Abstract
Objective
Exercise training has been shown to have beneficial effects on liver function in adults overweight or with fatty liver disease. To establish which exercise programme characteristics were likely to elicit optimal improvements.
Design
Systematic review and meta-analysis of randomised, controlled trials.
Data sources
PubMed, CINAHL and Cochrane controlled trials registry searched (1966 to 2 October 2015).
Eligibility criteria for selecting studies
Exercise intervention, with or without dietary intervention, versus usual care in adults undertaking, exercise training, who were overweight, obese or exhibited fatty liver disease (non-alcoholic fatty liver disease or non-alcoholic steatohepatitis).
Results
We included 21 randomised controlled trials, totalling 1530 participants. Exercise intervention studies with total exercise programme workload >10 000 kcal produced significant improvements in intrahepatic fat, −3.46% (95% CI −5.20% to −1.73%), p<0.0001, I2=73%; effect size (standardised mean difference, SMD) −1.77 (−3.11 to −0.42), p=0.01, I2=77%. When data from only exercise studies were pooled, there was a reduction in fasting free fatty acids (FFAs) −74.15 µmol/L (95% CI −118.47 to −29.84), p=0.001, I2=67% with a large effect size (SMD) −0.94 (−1.36 to −0.52), p<0.0001, I2=0%. When data from only exercise studies were pooled, there was a significant reduction in insulin MD −1.88 UL (95% CI −3.43 to −0.34), p=0.02, I2=31%. The liver enzymes, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transpeptidase, were not significantly altered with exercise.
Conclusions
Exercise training reduces intrahepatic fat and FFAs while increasing cardiorespiratory fitness. An aggregate exercise programme energy expenditure (>10 000 kcal) may be required to promote reductions in intrahepatic fat.
Keywords: Evidence based review, Liver, Meta-analysis, Prevention
Introduction
Central obesity is linked to raised serum liver enzymes and future risk of developing insulin resistance.1 Fatty liver disease (FLD) and non-alcoholic fatty liver disease (NAFLD) are also associated with increased serum levels of liver enzymes.2 FLD may be diagnosed through liver function tests and imaging, such as MRI or ultrasound of the liver, to detect excess intrahepatic fat. Exercise can greatly assist in reducing obesity and FLD, mainly by improving weight loss through dietary changes and exercise and reducing alcohol consumption.3
Obesity and FLD are risk factors for diabetes and cardiovascular disease. The prevalence of FLD varies greatly from a 10% to 35% prevalence rate in the USA with ∼2–5% of patients with non-alcoholic steatohepatitis (NASH),4 the more severe form of NAFLD.
A 2012 systematic review by Thoma et al5 examined the effects of diet and exercise on liver function, but although data pooling was not performed, the authors concluded that dietary-induced weight loss may offer greater liver health benefits than exercise. A 2012 systematic review, of randomised and non-randomised controlled studies in obese and FLD populations, by Keating et al examined intrahepatic liver fat content and alanine aminotransferase (ALT). The pooled analysis reported that the former, but not the latter, was reduced with exercise training of 4–26 weeks duration.6 A number of new studies, including two studies with >100 participants,7 8 have been published after Keating et al's analysis. Moreover, Keating et al pooled data from different modalities of exercise (aerobic and resistance) and did not adjust for the great variation in exercise programme durations (1–12 months), frequency (2–7 sessions weekly) and intensity. There are now sufficient randomised controlled trials available to conduct analyses of several additional outcome measures and to adjust for variation in exercise programme characteristics to identify those that are most beneficial in patients with obesity and FLD. Furthermore, liver enzymes may not be sensitive enough to be the only measure of liver function or liver fat,9 so new studies mean other markers can be examined.
The primary aim of this work was to conduct a systematic review and meta-analyses to establish the effect of exercise training on surrogate markers of liver function in adults who were overweight or exhibited FLD. The secondary aim was to establish if there is an exercise programme volume more likely to elicit optimal improvements in related health outcome measures.
Materials and methods
Search strategy
Potential studies were identified by conducting a systematic search using PubMed, http://www.ncbi.nlm.nih.gov/pubmed (1966 to 2 October 2015); the PubMed search strategy can be seen in supplementary files. CINAHL and the Cochrane controlled trials registry were also searched (1966 to 2 October 2015). The search strategy included the key concepts of overweight, obese, FLD, NAFLD, NASH, lifestyle therapy, physical training and exercise training. These were combined with a sensitive search strategy to identify randomised controlled trials (see online supplementary figure S1). Titles and abstracts were screened by NAS and GD, and full-text articles were downloaded for those meeting inclusion criteria. Reference lists of papers found were also scrutinised for new references. All identified papers were assessed independently by two reviewers (GD and NAS); a third reviewer (JRM) was consulted to resolve disputes. Searches of published papers were also conducted up until 2 October 2015.
bjsports-2016-096197supp001.pdf (31.3KB, pdf)
Inclusions
Randomised controlled trials of progressive aerobic, resistance or combined exercise training in adults (>18 years) who were overweight or obese or exhibited FLD (NAFLD or NASH) were included. The definition of obese/overweight was made by the individual studies, but this was verified by checking baseline data. Combined exercise training was defined as interventions that used both aerobic and resistance training simultaneously. We included studies comparing two types of exercise (eg, aerobic vs resistance) or exercise versus usual care. Included studies employed three sessions or more of exercise, in order to differentiate from acute exercise effects. Studies involving dietary control or intervention groups were included only if the diet was the same between exercise and control groups allowing the independent effects of exercise to be examined. There were no language restrictions.
Exclusions
Animal studies, review papers and non-randomised controlled trials were excluded. Studies with unmatched intervention versus control group participants were excluded. We also excluded studies of healthy, normal body mass participants as liver function was likely to be normal. Authors were contacted to provide missing data or to clarify if data were duplicated in multiple publications. Incomplete data, or data from an already included study, were excluded. Studies using interventions other than exercise (eg, electroacupuncture, ultrasound) were excluded.
