Abstract
Background
Lopinavir/ritonavir ‘pellets’ were recently tentatively approved for licensing. We describe their acceptability for infants and children up to 48 weeks.
Methods
CHAPAS-2 was a randomised, 2-period crossover trial comparing syrup and pellets in HIV-infected infants (n=19, group A, aged 3-<12 months) and children (n=26, group B, 1-<4yrs) and tablets and pellets in older children (n=32, group C, 4-<13yrs) from 2 clinics (“JCRC”, “PIDC”) in Uganda. At week 8, all groups chose which formulation to continue. Acceptability data were collected at weeks 0,4,8,12, and 48.
Results
For groups A and B overall, the proportion preferring pellets increased between week 0 and week 12 and decreased at week 48 (group A 37%, 72%, 44%; group B 12%, 64% and 36% respectively), although there were marked differences between clinics. For group C, pellets were progressively less preferred to tablets over time: 41%,19%,13% at weeks 0,12,48 respectively. During follow-up unpleasant taste was similarly reported among young children taking pellets and syrups (37%/43% group A; 29%/26% group B), whereas among older children, pellets tasted worse than tablets (40%/2%). No participants reported problems with storage/transportation for pellets (0%/0%) unlike syrups (23%/13%).
Conclusions
For children <4 years, pellets were more acceptable at week 12 but not week 48. Clinic differences could reflect bias among healthcare workers for different formulations. Pellets taste similar to syrup, are easier to store and transport than syrup, and represent an alternative formulation for young children unable to swallow tablets; improvements in taste and support for healthcare workers may help sustain acceptability.
Keywords: Lopinavir, ritonavir, pellets, minitabs, acceptability, children
Introduction
World Health Organization (WHO) HIV treatment guidelines recommend a ritonavir-boosted protease inhibitor (bPI) plus two nucleoside reverse transcriptase inhibitors (NRTIs) for first-line antiretroviral therapy (ART) in younger children (<3 years), and for second-line ART in children receiving 2 NRTIs with an NNRTI first-line.(1) Access to second-line ART is increasing, but still remains low (~4%) in many low and middle income settings.(2)
The major factors limiting the wider use of second-line ART and bPI-based first-line ART in children aged <3 years is the lack of affordable and appropriate bPI-based formulations for resource-limited settings. To date the only available combined bPI is ritonavir-boosted lopinavir (LPV/r), which is available as a liquid for younger children and paediatric tablets for older children. The syrup has an unpleasant taste and requires refrigeration, and is used relatively infrequently (~15,000 infants and children in 2013 (WHO Global Price Reporting Mechanism)) whilst tablets are large and must not be crushed/split or they lose bioavailability.(3)
We previously reported that LPV/r exposure from a minitab sprinkle formulation stored in capsules (hereafter referred to as “pellet”) was comparable with syrup, but lower than tablets, with no significant differences in subtherapeutic concentrations.(4) In the first 12 weeks of the study, pellets were more acceptable than syrups for younger children, but older children preferred tablets. The USA Food and Drug Administration (FDA) gave tentative approval for the LPV/r (40/10mg) pellets used in this study in May 2015.(5) However the pellets are yet to be rolled out widely in ART programmes.
Here we evaluated acceptability of pellets compared to syrup and tablets up to week 48. We also assessed virologic suppression at week 48 among the children on pellets in whom HIV viral load was assayed. These data will be useful for national programs intending to roll out the LPV/r pellets.
Methods
The CHAPAS-2 trial design has been described previously.(2) Briefly, it was an open, randomized, phase 1, 2-period crossover comparative bioavailability trial in HIV-infected infants and children aged 3 months to <13 years taking or about to start first-line or second-line ART with 2 NRTIs plus LPV/r. Seventy-seven children were recruited from two clinics in Kampala: the Joint Clinical Research Centre (JCRC); and the Mulago Hospital Paediatric Infectious Disease Clinic (PIDC).
