Abstract
People show systematic biases in perception, memory, and attention to favor information related to themselves over information related to other people. Researchers have examined these biases in order to throw light on the nature of the self. We review this evidence in memory, face recognition, and simple perceptual matching tasks through objective measures of self-biases. We argue that the self serves as a stable anchor across different forms of judgment and that referring a stimulus to ourselves enhances the binding of stimulus features at different stages of processing (e.g., in perception and in memory) and also the binding between processing stages. There is neural evidence that self-biases reflect an underlying neural network that interacts with but is independent of attentional control networks in the brain, and that damage to the self-related network disrupts the bias effects. We discuss the implications for understanding the nature of the self.
Keywords: self-representation, decision making, binding, redundancy gains, fMRI default network
Introduction
The concept of self is used to explain how we interact with the world and think about both our own behavior and the behavior of others. It is not surprising that the self as an ubiquitous topic in psychology has driven researchers for centuries and continues to drive interest in different research fields (e.g., in memory1–5 and attention6,7), specifically, in cognitive science and neuroscience in the last 2 decades. Arguments about the nature of the self have been put forward since the beginnings of psychology as a science. James8 distinguished between the physical, mental, and spiritual aspects of the self, arguing that physical representations could be extended to objects that are associated with the individual.8 This idea of self-expansion has more recently been incorporated in social psychological theories about “the expanding self,”9,10 where it has been proposed that the self forms the basis for representations of our own group, which can be incorporated into the self-representation,11,12 and also in a basis model of self-specificity in neuroscience that argues the function of self-specificity in associating the self with either internal or external stimuli by the brain’s spontaneous activity.13 Despite the importance placed by theorists on the concept of the self along this line of research, the nature of self-expansion/association, and what any such established representation does for us during self-expansion/association, has remained elusive. For example, what function might self-representation play in the different processes determining our cognitions? Are self-representations in some way special and do they depend on distinct neural circuitry? Answers to such questions are beginning to emerge, and they form the focus for this review.
We attempted to answer these questions through empirical measures of self-biases that can be sued as a proxy for self-representation. In the following, we begin by summarizing the evidence of self-biases in different domains and tasks using self-biases as objective measures (i.e., memory, face recognition, and perceptual matching tasks). Then, we discuss two properties of self-reference to account for the functions of the self in information processing: that the self serves as a stable anchor in decision making that is less affected by the external decision boundary, and that referring a stimulus to ourselves enhances the binding of stimulus features and also binding between different stages of information processing (e.g., in perception and in memory). Finally, we discuss neural networks underlying the functions of the self.
Self-bias as a proxy for self-representation
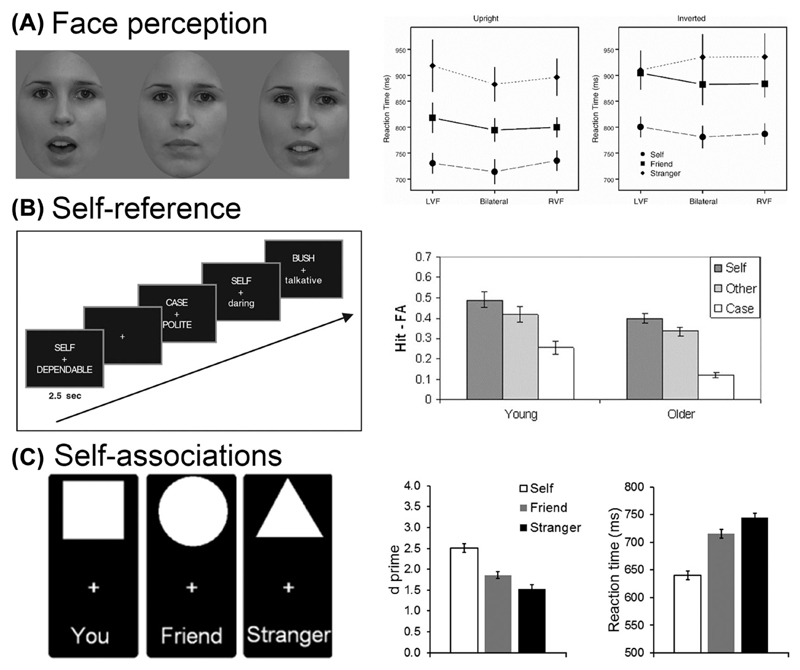
One of the difficulties in much of the early work about the self was that it was reliant on subjective report to derive self-related indices of quite general concepts, such as how independent or interdependent a person is relative to others. Such indices can be open to effects of task demands, they do not map easily onto current models of cognition, and the derived measures are likely to reflect a broad range of processes operating at different levels and over different time courses. In order to generate more objective indices, researchers have more recently focused on how referring a stimulus to ourselves systematically biases cognition. These biases are robust and open to various manipulations that help to constrain our understanding of the attributes of the self and the role that the self may play in cognition. In these studies, self-bias effects serve as a proxy for the otherwise difficult to measure and abstract concept of the self. The biases have been studied with a wide range of stimuli (e.g., faces, trait terms, even simple geometric shapes associated with the self and others) and in a variety of tasks stressing perception, memory, and decision making (see Fig. 1 for illustrations of the stimuli, tasks, and results), illustrating the ubiquitous nature of self-reference effects on information processing.
Figure 1.
Self-prioritization across different tasks. (A) Face recognition (data from Ref. 87), (B) self-reference memory (data from Refs. 15 and 72), and (C) self-associations in a shape–label matching task (see Refs. 27 and 75). For each task, the left panel illustrates the stimuli and procedures and the right panel shows the data.
Self-biases in memory
Some of the earliest studies into self-bias focused on the effects on memory of referring a stimulus to the self. Rogers et al. had participants make judgments to trait terms in relation to either themselves or others.5 Memory for the terms was better if participants had judged them in relation to themselves rather than other people, owing to organizational and elaborative processes in encoding, as argued in the early stage of the field.1–5 This result, enhanced memory after reference is made to the self, has now been found in numerous studies.3,14–17 Interestingly, very recently researchers proposed that the effect does not seem to rely on deeper, more elaborated processing being applied to self-related stimuli. For example, Leshikar et al.18 had participants make judgments about either the semantic meaning or the self-relevance of positive and negative adjectives. Despite being compared with a condition involving deep encoding (judging the semantic meaning of stimuli), there was better recollection for items judged in relation to the self. Sui and Humphreys19 examined the effects of self-reference on memory in an amnesic patient who showed no effects of depth of processing (when semantic encoding conditions were compared with encoding based on a judgment about the surface property of a stimulus (the physical size of a picture). The amnesic patient had enhanced memory for stimuli processed in relation to himself versus another person, and this effect was as large as that found for control participants. Thus, the effects of self-reference on memory can dissociate from the effects of deeper semantic encoding.
