Abstract
Optical methods for modulating cellular behavior are promising for both fundamental and clinical applications. However, most available methods are either mechanically invasive, require genetic manipulation of target cells, or cannot provide sub-cellular specificity. Here, we address all these issues by showing optical neuromodulation with free-standing coaxial p-type/intrinsic/n-type silicon nanowires. We revealed the presence of atomic gold on the nanowire surfaces, likely due to gold diffusion during the material growth. To evaluate how surface gold impacts the photoelectrochemical properties of single nanowires, we used modified quartz pipettes from a patch clamp and recorded sustained cathodic photocurrents from single nanowires. We show that these currents can elicit action potentials in primary rat dorsal root ganglion neurons through a primarily atomic gold-enhanced photoelectrochemical process.
Extracellular electrical stimulation of excitable cells is the basis for many implantable devices that treat a variety of diseases1. While these devices have been efficacious, they are often limited by their bulkiness, mechanical invasiveness, and inability to target single cells2,3. Thus, researchers have looked to optical stimulation techniques, where organic or inorganic photodiode substrates are used for photovoltaic neuronal stimulation4-6; however, these tools cannot be easily administered in a drug-like fashion. While some photothermally-modulating materials meet these criteria7,8, chronic cellular effects due to heat from such devices are unknown. Optogenetics has been promising for addressing these issues, but fundamentally requires genetic modifications9,10, which can be difficult to implement in vivo. As a result, there is still a need for exploring a non-genetic approach that can be administered in a drug-like fashion.
Silicon (Si) nanomaterials are promising to address these concerns as they have been widely used for many biophysical or biomedical applications due to their highly tunable electrical and chemical properties, ability to absorb a broad range of wavelengths of light, and biocompatibility3,8,11-18. Si nanowires (SiNWs) are particularly advantageous due to their unique one-dimensional structure, precisely tunable doping profiles during growth, nanoscale diameter that allows for high spatial specificity, and ability to be dispersed in a drug-like fashion19,20. Here, we use coaxial p-type/intrinsic/n-type (PIN) SiNWs, consisting of p-doped cores, and intrinsic and n-doped shells21, to wirelessly and photoelectrochemically modulate primary rat dorsal root ganglion neuron excitability (Fig. 1a). Upon light stimulation at a neuron-PIN-SiNW interface, electrons move towards the n-type shell and holes to the p-type core, inducing a cathodic process at the n-shell22 that can locally depolarize a target neuron (Fig 1a).
Figure 1. X-ray photoelectron spectroscopy and atom probe tomography reveal the presence of atomic Au on coaxial nanowire surfaces.
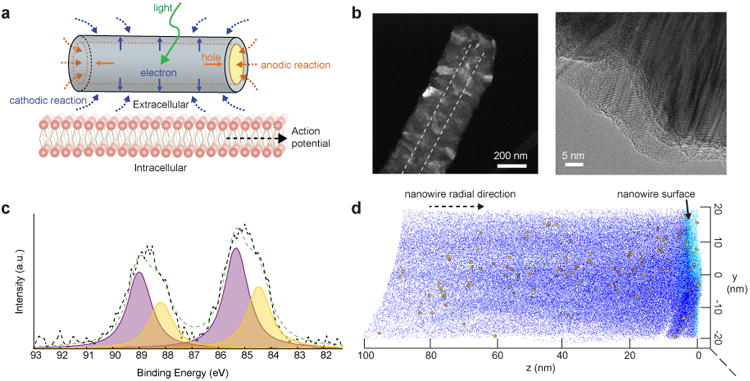
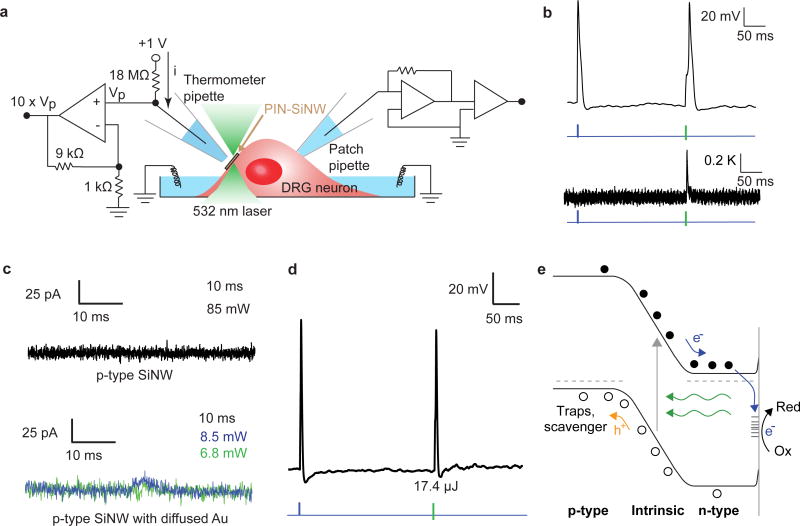
(a) Schematic of the Faradaic current produced by a PIN-SiNW at the neuronal cell membrane on light stimulation, inducing action potential generation in the neuron via membrane depolarization. Solid blue and orange lines represent movement of electrons and holes towards n-type and p-type Si, respectively, upon light stimulation. Blue and orange dotted lines represent the cathodic and anodic reactions, respectively. (b) HAADF STEM image of PIN-SiNW (left) with p-type core outlined by white dotted line. This is a representative image from one of a total of 64 PIN-SiNWs imaged from 2 independent experiments. TEM image of PIN-SiNW (right). This is a representative image from one of a total of 8 PIN-SiNWs imaged from 3 independent experiments. (c) XPS spectrum (black dotted line) and the fitted curve (green dotted line) of Au 4f signals from the PIN-SiNWs. Deconvoluted peaks of Au 4f 7/2 and Au 4f 5/2 at 84.46 eV and 88.13 eV (yellow) represent nanoclustered Au species. Au 4f 7/2 and Au 4f 5/2 peaks at 85.4 eV and 89 eV (purple) represent atomic-like Au species. (d) 3D chemical reconstruction of local electrode APT data from a single PIN-SiNW displaying Si atoms (dark blue dots, 10% of all Si atoms displayed), O atoms (light blue dots, 100% of all O atoms displayed), and Au atoms (yellow balls, 100% of all Au atoms displayed). This is representative data from one of 3 independent probes that were prepared for APT analysis.