Data extraction
Data on outcome measures were archived in a database; data were extracted by NAS and verified by GD; JRM was consulted if discrepancies occurred. The outcome measures were: change in intrahepatic fat (percentage change), ALT, aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), free fatty acids (FFAs), body mass and body mass index (BMI), insulin, total cholesterol and peak VO2 (table 1). We calculated energy expended by establishing oxygen consumption at the training intensity and using 5 kcal/L of oxygen consumed, thus establishing kcals expended per minute. We then multiplied kcals/min by session duration (min) and then multiplied this by total number of sessions to calculate aggregate energy expenditure for each exercise programme, this is an approach we have used previously.10 11 12 We also conducted analysis of effects on interhepatic fat (IHF) only in exercise programmes that expended an aggregate of >10 000 kcal.
Table 1.
List of outcome measures and other data extracted from included studies
| Data | Type | Units |
|---|---|---|
| Interhepatic fat | Outcome measure | Per cent of interhepatic fat |
| Body mass | Outcome measure | Mass in kg |
| Body mass index | Outcome measure | kg/m2 |
| Free fatty acids | Outcome measure | µmol/L |
| γ-Glutamyl transpeptidase | Outcome measure | Serum IU/L |
| Alanine aminotransferase | Outcome measure | Serum IU/L |
| Aspartate aminotransferase | Outcome measure | Serum IU/L |
| Peak VO2 | Outcome measure | mL O2/kg/min |
| Insulin | Outcome measure | Serum IU/L |
| Total cholesterol | Outcome measure | Serum mg/dL |
| Session duration | Covariate | Minutes |
| Programme duration (weeks) | Covariate | Weeks |
| Session frequency | Covariate | Sessions per week |
| Exercise mode | Covariate | Type of activity |
| Exercise intensity | Covariate | Per cent of maximum heart rate |
| Year | Year of publication | Year |
Data synthesis
The meta package in R V.3.2.1 was used to complete the meta-analysis and generate forest plots (Schwarzer G. Meta: General Package for Meta-Analysis, in R package. 2015) (Team RC. A language and environment for statistical computing. R Foundation for Statistical Computing. 2015: Vienna, Austria). Pooled data are presented as mean differences (MDs) and as the effect size Hedges' g, that is, standardised mean differences (SMDs) with 95% CI for intervals with a significant MD. We chose a random-effects model as we anticipated considerable heterogeneity. A minimum of three studies was required for meta-analysis to be undertaken. Multivariate meta-analysis (using the metafor package)13 with a random intercept for study was used for studies that compared more than one intervention group with a control.
Meta-analyses were completed for continuous data by using the change in the mean and SD of outcome measures. It is an accepted practice to only use postintervention data for meta-analysis, but this method assumes that random allocation of participants always creates intervention groups matched at baseline for age, disease severity, etc. Change in postintervention mean was calculated by subtracting baseline from postintervention values. Data required were either (1) 95% CI data for pre–post intervention change for each group or when this was unavailable; (2) actual p values for pre–post intervention change for each group or if only the level of statistical significance was available; (3) we used default p values, for example, p<0.05 becomes p=0.049, p<0.01 becomes p=0.0099 and p=not significant becomes p=0.05.
Effect sizes are interpreted in the usual way14 with an effect size of 0.2 defined as small, 0.5 defined as moderate and 0.8 or higher defined as large.
Analyses
Where sufficient studies were included, meta-analysis was performed for the following groups (1) aerobic versus resistance training studies, (2) exercise only versus control studies and (3) exercise plus diet versus diet control studies.
Several subanalyses were conducted as follows:
In order to avoid considerable heterogeneity,15 we only presented forest plots when I2 heterogeneity was <75%. In the exercise-only interventions, we conducted meta-analyses for:
FFAs, GGT, BMI, insulin and total cholesterol.
Sensitivity analysis
Heterogeneity
Heterogeneity was quantified using the I2 test,16 as it does not inherently depend on the number of studies considered. The I2 statistic and corresponding 95% CI were presented, to gauge the degree of heterogeneity present in sample.16 17 I2 values range from 0% (homogeneity) to 100% (greater heterogeneity); a CI that does not include 0% indicates that the hypothesis of homogeneity is rejected, and an inference of heterogeneity is merited.16
Meta-analysis and forest plots are presented only for those studies in which the heterogeneity (as measured by I2) was <75%. Heterogeneity beyond this level was deemed too heterogeneous15 to be sensibly pooled, and while interest was in trying to identify factors responsible for the heterogeneity, none of the subsets contained the recommended minimum number of studies (10) to allow this.
Publication bias
Egger bias tests (when at least 10 studies were included) and funnel plots18 were provided to assess the risk of publication bias (see online supplementary files).
Risk of bias assessment
Study quality was assessed by using the TESTEX scale19—the Tool for the assEssment of Study qualiTy and reporting in EXercise—a study quality and reporting assessment tool, designed specifically for use in exercise training studies. The main point of difference in TESTEX is that there are accommodations for: activity monitoring in control groups to measure crossover to exercise by sedentary control patients; assessment of the existence and method of activity monitoring in exercise intervention and sedentary controls; assessment of whether the relative exercise intensity remained constant and therefore potentially avoided de-training as participants initially adapt to new exercise programmes; assessment of whether periodic evidence-based adjustment of exercise intensity is reported exercise volume and exercise expenditure. Information on all exercise characteristics (intensity, duration, frequency and mode) is provided to calculate exercise volume and exercise energy expenditure.
This tool is a 15-point scale (5 points for study quality and 10 points for reporting) and addresses previously unmentioned quality assessment criteria specific to exercise training studies. Two reviewers (NAS and GD) conducted the risk of bias assessment; JRM was consulted if discrepancies occurred.