One other aspect of self-bias effects on memory is that they are associated with increased recall of episodic details about items judged in relation to ourselves, compared to items judged for meaning. For example, Craik et al.20 found that there was increased memory for the perceptual details of stimuli, even though these details were not strictly required for performance. Prior work also showed that self-reference is an efficient strategy in encoding to reduce memory impairment in aging by linking stimuli to personal knowledge and subsequently enhancing memory retrieval.21 In addition, young children also benefit self-referential encoding and demonstrate self-advantage in memory.22 For example, Cunningham et al.14 presented children with images of their own or another child’s face along with an object, with the task being to judge whether the child liked the object. On a subsequent memory test, the children not only demonstrated better performance on objects judged in relation to themselves but they also had performed better at judging which face the remembered object was presented with.22,23 These last results raise the question of whether self-reference does not simply improve memory performance but has a more specific effect on the binding of elements within an episode (including the binding of memory to its source, which judgment was made initially, and when stimuli were processed in relation to the self).
Self-biases in perceptual judgments
As well as affecting memory, there is evidence that self-reference influences perceptual judgments to stimuli. For example, judgments about whether a face is oriented toward or away from a viewer are made more quickly and with higher accuracy if a participant sees their own face than if they see someone else’s face—including the face of their best friend.24,25 Perceptual judgments are enhanced by self-reference not only with well-learned stimuli (own versus others’ faces) but also with stimuli that have arbitrary and newly learned relations to the self and other people. Sui et al.26,27 had participants learn new relationships between a stimulus (a color or a shape) and a label referring to the self or another person (e.g., circle–you, square–friend, triangle–stranger). Participants were then presented with the original pairing (a match trial) or with the stimuli re-paired (circle–friend, square–stranger, triangle–you: mismatch trials); the task was to decide whether an original or a re-paired stimulus was presented on a trial. There was a robust advantage for matching self-related stimuli compared with stimuli linked to other people. This self advantage also interacted with whether the stimuli were presented with high contrast or degraded—self-related stimuli showed smaller effects of contrast reduction compared with stimuli associated with other people. This last result is consistent with perceptual processing being enhanced for self-related items. We note that this evidence for rapid development of self-reference effects for stimuli arbitrarily associated with the self fit with the argument put forward by William James that aspects of self-representation can be transferred to external objects.
Self-biases in attention
At least some of the self-bias effects we have noted may reflect enhanced attention to stimuli associated with the self. There is evidence for attention being drawn to self-related stimuli. In a seminal study, Moray6 found that presentation of the participant’s own name in an unattended auditory channel could capture attention; participants were more likely to switch attention to the unattended channel and to report their own name than the name of another person. Own-name effects have also been studied in the attention blink (AB)—an attentional limitation in reporting a second target when a first target has recently been identified. The AB has been found to be less pronounced when the second target is the participant’s own name, compared with other names.28 Similar to this, inattentional blindness (the failure to notice an unattended but perceptible stimulus) is reduced for own-name stimuli,29 as is repetition blindness (the difficulty in reporting a repeated occurrence of a stimulus). Yang et al.30 have also reported that participants fixate earlier and make fewer saccades to a target that is their own name compared with the names of others. In each of these cases, own-name stimuli seem to be less subject to attentional limits, and even to dictate where attention falls, when compared with other stimuli.
Supporting neuropsychological evidence comes from Sui and Humphreys’s work in progress. Sui and Humphreys examined the phenomenon of extinction, where patients can report a single stimulus presented on the side of space opposite their lesion but fail to notice the same item when a second stimulus appears simultaneously on the ipsilesional side and competes for attention. Sui and Humphreys found that extinction was reduced on two-item trials when one stimulus was associated to the self, and that the presence of the self on the contralesional side even induced extinction of the ipsilesional stimulus. This result suggests that self-related stimuli can be encoded preattentively and then affect the subsequent deployment of attention. The neuropsychological evidence is also supported by an electrophysiological study with normal observers,31 where researchers assessed self-biases in face perception using a continuous flash suppression paradigm to present stimuli subliminally. They found that subliminal self-faces were associated with a greater probability of breaking into awareness under the continuous flash suppression conditions, and they also modulated the amplitude of the positive event-related component over the central midline cortex, compared with subliminally presented famous faces. These electrophysiological effects were distinct from those found when the stimuli were presented supraliminally and suggest that there is differential processing of self relative to other faces even outside awareness.
In all of the above-mentioned cases, the presence of self-related stimuli improved performance. The converse of this is that self-related items can disrupt performance if they are irrelevant to the task. For example, the central presentation of the participant’s own name can disrupt the detection of peripheral stimuli,32,33 and a participant hearing their own name in an irrelevant list has been found to reduce working memory capacity.34 Similarly, the presence of a participant’s own face can disrupt a primary task (e.g., judging which arm of a cross is longer) when the self-face appears as a background distractor.35,36
However, not all the evidence indicates that self-stimuli are processed independently of attention. Turk et al.37 reported that divided attention abolished the self-advantage effect in memory, though participants remembered more items after self-ownership judgments compared with other-ownership judgments when full attention was allocated. In this case, divided attention may disrupt elaborative encoding to self-referential items,16,38 reducing the self-advantage. The authors propose that there is a reduction in the memory enhancement to the self when insufficient resources are allocated to the stimuli. Other investigators have reported that the own-name effect decreases when stimuli are presented outside the focus of attention.39,40 In line with this study, Mattan et al.41 tested the role of self in perspective taking. They showed scenes containing a number of discs and an avatar. When the number of discs viewed by the avatar was incongruent with the number the viewer could see, viewers were slower at judging how many they could see.41 The effects of perspective incongruity were also reported in other studies.42,43 The avatar could also be associated with the self or another person. Though performance was accelerated when the avatar was associated with the self, the effects of viewing incongruent numbers of discs were comparable for both the self- and other-related avatar conditions. The authors argue that there is not automatic and exclusive attention to the information viewed by the self. Bundesen et al.39 briefly presented words in one of two colors and had participants report items from a particular color. Performance was not disrupted when the participant’s own name appeared in the distractor color. Harris and Pashler44 did report differential distraction from the participant’s own name compared with other names, but only when the own names were rare events—any distraction effect disappeared if the own name distractors occurred half the time. Harris and Pashler suggest that, rather than there being automatic attention capture by one’s own name, interference stems from the surprise of occasionally seeing your own name on trials where the distractor is attended.
To account for such results, Humphreys and Sui7 have argued that the bias to attend a self-related stimulus interacts with attentional control mechanisms. There can be some processing of self-related stimuli without attention. For example, a neuropsychology study reported that self-name can be processed by people with minimal consciousness.45 In contrast, self-related processing is differentially enhanced when items are attended.13,46 In addition, controlled attention to another stimulus can reduce any selective processing of the self when the self is irrelevant to the task.