Characterization of coaxial nanowire surfaces
The PIN-SiNWs were synthesized via a combination of gold (Au) nanoparticle (NP)-catalyzed vapor-liquid-solid growth of p-type SiNW cores, vacuum annealing, and a final vapor-solid growth of Si shells (Methods). High-angle annular dark field (HAADF) scanning transmission electron microscope (STEM) and transmission electron microscopy (TEM) show a final nanowire diameter of 200-250 nm with polycrystalline surfaces (Fig. 1b). NW shell synthesis of this kind at high temperatures and low pressures favors diffusion of the gold catalyst down the sidewalls of the NWs, resulting in atomic gold accumulation around the shells23,24. X-ray photoelectron spectroscopy (XPS) of PIN-SiNWs attached to their growth substrate indicates the presence of two types of Au within 7-10 nm from the surface of the NWs (Fig. 1c, Supplementary Table 1). XPS peaks with binding energies of 84.5 eV and 88.1 eV represent Au nanoclusters, while those with binding energies of 85.4 eV and 89.0 eV highlight the presence of atomic Au species (Fig. 1c, Supplementary Table 1, Supplementary Fig. 1). On average, 58.5% of the Au present is represented by the atomic Au and 41.5% is represented by the nanoclustered Au (Supplementary Fig. 1), and no metallic Au is identified. Energy dispersive x-ray spectroscopy (EDS) further confirms this result in single PIN-SiNWs. Spectra acquired displayed characteristic Au Lα1 and Lβ1 peaks at 9.7 and 11.4 keV X-ray energies respectively, thus confirming the presence of Au atoms in single PIN-SiNW nanowires (Supplementary Fig. 2).
To more definitively characterize the amount and spatial distribution of atomic Au in our NWs, we performed local electrode atom probe tomography (APT) on a single PIN-SiNW (Supplementary Fig. 3). The APT data demonstrate the existence of atomic Au in band-like domains with additional accumulation at the surface (Fig. 1d). We believe that the high temperature and low pressure conditions used for annealing and subsequent shell depositions allow for complete Au catalyst diffusion down the sidewalls of the nanowire cores23,24 and the subsequent Au migration into the shells through grain boundaries or gettering effect25. Despite the dominant argument that atomic Au generates deep traps in Si, the Au at the Si surface may also confer properties that can be useful in a photoelectrochemical processes during cellular excitability modulation. Specifically, surface atomic Au may alter the surface states of the Si in such a way that is beneficial for reducing NW impedance in aqueous solutions26, thus favoring the production of faradaic, not capacitive currents, as in traditional photoelectrochemical cells.
Estimation of the single nanowire photoelectrochemical behavior
We next developed a method to measure the photocurrents from single PIN-SiNWs in an interconnect free configuration, using a patch clamp setup under physiological conditions (modified Tyrode's solution), in response to a 532 nm light illumination, which was chosen according to previously measured action spectra for PIN-SiNWs27. We grew PIN-SiNWs with final diameters inside of quartz capillary tubes, pulled them into patch pipettes, and mounted them onto a patch clamp set-up. The laser was shone onto single NWs positioned so as to minimize any changes in pipette resistance due to increases in temperature produced by light absorption (Fig. 2a). All measurements were performed in voltage clamp mode, holding the voltage at zero in order to function as virtual ground. Keeping the power constant at 17 mW, we first altered the laser pulse duration (Fig. 2b). Measured pipette currents were characterized by a sharp and fast initial rise in current, likely limited by the system bandwidth, to peaks of 50.7 pA, 83.5 pA, and 101 pA for 0.5, 2, and 10 ms pulses, respectively (Fig. 2b). While the observed currents do not represent absolute values of photocurrents produced by single PIN-SiNWs, as some current will be inevitably shunted by the surrounding solution, much can still be garnered from the relative amplitudes and durations of the photocurrents measured. Notably, the current resulting from the 10 ms pulse was sustained at 101 pA for the whole illumination duration (Fig. 2b). This production of a sustained current is not characteristic of a capacitive current given that the NW-associated electrical capacitance is below the pF range28-30, and instead is suggestive of a faradaic process. This observed faradaic process, characterized by electron transfer between the NW and the electrolyte, may be particularly favorable in the context of biological systems as it mimicks basic cellular processes that inherently utilize redox reactions. Additionally, we observed light intensity-dependent current generation in another PIN-SiNW (Fig. 2c). For a laser pulse duration of 10 ms, the photocurrent peaks were measured to be 120 pA, 43.1 pA, and 22.3 pA for laser powers of 8.5 mW, 3.4 mW, and 1.7 mW, respectively. The polarity of the current suggests a photocathodic reaction over the PIN-SiNW surface. When the laser spot was moved off the nanowire onto plain glass, no current was recorded (Supplementary Fig. 4). The observed unipolar photoelectrochemical current, for up to 10 ms (i.e., a time scale relevant to neural excitation), suggests that the solution reaction kinetics for light-generated hole carriers is much slower than that for electrons, partially due to the much smaller exposed surface area for p-type Si core. We hypothesize that the surface atomic gold may also affect the observed non-equilibrium photoelectrochemical current production. Before determining whether this is the case, though, we wanted to understand whether the current from these PIN-SiNWs could modulate neuronal excitability.
Figure 2. Single nanowire recordings reveal that coaxial Si nanowires are photoelectrochemical current sources.
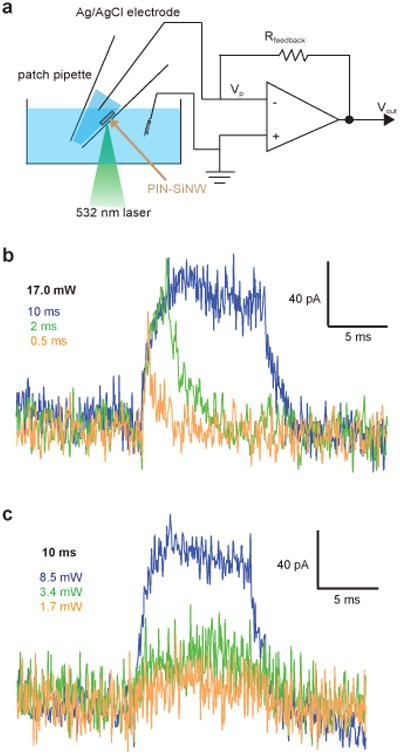
(a) Schematic of photocurrent measurement setup. Vp represents the pipette voltage; Rfeedback is the resistance of a feedback resistor; Vout denotes the output voltage. (b) Photocurrent traces from a single PIN-SiNW illuminated with a 532 nm laser at 17 mW for durations of 0.5 ms (orange), 2 ms (green), or 10 ms (blue). (c) Photocurrent traces from a single PIN-SiNW illuminated with a 532 nm laser at 8.5 mW (blue), 3.4 mW (green), or 1.7 mW (orange) for a duration of 10 ms. The traces in (b) and (c) are representative ones from a total of 40 current traces measured from 5 independent PIN-SiNWs.
