Abstract
Extracellular interleukin 1 alpha (IL-1α) released from keratinocytes is one of the endpoints for in vitro assessments of skin irritancy. Although cells dying via primary skin irritation undergo apoptosis as well as necrosis, IL-1α is not released in apoptotic cells. On the other hand, active secretion has been identified in interleukin-1 receptor antagonist (IL-1ra), which was discovered to be a common, upregulated, differentially-expressed gene in a microarray analysis performed with keratinocytes treated using cytotoxic doses of chemicals. This study examined whether and how IL-1ra, particularly extracellularly released IL-1ra, was involved in chemically-induced keratinocyte cytotoxicity and skin irritation. Primary cultured normal adult skin keratinocytes were treated with cytotoxic doses of chemicals (hydroquinone, retinoic acid, sodium lauryl sulfate, or urshiol) with or without recombinant IL-1ra treatment. Mouse skin was administered irritant concentrations of hydroquinone or retinoic acid. IL-1ra (mRNA and/or intracellular/extracellularly released protein) levels increased in the chemically treated cultured keratinocytes with IL-1α and IL-1β mRNAs and in the chemically exposed epidermis of the mouse skin. Recombinant IL-1ra treatment significantly reduced the chemically-induced apoptotic death and intracellular/extracellularly released IL-1α and IL-1β in keratinocytes. Collectively, extracellular IL-1ra released from keratinocytes could be a compensatory mechanism to reduce the chemically-induced keratinocyte apoptosis by antagonism to IL-1α and IL-1β, suggesting potential applications to predict skin irritation.
Keywords: IL-1ra, Keratinocyte apoptosis, Skin irritation
INTRODUCTION
Skin irritation is a common inflammatory reaction induced by skin contact. In response to chemical contact, resident skin cells and infiltrating inflammatory cells synthesize and release cytokines, inflammatory mediators. In fact, cytokine release, along with cell viability and cytotoxicity (cell membrane integrity), has been presented as an endpoint for the in vitro assessment of skin irritancy (Osborne and Perkins, 1991). Keratinocytes, in particular, play a crucial role in regulating inflammatory reactions of the skin. Interleukin 1 alpha (IL-1α) production and release from epidermal keratinocytes increase when an irritant is applied (Coquette et al., 1999), and the extracellular release of IL-1α is widely accepted as a predictor of chemical irritancy (Muller-Decker et al., 1994; Corsini and Galli, 1998; Perkins et al., 1999; Kidd et al., 2007). However, IL-1α is not released in apoptotic cells (Cohen et al., 2010), although cells dying by primary skin irritation undergo apoptosis as well as necrosis (Kanerva, 1990). In addition, the role of IL-1α as a representative marker in skin irritation remains disputed, particularly with chronic, repeat exposure to an irritant (De Jongh et al., 2006).
A microarray analysis was performed using primary, cultured normal adult skin keratinocytes treated with or without chemicals at a concentration of 80% cell survival, and the results suggest that interleukin-1 receptor antagonist (IL-1ra) was a common upregulated differentially expressed gene (Supplementary Table 1). IL-1ra, a member of the IL-1 family, is the first to be described as an endogenous antagonist that inhibits the activities of IL-1α and IL-1β via competitive binding to the surface IL-1 receptor (Arend et al., 1998). IL-1ra plays a critical role as an anti-inflammatory protein in numerous experimental animal models of disease and in human autoimmune and chronic inflammatory diseases. A loss-of-function mutation in IL-1ra allows an unopposed action by IL-1 and leads to life-threatening systemic inflammation, particularly in the skin and bone (Aksentijevich et al., 2009). Regarding homeostasis, a role could be expected for the anti-inflammatory cytokine, IL-1ra, in skin irritation. Among four isoforms of IL-1ra, one secreted (sIL-1ra) and three intracellular (icIL-1ra) (Arend and Guthridge, 2000), the intracellular form of IL-1ra is produced with IL-1α and IL-1β in keratinocytes. IL-1ra needs be secreted for competitive inhibition against binding of IL-1α and IL-1β to cell surface IL-1 receptors (Arend et al., 1998). Although no secretion of icIL-1ra due to a lack of a secretory signal peptide has been documented in keratinocytes (Corsini and Galli, 1998), the active secretion of the keratinocyte-derived icIL-1ra through a leaderless secretion pathway has been identified (Corradi et al., 1995). Nonetheless, the correlation between IL-1ra expression levels and skin irritation due to chemicals has been rarely examined (Bernhofer et al.,1999; Perkins et al., 2001), and its action mechanism is unknown.