Properties of self-reference effects
Given the ubiquitous effects of self-reference on memory, perception, and attention, we can begin to ask what the properties of self-reference effects are and what these properties tell us about self-representation. We highlight two properties here: the role of self-reference as a stable anchor point in decision making and the effects of self-reference on binding.47
Self-reference as an anchor in decision making
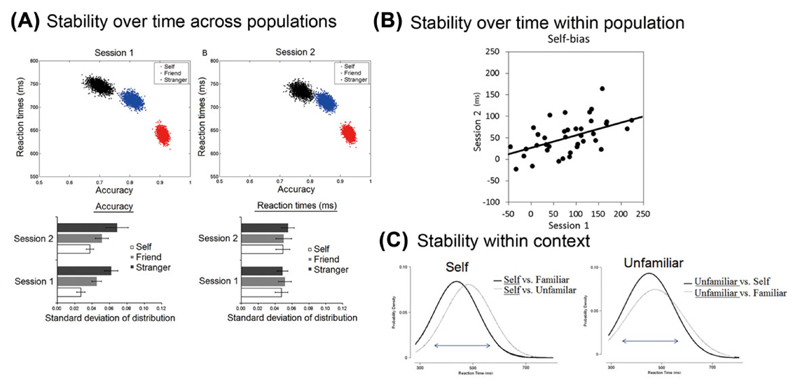
Self-bias effects on perceptual judgments to neutral stimuli are highly stable within individuals. Participants who show a strong (or weak) self-bias effects at time N show a strong tendency to show a strong (or weak) self-bias effect when the bias is assessed a month later.48 There is a trait-like stability for the self-bias effects on perceptual matching when individuals are tested on different occasions (see Fig. 2A).49 Self-related stimuli show greater stability within individuals across sessions (Fig. 2B).48,49
Figure 2.
Stability of self-processing. (A) Stability over time across populations of participants, here illustrating the distributions of data across participants (from Ref. 19). (B) Self-biased responses in one session correlate those in another session. Self-related processing over time also forms one unique factor compared with other-related processing, which form clusters based on sessions (from Refs. 49 and 88). (C) Stability within different decision contexts for self-related stimuli compared with other-related stimuli (from Ref. 50).
This within-subject stability is also apparent across different types of decision. Sui and Humphreys50 had participants make varying judgments to stimuli associated to the self or other people. The participants saw images of their own face, their friend’s face, or the face of a stranger. In one case, the task was to distinguish the self-face against the others (self versus other decisions). In a second case, participants had to discriminate familiar faces (self + friend) versus unfamiliar faces (other). That is, the decision boundary was varied across the stimuli in the two tasks. Performance was assessed using mathematical modeling of the reaction times based on the assumption that the underlying distribution reflected the combination of a Gaussian and an exponential function (an ex-Gaussian analysis; see Refs. 51 and 52), where the Gaussian function is represented in terms of parameters for the mean and standard deviation of the distribution (mu (μ) and sigma (σ)), while the exponential distribution is represented by a single parameter, tau (τ), which reflects both the mean and the standard deviation. Some authors have attempted to link the different distributions to contrasting underlying processes, though this remains somewhat controversial. For example, shifts in the mean of the Gaussian function may reflect the time to initiate stimulus processing or to retrieve stimulus–response associations53 or the noise operating under different conditions.51,54 In contrast, changes in the exponential function may reflect decision making,54 conflict resolution (e.g., on congruent versus incongruent trials in the Stroop task; see Ref. 55), and/or more attention-demanding processing of particular stimuli that consequently have a greater spread in the time taken to assign them to a perceptual category.56–58
The results in Sui and Humphreys50 were striking. Key parameters of the functions (the σ and τ components) were constant for self-stimuli across the two tasks (see Fig. 2C), and there was reduced variance in the distributions of the parameters for self-related stimuli compared with stimuli associated with other people. In contrast, for friend and stranger stimuli, the parameters not only differed from those found with the self, but they also varied across the tasks. The results suggest that there is stability in decision making in relation to the self, both when the task requires that a boundary is set between the self and other faces (i.e., an explicit self–other boundary is required) and when the boundary must be set between familiar faces and others (when an explicit self–other boundary is not required). Sui and Humphreys suggested that the self–other boundary was consistently maintained even when this distinction was not demanded by the task; that is, it is a stable boundary that is applied across different explicit distinctions.
Contrasting with the self-anchor effect observed when external criteria are made in decision making, prior work has shown that self-reference also serves as a stable anchor when no correct answer based on external circumstances is available.59 In this case, subjective preference, determined by self-reference, becomes an internal criterion in decision making. For example, researchers report that self-reference reduces the conflict by biasing one of two occupational choices in decision making compared with other reference.60 The self acting as an implicit anchor in decision making may reflect that the self always exists in our consciousness,61 especially when self-related stimuli are present.
Self-reference and binding
When discussing the effects of self-reference on memory, we noted that the effects were not confined to a general increase of memory performance, but there is a particular benefit on source memory—there is better integration of the stimuli to be memorized with the context in which they were encoded.14,19,22 That is, self-reference may improve the binding of stimuli to the episodic event in memory (Fig. 3A). This apparent effect of self-reference on binding is not confined to memory, and there is also evidence for improved binding of elements in perception and for binding between different stages of processing when stimuli are associated with the self.
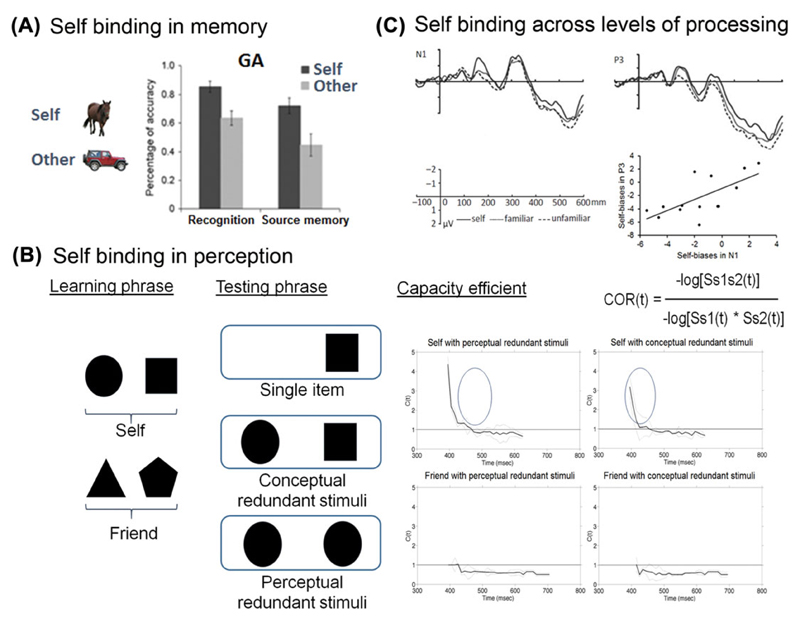
Figure 3.
Self-binding in different levels of information processing. (A) Self-binding in memory (from Ref. 13). (B) Self-binding in perception (from Ref. 62). (C) Self-binding across levels of processing (from Ref. 70).