Basic coaxial nanowire/neuron interfaces
We drop-casted PIN-SiNWs onto primary dorsal root ganglion (DRG) neurons cultured from neonatal rats (Fig. 3a-b). Scanning electron microscopy (SEM) shows that PIN-SiNWs form close interactions with neuronal membranes without being internalized into cells (Fig. 3c). We hypothesized that the photocathodic electrochemical effect produced from a single nanowire could cause membrane depolarization (by reducing the potential in a local extracellular region) and trigger action potentials (APs). We aligned a neuron/PIN-SiNW interface to a 532 nm laser spot, and used a whole cell current clamp set-up to record the neuronal membrane voltage before, during and after laser stimulation (Fig. 3a). We found that laser stimulation at the neuron/PIN-SiNW interface can elicit APs in neurons at an average minimum laser energy of 6.44 μJ for laser pulse durations ranging from 0.5 ms to 5 ms (Fig.3d and Supplementary Fig. 5). This laser energy is similar to or less than that required for other photothermally stimulating materials to elicit APs in neurons7,8, even though the direct cell contact area is at least 10 times smaller in the present case. Additionally, the same minimal energy necessary to trigger APs at several stimulating pulse durations suggests that the PIN-SiNW mimicks a classic external stimulation electrode without the mechanical invasiveness and bulkiness inherent to physical electrodes31. Control experiments using pure p-type and undoped SiNWs were unable to elicit APs, and only produced sub-threshold depolarizations upon laser stimulation, even at high energies of 100 μJ and 1 ms durations (Supplementary Fig. 5). In the absence of a NW, laser stimulation with an energy of up to 100 μJ at 1 ms laser pulse durations cannot depolarize the cell's membrane (Fig. 3d). We also confirmed via a live-dead cell viability assay that the presence of PIN-SiNWs in the neuron culture had a negligible effect on cell viability and that laser stimulation on the time scales explored here also had a negligible effect on cell viability (Supplementary Fig. 6).
Figure 3. Basic silicon nanowire-based neural interfaces.
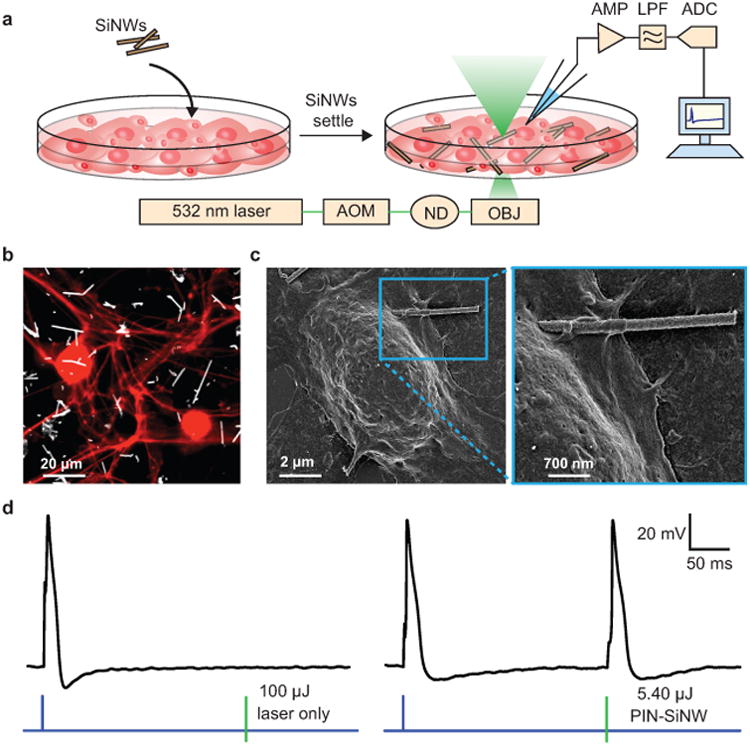
(a) Schematic of the set-up used to record DRG neuron APs in response to a 532 nm laser stimulation at a single neuron/SiNW interface. This set-up includes an ordinary electrophysiology capability for patch clamp experiments, with an amplifier (AMP), used in current clamp mode, a low pass filter (LPF), and an analog to digital converter (ADC). In addition the setup was implemented with a 532 nm laser beam controlled by an acoustic modulator (AOM) and various neutral density (ND) filters (to attenuate the power). The laser beam is aligned to the optical central axis of the objective lens (OBJ) of an inverted microscope. SiNWs were sonicated off of their growth substrate and drop casted onto the primary neuron culture. After 20 min of settling, the experiments were performed. (b) Confocal microscopy image of primary neonatal rat dorsal root ganglion (DRG) neurons stained with anti-β-tubulin III (red) co-cultured with PIN-SiNWs (white). This is a representative image from one of a total of 10 images taken from 2 independent experiments. (c) Scanning electron microscopy images of a single DRG neuron interacting with a single PIN-SiNW (left); zoomed in image displaying neuron-PIN-SiNW interface (right). This is a representative image from one of a total of 63 images taken from 4 independent experiments. (d) Patch clamp electrophysiology current clamp trace of membrane voltage in DRG neuron stimulated (blue pulse) or a laser pulse (green bar) of various energies as labeled at the neuron/SiNW interface. Two conditions are displayed: laser only with no SiNW (left) and PIN-SiNW (right). These are representative traces from one of 173 total action potential traces measured from 30 neurons with PIN-SiNWs, and one of 27 total traces from 2 neurons with PIN-SiNWs.
