagulant activity was 33.7±18.5% of the normal value (Figs. 1 and 2). Among participants who had a negative status for cross-reactive material, factor IX antigen values were 1.2 to 7.0% of the normal value (Table 1). In nine participants who had an anti–AAV-Spark100 neutralizing antibody titer that was less than 1:1 at baseline, the mean steady-state vector-derived factor IX coagulant activity was 35.5±18.7% of the normal value. Participant 6 had a baseline anti–AAV-Spark100 neutralizing antibody titer of 1:1 and a steady-state factor IX coagulant activity that was 18% of the normal value. Transgene-derived factor IX coagulant activity was also evaluated by means of chromogenic assay (Fig. S3 in the Supplementary Appendix).
Figure 1. Factor IX Activity after One Peripheral Infusion of SPK-9001 in the Eight Participants Who Did Not Have an Adeno-Associated Viral Capsid-Directed Immune Response.
The vector SPK-9001 was administered at a dose of 5×1011 vector genomes per kilogram of body weight.
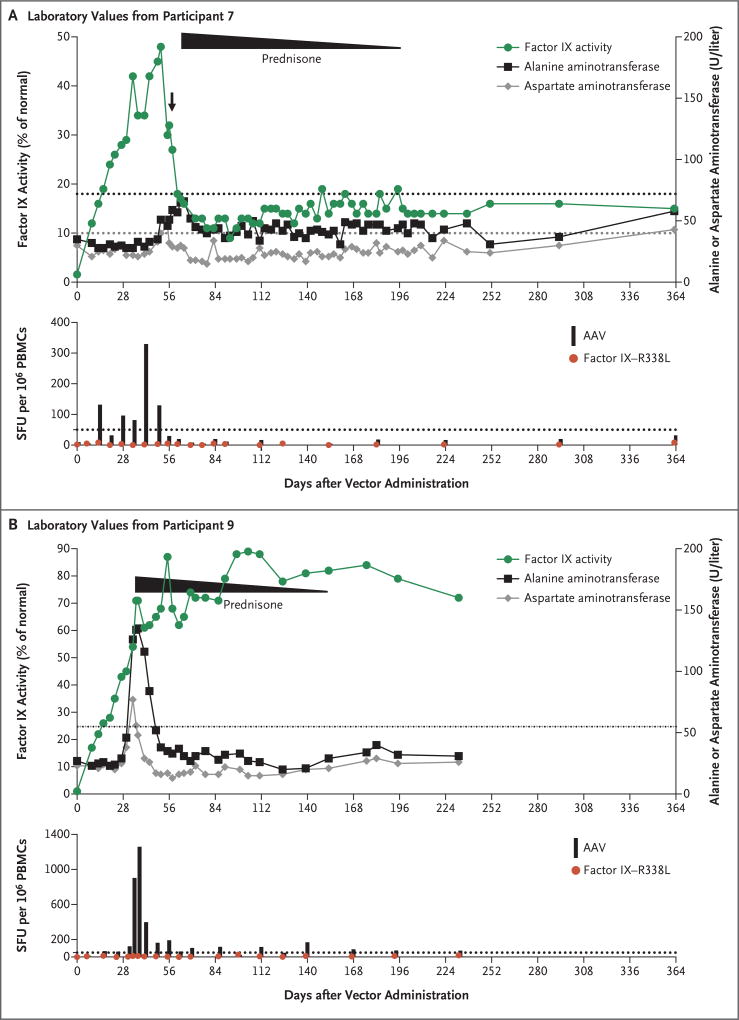
Figure 2. Laboratory Values in Two Participants Who Had an Immune Response to the Adeno-Associated Viral (AAV) Vector.
Evidence of an immune response was determined by monitoring each participant’s factor IX activity and alanine aminotransferase and aspartate aminotransferase levels and by evaluating the results of the interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assay regarding the reaction of the participant’s peripheral-blood mono nuclear cells (PBMCs) to the AAV capsid peptides and the transgene product (factor IX–R338L), as compared with a media control. The dotted lines in the top graph in each panel indicate the upper limit of the normal range for the alanine aminotransferase (black) and aspartate aminotransferase (gray) levels in each participant, according to the local laboratory. The arrow after day 56 in Panel A (showing values for Participant 7) indicates the receipt of a single dose of prednisone. The duration of a full course prednisone treatment (started a few days later) is shown, with the slope indicating tapering. The levels of alanine aminotransferase and aspartate aminotransferase returned to baseline levels in these two participants after prednisone initiation, and transgene expression was maintained in the two participants after prednisone was tapered and stopped. Results of the ELISPOT assay are shown as the number of spot-forming units (SFU) per 1 million PBMCs; values that are more than 50 SFU or that are above the media control (dotted line) by a factor of three are considered to be positive.