The enhanced binding of visual elements in perception has been reported by Sui et al.62 Sui et al. first had participants form associations between two geometric shapes and personal labels (you, friend; also see Ref. 27). Subsequently participants saw either a single shape on a trial or two shapes, both of which would correspond to the same person (the shapes could be identical (e.g., two squares, both you) or nonidentical (square + circle, both you)). The task was then to classify the shape(s) as you or your friend (Fig. 3B). When two stimuli are present that map onto the same classification response, there is typically a performance benefit relative to when only a single shape exemplar is present (there is a redundancy gain; see Refs. 63 and 64). One interesting aspect of such redundancy gains is that they can be assessed formally in relation to explicit models of performance; for example, performance can be tested against a model that assumes that there is perceptually independent processing of each of the exemplars.63 Under particular conditions, performance can be better in the redundant target condition relative to that expected from independent processing of the stimuli—participants seem able to respond to the perceptual whole more than the sum of the independent parts. Consistent with this last argument, redundancy gains are particularly strong when the stimulus elements are part of the same perceptual object.65
Sui et al.62 formally modeled shape classification using the capacity analysis proposed by Townsend and Eidels.66 This analysis assesses the probability of a response being made across different time bins in the contrasting conditions, taking the ratio for performance on redundant targets relative to the sum of that for single target trials. When this ratio is <1.0, there is evidence for interference between the processing of the elements on redundant trials (the probability of making the response within a given time window on redundant trials is less than the summed probability on single-item trials). When the ratio is equal to 1.0, then the shapes are said to be processing without capacity limitations. When the ratio is >1.0, then processing shows supercapacity, where, for given time windows, responses are completed with a higher probability than can be expected from the summed probability on single-item trials. The results are presented in Figure 3B, for conditions in which the stimuli had perceptual redundancy (two identical shapes) and when they had conceptual redundancy (two different shapes), for self-related items (top figures) and items related to the friend (bottom figures). The results indicate that there was supercapacity (ratio redundant/summed single shapes >1.0), especially across the short time windows (when the fastest responses were made). This effect occurred both when the shapes were identical and when they were nonidentical, consistent with the enhanced redundancy gain stemming from the conceptual association between the shape and the self. In contrast, friend-related shapes suffered interference on redundant shape trials, with the ratio for redundant shapes/summed single shapes <1.0.
These data suggest that there is enhanced perceptual binding of elements in self-related processing, compared with other-related processing. This enhanced perceptual binding generates super-redundancy gains when two self-related elements are present, over and above the performance expected from independent processing of the elements.
Besides self-related binding in perception, this enhanced binding, when stimuli are associated with the self, may contribute to the tendency to assimilate new information into our self-representations,9,10 including the representations of members of our own group.12 The self can expand because self-reference provides a form of “glue” that binds together different conceptual elements. Interestingly, there is a perceptual counterpart to this notion of self-expansion. Sui and Humphreys67 had participants carry out perceptual matching between personal labels (you, friend, stranger) and geometric shapes (square, circle, triangle). On a minority of trials, the shape could be smaller or larger than the shape that participants learned initially. Responses to self-related stimuli uniquely benefited when the shapes were shown at a larger size, and this size-enhancement effect was correlated with the degree of self-bias that participants showed with medium-sized shapes (participants who tended to have a large self-bias benefited most when self-related shapes were depicted at a larger size). The results suggest that presenting more information relating to self selectively facilitates identification responses, similar to the results on redundancy gains.62,68
The flip side of self-reference facilitating stimulus identification by enhancing the binding of visual features is that performance may be disrupted when self-enhanced binding must be discarded. This was investigated by Wang et al.69 Participants learned associations between shapes and personal labels (see above). After this, the shape–label relationships were switched, so that a shape previously associated with the self or a friend had then to be associated with the label stranger. Sui and Humphreys found that there was worse matching performance when a new association had to be made with the shape formerly linked to the self, compared with when the shape was formerly linked to the friend. There was cost from earlier self-related binding of the shape to the label, which led to new associations to the shape being difficult to form.69
In addition to the evidence for enhanced binding of elements in perception, there are also data indicating that self-reference increases the linkages between different stages of processing. Liu et al.70 assessed the effects of facial cues on responses to peripheral target stimuli. The face cues could cue left or right, and the target was then randomly presented on the left or right side of fixation. Target responses were enhanced when the face turned to match the location of the subsequent target, and this effect was particularly strong when the participant saw their own face as the cue. This increased effect of the facial cue was linked to two components of the visually evoked response, with a greater N1 component and a reduced P300 component for self-related stimuli. An enhanced N1 has been linked to increased attention to a perceptual stimulus, while reductions in the P300 component are associated with greater decision certainty. The self-bias effects on these two components were correlated—participants who showed a greater self-related N1 also showed a greater self-related reduction in the P300 (see Fig. 3C). The results indicate that self-reference mediated the association between attentional responses to the stimulus and greater certainty at the stage of decision making.
The neural basis of self-reference effects
The neural basis of these self-reference effects has been examined in several studies. One consistent finding is that, for a wide range of tasks (e.g., from trait judgments through to perceptual matching of neutral shapes), self-reference is associated with increased activation in the medial prefrontal cortex (mPFC; see Refs. 71–73). Activation of the mPFC has been found when participants are at rest, with activity then sometimes decreasing when participants are engaged in attentionally demanding tasks (Fig. 4A).74 That is, activity of the mPFC may reflect a form of default setting of brain activity. Northoff13 has proposed that this default activity actually reflects our representation of ourselves (Fig. 4B).
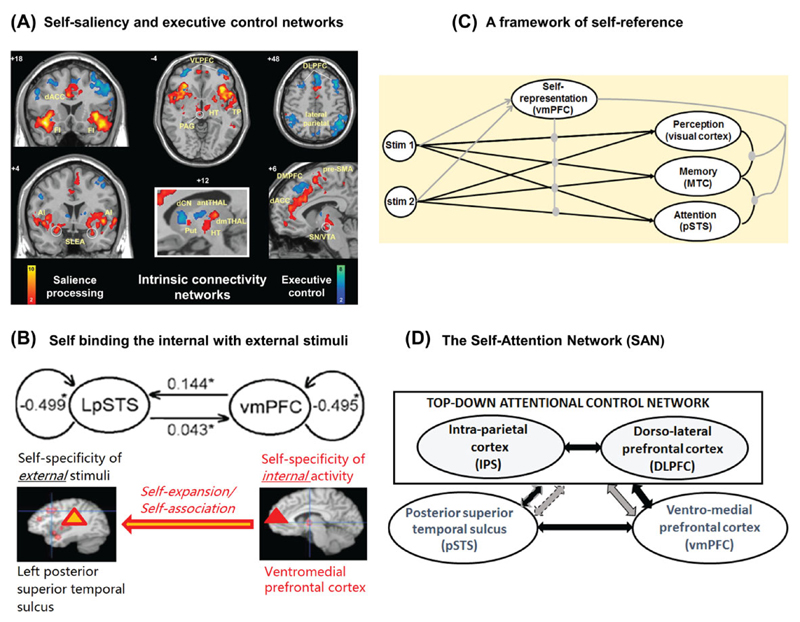
Figure 4.