Systematic neuromodulation studies
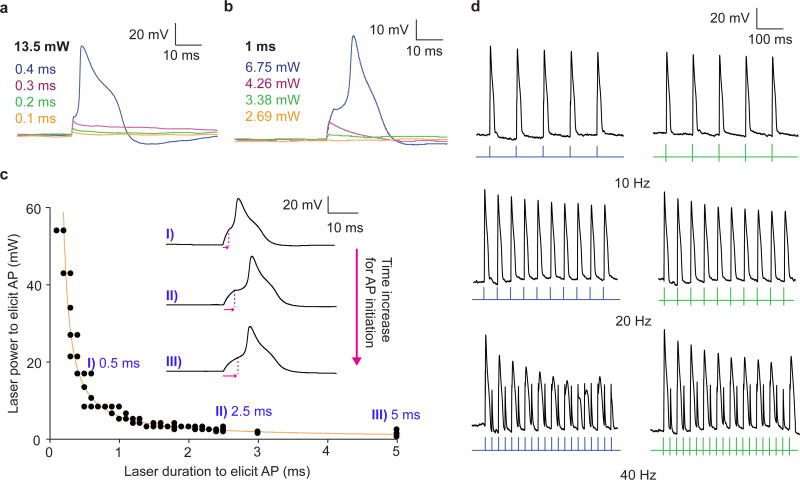
We further studied PIN-SiNW enabled neuron excitation by altering the laser power and pulse duration. At a laser power of 13.5 mW, we were able to produce sub-threshold depolarizations at increasing durations of 0.1, 0.2, and 0.3 ms, respectively (Fig. 4a). At a laser duration of 0.4 ms and energy of 5.4 μJ, an AP was generated (Fig. 4a). Keeping the stimulus duration constant at 1 ms, we were able to passively depolarize the membrane at increasing laser powers until reaching 6.75 mW and a total energy of 6.75 μJ, at which an AP was generated in the cell (Fig. 4b). We then constructed an excitability curve and found that as the duration of the laser stimulus increased, the amount of laser power required to generate an AP decreased (Fig. 4c). We fit the data to a hyperbolic function and found that the minimum total energy required to trigger an AP is independent of the pulse duration, as would be expected for current injecting electrodes 31 (Supplementary Fig. 7). This phenomenon can both be seen by directly plotting minimum total energy as a function of the laser pulse duration, as well as by the exponent in the denominator of the hyperbolic function, which is close to 1 (Supplementary Fig. 7). The rheobase of the fitted curve is 1.178 mW and chronaxie 2.597 ms, which compares well to literature chronaxie values for DRG neurons 31 (Fig. 4c). We also found that with increasing laser pulse durations, the time to AP initiation was increased (Fig. 4c). These results together demonstrate that PIN-SiNWs can elicit APs in a manner that is physiologically indistinguishable from those induced by classical external current injecting electrodes.
Figure 4. Photocurrent generated by coaxial nanowires can be harnessed to elicit action potentials in primary rat dorsal root ganglion neurons.
(a and b) Patch clamp electrophysiology current clamp traces of membrane voltage in DRG neurons illuminated by a 532 nm laser pulse at the neuron/PIN-SiNW interface at (a) 13.5 mW with durations of 0.1 ms (orange), 0.2 ms (green), 0.3 ms (pink), and 0.4 ms (blue) and (b) 2.69 mW (orange), 3.38 mW (green), 4.26 mW (pink), and 6.75 mW (blue) for 1 ms. These traces in (a) and (b) are representative traces from one of a total of 1398 traces from 30 independent neurons, many of which are sub-threshold depolarizations at various laser powers and durations. 173 traces out of the 1398 represent action potentials. (c) Excitability curve displaying 532 nm laser power and duration combinations that produce APs in neurons (N=6 neurons; total of N=78 replicates) interacting with a single PIN-SiNWs with specific traces and time to peak response for each of those traces (pink arrows) at I) 0.5 ms, II) 2.5 ms, and III) 5 ms durations highlighted. Error bars represent the standard error about the average. Some of the data points are overlaid. (d) AP traces from neurons interacting with single PIN-SiNWs pulsed at 10, 20 and 40 Hz with light (green bars) and injected current from patch amplifier (blue pulses). These are representative traces from a total of 6 pulse train traces for each frequency taken from 3 independent neurons.
We also pulsed the laser at varying frequencies at the neuron/PIN-SiNW interface and assessed the cellular response. We found that neurons are able to generate trains of APs at 10 and 20 Hz both with injected current and laser stimulation (Fig. 4d). At 40 Hz, the cell depicted failed to produce APs in response to every pulse of laser light or injected current (Fig. 4d). This neuron produced 10 APs in the case of the light stimulation and 9 in the case of the injected current out of 20 pulses at 40 Hz (Fig. 4d). At the lowest frequency that neurons begin to fail to generate one AP for every pulse of current or light, the percentage of failed APs tended to be the same when comparing the two stimuli (Supplementary Fig. 8). These results indicate that APs produced in neurons through PIN-SiNW-enabled optical stimulation are physiologically representative and follow the intrinsic limitation of the cell to fire trains of APs above a specific frequency.
To understand how effectively the PIN-SiNWs would be able to perform neuromodulation when not used fresh, we incubated PIN-SiNWs in culture media or Tyrode's buffer for 1- and 2-week time points and used them to trigger APs in primary rat DRG neurons. The minimum laser energy threshold necessary to elicit APs increased from the previously observed 6.44 μJ to an average of 28.33 μJ and 29.58 μJ for the 1-week timepoint in media and Tyrode's respectively, and 44.30 μJ and 43.10 μJ for the 2-week time point in media and Tyrode's respectively (Supplementary Fig. 9). We propose that the increased laser energy threshold is caused by oxidation/hydration induced degradation of both the Si heterojunction and atomic Au activity.
Probing of the neuromodulation mechanism
Having demonstrated the optical modulation of single primary neuron excitability with PIN-SiNWs, we next further studied the mechanism of this stimulation. Previous work has demonstrated that mesoporous silicon materials can elicit APs in neurons via a photothermal effect8. Thus, we wanted to understand the contribution of photothermal current generation to PIN-SiNW-enabled neuromodulation. We used a calibrated micropipette resistance method to measure the temperature change ∼ 2 μm away from the neuron-PIN-SiNW interface during laser induced AP generation in the neuron (Fig. 5a and Supplementary Fig. 10). Laser induced AP generation at a 5.36 μJ laser energy (minimum energy necessary to produce an AP with PIN-SiNWs) resulted in a 0.36 K increase in temperature at the neuron-PIN-SiNW interface (Fig. 5b). In comparison to other photothermally-stimulating materials that produce 2 K temperature increases at similar pipette distances, this temperature increase is minor, suggesting a minor photothermal contribution to the stimulation mechanism described here. No temperature increase was observed in the absence of a SiNW (Supplementary Fig. 10). Other control experiments further suggest that a photothermal effect is not the primary mechanism here (Supplementary Fig. 10).
Figure 5. The mechanism of coaxial nanowire photocurrent generation and neuronal modulation is primarily photoelectrochemical, aided by surface atomic Au.
(a) Schematic of temperature measurement setup for simultaneous measurement of temperature and neuronal APs produced by laser stimulation or injected current through patch amplifier. (b) Patch clamp electrophysiology current clamp trace of membrane voltage (top) in DRG neuron stimulated by injected current (blue pulse) and illuminated by a 532 nm laser pulse at the neuron-PIN-SiNW interface (green bar). Corresponding temperature measurement (bottom) taken 2 μm away from neuron/PIN-SiNW interface produced by calibrating the thermometer pipette resistance with temperature changes. This is a representative temperature measurement from one of 18 total traces from 3 independent neurons. (c) Photocurrent measurement taken from a single p-type SiNW illuminated with 532 nm laser light for 10 ms at a laser illumination power of 85 mW (top). This is a representative trace from one of a total of 71 traces measured from 4 independent p-type SiNWs. Photocurrent measurement taken from a p-type SiNW with diffused Au for 10 ms at laser illumination powers of 8.5 mW (blue) and 6.8 mW (green). These are representative traces from a total of 52 traces measured from 6 independent p-type SiNWs with diffused Au. (d) Patch clamp electrophysiology current clamp trace of membrane voltage (top) in DRG neuron stimulated by injected current (blue pulse) and illuminated by a 1 ms 17.4 μJ 532 nm laser pulse at the neuron-p-type SiNW with diffused Au interface (green bar). This is a representative trace from one of a total of 40 traces measured from 5 independent neurons. (e) Band diagram representing the redox reaction that occurs at the interface between the PIN-SiNW and the electrolyte solution. Kinetic barrier for photoelectrochemical reaction is lowered by the presence of atomic Au. Green arrows represent the light stimulus. Grey arrow shows the excitation of electrons from the valence to the conduction band. Grey dashed lines represent Fermi levels in dark.