Participants 7 and 9 had an asymptomatic, transient increase in the alanine aminotransferase values that was managed with a course of prednisone (Fig. 2, and the Supplementary Results section in the Supplementary Appendix). Concomitant positive interferon-γ ELISPOTs to AAV-Spark100 capsid peptides were observed. Participant 7 had a decline in factor IX coagulant activity from 48% to 30% on day 56 after the vector infusion and received a dose of prednisone (60 mg). On the basis of an apparently stable factor IX coagulant activity of 30%, glucocorticoids were discontinued until the activity again declined at day 62 to 18% and prompted a continuous prednisone course that prevented further loss of transgene expression (Fig. 2). Throughout, the alanine aminotransferase values in this participant remained within normal limits but rose above the baseline value at 52 days after the administration of vector and returned to the baseline value when he received prednisone. Participant 9 had a grade 1 elevation in the alanine aminotransferase level on day 34 after the administration of vector. Glucocorticoids were initiated, and the alanine aminotransferase values returned to baseline within 1 week thereafter. Despite the elevation in aminotransferase levels, the factor IX coagulant activity levels continued to increase and plateaued near 80% of the normal value. These two participants were able to take prednisone without associated adverse events and did not have a decline in factor IX coagulant activity after glucocorticoids were discontinued.
Of the 10 participants, 9 did not have a rise in the aminotransferase values to 1.5 or more times the upper limit of the normal range. Among the 8 participants who were not treated with prednisone, results of the capsid ELISPOT assay were negative or minimally positive, and no loss of factor IX coagulant activity or rise in the alanine aminotransferase level was observed (Fig. S2 in the Supplementary Appendix). In a finding that was consistent with results in previous studies, high-titer antibodies to the AAV capsid developed in all the participants after vector infusion (data not shown).
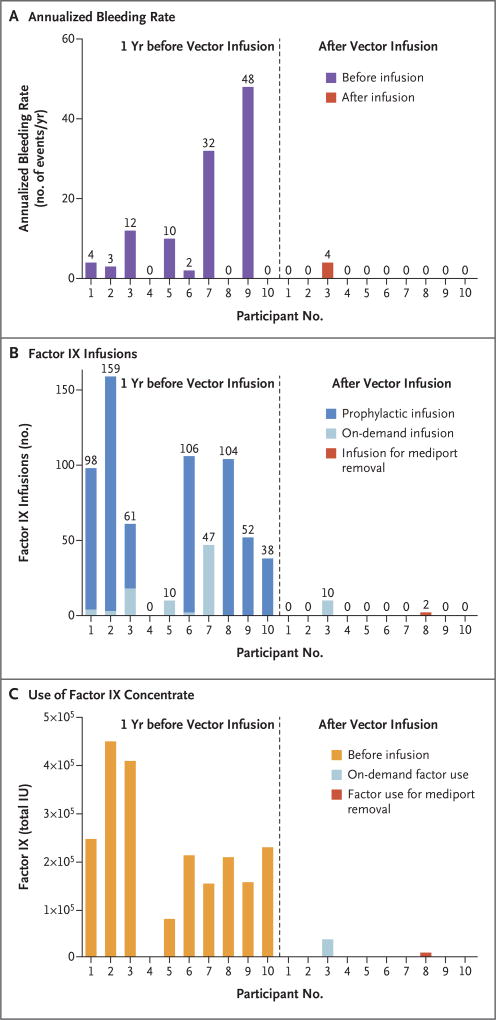
ANNUALIZED BLEEDING RATE AND EXOGENOUS FACTOR IX USE
Among all the participants, the annualized bleeding rate significantly decreased after vector administration (mean rate, 11.1 events per year [range, 0 to 48] before vector administration vs. 0.4 events per year [range, 0 to 4] after administration; P = 0.02; W = 28) (Table 1 and Fig. 3). Participant 3, who had extensive hemophilic arthropathy at baseline, reported four hemarthroses in two joints (one in the left ankle and three in the right knee) in the 52 weeks of study follow- up (Table 1, and the Supplementary Results section and Table S2 in the Supplementary Appendix). Participant 8 received two doses of factor for central catheter (mediport) removal that was deemed to no longer be necessary owing to cessation of factor use (Table 1 and Fig. 3). No other participants had bleeding or used factor.
Figure 3. Comparison of Annualized Bleeding Rate, Number of Exogenous Factor IX Infusions, and Use of Factor IX Concentrate in the 52 Weeks before Screening and after Vector Infusion.