(A) Intrinsic connectivity networks. The salience network (red–orange color bar) is anchored by paralimbic anterior cingulate and frontoinsular cortices and features extensive connectivity with subcortical and limbic structures. In the executive-control network (blue–green color bar), the dorsolateral frontal and parietal neocortices are linked, with more selective subcortical coupling (derived from Ref. 74). (B) Self-expansion in perception. The figure is about the relationship between self and perception and demonstrates how the internally generated self-related activity in the ventromedial prefrontal activity modulates subsequent externally based stimulus-induced activity as related to the left posterior superior temporal sulcus (from Ref. 13). (C) Neural mechanisms in self-binding: a framework of self-reference. The activation of self-representations modulates (gray nodes) the mapping between stimuli (Stim) and perception, memory, and decision making, and also between different stages of information processing (perception, memory, and decision making) (from Ref. 77). (D) The Self-Attention Network (SAN) distinguishes between a top-down attentional network (including the intraparietal sulcus (IPS) and the dorsolateral prefrontal cortex (DLPFC)) and a network that responds in a bottom-up fashion to self-related information (the posterior superior temporal sulcus (pSTS) and the ventromedial prefrontal cortex (vmPFC)). Black arrows indicate excitatory connections. Gray arrows indicate inhibitory connections. Dotted arrows (to and from the pSTS) highlight that there is currently little direct evidence for these functional corrections (from Ref. 7).
Sui et al.75 examined the relationships between activity in the mPFC and more posterior brain regions related to stimulus-driven attention to the environment (notably the left posterior superior temporal sulcus (LpSTS); see Ref. 76). Brain activity was measured using functional magnetic resonance imaging (fMRI) while participants carried out shape–label perceptual matching with stimuli related to the self, a best friend, and a stranger. They found increased activity to self-related stimuli in the mPFC and also the LpSTS, when compared with stimuli related to other people. Dynamic causal modeling of this activity indicated that self-association in particular increased functional connectivity from the mPFC to the LpSTS (see Fig. 4B). This result fits with the idea that self-bias can come about through upregulation of brain regions responding to stimulus-driven attention, perhaps after access to self-representations in the mPFC. To accommodate this last result, Sui and Humphreys77 speculated that there is rapid access to self-representations in the mPFC, and that this then enhances binding in memory, perception, attention, and decision making, perhaps through increases in excitatory neurotransmitters (Fig. 4C). This speculation awaits future testing.
Taking all of the foregoing considerations together, we propose that the activity of the ventro-medial prefrontal cortex (vmPFC) is related to the functions of self-anchor in decision making and of self-binding. The idea is empirically supported by a recent meta-analysis of neuroimaging studies showing that internally guided decision making (e.g., subjective preference, beliefs) consistently enhances the activity of the vmPFC, which couples with the perigenual anterior cingulate cortex, posterior cingulate cortex, and superior temporal gyrus as a distinct neural network of internally guided decision making.59 On the other hand, the binding function with the activity of the vmPFC is supported by the studies in self-referential memory20 and self-perception.75 For example, in a shape–personal label matching task, participants were assigned an external stimulus (e.g., geometric shape) with themselves, which increased the neural coupling between the vmPFC and the posterior superior temporal sulcus (pSTS), indicating the associations between self-related label and neutral geometric shapes.46 Here, we argue that one of the functions of the vmPFC is to bind external stimuli/objects with the established self-representation, and self-binding strength is indexed by the coupling strength from the vmPFC to the pSTS, reflecting the degree of self-expansion.
Interestingly, in the study of Sui et al.75 the brain regions typically linked to task-driven attentional control (the dorsal frontoparietal network; Ref. 78) were more activated when participants responded to associations to other people compared with associations to themselves. This suggests that stimulus-driven attention to self-associated stimuli can be distinguished from task-dependent attention, recruited for matches to other people (Fig. 4D).
A greater understanding of the mechanisms underlying the functions of the self will have widespread application in neuropsychological patients and psychiatric disorders by providing objective measures rather than traditionally subjective assessment. For example, it has been found that schizophrenic patients showed decreased activity in the vmPFC and ACC and their functional connectivity79 related to self-representation in a self-reflection task. This result indicated that the objective measures of self-function may provide important indices for psychiatric diagnosis.
Humphreys and Sui7 have recently discussed these results in relation to the framework of a self-attention network. They propose that self-related attention is dependent on a ventral network involving the mPFC and LpSTS, with activation of this network acting to drive attention to self-related stimuli in the environment. This self-related network interacts with more dorsal frontoparietal brain regions involved in task-based attentional control. For example, in simple shape–label perceptual matching tasks, activation of the ventral network speeds responses to self-related stimuli but may interfere with responses to stimuli related to other people, when self-related responses have to be overruled (this could occur owing to re-pairings of the self-related label to another shape, on mismatching trials; on subsequent matching trials, the shape may now partially activate the self-network). In tasks where self-related information is associated with distractors and has to be ignored,35 the dorsal network would need to inhibit the ventral self-network to enable self-related information to be ignored. However, when self-related stimuli are task relevant, then the dorsal network may boost the ventral network to enhance stimulus processing. Evidence consistent with this was reported by Sui and Humphreys.80 In this study, the probability of different stimuli was varied in a perceptual matching task. Self-bias increased when self-related stimuli had a high probability of occurrence.
These arguments for a distinction between a ventral, self-related network and a more dorsal attentional control network are in accord with brain imaging studies of resting-state activity that separate out contrasting neural networks based on patterns of intercorrelated brain activity. For example, Seeley et al.74 distinguished between what they term a saliency network involving paralimbic brain regions, the anterior cingulate cortex and frontoinsular cortices, and a more dorsal, frontoparietal network that they link to executive attentional control. We argue that the more ventral, saliency network is implicated in self-reference effects.
Neuropsychological evidence also supports the argument for the distinct, and sometimes antagonistic effects of self-attention and task-based, executive attention, and for the critical role of the mPFC and the LpSTS in self-based attention. Sui et al.81 report data from two patients, one with damage to the mPFC and one with more posterior damage involving the LpSTS. They found that self-bias effects on perceptual matching were reduced in the patient with the mPFC lesion, consistent with damage to the self-representation critical to “drive” attention to self-related items. On the other hand, the patient with LpSTS damage showed an increased self-bias effect. This last result can be understood if the top-down activation from self-representations (in the unlesioned mPFC) has a greater than normal effects when attentional processes are disrupted (due to the LpSTS lesion).
Is the self special?