In further delving into the neuro-excitation mechanism, we wanted to evaluate if the surface atomic gold plays a role in the observed photoelectrochemical current generation and neuromodulation. Since Au diffusion inherently occurs during PIN-SiNW shell deposition, we were unable to grow PIN-SiNWs without diffused Au. Thus, we first reduced the atomic Au distribution at the PIN-SiNW surfaces by promoting Au diffusion into the Si growth substrate (Supplementary Fig. 11). We found that the average minimum laser energy necessary to elicit APs became 19.26 μJ, ∼ 3 times that needed for typical PIN-SiNWs used in this work.
In a second approach, we compared the photoelectrochemical behaviors of pure 200-250 nm p-type SiNWs, and 200-250 nm p-type SiNWs with intentional Au diffusion (Fig. 5c-d, Methods). We chose p-type Si, instead of n-type Si to serve as controls, as p-type semiconductors in contact with electrolyte solutions experience band bending in such a manner that drives photogenerated electrons towards the semiconductor-electrolyte interface22,32. Thus, light illumination would allow for electron injection from the nanowire into the solution, yielding similar cathodic reaction as what occurs in the case of PIN-SiNWs. Indeed, both p-type and p-type/n-type (n- is the exposed end) diode Si devices have been used for photocathodes in electrochemical cells22,32,33.
We measured photocurrents generated by these different nanowires as we did with the PIN-SiNWs. Currents could not be detected when the laser spot was shone onto a single p-type SiNW (Fig. 5c). However, currents from p-type nanowires with Au catalyst diffused at 750°C for 30 min after growth were recorded at a laser duration of 10 ms (Fig. 5c). These current peaks were measured to be 28.8 pA and 15.0 pA for laser powers of 8.5 mW and 6.8 mW, respectively (Fig. 5c). Subsequent neuron excitation experiments showed that Au-diffused p-type SiNWs and not p-type SiNWs were able to elicit APs with a minimum laser pulse energy of 17.4 μJ at a 1 ms pulse duration (Fig. 5d). The fact that diffused Au along p-type SiNW enhances photoelectrochemical current generation suggests its catalytic role in the interfacial chemical reaction. This is feasible given Au is more electronegative than Si; therefore, the photogenerated electrons can accumulate near the surface Au sites for cathodic reaction even under physiological condition. The exact chemical species that promote the cathodic reaction is unknown given the heterogenous nature of the culture medium used in the present study. Additionally, the atomic Au covered p-type SiNWs still yield lower amplitudes of currents and require a greater energy threshold to elicit APs in neurons upon light stimulation when compared with those recorded from PIN-SiNWs at similar laser powers and durations (Fig. 2b-c, 5b and Supplementary Fig. 5).
Taken together, these results indicate the combined importance of the diffused Au in promoting the interfacial reaction at the PIN-SiNW surface and charge separation at the diode junction, both enabling photoelectrochemical current generation. More specifically, upon light illumination, holes migrate to the p-type core and electrons to the n-type shell (Fig. 5e). The electrons in the n-type shell are injected through surface state34 (i.e., atomic Au and other surface defects)-enhanced processes into the electrolyte solution and are able to participate in cathodic reactions (e.g., reduction of protons) (Fig. 5e). The photogenerated holes, however, are swept into a spatially separated region, and consumed by recombination within Si or chemical scavengers at the exposed ends. The anodic reaction is expected to be slower given the exposed p-type surfaces (of the coaxial nanowire) have much smaller surface areas and contain no catalyst, yielding the unipolar photocurrent recording at a timescale relevant to neural excitation. In this way, the PIN-SiNW behaves similarly to a wireless, nanoscale photoelectrochemical cell, with atomic Au to promote the cathodic process, which locally modulates neuronal function (Fig. 5e).
Outlook
This work represents the first study of single nanowire-based photoelectrochemical modulation of cellular excitability in a non-invasive, non-genetic, drug-like manner. Our results have implications for both fundamental studies and clinical therapeutics. For fundamental studies, coaxial p-type/i-type/n-type silicon nanowires are advantageous because gene transfection is not required for their use in neuromodulation, they can be administered in a drug-like fashion, their length scale allows for high spatial specificity, and their surfaces can be modified easily to allow for high affinity binding to specific cell types. Additionally, atomic Au plays the role that a catalyst would play in traditional photoelectrochemical devices in that it reduces the kinetic barrier necessary for photoelectrochemical current generation. The introduction of an even more potent “catalyst”22,32 or internalization of these nanowires into neurons19,35 could be used to reduce the optical power density necessary for stimulation, allowing for the use of an LED instead of a laser. These optimizations could be advantageous for expanding the possibilities of target organs for in vivo stimulation.
In clinical therapeutics, the potential degradability of silicon nanowires in vivo can be advantageous for temporally dependent applications and can be tuned by surface functionalization. Moreover, the ability of Si to absorb light in the near infrared regime can be useful for penetrating tissue. Due to the light penetration depth in tissue, injecting these nanowires to target peripheral nerves could be a non-invasive treatment for diseases such as diabetic peripheral neuropathy, which are characterized by severe neuropathic pain. Collectively, our findings demonstrate a new nanotechnology for cellular membrane potential and excitability control, which may be broadly applicable to both fundamental single cell bioelectric studies36,37 and photoresponsive therapeutics38 in the clinic.