Results
Studies included in the review
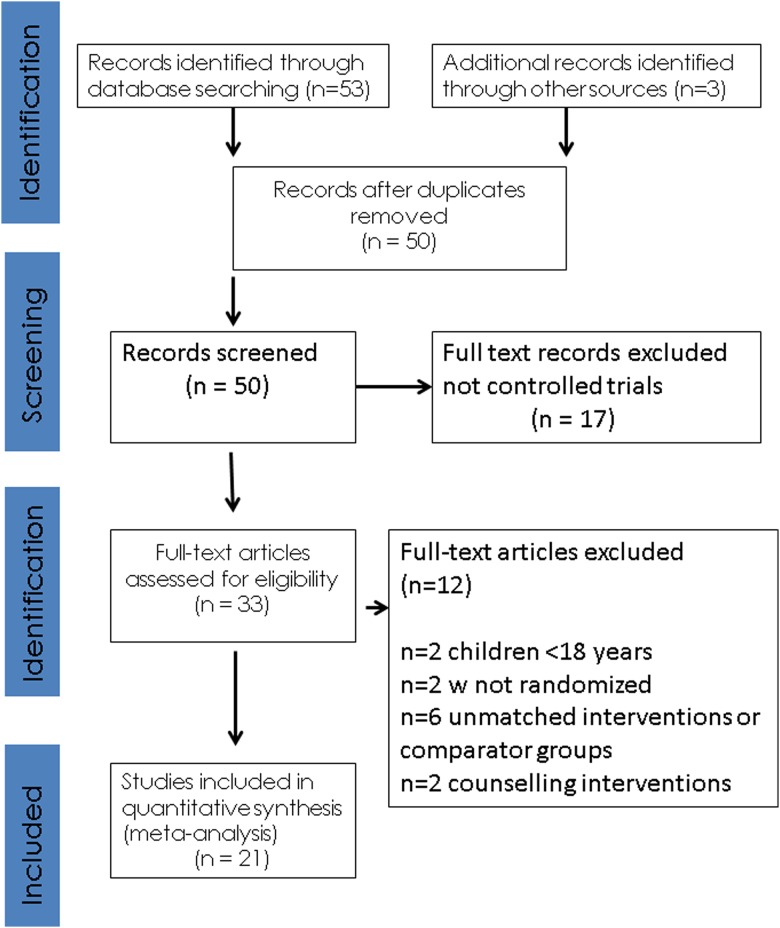
Our initial search identified 53 manuscripts, hand searching of reference lists of included studies and key articles such as related reviews and the latest editions of relevant journals yielded a further 3 manuscripts. Out of 56 studies, 6 were excluded at first inspection as duplicates, 17 were not controlled trials of exercise therapy, 2 were excluded as they had participants <18 years, 2 were excluded as they were not randomised trials, 6 used unmatched interventions or comparator groups and 2 were counselling interventions encouraging exercise participation, leaving 21 included studies for analysis (figure 1).
Figure 1.
PRISMA statement.
Description of included studies
Our 21 included randomised controlled trials (25 intervention groups) had an aggregate of 1530 participants, 884 exercise participants and 646 controls. Table 2 summarises included studies.7 8 20–38 Online supplementary table S1 in the supplementary files summarises the excluded controlled trials.39–50
Table 2.
Included studies—exercise programming details
| Study | Study duration, frequency and session times | Intervention | Exercise intensity | Total exercise programme energy expenditure (kcal) | Number of participants exercise (Con) | Method of IHF assessment | Base ALT (IU/L) mean (SD) | Change in body mass (kg) |
|---|---|---|---|---|---|---|---|---|
| Exercise vs Con or exercise vs exercise | ||||||||
| Al-Jiffri et al38 | 12 weeks 3 times/week 30 min |
A vs Con | A: 65–75% maximum HR | NR | A: 50 C: 50 |
Liver biopsy | A: 74.6 (9.3) C: 73.3 (8.9) |
NR |
| Bacchi et al20 | 16 weeks 3 times/week 60 min |
A vs R | A: 60–65% HRR R: 70–80% IRM |
NR | A: 13 R: 17 |
MRI | A: 24.7 (11.6) R: 32.4 (25.6) |
NR |
| Balducci 2014 | 52 weeks 2 times/week NR |
C vs Con | 55–75% peak VO2 60% 1RM |
NR | C: 303(303) | NR | C: 31.8 (16.9) Con: 29.7 (14.8) |
NR |
| Bonekamp et al21 | 26 weeks 3 times/week 45 min |
C vs Con | A: moderate R: 50% 1RM |
13 650 | 28 (17) | MRS | NR | NR |
| Hallsworth et al24 | 8 weeks 3 times/week 45–60 min |
R vs Con | 50–70% 1RM | NR | 11 (8) | MRI | R: 59.6 (38.6) Con: 61.6 (41.4) |
R: 0.0 Con: +0.6 |
| Johnson et al25 | 4 weeks 3 times/week 30–45 min |
A vs Con | 50–70% peak VO2 | 3800 | 12 (7) | MRI | R: 35.7 (19.5) Con: 37.7 (11.3) |
A: −0.3 Con: −0.2 |
| Keating et al26 | 8 weeks LO:HI: 3 days/week 60 min, 180–240 min/week HI:LO and LO:LO: 3 days/week 45 min, 90–135 min/week |
LO:HI, HI:LO and LO:LO vs Con | LO:HI 50% peak VO2 HI:LO 70% peak VO2 LO:LO 50% peak VO2 |
LO:HI: 11.474 HI:LO: 8443 LO:LO: 4838 |
LO:HI: 12 HI:LO: 12 LO:LO: 12 Con: 12 |
MRS | LO:HI: 21.8 (3.6) HI:LO: 24.3 (3.0) LO:LO: 21.8 (3.6) Con: 32.3 (6.8) |
LO:HI: −1.4 HI:LO: −1.3 LO:LO: +0.1 Con: +0.8 |
| Levinger et al28 | 10 weeks 3 times/week 2–6 sets, 12–15 rep |
R vs Con | 75–85% 1RM | NR | HiMF: 15 (15) LoMF: 12 (13) |
NA | NR | NR |
| Shojaee-Moradie et al30 | 6 weeks 3 times/week 20 min |
A vs Con | 60–85% peak VO2 | 3586 | 10 (7) | MRS | NR | A: +0.2 Con: −0.8 |
| Slentz et al7 | 34 weeks 3 times/week 19.2 km/week |
1. A vs R 2. A vs C |
A: 75% peak VO2 R: 70% 1RM |
NA | A: 48 R: 52 C: 44 |
CT | A: 31.7 (17.7) R: 29.3 (13.7) C: 31.5 (13.9) |
A: −2.0 R: +0.7 C: −2.1 |
| Sullivan et al33 | 16 weeks 5 times/week 30–60 min |
A vs Con | 45–55% peak VO2 | 21 325 | 12 (6) | MRS | A: 45.6 (29.8) Con: 33.7 (14.7) |
A: −0.2 Con: +0.2 |
| Thompson et al35 | 24 weeks 4 times/week 30–60 min |
A vs Con | 50–70% peak VO2 | NR | 20 (21) | NA | A: 30.2 (16.2) Con: 30.4 (12.9) |
A: −1.6 Con: +0.2 |
| Zelber-Sagi et al37 | 12 weeks 3 times/week 40 min |
R vs Con | NR | NR | 33 (31) | Ultrasound | R: 53 (36.6) Con: 50 (37.2) |
R: −0.39 Con: +0.33 |
| Exercise and diet vs diet | ||||||||
| Al-Jiffri et al38 | 12 weeks 3 times/week 30 min |