One long-standing question, raised by the evidence on self-bias effects, is whether the effects of the self on the stability of decision making and on binding is in some way special, dissociated from other general factors that may modulate memory, perception, and attention. One potentially important factor is familiarity. Especially in experiments using face stimuli, it may be that performance is modulated by differential familiarity of one’s own face relative to the faces of other people. People tend to see their own images more often in the mirror than other’s images. It has been argued that the self-bias effects in the presence of one’s own mirror image relative to the face images of others may reflect the familiarity of stimuli. In contrast to the account of familiarity in self-bias effects, there is also evidence showing the qualitative difference between the processing of one’s own and close other face images. For example, researchers have reported that, when classifying the face images of self, friend, or stranger, participants made faster responses to the self faces than the faces of others, no matter whether the face images were presented upright or upside down. In contrast, the familiarity effect for the friend (versus stranger) face was exclusively observed when the face images were inverted. The results indicate that the self-biased responses cannot be reduced to greater familiarity of stimuli. In addition, differential familiarity with the stimulus cannot be the critical factor in studies using neutral shapes, especially when simple shape identification is required.46,68 Similarly, studies have demonstrated a self-advantage even when other people are highly familiar (e.g., the participant’s mother or his/her best friend; see Ref. 82). At the very least, there seems to be a nonlinear effect of familiarity in relation to the self.
Other possibilities are that self-biases reflect differences in the reward and/or emotional value of self-related stimuli. Northoff and Hayes,83 for example, argue that self-related stimuli are inherently rewarding, and so the effects of self-bias may specifically stem from the effects of reward on memory, perception, and attention. Alternatively, Ma and Han24 proposed that self-related stimuli generate a positive emotional response and that this may modulate the observed effects. Note that there are effects of reward and emotional valence on cognition, which are unrelated to the self,84–86 so effects of reward and emotion linked to the self would not necessarily stem from some special properties of the self.
Though there remains much work to be done on the relationships between the self, reward, and emotion, there are results indicating that not all aspects of self-bias arise from these factors. For example, redundancy gains in shape identification tasks are stronger for self-related stimuli, and only self-related stimuli manifest supercapacity when multiple exemplars are presented.62 Self- but not reward-related stimuli are sensitive to changes in the size of the associated shapes in shape-matching tasks.67 Also, while both self- and reward biases are stable across individuals over time (see Fig. 2), the two biases do not strongly correlate across individuals, and self- and high-reward associations do not cluster together when variations across individuals are examined. Such results indicate that self- and reward biases can at least be partially dissociated. Similarly, there are not strong associations between self-bias effects and biases to positive emotion,49 consistent with some differentiation between self- and emotion-related biases. An important issue for future research is to delineate whether at least some aspects of self-bias reflect these underlying factors and whether other aspects relate to self-specific representation.
Conclusions
In this review, we focused on the functions of the self and how they arise across diverse situations. Through an empirical measure of self-biases, we have reviewed evidence indicating the pervasive influence of self-reference on information processing from perception, through attention, to memory. Furthermore, we have argued that the functions of self-reference are linked to specific processing changes—(1) the self being used as a stable anchor point in decision making and (2) self-reference modulating binding. The functions of self-anchoring and self-binding are mediated by the activity of the vmPFC, which interacts with other brain regions to generate self-biased responses in a given situation. We argue that, by delineating the two properties of self-reference through self-bias effects, we begin to learn about the functional role of self-representation at particular stages of cognition, and, through this, what self-representation does for us.
Acknowledgments
This paper is dedicated to the memory of Glyn W. Humphreys (1954–2016). This work was supported by grants from the Economic and Social Research Council (UK) (ES/K013424/1) and a Wellcome Trust Senior Investigator Award (WT 106164MA).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Klein S, Kihlstrom JF. Elaboration, organization, and the self-reference effect in memory. J Exp Psychol Gen. 1986;115:26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- 2.Klein S, Loftus J, Kihlstrom J. Self-knowledge of an amnesic patient: toward a neuropsychology of personality and social psychology. J Exp Psychol Gen. 1996;125:250–260. doi: 10.1037//0096-3445.125.3.250. [DOI] [PubMed] [Google Scholar]
- 3.Conway M, Pleydell-Pearce C. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Rathbone CJ, Conway MA, Moulin CJA. Remembering and imagining: the role of the self. Conscious Cogn. 2011;20:1175–1182. doi: 10.1016/j.concog.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- 6.Moray N. Attention in dichotic listening: affective cues and the influence of instructions. Q J Exp Psychol. 1959;11:56–60. [Google Scholar]
- 7.Humphreys GW, Sui J. Attentional control and the self: the Self-Attention Network (SAN) Cogn Neurosci. 2015;7:5–17. doi: 10.1080/17588928.2015.1044427. [DOI] [PubMed] [Google Scholar]
- 8.James W. The Principles of Psychology. London: Macmillan; 1890. [Google Scholar]
- 9.Aron A, Aron EN. Self and self expansion in relationships. In: Fletcher GJO, Fitness J, editors. Knowledge Structures in Close Relationships: A Social Psychological Approach. Mahway, NJ: Lawrence Erlbaum Associates; 1996. pp. 325–344. [Google Scholar]
- 10.Aron A, Aron EN, Norman C. Self expansion model of motivation and cognition in close relationships and beyond. In: Clark M, Fletcher G, editors. Blackwell’s Handbook of Social Psychology. Vol. 2: Interpersonal processes. Oxford: Blackwell; 2001. pp. 478–501. [Google Scholar]
- 11.Smith E, Henry S. An in-group becomes part of the self: response time evidence. Pers Soc Psychol Bull. 1996;22:635–642. [Google Scholar]
- 12.Swann W, Jetten J, Gomez A, et al. When group membership gets personal: a theory of identity fusion. Psychol Rev. 2012;119:441–456. doi: 10.1037/a0028589. [DOI] [PubMed] [Google Scholar]