Methods
Nanowire synthesis
Coaxial pin silicon nanowires (PIN-SiNWs) were synthesized using a gold (Au) nanocluster-catalyzed chemical vapor deposition (CVD) process. Citrate-stabilized Au colloidal nanoparticles (Ted Pella Inc. 50 nm diameter) were deposited onto silicon (Si) <100> substrates (Nova Electronic Materials, n-type, 0.001-0.005 Ωcm) and used as catalysts. During the nanowire growth, silane (SiH4) was used as the Si reactant, diboron (B2H6, 100 ppm in H2) as the p-type dopant, phosphine (PH3, 1000 ppm in H2) as the n-type dopant, and hydrogen (H2) as the carrier gas. For the p-type core nanowire growth, SiH4, B2H6, and H2 were delivered at flow rates of 2, 10, and 60 standard cubic centimeters per minute (sccm), respectively. For the intrinsic Si shell (i-shell) deposition, SiH4 and H2 were delivered at 0.3 and 60 sccm, respectively. Flow of PH3 gas was then added for the n-type outer shell deposition at a flow rate of 1.5 sccm. The core growth was carried out at 470°C at a pressure of 40 torr for 30 min. Prior to i-shell deposition, growth was paused in a vacuum for 20 minutes until the CVD furnace temperature was stabilized at 750°C in preparation for shell deposition. The shell depositions were performed at 750°C at a pressure of 20 torr for 15 min per shell.
P-doped Si nanowires (p-type SiNWs) were synthesized also using 250 nm Au nanocluster-catalyzed CVD. SiH4, B2H6, and H2 were delivered at flow rates of 2, 10, and 60 sccm, respectively, at 470°C at a pressure of 40 torr for 30 min.
Undoped Si nanowires were synthesized also using 250 nm Au nanocluster-catalyzed CVD. SiH4 and H2 were delivered at flow rates of 2 and 60 sccm, respectively, at 475°C at a pressure of 40 torr for 30 min.
P-doped Au diffused Si nanowires were synthesized also using 250 nm Au nanocluster-catalyzed CVD. SiH4, B2H6, and H2 were delivered at flow rates of 2, 10, and 60 sccm, respectively, at 470°C at a pressure of 40 torr for 30 min. Au catalyst was allowed to diffuse down the synthesized nanowires in a vacuum at 750°C for 30 min.
X-ray photoelectron spectroscopy
PIN-SiNWs still attached to their growth substrate were cleaned in 10% HF for 90 sec, rinsed in DI H2O for 30 sec, and dried with N2 gas. X-ray photoelectron spectroscopy (XPS) was performed on the nanowire samples using a monochromatic Al Kα X-ray source (AXIS Nova Kratos Analytical) that probes elemental composition 7-10 nm from the surface of the sample. The Al anode was powered at 10 mA and 15 kV. The instrument work function was calibrated to give an Au 4f7/2 metallic gold binding energy (BE) of 83.95 eV. Instrument base pressure was ca. 1×10−9 Torr. The analysis area size was 0.3 × 0.7 mm2. For calibration purposes, the binding energies were referenced to Si 2p peak at 99.8 eV and/or C 1s peak at 285.5 eV. To improve reliability of the calibration, Pt metal was also introduced to the surface of some samples and Pt 4f signal 71.0 eV was used for cross-checking the calibration. Survey spectra were collected with a step size of 1 eV and 160 eV pass energy. The high-resolution spectra of Si 2p and Au 4f were collected with a pass energy of 20 eV and 0.1 eV step size using 3 and 20 sweeps of 120 s for each sweep, respectively. XPS peaks were fitted with an asymmetric Gaussian/Lorentzian peak shape with linear background correction. Initial peak approximation model was based on the Au 4f peak modeling of the pure gold sample in order to better evaluate the asymmetric nature of the peak profile and the fit envelope.
Transmission electron microscopy
PIN-SiNWs synthesized from 50 nm AuNPs were sonicated off of their growth substrate in IPA, and then dropcasted onto copper grids (Ted Pella Inc., USA, Lacey Formvar/Carbon, 200 mesh) for transmission electron microscopy (TEM) (FEI, USA, Tecnai F30) and for high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM). STEM images were recorded using an aberration corrected STEM (JEOL, Japan, JEM-ARM200CF).
Energy Dispersive X-ray Spectroscopy
PIN-SiNWs synthesized from 50 nm AuNPs were sonicated off of their growth substrate in IPA, and then dropcasted onto copper grids (Ted Pella Inc., USA, Lacey Formvar/Carbon, 200 mesh) for TEM (JEOL, Japan, JEM-3010) energy dispersive x-ray spectroscopy (EDS) (Thermo Fisher Scientific, USA, Thermo Noran Vantage XEDS). 840 second EDS measurements were taken for each nanowire and elemental peaks were assigned.
Atom probe tomography (APT)
200-350 nm PIN-SiNW were synthesized from 50 nm AuNPs and coated with 50 nm of Ni layers using an e-beam evaporator (AJA International, USA). The Ni-protected SiNWs were transferred onto Si microposts using a micromanipulator inside a focused ion beam (FIB) system (FEI, USA, Nova 600 NanoLab). Samples were then milled and sharpened into needle-like microtip specimens for APT characterization. The APT was run in an ultraviolet (UV) laser-assisted local-electrode atom-probe instrument (Cameca, USA, LEAP 400XSi). The surface atoms from each microtip were evaporated with an applied voltage of 1-6 kV and 20 pJ of 355 nm UV laser pulsing at a frequency of 250 kHz. The mass-to-charge (m/z) ratios of individual evaporated ions and their corresponding (x, y, z) coordinates in space were recorded with a position sensitive detector. The samples were held at 30 K and 2×10-11 Torr during APT experiments. The 3D reconstructions and data analyses were performed using Cameca's Integrated Visualization and Analysis Software (IVAS) 3.6 code. The proximity histogram was created with respect to the 80% Si isoconcentration surface.
Cell culture protocol
Dorsal root ganglia (DRG) were excised from P1-P3 neonatal rats into DMEM-F12 on ice. They were then digested in 2.5 mg/mL trypsin (Worthington) in EBSS for 20 min in a 37°C shaker. Following trypsinization, digested DRGs were resuspended into EBSS + 10% FBS in order to inhibit further digestion by any remaining trypsin. Digested ganglia were then mechanically triturated via three glass pipettes decreasing in size. The resulting dispersed DRG cells were then resuspended into DMEM + 5% FBS + 100 U/mL penicillin + 100 μg/mL streptomycin and seeded onto glass bottom dishes previously treated with 0.01% poly L lysine.