A vs Con | 65–75% MHR | NR | 50 (50) | NR | A: 46.9 (5.4) C: 47.2 (6.1) |
NR |
| Bozetto et al22 | 8 weeks 2 times/week 45 min |
Diet 1 and A vs diet 1 Diet 2 and A vs diet 2 |
70% peak VO2 | 3320 | Diet 1 and A: 10 (9) Diet 2 and A: 9 (8) |
MRS | Diet 1 and A: 263 (22) Diet 1: 22 (8) Diet 2 and A: 28 (8) Diet 2: 34 (34) |
Diet 1 and A: 0 Diet 1: 0 Diet 2 and A: 0 Diet 2: 0 |
| Goodpaster et al23 | 52 weeks A or 26 weeks Con 5 times/week 60 min |
Diet and A vs diet | Diet and A: 12 m moderate Diet: 6 m moderate |
NR | Diet and A: 67 (63) | CT | Diet and A: 30.58 (NR) Diet: 30.3 (NR) |
Diet and A: −10.9 Diet: −8.16 |
| Larson-Meyer et al27 | 26 weeks 5 times/week Individualised |
Diet and A vs diet | NR | Individualised | Diet and A: 11 (12) | MRS | Diet and A: 19.6 (9.7) Diet: 23.8 (13.8) |
Diet and A: −8.2 Diet: −8.1 |
| Promrat et al31 | 10 000 steps/day | Diet and A vs diet | Moderate | NA | Diet and A: 21 (10) | NR | Diet and A: 85.6 (38.8) Diet: 85.5 (36.5) |
Diet and A: −8.7 Diet: −0.5 |
| Shah et al29 | 26 weeks 3 times/week 90 min |
Diet and C vs diet | 70–85% MHR or 65–80% 1RM | 23 400 | Diet and C: 9 (9) | MRS | Diet and C: 28.4 (3.8) Diet: 32.6 (4.1) |
Diet and C: −8.3 Diet: −9.2 |
| Straznicky et al32 | 12 weeks 3.5 times/week 40 min |
Diet and A vs diet | 65% MHR | 15 294 | Diet and A: 19 (20) | NA | Diet and A: 26 (NR) Diet: 24 (NR) |
Diet and A: −8.7 Diet: −7.1 |
| Tamura et al34 | 2 weeks 5–6 times/week 2–3×30 min |
Diet and A vs diet | 50–60% peak VO2 | NR | Diet and A: 7 (7) | MRS | Diet and A: 27.9 (8.5) Diet: 31.3 (6.6) |
NR |
| Yoshimura et al36 | 12 weeks 3 times/week 60 min plus 120 min/week walking ≥300 min/week |
Diet and A vs diet | Moderate | 3067 | Diet and A: 15 (18) | CT | NR | Diet and A: −5 Diet: −5.4 |
1RM, 1 repetition maximum; A, aerobic exercise; ALT, alanine aminotransferase; BMI, body mass index; C, combined aerobic plus resistance exercise; Con, control; HI:LO, high intensity:low volume aerobic exercise; HiMF, high metabolic risk factors; HRR, heart rate reserve; IHF, interhepatic fat; LO:HI, low intensity:high volume aerobic exercise; LO:LO, low intensity:low volume aerobic exercise; LoMF, low metabolic risk factors; MHR, maximum heart rate; MRS, magnetic resonance spectroscopy; NA, not applicable; NR, not reported; R, resistance exercise.
Thirteen studies used only an exercise intervention and eight studies used both exercise and dietary interventions. Five studies used resistance exercise and 15 used aerobic exercise, and 4 studies also used combined aerobic and resistance exercise. Study duration ranged from 4 to 52 weeks, and training frequency ranged from 2 to 5 times weekly with 20–60 min session duration. Aerobic training intensity ranged from 45% to 85% of peak VO2. Control groups were classified as sedentary, although some used a stretching routine.
Measurement of study outcomes
Of note is that only one of the included studies38 used the gold standard liver biopsy method to assess liver function. Instead of direct assessment of liver function, several studies used plasma concentrations of liver enzyme as surrogate markers of liver function. Moreover, a number of different techniques were used to quantify liver enzymes.
Study quality assessment
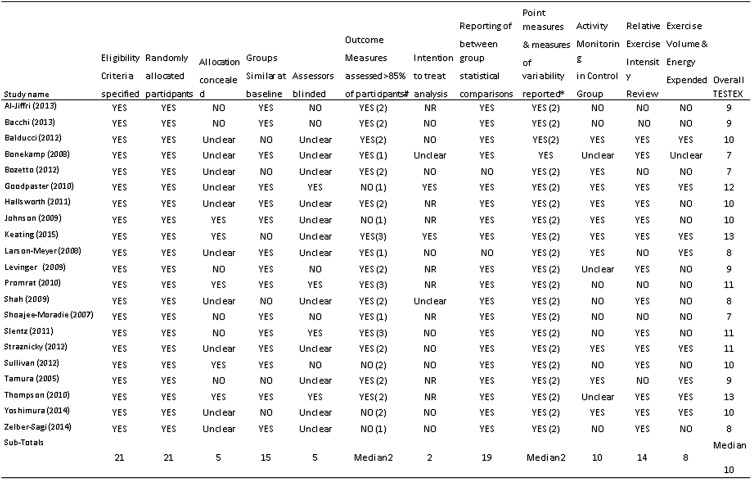
Median TESTEX score was assessed as 10 out of 15 by two reviewers (NAS and GD); three studies each scored 7, 8 or 11. Four studies scored 9, five studies scored 10, one study scored 12 and two studies scored 13 (figure 2). Of the TESTEX items, the following were done particularly poorly in general: allocation concealment only 5/21 studies; blinding of assessors 5/21; physical activity monitoring in the control groups to check with sedentary controls crossed over to exercise 10/21; assessment of energy expended during exercise 10/21.