- 13.Northoff G. Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain’s spontaneous activity. Cogn Neurosci. 2016;7:203–222. doi: 10.1080/17588928.2015.1111868. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham S, Turk D, Macdonald L, et al. Yours or mine? Ownership and memory. Conscious Cogn. 2008;17:312–318. doi: 10.1016/j.concog.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Gutchess A, Kensinger E, Yoon C, et al. Ageing and the self-reference effect in memory. Memory. 2007;15:822–837. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- 16.Symons C, Johnson B. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- 17.Turk D, van Bussel K, Brebner J, et al. When “it” becomes “mine”: attentional biases triggered by object ownership. J Cogn Neurosci. 2011;23:3725–3733. doi: 10.1162/jocn_a_00101. [DOI] [PubMed] [Google Scholar]
- 18.Leshikar ED, Dulas MR, Duarte A. Self-referencing enhances recollection in both young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:388–412. doi: 10.1080/13825585.2014.957150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sui J, Humphreys GW. Self-referential processing is distinct from semantic elaboration: evidence from long-term memory effects in a patient with amnesia and semantic impairments. Neuropsychologia. 2013;51:2663–2673. doi: 10.1016/j.neuropsychologia.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Craik F, Moroz T, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- 21.Kalenzaga S, Sperduti M, Anssens A, et al. Episodic memory and self-reference via semantic autobiographical memory: insights from an fMRI study in younger and older adults. Front Behav Neurosci. 2015;8:449. doi: 10.3389/fnbeh.2014.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham S, Brebner J, Quinn F, et al. The self-reference effect on memory in early childhood. Child Dev. 2014;85:808–823. doi: 10.1111/cdev.12144. [DOI] [PubMed] [Google Scholar]
- 23.Sui J, Zhu Y. Five-year-olds can show the self-reference advantage. Int J Behav Dev. 2005;29:382–387. [Google Scholar]
- 24.Ma Y, Han S. Why we respond faster to the self than to others? An implicit positive association theory of self-advantage during implicit face recognition. J Exp Psychol Hum Percept Perform. 2010;36:619–633. doi: 10.1037/a0015797. [DOI] [PubMed] [Google Scholar]
- 25.Sui J, Han S. Self-construal priming modulates neural substrates of self-awareness. Psychol Sci. 2007;18:861–866. doi: 10.1111/j.1467-9280.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 26.Sui J, Liu C, Wang L, et al. Attentional orientation induced by temporarily established self-referential cues. Q J Exp Psychol. 2009;62:844–849. doi: 10.1080/17470210802559393. [DOI] [PubMed] [Google Scholar]
- 27.Sui J, He X, Humphreys GW. Perceptual effects of social salience: evidence from self-prioritization effects on perceptual matching. J Exp Psychol Hum Percept Perform. 2012;38:1105–1117. doi: 10.1037/a0029792. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro K, Caldwell J, Sorensen R. Personal names and the attentional blink: a visual “cocktail party” effect. J Exp Psychol Hum Percept Perform. 1997;23:504–514. doi: 10.1037//0096-1523.23.2.504. [DOI] [PubMed] [Google Scholar]
- 29.Mack A, Rock I. Inattentional Blindness. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 30.Yang H, Wang F, Gu N, et al. The cognitive advantage for one’s own name is not simply familiarity: an eye-tracking study. Psychon Bull Rev. 2013;20:1176–1180. doi: 10.3758/s13423-013-0426-z. [DOI] [PubMed] [Google Scholar]
- 31.Geng H, Zhang S, Li Q, et al. Dissociations of subliminal and supraliminal self-face from other-face processing: behavioral and ERP evidence. Neuropsychologia. 2012;50:2933–2942. doi: 10.1016/j.neuropsychologia.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Bargh J, Pratto F. Individual construct accessibility and perceptual selection. J Exp Soc Psychol. 1986;22:293–311. [Google Scholar]
- 33.Wood N, Cowan N. The cocktail party phenomenon revisited: attention and memory in the classic selective listening procedure of Cherry (1953) J Exp Psychol Gen. 1995;124:243–262. doi: 10.1037//0096-3445.124.3.243. [DOI] [PubMed] [Google Scholar]
- 34.Roer J, Bell R, Buchner A. Self-relevance increases the irrelevant sound effect: attentional disruption by one’s own name. J Cogn Psychol. 2013;25:925–931. [Google Scholar]
- 35.Sui J, Chechlacz M, Humphreys G. Dividing the self: distinct neural substrates of task-based and automatic self-prioritization after brain damage. Cognition. 2012;122:150–162. doi: 10.1016/j.cognition.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Sui J, Chechlacz M, Rotshtein P, et al. Lesion-symptom mapping of self-prioritization in explicit face categorization: distinguishing hypo- and hyper-self-biases. Cereb Cortex. 2013;25:374–383. doi: 10.1093/cercor/bht233. [DOI] [PubMed] [Google Scholar]
- 37.Turk D, Brady-van den Bos M, Collard P, et al. Divided attention selectively impairs memory for self-relevant information. Mem Cogn. 2013;41:503–510. doi: 10.3758/s13421-012-0279-0. [DOI] [PubMed] [Google Scholar]
- 38.Gardiner J, Gregg V, Mashru R, et al. Impact of encoding depth on awareness of perceptual effects in recognition memory. Mem Cogn. 2001;29:433–440. doi: 10.3758/bf03196394. [DOI] [PubMed] [Google Scholar]
- 39.Bundesen C, Kyllingsbaek S, Houmann K, et al. Is visual attention automatically attracted by one’s own name? Percept Psychophys. 1997;59:714–720. doi: 10.3758/bf03206017. [DOI] [PubMed] [Google Scholar]
- 40.Devue C, Bredart S. Attention to self-referential stimuli: can I ignore my own face? Acta Psychol. 2008;128:290–297. doi: 10.1016/j.actpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Mattan B, Quinn K, Apperly I, et al. Is it always me first? Effects of self-tagging on third-person perspective-taking. J Exp Psychol Learn Mem Cogn. 2015;41:1100–1117. doi: 10.1037/xlm0000078. [DOI] [PubMed] [Google Scholar]
- 42.Samson D, Apperly I, Braithwaite J, et al. Seeing it their way: evidence for rapid and involuntary computation of what other people see. J Exp Psychol Hum Percept Perform. 2010;36:1255–1266. doi: 10.1037/a0018729. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi A, Apperly I, Samson D. Executive function is necessary for perspective selection, not level-1 visual perspective calculation: evidence from a dual-task study of adults. Cognition. 2010;117:230–236. doi: 10.1016/j.cognition.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Harris C, Pashler H. Attention and the processing of emotional words and names—not so special after all. Psychol Sci. 2004;15:171–178. doi: 10.1111/j.0956-7976.2004.01503005.x. [DOI] [PubMed] [Google Scholar]
- 45.Perrin F, Schnakers C, Schabus M, et al. Brain response to one’s own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch Neurol. 2006;63:562–569. doi: 10.1001/archneur.63.4.562. [DOI] [PubMed] [Google Scholar]
- 46.Sui J, Liu M, Mevorach C, et al. The salient self: the left intraparietal sulcus responds to social as well as perceptual-salience after self-association. Cereb Cortex. 2013;25:1060–1068. doi: 10.1093/cercor/bht302. [DOI] [PubMed] [Google Scholar]
- 47.Sui J. Self-reference acts as a golden thread in binding. Trends Cogn Sci. 2016;20:482–483. doi: 10.1016/j.tics.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphreys G, Sui J. The salient self: social saliency effects based on self-bias. J Cogn Psychol. 2015;27:129–140. [Google Scholar]
- 49.Stolte M, Humphreys GW, Yankouskaya A, et al. Dissociating biases towards the self and positive emotion. Q Exp Psychol. 2016;29:1–12. doi: 10.1080/17470218.2015.1101477. [DOI] [PubMed] [Google Scholar]