Hydroquinone (HQ) and retinoic acid (RA) have not been classified as irritants. However, skin irritation is one of the most common adverse reactions to HQ and RA, popular chemicals that ameliorate hyperpigmentation (Noble and Wagstaff, 1995; Matsubayashi et al., 2003; Samuel et al., 2005; Stratigos and Katsambas, 2005). In this study, we examined whether IL-1ra, particularly extracellularly released IL-1ra, could be involved in keratinocyte apoptosis and skin irritation due to chemicals, including these depigmenting agents. IL-1ra mRNA and/or protein expression increased time- and/or dose-dependently in primary cultured normal human keratinocytes and mouse skin after applying different cytotoxic concentrations of depigmenting chemicals (HQ and RA), a representative irritant (SLS), or a representative sensitizer (urshiol). Recombinant human IL-1ra (rhIL-1ra) reduced the chemical-induced apoptosis of keratinocytes with the inhibition of IL-1α and IL-1β expression, suggesting the potential applicability of extracellular IL-1ra in predicting skin irritation.
MATERIALS AND METHODS
Cell culture
Adult skin specimens were obtained from Caesarean sections and circumcisions to establish cells in culture. For keratinocyte culture, individual epidermal cells were suspended in EpiLife Medium (Invitrogen, Carlsbad, CA, USA) supplemented with bovine pituitary extract, bovine insulin, hydrocortisone, human epidermal growth factor and bovine transferrin (Invitrogen). The harvested cells were resuspended at 7.5×104 cells/mL in each culture medium and were seeded at 1.5×105 cells/well into six-well plates. One day after seeding, appropriate concentrations of each chemical were added. After two days, the keratinocyte was harvested and subjected to a cell viability assay, TUNEL assay, real-time PCR, Western blot analysis, and ELISA.
Mouse preparation
Eight-week-old female C57BL/6 mice purchased from Orient Bio (Gyeonggi-do, Korea) were maintained in an uncontrolled, conventional air environment in the Laboratory Animal Facilities at the Dongguk University School of Medicine. The Dongguk University Institutional Animal Care and Use Committee (IACUC) approved all described protocols (Protocol number 20131194). The hair on the backs of the mice was shaved using an electric shaver, followed by treatment with a skin-hair-remover (Ildong, Seoul, Korea). One day later, a patch was applied using a 2 cm-diameter gauze patch, impregnated with the appropriate concentration of each chemical, on the shaved dorsal skin. These procedures were repeated twice for 48 hrs. Chemically-treated full thickness skin specimens were biopsied and stored at −80°C until use for immunohistochemistry.
Chemicals and reagents
For the in vitro studies, HQ (Sigma Aldrich, St. Louis, MO, USA) and RA (Sigma Aldrich) were prepared as stock solution using dimethylsulfoxide (DMSO; Sigma Aldrich). SLS (Sigma Aldrich) was solved in distilled water (DW) to produce a stock solution. For the in vivo studies, HQ was prepared as 10% and 20% and RA as 0.1% and 0.5% in a petrolatum base. Recombinant human interleukin-1 receptor antagonist (rhIL-1ra; R&D Systems, Minneapolis, MA, USA) was diluted using phosphate buffered saline (PBS).
Cell viability test
The cell viability was evaluated using the MTT reduction method. The cells were stained with MTT for 4 hrs. The precipitated formazan was then dissolved in distilled water (DW), DMSO, and the optical density was measured at 570 nm with background subtraction at 630 nm using a spectrophotometer. The effects that HQ, RA, SLS and urshiol (Sigma, St. Louis, MO, USA) had on cell growth were calculated from the ratio of the cell viability relative to that with solvent, DW or DMSO. The irritant concentrations were determined as the range of concentrations showing approximately 40–50% and 70–80% of cell viability ratios in two days, which were 20 and 40 µM in HQ, 0.5 and 1.5 µM in RA, 8 and 12 µM in SLS, and 1 and 2 µM in urshiol in keratinocyte monocultures.