Figure 2.
Study quality assessment. Key: total out of 15 points. Legend: #three points possible—one point if adherence >85%, one point if adverse events reported, one point if exercise attendance is reported. *Two points possible—one point if primary outcome is reported, one point if all other outcomes reported. TESTEX, Tool for the assEssment of Study qualiTy and reporting in Exercise.
Summary of change in outcome measures
Only two included studies compared aerobic versus resistance training, as such this training group is not considered further.
Change in intrahepatic fat
Data on IHF were available from nine studies (12 intervention groups) of exercise, with or without dietary intervention. Six study groups showed significant IHF reductions; one study showed a significant increase and five studies showed no change.
Exercise-only studies had high heterogeneity (I2 84.4%, 95% CI 70.1% to 91.4%) and there were too few studies (6) to allow for investigation of the cause of the heterogeneity beyond the following subgroup analysis.
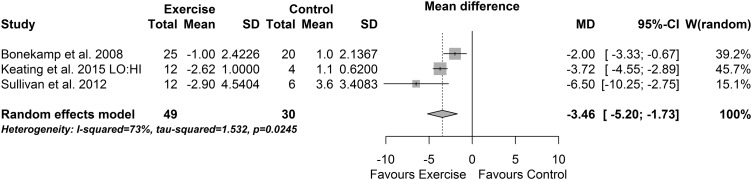
When data from the exercise-only versus control programmes that expended an aggregate of >10 000 kcal were pooled, there was a reduction in IHF (−3.46% (95% CI −5.20% to −1.73%), p<0.0001, I2=73% (95% CI 9% to 92%), figure 3). The effect size was large (SMD −1.77 (−3.11 to −0.42), p=0.01, I2=77%).
Figure 3.
Percentage change in intrahepatic fat: exercise programmes >10 000 kcal energy expenditure.
Diet plus exercise studies had no evidence of heterogeneity (I2 0%, 95% CI 0% to 84.2%) and indicated no evidence of a change in IHF between diet and exercise versus diet alone studies (MD 0.87% (95% CI −0.07% to 1.81%), p=0.07 (from a multivariate model)). Effect size (SMD) was small to moderate (0.29, 95% CI (−0.19 to 0.78), p=0.24).
Change in body mass and BMI
Fourteen exercise studies with or without dietary intervention (17 intervention groups) reported change in body mass. Nine groups reported a significant fall in body mass, one group reported a significant rise and seven groups were equivocal.
The exercise-only body mass studies (n=7) exhibited substantial heterogeneity (I2 95.1%, 95% CI 92.6% to 96.8%) even in the multivariate analysis. The small number of studies precluded investigation of the heterogeneity. The diet and exercise studies showed less heterogeneity (I2 71.3%, 95% CI 40.8% to 86.1%) but no evidence of a change in body mass in the multivariate meta-analysis model (MD −2.94 kg, 95% CI (−5.93 to 0.04)); effect size (SMD) was moderate (−0.34, 95% CI (−0.55 to −0.13), p=0.001).
Eleven exercise study groups, with (n=5) or without (n=6) dietary intervention, reported change in BMI, with only four groups reporting a significant improvement, while the other seven groups showed no change.
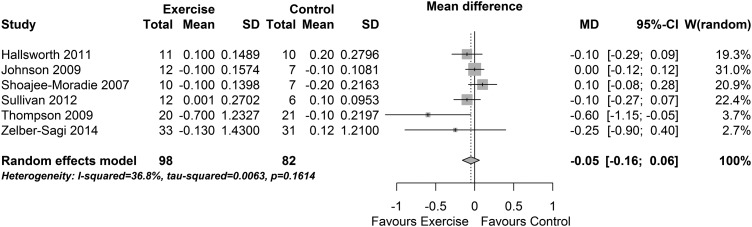
Meta-analysis of the five diet and exercise BMI studies indicated substantial heterogeneity (I2 83% (95% CI 62% to 93%). The small number of studies precluded investigation of the heterogeneity. Meta-analysis of the effect of exercise-only intervention studies on BMI found a no evidence of a change (MD −0.05 kg/m2 (95% CI −0.16 to 0.06), p=0.38, I2=37% (95% CI 0% to 75%), see figure 4). The effect size was small to moderate (SMD −0.26 (−0.56 to 0.04), p=0.09, I2=0%) and also indicated no change in BMI.
Figure 4.
Change in body mass index: exercise interventions.
Fasting FFAs
When data from all nine exercise studies with or without dietary intervention (11 intervention groups) were examined, only 5 groups reporting a significant improvement, while the other 6 groups showed no change.
When data from only exercise studies were pooled using a multivariate meta-analysis model, there was a reduction in fasting FFA (−74.15 µmol/L (95% CI −118.47 to −29.84), p=0.001, I2=67% (95% CI 32% to 85%), figure 5). The effect size (SMD) indicated a large change in FFA (−0.94 (−1.36 to −0.52), p<0.0001, I2=0%). The three pooled diet and exercise studies exhibited substantial heterogeneity (I2 88%, 95% CI (65% to 96%)) and were not considered further.
Figure 5.
Change in fasting free fatty acids: exercise interventions.
Liver enzymes
Alanine aminotransferase
Examination of data from 16 exercise studies with or without dietary intervention (20 intervention groups) revealed that ALT was not significantly altered in 10 groups and was significantly reduced (improved) in 5 groups and increased in 5 groups.
The nine pooled exercise-only studies exhibited substantial heterogeneity (I2 91%, 95% CI 87% to 94%) and were not considered further. The seven pooled diet and exercise studies exhibited high levels of heterogeneity (I2 73%, 95% CI 45% to 87%) with no evidence of a difference in effect between diet and exercise and diet control groups (MD 0.96 IU/L (95% CI −2.84 to 4.76)). Effect size (SMD) was negligible (0.01, 95% CI (−0.40 to 0.42), p=0.95).
Aspartate aminotransferase
When data from nine exercise studies with or without dietary intervention (12 intervention groups) were examined, there was no change in AST in 6 groups, while 3 groups showed a significant reduction and 3 groups showed a significant increase.