Panel A shows the annualized bleeding rate for each participant, Panel B the number of factor IX infusions, and Panel C the total number of international units (IU) of factor IX concentrate that were infused. The left side of each graph shows the values before vector administration (dashed line), and the right side the values after infusion. Values were normalized for each participant’s follow-up period. Significant reductions in the annualized bleeding rate (P = 0.02, W = 28), in the number of infusions (P = 0.004, W = 45), and in the amount of factor used (P = 0.004, W = 45) were observed in each participant after vector infusion.
The data regarding the reduced annualized bleeding rate are noteworthy given the termination of prophylaxis after vector infusion and the degree of baseline hemophilic arthropathy represented among the participants (mean number of target joints, 1.8±1.9). Concurrent with reduced bleeding, the consumption of factor was significantly reduced (mean value, 2908 IU per kilogram [range, 0 to 8090] before vector administration vs. 49.3 IU per kilogram [range, 0 to 376] after administration; P = 0.004; W = 45), as was the number of infusions (mean value, 67.5 [range, 0 to 159] before vector administration vs. 1.2 [range, 0 to 10] after administration; P = 0.004; W = 45) (Table 1 and Fig. 3). During the cumulative 492-week follow-up interval, a 100% reduction in the use of exogenous factor IX was noted in 8 of 10 participants, and a 91% reduction was noted in the 1 participant who reported bleeding after vector infusion (Table 1 and Fig. 3). Collectively, this amounted to a reduction in the use of factor IX concentrate of 1.95 million IU or an estimated savings of $3.6 million, according to 2016 pricing of factor IX.
DISCUSSION
We found that the use of a high-specific-activity transgene, factor IX–R338L, and codon-optimized expression cassette permitted the safe use of a low dose of vector (5×1011 vg per kilogram) and yielded high levels of sustained vector-derived factor IX coagulant activity and a low incidence of a capsid-directed immune response.7,10,11,20–22 These data support our hypothesis that a highly efficient vector can be administered at low doses to drive therapeutic factor IX expression while minimizing the risk of an AAV capsid-directed immune response.
We found that SPK-9001 was safe, a finding that is consistent with the results in previously published AAV gene-therapy trials.7,23 In addition, inhibitors to nonmutant FIX protein did not develop in any participants, and no marked ELISPOT response to factor IX–R338L was seen — findings that support the conclusions that expression of factor IX–R338L did not result in neoantigen formation and that sustained factor IX–R338L expression was safe over the time course that was examined in participants with either positive or negative results for cross-reacting material.
Furthermore, transgene-derived factor IX–specific activity was consistent with results from preclinical studies.13,24 The long-term safety of AAV gene transfer remains to be determined; however, to date, no genotoxic or gene-silencing events have been noted in human participants, including those who have been followed since the first AAV trials were reported in 1998.25 Transgene DNA is stabilized predominantly episomally. Thus, an advantage of AAV-mediated gene transfer is the reduced risk of insertional mutagenesis. Several features of the vector and the study design minimized the risks of insertional mutagenesis that have been defined in nonclinical studies, including the use of a vector dose that was lower by a factor of 4 to 120 times than doses used in other ongoing trials of gene therapy for hemophilia, the targeting of adult postmitotic hepatocytes, and the use of a liver-specific hAAT promoter.7,11,21,22,26 Although the long-term efficacy is unknown, stable expression after liver-directed AAV-mediated factor IX gene transfer has been reported for 4 years in a clinical trial and for 8 years in canine hemophilia B models.11,27 Continued follow-up of the participants will be necessary to determine long-term safety and durability of expression and to monitor vector shedding as it relates to any possible consequence to contacts of participants.
A major impediment to the advancement of in vivo AAV gene transfer is the lack of animal models that recapitulate the human cellular immune response to the AAV capsid; thus, the study of this phenomenon is limited to clinical investigation.7,28 Two participants were safely and successfully treated with glucocorticoids that were aimed to control an AAV capsid-directed cellular immune response; transgene expression and therapeutic efficacy were maintained in these participants. Our data suggest that the cellular immune response to this AAV vector is controllable with oral glucocorticoids. This favorable response may reflect properties of the vector, the dose, or both. The occurrence of the capsid-directed immune response highlights the need for sensitive immunomonitoring techniques, which we used during the at-risk window. Our data indicate that the combined evaluation of liver-function studies and assays to assess factor IX coagulant activity aid in the timely detection and appropriate management of a capsid-directed immune response and can be completed in any clinical laboratory. ELISPOT assay results provided meaningful data to confirm the cause of elevations in the aminotransferase levels but would be impractical for use in widespread adoption of this therapy.