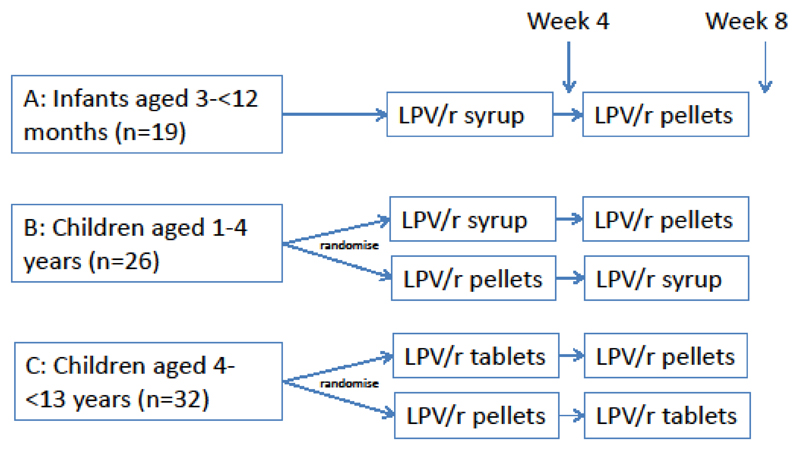
Children were randomised or allocated according to age-groups as in Figure 1: infants aged 3 to <12 months (group A) were included in a non-randomised two period crossover design, comparing syrup with novel pellets. Children aged 1-<4 years (group B) and children aged 4 to <13 years weighing <25kg (group C) were randomised 1:1 to syrup or pellets, and tablets or pellets, respectively. Carers chose which formulation to continue from week 8 to 48 (final follow-up). LPV/r was dosed twice daily according to WHO 2013 paediatric weight bands.(1)
Figure 1. Design of CHAPAS-2 Trial.
As part of a secondary objective of the main trial, data on caregiver acceptability of each LPV/r formulation were collected from standardized questionnaires at baseline and weeks 4, 8, 12 and 48. Caregivers were asked to comment on the type of formulation that they thought they would prefer if they had not used both formulations before, or what they did prefer if they had used both.
Viral load data were collected at week 48 for children who had this assayed as part of site protocol. Viral load was measured by Abbott m3000sp/rt with lower limit of detection 50c/ml. Descriptive statistics were used to summarise the data by age group, and all analyses were conducted using STATA 13 (Statacorp, College Station, Texas).
Results
Nineteen infants (group A, median (interquartile range) age 0.5 (0.4, 0.7) years) were enrolled; 88% (15/17), 84% (15/18), 78% (14/18), 78% (14/18), and 11% (2/18) were breastfed at 4, 8, 12, 24 and 48 weeks respectively. There were 26 young children (group B, median age 2.0 (1.8, 2.8)) and 32 older children (group C, median age 6.2 (5.8, 7.8) years).
Overall 51% were male, and all age groups were moderately wasted and stunted (median weight and height-for-age z-scores were -1.11 (-0.76, -2.42) and -1.99 (-1.02, -2.66) in infants, -1.19 (-0.18, -2.11) and -2.05 (-1.37, -3.43) in young children, and -0.82 (-0.18, -1.70) and 1.24 (-0.30, -2.05) in older children).(4) Table 1 shows caregiver preferences for pellets at enrolment, week 12 and week 48 by clinic. Overall across both clinics, for infants and 1-<4 year olds, the proportion of caregivers preferring pellets increased between enrolment and week 12 and decreased thereafter (37%, 72%, 44% and 12%, 64%, 36% respectively), whilst for older children, preference for pellets fell throughout follow-up(41%, 19%, 13%). Although patterns were similar for both clinics, preference for pellets was higher than alternative formulations at week 48 in both younger and older children at JCRC (70% and 17% at JCRC versus 13% and 0% respectively at PIDC).