TUNEL assay
A TUNEL assay was performed to detect apoptotic cells using the Apo-BrdU TUNEL assay kit (A23210; Invitrogen) processed following the manufacturer’s instructions. The cells were fixed in 2% paraformaldehyde and 70% ice-cold ethanol. The stained cells were then observed under a fluorescence microscope. To conduct a quantitative analysis of the apoptosis, four random fields were photographed from the apoptotic samples, and the total number of cells was counted.
Real-time PCR
cDNA was synthesized from the total RNA using the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Boehringer Mannheim, Germany). The amount of target mRNAs was quantified via real-time PCR using a Light Cycler real-time PCR machine (Roche, Penzberg, Germany). The relative amount of mRNAs was calculated by the ratio of each target relative to the amount of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences for real-time PCR were as follows: IL-1α 5′-GGTTGAGTTTAAGCCAATCCA-3′ (forward) and 5′-TGCTGACCTAGGCTTGATGA-3′ (reverse); IL-1β 5′-CCGGGACTCACAGCAAAA-3′ (forward) and 5′-GGACATGGAGAACACCACTTG-3′ (reverse); IL-1ra 5′-TGGCTTTAGCTGACTTGTATGAAG-3′ (forward) and 5′-TTGCTGGATTTTCTCCCAGA-3′ (reverse); GAPDH 5′-TCCACTGGCGTCTTCACC-3′ (forward) and 5′-GGCAGAGATGATGACCCTTT-3′ (reverse).
Western blot analysis
Equal amounts of extracted proteins were resolved and transferred to nitrocellulose membranes. The membranes were incubated with antibodies to IL-1α, IL-1β (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and IL-1ra, cleaved caspase-3 (Cell Signaling Technology, Beverly, MA). After incubating with appropriate anti-mouse or anti-rabbit horseradish peroxidase–conjugated antibodies (Thermo Fisher Scientific, Rockford, IL, USA) and enhanced chemiluminescence solution (Thermo Fisher Scientific), the signals were captured on an Image Reader (LAS-3000; Fuji Photo Film, Tokyo, Japan). To monitor the amount of protein loaded in each lane, the membranes were reprobed with a mouse monoclonal anti-β-actin antibody (Sigma) and were processed as described above. The protein bands were then analyzed via densitometry.
ELISA
Supernatant of cultured keratinocytes was collected at all steps of previous experiments. The concentration of human IL-1ra, IL-1α, and IL-1β in the supernatants was measured using ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Immunohistochemistry
For immunofluorescence staining of IL-1ra, fixed depigmented and normally pigmented epidermis samples were embedded in paraffin wax and sectioned at 4 mm. After deparaffinization and rehydration, the sections were boiled in a 100 mM citrate solution (pH 6.0) for 10 minutes on a hotplate. The sections were preincubated with 3% bovine serum albumin for 1 hr at room temperature and were then reacted sequentially with 1:100 anti-IL-1ra antibody for overnight at 4°C and Alexa Fluor labeled goat anti-rabbit IgG (488; Molecular Probes, Eugene, OR, USA) for 1 hr at room temperature. The nuclei were counterstained with Hoechst 33258 (Sigma Aldrich). The stained specimens were observed using an image analysis system (Dp Manager 2.1; Olympus Optical Co., Tokyo, Japan).
Statistical analysis
The statistical significance was assessed using Student’s t tests, and a p-value of less than 0.05 is considered statistically significant. All results are presented as means ± SD of the combined data from 3 to 5 independent experiments.