Neuron electrophysiology experiments
DRG neurons were patch clamped in whole cell current clamp configuration using an Axopatch 200B amplifier (Molecular Devices). The output voltage signal was digitized at 16-bit resolution by an Innovative Integration SBC-6711-A4D4 data acquisition board. The digital analogue converter (DAC) of the data acquisition board supplied the command voltage to the amplifier. DRG neurons were mounted onto a Zeiss IM 35 microscope (Carl Ziess Microscopy) and visualized through a 40× (0.55 NA) microscope objective lens. Si nanowires were sonicated for 10 seconds off of the growth substrate into a modified Tyrode's bath solution (NaCl 132 mM, KCl 4 mM, MgCl2 1.2 mM, CaCl2 1.8 mM, HEPES 10 mM, glucose 5.5 mM, pH 7.4). These wires were then dropcasted on top of the cultured neurons and allowed to settle for 20 minutes. Cells visually interacting directly with a single nanowire were then chosen to be tested for the generation of action potentials by laser pulses. Borosilicate glass pipettes pulled on a CO2 laser micropipette puller (Sutter Instruments P-2000) and flame polished using a custom microforge to produce 2 MΩ resistances when filled with internal pipette solution (NaCl 10 mM, KF 130 mM, MgCl2 4.5 mM, HEPES 10 mM, EGTA 9 mM, ATP 2 mM, pH 7.3) were used as patch pipettes. The 40× objective lens was used to focus a 532 nm DPSS laser (UltraLasers) spot (spot size: ∼5 μm) onto the cell nanowire interface. This laser beam was modulated with an acousto-optic modulator (NEOS Technologies, Gooch & Housego, PLC) and power adjusted via a series of neutral density filters. Current injections were performed with amplitudes varying from 500 to 1000 pA depending on the current amplitude necessary to generate an action potential in each cell at a duration of 1 ms. Laser pulse durations at the neuron-SiNW interface were varied from 0.1 to 10 ms and powers varied from 1 to 85 mW as described in the results. The University of Chicago Animal Care and Use Committee approved all animal protocols used in this work.
Scanning electron microscopy
DRG neurons were cultured onto a glass coverslip. PIN-SiNWs were sonicated into culture medium, drop casted onto the cells, and left to be co-cultured with the cells for 24 hours. The cell/NW co-culture was then fixed with 4% paraformaldehyde and then stained with 4% osmium tetroxide for 1 hour at room temperature. The culture was then dehydrated with ethanol and critical point dried before being sputter coated with 8 nm of Platinum/Palladium metal. Images were taken on a Carl Zeiss Merlin FE-SEM.
Fluorescent microscopy
DRG neurons were cultured onto a glass coverslip. PIN-SiNWs were sonicated into culture medium, drop casted onto the cells, and left to be co-cultured with the cells for 24 hours. Neurons were fixed in 4% paraformaldehyde and stained with a rabbit anti-rat anti-β tubulin III primary antibody (Abcam ab18207) and a goat anti-rabbit Texas Red secondary antibody (Abcam ab6719). Cells were visualized on an inverted fluorescent microscope under a Texas Red filter and nanowires visualized via SEPC as demonstrated previously.
Temperature measurements
Thermometer pipettes with resistances of 2 MΩ were filled with bath solution and placed 2 μm away from the neuron/SiNW interface being tested. Pipette resistance was monitored as part of a tension divider using a voltage amplifier during action potential generation in the nearby cell. Conversion of pipette resistance to temperature was achieved by using a calibration curve produced individually for each pipette by pairing resistance values with a broad range of temperatures as a solution starting at 40° C was cooled down passively to room temperature. The temperature was simultaneously recorded by a thermocouple placed very close to the pipette tip during the calibration procedure.
Photocurrent measurements
Si nanowires were synthesized as described above using a gold (Au) nanocluster-catalyzed chemical vapor deposition (CVD) process. Citrate-stabilized Au colloidal nanoparticles (Ted Pella Inc. 50 nm diameter) were deposited into quartz glass capillary tubes (Sutter Instruments) and used as catalysts. Nanowire growth was performed under the same conditions as described above. Quartz capillary tubes containing silicon nanowires were pulled to produce pipettes with 14-20 MΩ resistances (pipette tip diameter, ∼ 1 μm) and filled with bath solution. These pipettes were then mounted onto the aforementioned electrophysiology setup and current recordings were performed in voltage-clamp mode at 0 mV with the 532 nm laser focused onto single nanowires positioned ∼10-30 μm from the tip of the pipettes, thus minimizing any changes in pipette resistance due to increases in temperature produced by light absorption. Laser pulses between 0.5 and 10 ms durations and 1 to 20 mW powers were used. Raw traces were filtered by averaging every 10 points of data.
Cell viability assay
DRG neurons were cultured onto glass bottom petri dishes. PIN-SiNWs were sonicated into culture medium, drop casted onto the cells, and left to be co-cultured with the cells for 24 hours. For experiments without light stimulation, cells were stained with a LIVE/DEAD cell viability assay kit (ThermoFisher Scientific) and the numbers of live cells in culture with and without nanowires were counted. For experiments with light stimulation, cells were stimulated via the 592 nm depletion laser on an SP5 laser scanning confocal microscope (Leica, USA, SP5 II STED-CW) under a 40× objective (Leica, USA, HCX PL APO) at various frequencies for various durations at a total energy density of 0.31 μJ/μm2 for each pulse. After stimulation, cells were stained with a LIVE/DEAD cell viability assay kit (ThermoFisher Scientific) and the numbers of live stimulated neurons, neurons neighboring the stimulated neurons, and unstimulated neurons were counted.
Supplementary Material
Acknowledgments
We thank Dr. Fengyuan Shi at the University of Illinois Chicago for her help in collecting the EDS data. This work is supported by the Air Force Office of Scientific Research (AFOSR FA9550-14-1-0175, FA9550-15-1-0285), the National Science Foundation (NSF CAREER, DMR-1254637; NSF MRSEC, DMR 1420709), the Alfred P. Sloan Foundation Fellowship (FG-2016-6805), the Searle Scholars Foundation, the National Institute of Health (NIH GM030376, NIH F30AI138156, and NS101488), MSTP Training Grant (T32GM007281), and the Paul and Daisy Soros Foundation.
Footnotes
Author contribution: R.P. grew SiNWs for all experiments and performed all neuron electrophysiology experiments. R.P. and M.J.B. analyzed neuron electrophysiology data. Y.J. performed and analyzed STEM data. Y.J., J.Y. and R.P. prepared samples for, performed, and analyzed atom probe tomography experiments. K.K. performed and analyzed XPS experiments. R.P. and A.W.P. prepared samples for and performed SEM on neuron/SiNW samples. R.P. and M.J.B. performed and analyzed live/dead and fluorescence microscopy experiments. J.L.C.-d.-S. and R.P. set up equipment for all neuron electrophysiology experiments, temperature recordings, and photocurrent recordings. R.P., J.F.Z. and J.L.C.-d.-S. developed the single NW photocurrent recording method. R.P. performed and analyzed all photocurrent and temperature recordings. E.J.A. provided support and input on all experiments. B.T. and F.B. directed the research. R.P. and B.T. co-wrote the paper. All authors read and commented on the manuscript.
Competing financial interest: The authors declare no competing financial interests.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional Information: Supplementary information is available in the online version of the paper. Reprints and permission information is available online at www.nature.com/reprints. Correspondence and requests for materials should be addressed to B.T. and F.B.