Table 1. Caregiver preference for pellets, and pellet use in children, at enrolment, week 12 and week 48, by group.
| JCRC (n=41) | PIDC (n=36) | Total (n=77) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (3-<12m) n=6 | B (1-<4 yrs) n=11 | C (4-<13yrs) n=24 | A (3-<12m) n=13 | B (1-<4 yrs) n=15 | C (4-<13yrs) n=8 | A (3-<12m) n=19 | B (1-<4 yrs) n=26 | C (4-<13yrs) n=32 | ||||||||||
| n/total | % | n/total | % | n/total | % | n/total | % | n/total | % | n/total | % | n/total | % | n/total | % | n/total | % | |
| Caregiver preference for pellets | ||||||||||||||||||
| Enrolment | 2/6 | 33% | 2/11 | 18% | 9/24 | 38% | 5/13 | 38% | 1/15 | 7% | 4/8 | 50% | 7/19 | 37% | 3/26 | 12% | 13/32 | 41% |
| Week 12 | 3/5 | 60% | 7/10 | 70% | 5/23 | 22% | 10/13 | 77% | 9/15 | 60% | 1/8 | 13% | 13/18 | 72% | 16/25 | 64% | 6/31 | 19% |
| Week 48 | 2/5 | 40% | 7/10 | 70% | 4/24 | 17% | 6/13 | 46% | 2/15 | 13% | 0/7 | 0% | 8/18 | 44% | 9/25 | 36% | 4/31 | 13% |
| Childrens’ pellet use, last 4 wks | ||||||||||||||||||
| Week 12 | 3/5 | 60% | 8/10 | 80% | 5/23 | 22% | 10/12 | 83% | 10/15 | 67% | 2/8 | 25% | 13/17 | 76% | 18/25 | 72% | 7/31 | 23% |
| Week 48 | 2/5 | 40% | 9/11 | 82% | 4/24 | 17% | 2/13 | 15% | 3/15 | 20% | 0/7 | 0% | 4/18 | 22% | 12/26 | 46% | 4/31 | 13% |
Table 1 also shows the proportion of children taking pellets in the last 4 weeks, by age group, time point and clinic. The proportion taking pellets closely followed the trends in caregiver preferences. Thus, children at JCRC were more likely to be on pellets at week 48 compared to children at PIDC (40%, 82% and 17% in infants, younger and older children respectively at JCRC, compared to 15%, 20% and 0% at PIDC.
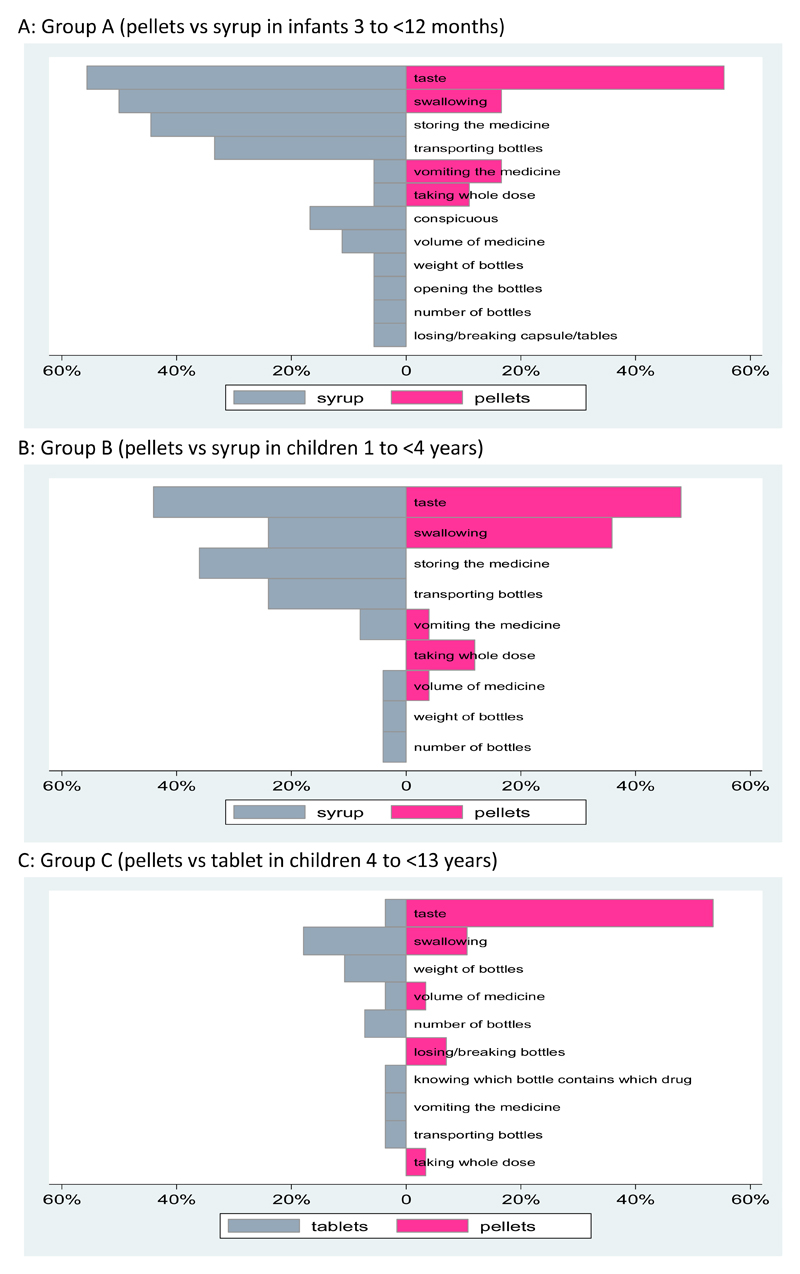
Figure 2 shows problems that carers reported with different formulations during follow-up. In infants and younger children, the proportion reporting unpleasant taste was similar for syrup and pellets, whilst for older children, pellets were reported to have a worse taste than tablets. Carers reported more problems with storing medicine and transporting bottles for their infants and young children when taking syrups than pellets. Of note, swallowing problems were more common for syrups than pellets among younger children aged 1-<4 years. Overall, problems were less in older children; apart from taste problems associated with pellets, other reported problems were reported by <20% of the group.