Despite varied trial courses among the 10 participants, the mean steady-state factor IX coagulant activity was 33.7±18.5% of the normal value, and a therapeutic effect at this dose was observed in all the participants, even those who had preexisting (low titer) neutralizing antibodies or cellular immune responses that occurred after infusion. It is not clear what the basis was for the finding that the factor IX coagulant activity in Participant 9 was approximately 2 times as high as that in the other participants. Although the mechanism of transduction with AAV vectors has been extensively studied, many aspects, including binding to cell-surface receptors, entry by means of endosomal pathways, endosomal escape, uncoating, and entry into the nucleus, are poorly understood.29,30 The range of levels of factor IX coagulant activity that we observed may represent the inherent variability in transgene-derived protein levels with this new class of therapeutic agents. The two participants who had a capsid-directed immune response also had the most rapid initial rise in factor IX coagulant activity. More extensive experience will be required in order to determine whether these events occurred by chance or represent a useful predictor of immune response. It is also unclear whether the immune response somehow facilitated vector expression. In addition, the plateau in the factor IX coagulant activity in Participant 6, which was lower than that in the other participants, is consistent with an expected reduction in hepatocyte transduction in the presence of the 1:1 titer for neutralizing antibody (anticipated to neutralize 50% of vector) to AAV (Table 1); this finding supports the hypothesis that our AAV neutralizing antibody assay is sensitive. A current limitation of in vivo AAV gene transfer is the existence of AAV neutralizing antibodies in approximately 30 to 40% of the population, who would not be eligible for this therapy as it is currently formulated.31 A sensitive AAV neutralizing antibody assay is essential for the identification of patients with a negative titer and those with low titers, who may still benefit from this therapy but who would be predicted to have a transgene expression level that is lower than the level in patients with a negative titer, given the current vector formulation.32
We found consistent clinical outcomes despite the baseline heterogeneity of the participants, including the extent of hemophilic arthropathy, age, and coexisting conditions, such as previous HCV infection, which affects more than 90% of men with severe hemophilia B who are older than 40 years of age.33 The long-term outcomes regarding sustained, stable factor IX coagulant activity on joint health and quality of life are not yet known. Recent preclinical work has shown that factor IX products with an extended half-life improved synovial and osteochondral healing after hemarthrosis in mice with hemophilia B, whereas short-term factor IX replacement for hemostasis did not prevent arthropathy.34 This information suggests that sustained factor IX coagulant activity, such as the results we observed, may function to prevent acute hemarthrosis and to minimize the long-term sequelae of joint disease. Finally, even in a small cohort with limited follow-up, the reduction in the use of factor IX concentrate represented a marked cost savings.
In conclusion, we observed that a one-time intravenous infusion of SPK-9001 safely resulted in a sustained level of factor IX coagulant activity of approximately 30% that consistently permitted the termination of prophylaxis, prevented bleeding, and nearly completely eliminated the need for exogenous factor in 10 men with hemophilia B. This early success requires confirmation in a larger cohort and long-term monitoring of safety and efficacy.
Supplementary Material
Acknowledgments
Supported by Spark Therapeutics and Pfizer.
We thank Dr. Rodney Camire for review of an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Lindsey A. George, M.D., Spencer K. Sullivan, M.D., Adam Giermasz, M.D., Ph.D., John E.J. Rasko, M.B., B.S., Ph.D., Benjamin J. Samelson-Jones, M.D., Ph.D., Jonathan Ducore, M.D., M.P.H., Adam Cuker, M.D., Lisa M. Sullivan, M.D., Suvankar Majumdar, M.D., Jerome Teitel, M.D., Catherine E. McGuinn, M.D., Margaret V. Ragni, M.D., M.P.H., Alvin Y. Luk, Ph.D., Daniel Hui, Ph.D., J. Fraser Wright, Ph.D., Yifeng Chen, M.D., Yun Liu, Ph.D., Katie Wachtel, M.S., Angela Winters, M.P.H., Stefan Tiefenbacher, Ph.D., Valder R. Arruda, M.D., Ph.D., Johannes C.M. van der Loo, Ph.D., Olga Zelenaia, Ph.D., Daniel Takefman, Ph.D., Marcus E. Carr, M.D., Ph.D., Linda B. Couto, Ph.D., Xavier M. Anguela, Ph.D., and Katherine A. High, M.D.