The four pooled exercise-only studies exhibited substantial heterogeneity (I2 88%, 95% CI 77% to 94%) and were not considered further. The five pooled diet and exercise studies exhibited high levels of heterogeneity (I2 61%, 95% CI 5% to 84%) with no evidence of a difference in effect between diet and exercise and diet control groups (MD −0.68 IU/L (95% CI −2.54 to 1.18)). Effect size (SMD) was small (−0.20, 95% CI (−0.73 to 0.34), p=0.47).
γ-Glutamyl transpeptidase
Data from six exercise studies with or without dietary intervention (seven intervention groups) showed there was no significant change in GGT in six groups; only one group showed a significant reduction. Only two diet and exercise studies were included; thus, pooling was not performed for that group.
When data from the four exercise-only studies were pooled using a multivariate meta-analysis model, there was no significant reduction in GGT (MD −3.52 IU/L (95% CI −8.37 to 1.34), p=0.16, I2=71% (95% CI 26% to 89%), see figure 6). The effect size (SMD) was moderate, but the interval suggested no significant effect (−0.30 (−0.69 to 0.09), p=0.13, I2=74%).
Figure 6.
Change in γ-glutamyl transpeptidase: exercise interventions.
Peak VO2
Data from eight exercise studies with or without dietary intervention (11 intervention groups) were examined; there was a significant improvement in cardiorespiratory fitness in six groups but not in five groups.
The five pooled exercise-only studies exhibited substantial heterogeneity (I2 86%, 95% CI 75% to 92%) and were not considered further. The three pooled diet and exercise studies exhibited high levels of heterogeneity (I2 68%, 95% CI 8% to 89%) with no evidence of a difference in effect between diet and exercise and diet control groups (MD −0.05 mL O2/kg/min (95% CI −0.22 to 0.12)). Effect size (SMD) was small (0.1430, 95% CI (−0.5377 to 0.8236), p=0.68).
Insulin
Data from 11 exercise studies (12 intervention groups) with or without dietary intervention was analysed; there was a significant insulin reduction in 5 groups but no change in 6 groups, and 1 group showed an increase in insulin.
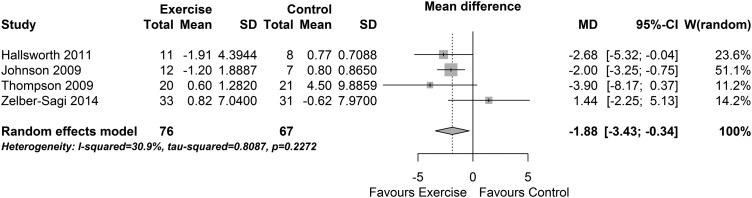
When data from the four exercise-only studies were pooled, there was a significant reduction in insulin (MD −1.88 IU/L (95% CI −3.43 to −0.34), p=0.02, I2=31% (95% CI 0% to 75%), figure 7). The effect size was moderate but was not significant (SMD −0.47 (−1.07 to 0.13), p=0.13, I2=63%). Pooling the seven diet and exercise studies resulted in substantial heterogeneity (I2 78% (95% CI 57% to 89%)); hence, these results are not considered further.
Figure 7.
Change in insulin: exercise interventions.
Total cholesterol
Data from 12 exercise studies (12 intervention groups) with or without dietary intervention were considered; there was a significant total cholesterol reduction in 6 groups but no change in 7 groups.
When data from the six exercise-only studies were pooled, there was a significant reduction in total cholesterol (MD −7.04 mg/dL (−11.96 to −2.13), p=0.005, I2=44% (95% CI 0% to 78%), figure 8 (top)). The effect size was moderate (SMD −0.49 (−0.95 to −0.03), p=0.035, I2=50%).
Figure 8.
Change in total cholesterol: exercise interventions.
When data from the six diet and exercise studies were pooled using a multivariate meta-analysis model, there was a significant reduction in total cholesterol (MD −2.47 mg/dL (−4.55 to −0.39), p=0.020, I2=44% (95% CI 0% to 78%), figure 8 (bottom)). The effect size was moderate (SMD −0.49 (−0.85 to −0.13), p=0.008, I2=52%).
Risk of bias assessment
The similarity in sample and effect sizes between randomised, controlled trials used in the analysis and those trials that were excluded from analysis suggests selection bias is minimal. The sample sizes are small; however, there is no apparent bias evident in the Egger plots (see online supplementary figures).
Discussion
This work analysed the effects of exercise training, with or without dietary intervention, on intrahepatic fat, body mass, BMI, FFAs, insulin liver enzymes, lipids and cardiorespiratory fitness (peak VO2). Our primary findings show that exercise training, in isolation from dietary intervention, has beneficial effects on some of these aforementioned outcome measures. We were able to establish that intrahepatic fat loss may be optimised by usage of greater total exercise training energy expenditure. It appears that the addition of recently published work has added diversity to the current evidence base and increasingly varied study designs have necessitated a more cautious approach to data pooling and subsequent meta-analysis.
Our work failed to show changes in any of the three primary liver enzymes with exercise training. The number of participants included in these three analyses varied between 797 and 1109, so it is unlikely that these analyses were underpowered. There were, however, several confounding variables in the included studies that may have prevented the isolated effects of exercise to be examined. There were eight studies that, in addition to exercise training, also provided various dietary interventions. Moreover, the exercise training programmes varied greatly between studies with respect to exercise intensity, duration, frequency and modality (eg, aerobic vs resistance training). Whatever the reason for the absence of significant changes, our work confirms the previous findings that liver enzymes have limited sensitivity in detecting early liver disease.9 It appears that liver enzymes are, at best, a blunt tool for assessing change in liver function after exercise training.
Our analysis suggested that intrahepatic fat is reduced with exercise training, in as little as 4 weeks,25 but aerobic exercise programmes of total energy expenditure >10 000 kcal are likely to elicit greater improvements than programmes with lower exercise energy expenditure. Our findings in this respect advance those of Keating et al6 who conducted pooled analyses of all exercise modalities.