References
- 1.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. A jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Transactions on Biomedical Engineering. 2004;51:896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- 3.Zhou W, Dai XC, Lieber CM. Advances in nanowire bioelectronics. Reports on Progress in Physics. 2017;80 doi: 10.1088/0034-4885/80/1/016701. [DOI] [PubMed] [Google Scholar]
- 4.Ghezzi D, et al. A hybrid bioorganic interface for neuronal photoactivation. Nature Communications. 2011;2 doi: 10.1038/ncomms1164. [DOI] [PubMed] [Google Scholar]
- 5.Ghezzi D, et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nature Photonics. 2013;7:400–406. doi: 10.1038/nphoton.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathieson K, et al. Photovoltaic retinal prosthesis with high pixel density. Nature Photonics. 2012;6:391–397. doi: 10.1038/nphoton.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho-de-Souza JL, et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207–217. doi: 10.1016/j.neuron.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang YW, et al. Heterogeneous silicon rnesostructures for lipid-supported bioelectric interfaces. Nature Materials. 2016;15:1023–1030. doi: 10.1038/nmat4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nature Neuroscience. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman J, Parameswaran R, Tian BZ. Nanoscale semiconductor devices as new biomaterials. Biomaterials Science. 2014;2:619–626. doi: 10.1039/C3BM60280J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers JA, Someya T, Huang YG. Materials and mechanics for stretchable electronics. Science. 2010;327:1603–1607. doi: 10.1126/science.1182383. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SW, et al. A Physically Transient Form of Silicon Electronics. Science. 2012;337:1640–1644. doi: 10.1126/science.1226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straub B, Meyer E, Fromherz P. Recombinant maxi-K channels on transistor, a prototype of iono-electronic interfacing. Nature Biotechnology. 2001;19:121–124. doi: 10.1038/84369. [DOI] [PubMed] [Google Scholar]
- 15.Chiappini C, et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nature Materials. 2015;14:532–539. doi: 10.1038/nmat4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priolo F, Gregorkiewicz T, Galli M, Krauss TF. Silicon nanostructures for photonics and photovoltaics. Nature Nanotechnology. 2014;9:19–32. doi: 10.1038/nnano.2013.271. [DOI] [PubMed] [Google Scholar]
- 17.Xie P, Xiong QH, Fang Y, Qing Q, Lieber CM. Local electrical potential detection of DNA by nanowire-nanopore sensors. Nature Nanotechnology. 2012;7:119–125. doi: 10.1038/nnano.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan RX, et al. Nature Nanotechnology. 2012;7:191–196. doi: 10.1038/nnano.2011.226. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman JF, et al. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Science Advances. 2016;2 doi: 10.1126/sciadv.1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang AQ, Lieber CM. Nano-Bioelectronics. Chemical Reviews. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian BZ, et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature. 2007;449:885–888. doi: 10.1038/nature06181. [DOI] [PubMed] [Google Scholar]
- 22.Su YD, et al. Single-nanowire photoelectrochemistry. Nature Nanotechnology. 2016;11:609–612. doi: 10.1038/nnano.2016.30. [DOI] [PubMed] [Google Scholar]
- 23.Hannon JB, Kodambaka S, Ross FM, Tromp RM. The influence of the surface migration of gold on the growth of silicon nanowires. Nature. 2006;440:69–71. doi: 10.1038/nature04574. [DOI] [PubMed] [Google Scholar]
- 24.Luo ZQ, et al. Atomic gold-enabled three-dimensional lithography for silicon mesostructures. Science. 2015;348:1451–1455. doi: 10.1126/science.1257278. [DOI] [PubMed] [Google Scholar]
- 25.Seibt M, et al. Gettering in silicon photovoltaics: current state and future perspectives. Physica Status Solidi a-Applications and Materials Science. 2006;203:696–713. [Google Scholar]
- 26.Yuan GB, et al. Understanding the origin of the low performance of chemically grown silicon nanowires for solar energy conversion. Angewandte Chemie-International Edition. 2011;50:2334–2338. doi: 10.1002/anie.201006617. [DOI] [PubMed] [Google Scholar]
- 27.Kim SK, et al. Tuning light absorption in core/shell silicon nanowire photovoltaic devices through morphological design. Nano Letters. 2012;12:4971–4976. doi: 10.1021/nl302578z. [DOI] [PubMed] [Google Scholar]
- 28.Garnett EC, et al. Dopant profiling and surface analysis of silicon nanowires using capacitance-voltage measurements. Nature Nanotechnology. 2009;4:311–314. doi: 10.1038/nnano.2009.43. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, et al. Anomalous high capacitance in a coaxial single nanowire capacitor. Nature Communications. 2012;3 doi: 10.1038/ncomms1833. [DOI] [PubMed] [Google Scholar]
- 30.Vogel EM. Technology and metrology of new electronic materials and devices. Nature Nanotechnology. 2007;2:25–32. doi: 10.1038/nnano.2006.142. [DOI] [PubMed] [Google Scholar]
- 31.Mogyoros I, Kiernan MC, Burke D, Bostock H. Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain. 1998;121:851–859. doi: 10.1093/brain/121.5.851. [DOI] [PubMed] [Google Scholar]
- 32.Walter MG, et al. Solar Water Splitting Cells. Chemical Reviews. 2010;110:6446–6473. doi: 10.1021/cr1002326. [DOI] [PubMed] [Google Scholar]
- 33.Reece SY, et al. Wireless Solar Water Splitting Using Silicon-Based Semiconductors and Earth-Abundant Catalysts. Science. 2011;334:645–648. doi: 10.1126/science.1209816. [DOI] [PubMed] [Google Scholar]
- 34.Sachsenhauser M, Sharp ID, Stutzmann M, Garrido JA. Surface State Mediated Electron Transfer Across the N-Type SiC/Electrolyte Interface. Journal of Physical Chemistry C. 2016;120:6524–6533. [Google Scholar]
- 35.Lee JH, Zhang AQ, You SS, Lieber CM. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Letters. 2016;16:1509–1513. doi: 10.1021/acs.nanolett.6b00020. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Colon BC, Ziesack M, Silver PA, Nocera DG. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science. 2016;352:1210–1213. doi: 10.1126/science.aaf5039. [DOI] [PubMed] [Google Scholar]
- 37.Sakimoto KK, Wong AB, Yang PD. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science. 2016;351:74–77. doi: 10.1126/science.aad3317. [DOI] [PubMed] [Google Scholar]
- 38.Silva GA. Neuroscience nanotechnology: Progress, opportunities and challenges. Nature Reviews Neuroscience. 2006;7:65–74. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.