RESULTS
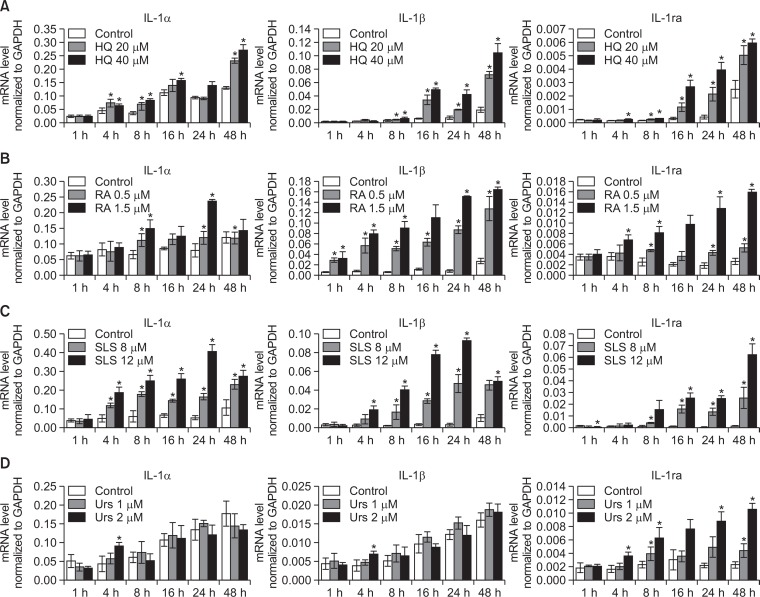
The cytotoxic doses of chemicals increased IL-1ra mRNA expression in a time- and dose-dependent manner in cultured human keratinocytes
Cultured normal human keratinocytes were treated with HQ, RA, SLS, and urshiol with two different doses showing 70–80% and 40–50% cell survival in an MTT assay. Sequential mRNA levels of IL-1ra were examined with that of IL-1α and IL-1β for 48 hrs. Real-time PCR showed the absolute IL-1ra mRNA levels normalized to GAPDH increased a time- and dose-dependent manner in these chemicals (Fig. 1). The relative increase in IL-1ra for each chemical compared to the corresponding solvent were usually higher than that of IL-1α and IL-1β, although the absolute mRNA levels for IL-1ra were lower than those of IL-1α and IL-1β. The relative increase was more consistent in IL-1ra mRNA compared to IL-1α and IL-1β mRNAs, which was more remarkable in urshiol-treated cells.
Fig. 1.
Cytotoxic doses of the chemicals increased IL-1ra mRNA expression in a time- and dose-dependent manner in cultured human keratinocytes. A time-course of IL-1ra with IL-1α and IL-1β mRNA levels via real time-PCR in primary cultured normal human keratinocytes treated with (A) HQ, (B) RA, (C) SLS, or (D) urshiol using two different doses of 70–80% and 40–50% cell survival in MTT assay. Data in the graph represents mean ± SD of absolute values from 3 independent experiments (*p<0.05 vs. solvent-treated control of corresponding time point).
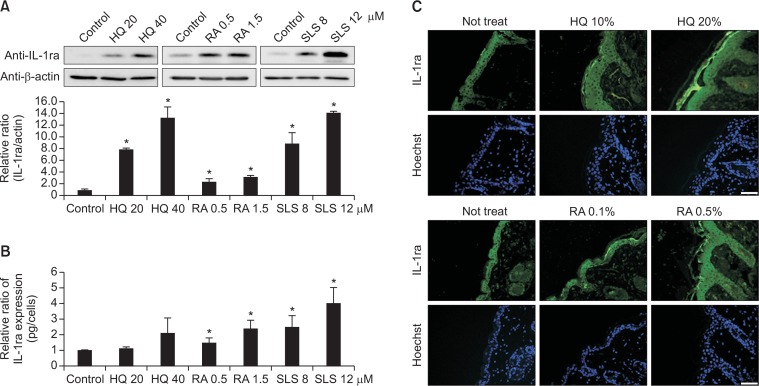
Cytotoxic doses of chemicals increased both intracellular and extracellular IL-1ra protein expression
Based on the results of the mRNA expression, Western blot analysis for icIL-1ra protein levels in cell lysates and ELISA for extracellularly released IL-1ra protein in culture supernatants were performed at 48 hrs after treatment of the same cytotoxic doses (70–80% and 40–50% cell survival in MTT assay) of HQ, RA, and SLS. The expression level of icIL-1ra protein increased dose-dependently (p<0.05; Fig. 2A). The level of released IL-1ra protein also increased dose-dependently in culture supernatants (p<0.05; Fig. 2B). In addition, immunofluorescence staining in mice skin specimens, which were treated with different concentrations of HQ or RA to induce mild and definite erythema, showed stronger staining intensities of IL-1ra in epidermis including stratum corneum with more irritation (Fig. 2C).
Fig. 2.
Cytotoxic doses of chemicals increased both intracellular and extracellular IL-1ra protein expression. (A) Western blot analysis of icIL-1ra protein expression in cell lysates (B) ELISA of secretory IL-1ra protein in culture supernatants at 48 hrs after treatment with HQ, RA, or SLS using the same doses as in Fig. 1. Data in the graph represents mean ± SD of relative values compared to solvent-treated control from 3 to 5 independent experiments (*p<0.05 vs. solvent-treated control). (C) Representative immunofluorescence staining using anti-IL-1ra antibody in mice skin specimens treated with two concentrations inducing mild erythema and definite erythema. Nuclei were counter-stained with Hoechst 33258 (scale bar=50 μm).