Figure 2. Percentage of Caregivers Reporting Problems with Different Formulations at any Point during Follow-up to 48 Weeks.
Caregivers provided multiple comments about the formulations at each visit. For those preferring syrups, key issues with pellets were their bitter taste, problems with masking this taste with food and food refusal, needing to sweeten food with sugar or honey (which is expensive), and concerns about not giving the whole dose. Interestingly, although most infants were breastfeed during the first 12 weeks, comments about taking pellets were generally positive at these time points with carers (mainly mothers) reporting that they were easy to administer.
Viral load was measured around week 48 in only a minority of children (not a protocol requirement). Among 19/77 (25%) children with viral load assayed at week 48, 14 (74%) were suppressed <50c/ml and all were <1000c/ml.
Discussion
The LPV/r (40/20mg) pellets used in this study have recently been tentatively approved by the USA FDA,(5) hence these are the first data assessing their longer term acceptability. Standardised acceptability questionnaire data over time, together with information on formulation preference and comments from caregivers regarding administration of the different drug formulations of LPV/r are valuable in order to help future healthcare workers anticipate problems with pellets, counsel carers appropriately and support them in their task of administering LPV/r-based ART regiments to infants and young children. In our trial, although some instruction was provided (eg to give pellets rapidly with their chosen food in order to minimise the taste permeating the food), additional ways to maximise acceptability of the drug for young children were encouraged, including trying different foods with pellets. Of note, caregivers commented that strong tasting food was required, hence the use of honey to attempt to mask the taste.