The authors’ affiliations are as follows: the Division of Hematology (L.A.G., B.J.S.-J., A.W., V.R.A.) and the Raymond G. Perelman Center for Cellular and Molecular Therapeutics (L.A.G., B.J.S.-J., A.W., V.R.A., J.C.M.L., O.Z.), Children’s Hospital of Philadelphia, the Departments of Pediatrics (L.A.G., B.J.S.-J., V.R.A.) and Medicine (A.C.), Perelman School of Medicine at the University of Pennsylvania, and Spark Therapeutics (A.Y.L., D.H., J.F.W., Y.C., Y.L., K.W., D.T., M.E.C., L.B.C., X.M.A., K.A.H.) — all in Philadelphia; the Department of Pediatrics, Mississippi Center for Advanced Medicine, Madison (S.K.S.), and the Departments of Pathology (L.M.S.) and Pediatrics (S.M.), University of Mississippi Medical School, Jackson; the Departments of Medicine (A.G.) and Pediatrics (J.D.), University of California–Davis Medical School, Sacramento; the Department of Medicine, Sydney Medical School, and the Gene and Stem Cell Therapy Program, Centenary Institute (J.E.J.R.), University of Sydney, and Cell and Molecular Therapies, Royal Prince Alfred Hospital (J.E.J.R.) — both in Camperdown, NSW, Australia; the Department of Medicine, University of Toronto Faculty of Medicine and St. Michael’s Hospital, Toronto (J.T.); the Department of Pediatrics, Weill Cornell Medical College, New York (C.E.M.); the Department of Medicine, University of Pittsburgh, Pittsburgh (M.V.R.); and Colorado Coagulation, Laboratory Corporation of America Holdings, Englewood, CO (S.T.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Mannucci PM, Tuddenham EGD. The hemophilias — from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 3.Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17:849–53. doi: 10.1111/j.1365-2516.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 4.Roth DA, Tawa NE, Jr, O’Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–42. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- 5.Powell JS, Ragni MV, White GC, II, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–45. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- 6.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–61. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 7.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 8.George LA, Fogarty PF. Gene therapy for hemophilia: past, present and future. Semin Hematol. 2016;53:46–54. doi: 10.1053/j.seminhematol.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 9.High KA, Anguela XM. Adeno-associated viral vectors for the treatment of hemophilia. Hum Mol Genet. 2016;25:R36–R41. doi: 10.1093/hmg/ddv475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monahan PE, Walsh CE, Powell JS, et al. Update on phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy for program for hemophilia B. J Thromb Haemost. 2015;13(Suppl 2):87. abstract. [Google Scholar]
- 11.Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua) N Engl J Med. 2009;361:1671–5. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 13.Crudele JM, Finn JD, Siner JI, et al. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–61. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuettrumpf J, Zou J, Zhang Y, et al. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol Ther. 2006;13:88–97. doi: 10.1016/j.ymthe.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Meliani A, Leborgne C, Triffault S, Jeanson-Leh L, Veron P, Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum Gene Ther Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anguela X, Toso R, Couto L, et al. Safety and efficacy of a novel AAV vector for treatment of hemophilia B. J Thromb Haemost. 2015;13(Suppl 2):324–5. abstract. [Google Scholar]
- 17.Wright JF, Le T, Prado J, et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther. 2005;12:171–8. doi: 10.1016/j.ymthe.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Sommer JM, Smith PH, Parthasarathy S, et al. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol Ther. 2003;7:122–8. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Altman DG. Practical statistics for medical research. London: Chapman and Hall; 2001. Comparing two groups of continuous data; pp. 189–205. [Google Scholar]
- 20.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasi J, Wong W, Rangarajan S, et al. Interim results of an open-label, phase 1/2 study of BMN 270, an AAV5-FVIII gene transfer in severe hemophilia A. Haemophilia. 2016;22(Suppl 4):151. abstract. [Google Scholar]
- 22.Leebeek FW, Tangelder M, Meijer K, et al. Interim results from a dose escalating study of AMT-060 (AAV5-hFIX) gene transfer in adult patients with severe hemophilia B. Blood. 2016;128 abstract. [Google Scholar]
- 23.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–60. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 24.Cheung WF, van den Born J, Kühn K, Kjellén L, Hudson BG, Stafford DW. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci U S A. 1996;93:11068–73. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–3. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 26.Chandler RJ, LaFave MC, Varshney GK, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125:870–80. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols T, Whitford MH, Arruda VR, Stedman HH, Kay MA, High KA. Translational data from AAV-mediated gene therapy of hemophilia B in dogs. Hum Gene Ther Clin Dev. 2014;26:5–14. doi: 10.1089/humc.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–22. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 29.Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74:992–6. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–45. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. 2016;127:3073–81. doi: 10.1182/blood-2015-10-675140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Hua B, Livingston EW, et al. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood. 2017;129:2161–71. doi: 10.1182/blood-2016-08-734053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.