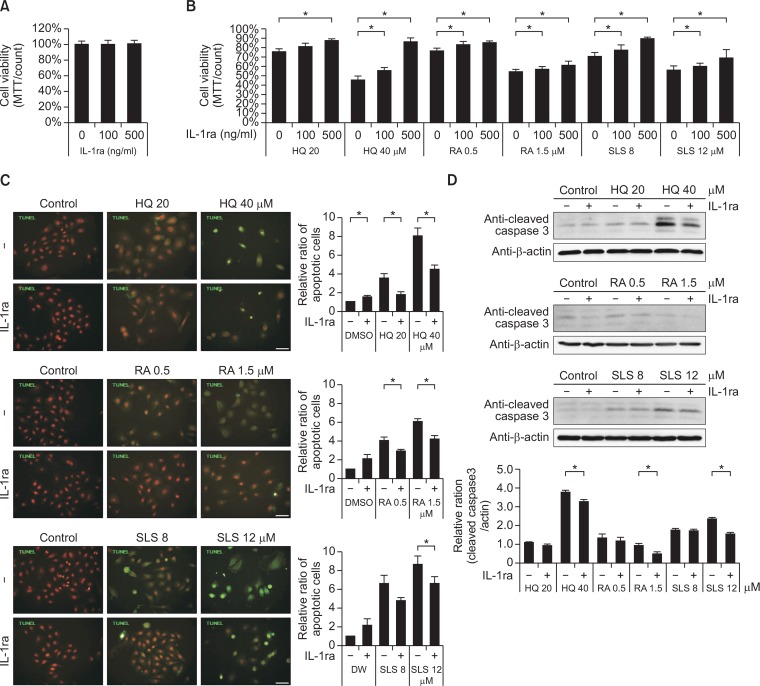
Recombinant IL-1ra treatment reduced keratinocytes apoptosis, which were induced by the chemical irritation
The level of IL-1ra proteins, including extracellularly released protein, increased due to the cytotoxic doses of chemicals in cultured keratinocytes and in mouse skin (Fig. 2). Since IL-1ra exerts action by binding to cell surface IL-1 receptors, the effect of rhIL-1ra on the survival of keratinocytes was examined. A wide concentration range of up to 500 ng/ml of rhIL-1ra (Conti et al., 1997) did not reduce the number of viable cells in primary cultured human keratinocytes (Fig. 3A). The rhIL-1ra increased the number of viable cells, which were reduced by cytotoxic doses of chemicals in a dose-dependent manner (p<0.05; Fig. 3B). On the other hand, number of TUNEL positive keratinocytes, which increased with cytotoxic doses of chemicals, decreased with rhIL-1ra (p<0.05; Fig. 3C). Expression levels of cleaved caspase-3 also decreased in the presence of rhIL-1ra (p<0.05; Fig. 3D).
Fig. 3.
Recombinant IL-1ra treatment reduced cell apoptosis, which was induced by the chemical irritation. (A, B) MTT assay in keratinocytes treated without (A) or with HQ, RA, or SLS (B) of the same doses as in Figure 1 in the presence of absence of different doses of rhIL-1ra. Data in the graph represents mean ± SD of relative values compared to solvent-treated control from 3 to 5 independent experiments (*p<0.05 vs. solvent-treated control of corresponding chemical concentration). (C) TUNEL assay using Apo-BrdU TUNEL assay kit (green fluorescence) and (D) Western blot analysis of cleaved csapase-3 in keratinocytes treated with HQ, RA, or SLS using the same doses as in Fig. 1 in the presence of absence of 500 ng/ml of rhIL-1ra. Nuclei were counter-stained with Hoechst 33258 (scale bar=50 μm). Data in the graph represents mean ± SD of relative values compared to solvent-treated control from 3 independent experiments (*p<0.05 vs. corresponding chemical concentration without rhIL-1ra treatment).