It is interesting that for infants and younger children, LPV/r pellets were more acceptable than LPV/r syrups near the start of administrations but that this preference waned over time. As the study could not be blinded, reasons such as expectation that pellets would be better cannot be ruled out. It may also be that initially, children enjoyed having new masking foods such as honey, but that over time, they came to associate these foods with the bad taste.
Data from older children in CHAPAS 2 are similar to those of the ARROW trial in Uganda and Zimbabwe, in which solid formulations were preferred to liquid after around 3 years of age. However, in ARROW, solid adult scored (tablet) formulations of combinations of abacavir, lamivudine, zidovudine and nevirapine were relatively small, were dispersible and had tolerable taste; in contrast LPV/r tablets should be swallowed whole to preserve bioavailability and this cannot easily be done in children less than 5-6 years of age. Also in ARROW solid formulations were compared with administration of multiple individual drug syrup formulations, so it is perhaps unsurprising that tablets were preferred at a younger age than in our trial.(6) Similar findings have been reported from another Ugandan study which found better adherence to fixed dose combination dispersible tablet formulations when compared to syrups in children from 6 months of age. (7)
In both CHAPAS 2 and ARROW studies, caregivers cited problems related to volume of drug, transportation, storage and handling associated with syrups; these appeared to be key reasons for initial preference of solid formulations. In CHAPAS 2 this is extended to pellets as well when compared with liquid formulations. However, these reasons appeared to wane in CHAPAS 2 and by week 48, pellets were less acceptable than syrups for young children overall. There were, however, substantial clinic differences, with caregivers at JCRC preferring and using pellets much more than those at PIDC. Reasons for this are unclear and could reflect bias among healthcare workers at each clinic, with their own preferences possibly influencing those of the caregivers. This highlights the need to ensure adequate and ongoing training of healthcare workers to familiarise them with different formulations; for example, given that CHAPAS 2 started as an intensive, primarily PK trial for 12 weeks, and that liquid LPV/r had already been introduced to both clinics before CHAPAS 2, during the more standard of care follow-up phase from 12 to 48 weeks, families may have seen healthcare workers who were less familiar with pellets and could have influenced preferences of carers. This proved to be difficult to investigate further after the end of the 48 week follow-up. However, from the many comments made by carers, it also appears that taste of the pellets and cost of foods to mask that taste likely also played a role for some caregivers reverting to taking syrup in this study. Of note, the observation from some caregivers that accompanying food needed to be strong tasting, was expensive (honey) and that, if given with staple porridge, some children subsequently refused this food, may have also contributed to decisions to stop pellets.
It is important to note that overall and at each of the clinics, at week 12 and at week 48, the proportion of children taking the syrups was reflected in the caregiver preferences, underscoring the importance of caregivers’ views in ensuring adherence to ART, as well as the need for training of healthcare workers and support of caregivers in their efforts to administer the pellets. This has also been observed in previous research, including in a recent qualitative study in Cuba, where caregivers were unlikely to give medications to their children because of anticipated problems such as vomiting,(8) and in a study of HIV-infected children in Togo, West Africa, where the caregiver’s perceived difficulty of ART administration predicted the child’s non-adherence.(9)
Among the infants, a high proportion were breastfeeding at the beginning of the study, with some below 6 months of age exclusively breastfeeding. Mothers exclusively breast feeding generally administered pellets with expressed breast milk on a spoon. It is interesting that, if anything, they reported fewer problems with pellets than syrups at this stage, although numbers are small (total 10 in the trial <6 months of age. Acceptability at this young age maybe more related to inability of young infants to express preference.