In mild FLD, the first choice therapy is lifestyle modification, although medications that decrease insulin resistance, hyperlipidaemia and those that induce weight loss have been shown to improve liver function.51 Our analysis reinforces the fact that even short-term exercise training is effective in reducing intrahepatic fat. These findings suggest that exercise may benefit liver health through a calorie expenditure mechanism, sufficient levels of which are acquired more rapidly with vigorous or high-intensity exercise training. Data from heart failure studies suggest that high-intensity exercise may promote superior health benefits.11
It has been recognised that FFAs are the vehicle by which triacylglycerol is stored in adipose tissue and is transported to its site of usage.52 FFA turnover is rapid with a half-life of 2–4 min.52 In our analysis, three studies using >10 000 kcal total exercise programme energy expenditure appear to elicit greater reductions in intrahepatic fat. High volume exercise energy expenditure provides a variety of benefits related to metabolic disorders; we propose that one clinical benefit directly related to liver function is that high volume aerobic exercise elicits triacylglycerol consummation. Of course other exercise-induced benefits will accrue and primarily affect other organs, besides the liver, such as improved flow-mediated vascular dilation,53 cardiac function,54 peak VO253 54 and weight loss which was minimal in our analysis.
Our analyses suggest that it is calorie burning that elicits reductions in liver fat, reduced fat storage, possibly reduced liver enzymes and the sum of all of this is improved glycaemic control. Of perhaps most interest is that the above changes are achieved almost totally independent of body mass changes. To illustrate this, we chose the study of Shoajee-Moradie et al,30 because this work was the only included study to use high-intensity exercise and measure peak VO2, allowing calorie expenditure to be calculated. The exercise participants in Shoajee-Moradie et al's30 study expended 9.8 kcal per minute, or 590 kcal each week, the study only ran for 6 weeks. Exercise participants therefore only expended 3500 kcal during the whole study, equivalent to 0.5 kg of fat. Not surprisingly, Shoajee-Moradie et al30 reported no change in body mass in either exercise or control groups. Nevertheless Johnson et al's25 study reported a similar aggregate exercise programme energy expenditure (3800 kcal) but as it was delivered in a shorter 4-week period, changes in IHF were noted.
Limitations
A major limitation of this work was that considerable heterogeneity meant that data pooling was unjustified in a number of meta-analyses. We systematically attempted to identify reasons for heterogeneity by grouping studies according to similarities in interventions, exercise programmes, patient populations, but we failed to identify definitive conclusions. We were able to reduce heterogeneity somewhat by limiting data pooling to studies that did not use concurrent dietary interventions. Some patients were overweight/obese, while others had definitive liver disease; some of the former may well have also exhibited clinical NAFLD. The likelihood is that the potential for non-clinical NAFLD participants to ‘regress to the mean’ was probably less than in those with diagnosed NAFLD.
As discussed previously, liver enzymes have limited predictive value for liver function, and it is notable that only one of the included studies used the gold standard liver biopsy method to assess liver function. There were studies that, in addition to exercise training, also provided various dietary interventions. The exercise training programmes varied greatly between studies with respect to exercise intensity, duration, frequency and modality (eg, aerobic vs resistance training). The normal distribution of the Egger plots evidenced minimal risk of publication bias.
In the case of the liver enzymes, some heterogeneity may be explained by the naturally occurring interparticipant variation that exists with these enzymes. Measures of lean, and fat, body mass would have shed more light onto the role that body composition plays in improving liver function through exercise. We would like to have conducted subgroup analyses of those with normal and impaired liver function but group level (as opposed to individual patient data) precluded this. We considered programme duration, modality and frequency subanalyses, but the variation in included study data precluded this.
Metaregression between change in liver function and body mass was considered, but change in body mass was not reported in all studies.
The current number of published studies does not allow meta-analyses to compare: aerobic versus resistance exercise, high-intensity versus low-intensity or moderate-intensity exercise, and effects of weekly exercise duration. All of these comparisons may influence change in current guidelines.
The risk of bias assessment identified that allocation concealment to investigators was conducted in less than one-quarter if studies and investigator bias is quite likely. Moreover, assessor blinding was uncommon (only 5 of 21 studies), so this would not mitigate allocation concealment. The lack of physical activity monitoring in 50% of the studies implies that there was likelihood that sedentary controls crossed over to exercise in some studies. The lack of assessment of energy expended during exercise in 10 studies meant that our meta-analyses of aggregate energy expenditure may not be fully representative.
Future studies should aim to at least blind the outcome assessors to the participants' intervention to minimise outcome assessment bias. Future work may also wish to focus on exercise-induced calorie expenditure and the presence or absence of associated weight loss, in order to clarify the mechanism of benefit. Use of gold standard biopsy, rather than reliance on liver enzymes, assessment of liver function is recommended.
Conclusions
Exercise training reduces intrahepatic fat and FFAs while increasing cardiorespiratory fitness. An aggregate exercise programme energy expenditure (>10 000 kcal) may be required to promote reductions in intrahepatic fat.
What are the findings?
Several new published randomised, controlled trials of exercise intervention suggest, for the first time, non-esterified fatty acids are reduced with exercise intervention.
Changes in intrahepatic may be possible, but only in exercise programmes that elicit >10 000 kcal of energy expenditure.
Varied study interventions render it difficult to separate the relative effects of diet and exercise on liver function.
How might it impact on clinical practice in the future?
Imaging, rather than liver enzyme concentrations, should be used to assess liver status.
Preferably 3 months of adherent exercise training, after baseline assessment, should ensue before liver imaging is repeated.
Footnotes
Contributors: NK is a postdoctoral lecturer who conducted some of the data extraction, study quality assessment and manuscript writing. GD assisted with data searching, extraction, data checking, study quality assessment, and writing and editing of the manuscript. JRM assisted with the literature search, assessment of study quality, and verified the accuracy of the main text and online supplementary files section as well as contributing to writing and editing the main text. PLG verified accuracy of data extraction, conducted analyses especially regarding heterogeneity, provided comprehensive statistical advice for the response to reviewers as well as contributing to the editing of the rebuttal and final revised text and provision of some of the figures. NAS was the team leader, who also assisted with study inclusion/exclusion, data extraction/analysis and writing and editing of the main text and supplementary files.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Supplementary files are available which consists of PubMed search strategy, Funnel plots, table of excluded RCTS.