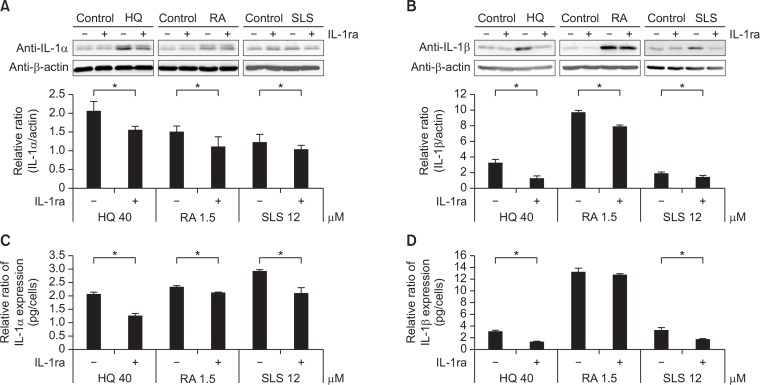
Recombinant IL-1ra reduced chemical irritation-induced IL-1α and IL-1β expression
IL-1ra inhibits IL-1α and IL-1β activities via competitive inhibition of cell surface IL-1 receptor binding (Arend et al., 1998). Therefore, the effect of rhIL-1ra on intracellular and extracellularly released IL-1α and IL-1β expression was examined. A western blot analysis suggested the 500 ng/ml of rhIL-1ra decreased intracellular levels of IL-1α and IL-1β, which increased with cytotoxic doses of the chemicals (p<0.05; Fig. 4A, 4B). ELISA also showed a decrease in released IL-1α and IL-1β with rhIL-1ra (p<0.05; Fig. 4C, 4D).
Fig. 4.
Recombinant IL-1ra reduced chemical irritation-induced IL-1α and IL-1β expression. Western blot analysis of (A) IL-1α and (B) IL-1β in cell lysates and ELISA of (C) IL-1α and (D) IL-1β in culture supernatants at 48 hrs after treatment with HQ, RA, or SLS using the same doses as in Fig. 1. Data in the graph represents mean ± SD of relative values compared to solvent-treated control from 3 independent experiments (*p<0.05 vs. corresponding chemical concentration without rhIL-1ra treatment).
DISCUSSION
Cytotoxic doses of the chemicals, either a representative irritant SLS or a representative sensitizer urshiol, increased IL-1ra mRNA in a dose- and time-dependent manner (Fig. 1A–1C) in monolayer-cultured keratinocytes. This result suggests a correlation between IL-1ra production and chemical-induced keratinocyte cytotoxicity. Although extracellular IL-1α has been accepted as an indicator of chemical-induced irritancy (Muller-Decker et al., 1994; Corsini and Galli, 1998; Coquette et al., 1999; Perkins et al., 1999; Kidd et al., 2007), the time-course changes in IL-1α mRNA were less consistent than those in IL-1ra mRNA (Fig. 1). No significant changes in IL-1α mRNA levels were particularly clear in urshiol-treated cells (Fig. 1D), but the reason remains unknown at this moment. A dose-dependent increase in expression of icIL-1ra protein and extracellularly released IL-1ra (Fig. 2A, 2B) indicated a change in IL-1ra protein levels similar to that in the mRNA levels. The increase in extracellularly released IL-1ra protein concentration, which requires binding to cell surface receptors to inhibit IL-1 function, indicated that IL-1ra may be involved in chemical-induced cytotoxicity to keratinocytes. In addition, the increased IL-1ra proteins in mice epidermis were proportional to the irritation intensities (Fig. 2C), showing a correlation between in vitro and in vivo results. Stronger staining intensities of IL-1ra in the irritation-induced mice stratum corneum as the other portion of epidermis (Fig. 2C) may provide a rationale to determine the IL-1ra cytokine concentration in stratum corneum as an indicator of human skin irritation (Perkins et al., 2001; De Jongh et al., 2006).
The biological effects of keratinocyte-derived IL-1ra, particularly on keratinocyte apoptosis or survival, have not been studied extensively. In this study, the effect of rhIL-1ra on cell survival was examined based on the opposite correlation between cell survival and IL-1ra, particularly extracellularly released IL-1ra in culture supernatants (Fig. 2B). The results of the MTT assay show that rhIL-1ra attenuated the chemical-induced keratinocytes death in a dose-dependent manner in the range of up to 500 ng/ml without effecting any changes in unstimulated keratinocytes (Fig. 3A, 3B), and this suggests the role of IL-1ra in protecting keratinocytes from death. In addition, the result of the TUNEL-positive cell number and cleaved caspase-3 level, which increased with cytotoxic doses of chemicals (Fig. 3C, 3D), decreased with rhIL-1ra treatment, supporting an inhibitory role of IL-1ra in chemical-induced keratinocyte apoptosis.