- 50.Sui J, Humphreys G. The boundaries of self face perception: response time distributions, perceptual categories, and decision weighting. Vis Cogn. 2013;21:415–445. [Google Scholar]
- 51.Balota D, Yap M, Cortese M, et al. Beyond mean response latency: response time distributional analyses of semantic priming. J Mem Lang. 2008;59:495–523. [Google Scholar]
- 52.Heathcote A, Brown S, Mewhort D. Quantile maximum likelihood estimation of response time distributions. Psychon Bull Rev. 2002;9:394–401. doi: 10.3758/bf03196299. [DOI] [PubMed] [Google Scholar]
- 53.Moutsopoulou K, Waszak F. Across-task priming revisited: response and task conflicts disentangled using ex-Gaussian distribution analysis. J Exp Psychol Hum Percept Perform. 2012;38:367–374. doi: 10.1037/a0025858. [DOI] [PubMed] [Google Scholar]
- 54.Hohle R. Inferred components of reaction times as functions of foreperiod duration. J Exp Psychol. 1965;69:382–386. doi: 10.1037/h0021740. [DOI] [PubMed] [Google Scholar]
- 55.Tse C, Altarriba J. The effects of first- and second-language proficiency on conflict resolution and goal maintenance in bilinguals: evidence from reaction time distributional analyses in a Stroop task. Biling-Lang Cogn. 2012;15:663–676. [Google Scholar]
- 56.Balota D, Spieler D. Word frequency, repetition, and lexicality effects in word recognition tasks: beyond measures of central tendency. J Exp Psychol Gen. 1999;128:32–55. doi: 10.1037//0096-3445.128.1.32. [DOI] [PubMed] [Google Scholar]
- 57.Schmiedek F, Oberauer K, Wilhelm O, et al. Individual differences in components their relations to working of reaction time distributions and memory and intelligence. Exp Psychol Gen. 2007;136:414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- 58.Staub A. Response time distributional evidence for distinct varieties of number attraction. Cognition. 2010;114:447–454. doi: 10.1016/j.cognition.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Nakao T, Ohira H, Northoff G. Distinction between externally vs. internally guided decision-making: operational differences, meta-analytical comparisons and their theoretical implications. Front Neurosci. 2012;6:31. doi: 10.3389/fnins.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakao T, Osumi T, Ohira H, et al. Medial prefrontal cortex–dorsal anterior cingulate cortex connectivity during behavior selection without an objective correct answer. Int J Psychophysiol. 2010;77:323. doi: 10.1016/j.neulet.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 61.Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 62.Sui J, Yankouskaya A, Humphreys G. Super-capacity me! Super-capacity and violations of race independence for self- but not for reward-associated stimuli. J Exp Psychol Hum Percept Perform. 2015;41:441–452. doi: 10.1037/a0038288. [DOI] [PubMed] [Google Scholar]
- 63.Miller J. Divided attention: evidence for coactivation with redundant signals. Cogn Psychol. 1982;14:247–279. doi: 10.1016/0010-0285(82)90010-x. [DOI] [PubMed] [Google Scholar]
- 64.Raab D. Statistical facilitation of simple reaction times. Trans N Y Acad Sci. 1962;24:574–590. doi: 10.1111/j.2164-0947.1962.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 65.Mordkoff J, Danek R. Dividing attention between color and shape revisited: redundant targets coactivate only when parts of the same perceptual object. Atten Percept Psychophys. 2011;73:103–112. doi: 10.3758/s13414-010-0025-2. [DOI] [PubMed] [Google Scholar]
- 66.Townsend J, Eidels A. Workload capacity spaces: a unified methodology for response time measures of efficiency as workload is varied. Psychon Bull Rev. 2011;18:659–681. doi: 10.3758/s13423-011-0106-9. [DOI] [PubMed] [Google Scholar]
- 67.Sui J, Humphreys G. Super-size me: self biases increase to larger stimuli. Psychon Bull Rev. 2015;22:550–558. doi: 10.3758/s13423-014-0690-6. [DOI] [PubMed] [Google Scholar]
- 68.Sui J, Humphreys GW. More of me! Distinguishing self- and reward bias using redundancy gains. Atten Percept Psychophys. 2015;77:2549–2561. doi: 10.3758/s13414-015-0970-x. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Humphreys GW, Sui J. Expanding and retracting from the self: gains and costs in switching self-associations. J Exp Psychol Hum Percept Perform. 2015;42:247–256. doi: 10.1037/xhp0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu M, He X, Rotshtein P, et al. Dynamically orienting your own face facilitates the automatic attraction of attention. Cogn Neurosci. 2016;7:37–44. doi: 10.1080/17588928.2015.1044428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Kelley W, Macrae C, Wyland C, et al. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 73.Murray R, Schaer M, Debbane M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Seeley W, Menon V, Schatzberg A, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sui J, Rotshtein P, Humphreys G. Coupling social attention to the self forms a network for personal significance. Proc Natl Acad Sci USA. 2013;110:7607–7612. doi: 10.1073/pnas.1221862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samson D, Apperly I, Chiavarino C, et al. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- 77.Sui J, Humphreys G. The integrative self: how self-reference integrates perception and memory. Trends Cogn Sci. 2015;12:719–728. doi: 10.1016/j.tics.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 79.Holt D, Cassidy B, Andrews-Hanna J, et al. An anterior-to-posterior shift in mid line cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sui J, Sun Y, Peng K, et al. The automatic and the expected self: separating self- and familiarity biases effects by manipulating stimulus probability. Atten Percept Psychophys. 2014;76:1176–1184. doi: 10.3758/s13414-014-0631-5. [DOI] [PubMed] [Google Scholar]
- 81.Sui J, Enock F, Ralph J, Humphreys GW. Dissociating hyper- and hypo-self biases to a core self-representation. Cortex. 2015;70:202–212. doi: 10.1016/j.cortex.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y, Zhang L, Fan J, et al. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34:1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 83.Northoff G, Hayes D. Is our self nothing but reward? Biol Psychiatry. 2011;69:1019–1025. doi: 10.1016/j.biopsych.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PLoS One. 2011;6:e27926. doi: 10.1371/journal.pone.0027926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chelazzi L, Estocinova J, Calletti R, et al. Altering spatial priority maps via reward-based learning. J Neurosci. 2014;34:8594–8604. doi: 10.1523/JNEUROSCI.0277-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ethofer T, De Ville D, Scherer K, et al. Decoding of emotional information in voice-sensitive cortices. Curr Biol. 2009;19:1028–1033. doi: 10.1016/j.cub.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 87.Keyes H, Brady N. Self-face recognition is characterized by “bilateral gain” and by faster, more accurate performance which persists when faces are inverted. Q J Exp Psychol. 2010;63:840–847. doi: 10.1080/17470211003611264. [DOI] [PubMed] [Google Scholar]
- 88.Sui J, Ohrling E, Humphreys GW. Negative mood disrupts self- and reward-biases in perceptual matching. Q J Exp Psychol. 2016;69:1438–1448. doi: 10.1080/17470218.2015.1122069. [DOI] [PMC free article] [PubMed] [Google Scholar]