In conclusion, in this study comparing preferences for three formulations of LPV/r across age groups, preference varied considerably by age group. For older children ≥4 years already able to swallow tablets whole, tablets were preferred, mainly because of the bitter taste of the pellets. In infants, and younger children, pellets were preferred initially but this preference tended to wane with time, particularly in the 1-<4 year old age group. However, the logistic advantages of pellets for transportation and storage were commented on by many carers, and they represent an important alternative formulation choice for young children unable to swallow tablets. The acceptability of this formulation will hopefully be greatly improved. Countries following WHO guidance and providing LPV/r for first-line ART for children <3 years, according to WHO 2013 guidelines, should consider using pellet formulations now that the USA FDA has approved their use.(1) An implementation study of the LPV/r pellets is starting in several African countries; results from this acceptability and preference study should help to inform strategies for administration and healthcare worker training to ensure optimal to support for caregivers giving pellets to their children.
Acknowledgements
We thank the families and children participating in the CHAPAS-2 trial, and the study teams of: the Joint Clinical Research Centre, Kampala, Uganda (Bernard Bainomuhwezi, Sophia Kasuswa, Rosette Keishanyu, Priscilla Kyobutungi, Grace Mirembe, Peter Mugyenyi, Disan Mulima, Phyllis Mwesigwa, Eva Natukunda, Paul Ocitti, Florence Odongo, Wilfred Opilo, Constance Tumusiime, Priscilla Wavamunno); Baylor-Uganda, Paediatric Infectious Disease Clinic, Mulago Hospital, Kampala, Uganda (Grace Akello, Annet Babirye, Philip Kasirye, Muzamil Kisekka, Sabrina Kitaka, Violet Korutaro, Moses Matovu, Sarah Nakalanzi, Rachel Namuddu, Grace Ngamita); Medical Research Council Clinical Trials Unit, London, UK (Adam Glabay, Lindsay Kendall, Bethany Naidoo-James, Nadine Van Looy, Natalie Young); and Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands (David Burger, Quirine Fillekes).
Funding CHAPAS-2 is funded by the Monument Trust, UK and also Drugs for Neglected Diseases initiative (DNDi), Geneva, Switzerland, via grants from UNITAID and MSF Norway. Drugs are provided by Cipla Pharmaceuticals, Mumbai, India
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. Jun, 2013. [PubMed] [Google Scholar]
- 2.UNICEF and UNAIDS. Global update on HIV treatment 2013: results, impact and opportunities. Geneva: World Health Organization; 2013. [Google Scholar]
- 3.Best BM, Capparelli EV, Diep H, Rossi SS, Farrell MJ, Williams E, et al. Pharmacokinetics of Lopinavir/Ritonavir Crushed versus Whole Tablets in Children. J Acquir Immune Defic Syndr Hum Retrovirol. 2011 doi: 10.1097/QAI.0b013e318232b057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66(2):148–54. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. NDA 205425 tentative approval 2015. [13 August 2015]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/205425Orig1s000TAltr.pdf.
- 6.Nahirya-Ntege P, Cook A, Vhembo T, Opilo W, Namuddu R, Katuramu R, et al. Young HIV-infected children and their adult caregivers prefer tablets to syrup antiretroviral medications in Africa. PLoS One. 2012;7(5):e36186. doi: 10.1371/journal.pone.0036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagenda A, Barlow-Mosha L, Bagenda D, Sakwa R, Fowler MG, Musoke PM. Adherence to tablet and liquid formulations of antiretroviral medication for paediatric HIV treatment at an urban clinic in Uganda. Ann Trop Paediatr. 2011;31(3):235–45. doi: 10.1179/1465328111Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 8.Castro M, Gonzalez I, Perez J. Factors related to antiretroviral therapy adherence in children and adolescents with HIV/AIDS in Cuba. MEDICC review. 2015;17(1):35–40. doi: 10.37757/MR2015.V17.N1.8. [DOI] [PubMed] [Google Scholar]
- 9.Polisset J, Ametonou F, Arrive E, Aho A, Perez F. Correlates of adherence to antiretroviral therapy in HIV-infected children in Lome, Togo, West Africa. AIDS Behav. 2009;13(1):23–32. doi: 10.1007/s10461-008-9437-6. [DOI] [PubMed] [Google Scholar]