IL-1α, present intracellularly, has been involved in keratinocyte apoptosis (Cohen et al., 2010; Wang et al., 2011). The role of IL-1β on keratinocyte apoptosis has been rather well reported compared to that of IL-1α (Nisticò et al., 2010; Grimstad et al., 2013; Yoshihisa et al., 2014). Considering that recombinant IL-1ra is supposed to exert action via cell surface IL-1 receptor binding (Arend et al., 1998), IL-1ra was expected to be involved in keratinocyte survival via IL-1 antagonism. However, the role of IL-1ra in cell survival by IL-1 antagonism has not been presented for skin keratinocytes, but in cells residing in different organs damaged by, for example, acute pancreatitis, hepatic failure, or ischemic heart disease (Fink and Norman, 1997; Vecile et al., 2013; Zheng et al., 2013). In this study, rhIL-1ra decreased IL-1α and IL-1β expression in cell lysates (Fig. 4A, 4B) as well as in culture supernatants (Fig. 4C, 4D). The result suggests that extracellular IL-1ra released from keratinocytes could reduce keratinocyte apoptosis through the inhibition of both IL-1α and IL-1β production and release as an endogenous antagonist. Extracellular release of IL-1α, an endpoint of in vitro assessment for skin irritation (Osborne and Perkins, 1991), is supposed to occur in necrotic, but not apoptotic, keratinocytes (Cohen et al., 2010), and it is unclear how IL-1β is secreted from the cell (Lopez-Castejon and Brough, 2011). Although isoforms of IL-1ra were not examined in this study, active secretion of the keratinocyte-derived icIL-1ra suggested that certain portions of the extracellular IL-1ra could originate from icIL-1ra. Since apoptotic cell death also develops by primary skin irritation (Kanerva, 1990), extracellular IL-1ra from keratinocytes may be a potential biomarker to predict in vitro skin irritation.
In summary, IL-1ra production and release, which were induced by administering cytotoxic doses of chemicals, could be a compensatory mechanism to reduce chemical-induced keratinocyte apoptosis through the antagonism of IL-1α and IL-1β.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN15C0102), by the National Research Foundation of Korea (NRF) grant, funded by the Korean Government (MSIP) (No. NRF-2017R1A2A2A09069507), and by a grant from Ministry of Food and Drug Safety (Grant No. 16182MFDS525), Republic of Korea.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis. 2000;59:i60–i64. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhofer LP, Barkovic S, Appa Y, Martin KM. IL-1alpha and IL-1ra secretion from epidermal equivalents and the prediction of the irritation potential of mild soap and surfactant-based consumer products. Toxicol In vitro. 1999;13:231–239. doi: 10.1016/S0887-2333(98)00088-5. [DOI] [PubMed] [Google Scholar]
- Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P, Barbacane RC, Trakatellis M, Placido FC, Cataldo I, Reale M. Influence of interleukin-1 receptor antagonist on [3H]serotonin and histamine release by rat basophilic leukemia-2H3 cells. Ann N Y Acad Sci. 1997;832:223–232. doi: 10.1111/j.1749-6632.1997.tb46250.x. [DOI] [PubMed] [Google Scholar]
- Coquette A, Berna N, Vandenbosch A, Rosdy M, Poumay Y. Differential expression and release of cytokines by an in vitro reconstructed human epidermis following exposure to skin irritant and sensitizing chemicals. Toxicol In vitro. 1999;13:867–877. doi: 10.1016/S0887-2333(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Corradi A, Franzi AT, Rubartelli A. Synthesis and secretion of interleukin-1 alpha and interleukin-1 receptor antagonist during differentiation of cultured keratinocytes. Exp Cell Res. 1995;217:355–362. doi: 10.1006/excr.1995.1097. [DOI] [PubMed] [Google Scholar]
- Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol. Lett. 1998;102–103:277–282. doi: 10.1016/S0378-4274(98)00323-3. [DOI] [PubMed] [Google Scholar]