References
- 1.Gray B, Muhlhausler BS, Davies PS, et al. Liver enzymes but not free fatty acid levels predict markers of insulin sensitivity in overweight and obese, nondiabetic adults. Nutr Res 2013;33:781–8. [DOI] [PubMed] [Google Scholar]
- 2.Pacifico L, Bonci E, Andreoli G, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis 2014;24:737–43. [DOI] [PubMed] [Google Scholar]
- 3.GESA. Information about fatty liver disease. 2011. (cited 3 September 2012). http://www.gesa.org.au/files/editor_upload/File/GESA%20Fatty%20Liver.pdf (accessed 03 Sep 2012).
- 4.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 5.Thoma C, CDay CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012;56:255–66. 10.1016/j.jhep.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 6.Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157–66. [DOI] [PubMed] [Google Scholar]
- 7.Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 2011;301:E1033–9. 10.1152/ajpendo.00291.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balducci S, Zanuso S, Cardelli P, et al. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care 2012;35:1347–54. 10.2337/dc11-1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guha IN, Parkes J, Roderick PR, et al. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut 2006;55:1650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail H, McFarlane JR, Dieberg G, et al. Exercise training program characteristics and magnitude of change in functional capacity of heart failure patients. Int J Cardiol 2014;171:62–5. 10.1016/j.ijcard.2013.11.045 [DOI] [PubMed] [Google Scholar]
- 11.Ismail H, McFarlane JR, Nojoumian AH, et al. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC Heart Fail 2013;1:514–22. 10.1016/j.jchf.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Smart NA, Meyer T, Butterfield JA, et al. Individual patient meta-analysis of exercise training effects on systemic brain natriuretic peptide expression in heart failure. Eur J Prev Cardiol 2012;19:428–35. 10.1177/1741826711409171 [DOI] [PubMed] [Google Scholar]
- 13.Wolfgang V. Conducting meta-analyses in R with the metafor package. J Stat Software 2010;36:1–48. [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2011. [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analysis. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F. et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 2015;13:9–18. 10.1097/XEB.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 20.Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013;58:1287–95. 10.1002/hep.26393 [DOI] [PubMed] [Google Scholar]
- 21.Bonekamp S, Barone BB, Clark J, et al. The effect of an exercise training intervention on hepatic steatosis. Hepatology 2008;48:1119. [Google Scholar]
- 22.Bozetto L, Prinster A, Annuzzi G, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012;35:1429–35. 10.2337/dc12-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795–802. 10.1001/jama.2010.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–83. 10.1136/gut.2011.242073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. 10.1002/hep.23129 [DOI] [PubMed] [Google Scholar]
- 26.Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 2015;63:174–82. 10.1016/j.jhep.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 27.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–62. 10.1038/oby.2008.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levinger I, Goodman C, Peake J, et al. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet Med 2009;26:220–7. 10.1111/j.1464-5491.2009.02679.x [DOI] [PubMed] [Google Scholar]
- 29.Shah K, Stufflebam A, Hilton TN, et al. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–8. 10.1038/oby.2009.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shojaee-Moradie F, Baynes KC, Pentecost C, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 2007;50:404–13. 10.1007/s00125-006-0498-7 [DOI] [PubMed] [Google Scholar]
- 31.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121–9. 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straznicky NE, Lambert EA, Grima MT, et al. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabetes Obes Metab 2012;14:139–48. 10.1111/j.1463-1326.2011.01497.x [DOI] [PubMed] [Google Scholar]
- 33.Sullivan S, Kirk EP, Mittendorfer B, et al. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012;55:1738–45. 10.1002/hep.25548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura Y, Tanaka Y, Sato F, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005;90:3191–6. 10.1210/jc.2004-1959 [DOI] [PubMed] [Google Scholar]
- 35.Thompson D, Markovitch D, Betts JA, et al. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol 2010;108:769–79. 10.1152/japplphysiol.00822.2009 [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura E, Kumahara H, Tobina T, et al. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes 2014;2014:197216 10.1155/2014/197216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelber-Sagi S, Buch A, Yeshua H, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 2014;20:4382–92. 10.3748/wjg.v20.i15.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, et al. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci 2013;13:667–72. 10.4314/ahs.v13i3.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsalani R, Riesco E, Lavoie JM, et al. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric 2013;16:88–95. 10.3109/13697137.2012.662251 [DOI] [PubMed] [Google Scholar]
- 40.Chen SM, Liu CY, Li SR, et al. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc 2008;71:551–8. 10.1016/S1726-4901(08)70168-0 [DOI] [PubMed] [Google Scholar]
- 41.de Piano A, de Mello MT, Sanches Pde L, et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol 2012;24:1313–24. 10.1097/MEG.0b013e32835793ac [DOI] [PubMed] [Google Scholar]
- 42.Haus JM, Solomon TP, Marchetti CM, et al. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 2010;95:323–7. 10.1210/jc.2009-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156–63. 10.2337/dc10-0856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Bacha F, Hannon T, et al. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 2012;61:2787–95. 10.2337/db12-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing non-alcoholic fatty liver disease: a retrospective study. Hepatology 2015;61:1205–15. 10.1002/hep.27544 [DOI] [PubMed] [Google Scholar]
- 46.Pugh CJ, Cuthbertson DJ, Sprung VS, et al. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 2013;305:E50–8. 10.1152/ajpendo.00055.2013 [DOI] [PubMed] [Google Scholar]
- 47.Pugh CJ, Spring VS, Kemp GJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol 2014;307(9):H1298–306. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Munoz V, Salas-Romero R, Del Villar-Morales A, et al. [Decrease of liver fat content by aerobic exercise or metformin therapy in overweight or obese women]. Rev Invest Clin 2013;65:307–17. [PubMed] [Google Scholar]
- 49.St George A, Bauman A, Johnston A, et al. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 2009;24:399–407. 10.1111/j.1440-1746.2008.05694.x [DOI] [PubMed] [Google Scholar]
- 50.Tsekouras YE, Magkos F, Kellas Y, et al. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am J Physiol Endocrinol Metab 2008;295:E851–8. 10.1152/ajpendo.90545.2008 [DOI] [PubMed] [Google Scholar]
- 51.Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician 2006;73:1961–8. [PubMed] [Google Scholar]
- 52.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–9. 10.2337/db11-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjonna AE, Lee SJ, Rognmo A, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 2008;118:346–54. 10.1161/CIRCULATIONAHA.108.772822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007;115:3086–94. 10.1161/CIRCULATIONAHA.106.675041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2016-096197supp001.pdf (31.3KB, pdf)