- De Jongh CM, Verberk MM, Withagen CE, Jacobs JJ, Rustemeyer T, Kezic S. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Derm. 2006;54:325–333. doi: 10.1111/j.0105-1873.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997;9:1023–1027. doi: 10.1006/cyto.1997.0260. [DOI] [PubMed] [Google Scholar]
- Grimstad Ø, Husebye H, Espevik T. TLR3 mediates release of IL-1β and cell death in keratinocytes in a caspase-4 dependent manner. J Dermatol Sci. 2013;72:45–53. doi: 10.1016/j.jdermsci.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Kanerva L. Electron microscopic observations of dyskeratosis, apoptosis, colloid bodies and fibrillar degeneration after skin irritation with dithranol. J Cutan Pathol. 1990;17:37–44. doi: 10.1111/j.1600-0560.1990.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Kidd DA, Johnson M, Clements J. Development of an in vitro corrosion/irritation prediction assay using the EpiDerm skin model. Toxicol In vitro. 2007;21:1292–1297. doi: 10.1016/j.tiv.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi T, Sakaeda T, Kita T, Kurimoto Y, Nakamura T, Nishiguchi K, Fujita T, Kamiyama F, Yamamoto A, Okumura K. Intradermal concentration of hydroquinone after application of hydroquinone ointments is higher than its cytotoxic concentration. Biol Pharm Bull. 2003;26:1365–1367. doi: 10.1248/bpb.26.1365. [DOI] [PubMed] [Google Scholar]
- Muller-Decker K, Furstenberger G, Marks F. Keratinocyte-derived proinflammatory key mediators and cell viability as in vitro parameters of irritancy: a possible alternative to the Draize skin irritation test. Toxicol Appl Pharmacol. 1994;127:99–108. doi: 10.1006/taap.1994.1144. [DOI] [PubMed] [Google Scholar]
- Nisticò S, Paolillo N, Minella D, Piccirilli S, Rispoli V, Giardina E, Biancolella M, Chimenti S, Novelli G, Nisticò G. Effects of TNF-α and IL-1β on the activation of genes related to inflammatory, immune responses and cell death in immortalized human HaCat keratinocytes. Int J Immunopathol Pharmacol. 2010;23:1057–1072. doi: 10.1177/039463201002300410. [DOI] [PubMed] [Google Scholar]
- Noble S, Wagstaff AJ. Tretinoin. A review of its pharmacological properties and clinical efficacy in the topical treatment of photodamaged skin. Drugs Aging. 1995;6:479–496. doi: 10.2165/00002512-199506060-00008. [DOI] [PubMed] [Google Scholar]
- Osborne R, Perkins MA. In vitro skin irritation testing with human skin cell cultures. Toxicol In vitro. 1991;5:563–567. doi: 10.1016/0887-2333(91)90094-T. [DOI] [PubMed] [Google Scholar]
- Perkins MA, Osborne R, Rana FR, Ghassemi A, Robinson MK. Comparison of in vitro and in vivo human skin responses to consumer products and ingredients with a range of irritancy potential. Toxicol Sci. 1999;48:218–229. doi: 10.1093/toxsci/48.2.218. [DOI] [PubMed] [Google Scholar]
- Perkins MA, Osterhues MA, Farage MA, Robinson MK. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res Technol. 2001;7:227–237. doi: 10.1034/j.1600-0846.2001.70405.x. [DOI] [PubMed] [Google Scholar]
- Samuel M, Brooke RC, Hollis S, Griffiths CE. Interventions for photodamaged skin. Cochrane Database Syst. Rev. 2005. p. CD001782. [DOI] [PubMed]
- Stratigos AJ, Katsambas AD. The role of topical retinoids in the treatment of photoaging. Drugs. 2005;65:1061–1072. doi: 10.2165/00003495-200565080-00003. [DOI] [PubMed] [Google Scholar]
- Vecile E, Dobrina A, Salloum FN, Van Tassell BW, Falcione A, Gustini E, Secchiero S, Crovella S, Sinagra G, Finato N, Nicklin MJ, Abbate A. Intracellular function of interleukin-1 receptor antagonist in ischemic cardiomyocytes. PLoS ONE. 2013;8:e53265. doi: 10.1371/journal.pone.0053265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Coleman DJ, Bajaj G, Liang X, Ganguli-Indra G, Indra AK. RXRα ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol. 2011;131:177–187. doi: 10.1038/jid.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa Y, Rehman MU, Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp Dermatol. 2014;23:178–183. doi: 10.1111/exd.12347. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.