Abstract
Although behavioral and experimental studies have shown links between victimization and antisocial behavior, the neural correlates explaining this link are relatively unknown. In the current study, we recruited adolescent girls from a longitudinal study that tracked youths’ reports of peer victimization experiences annually from the 2nd through 8th grades. Based on these reports, 46 adolescents were recruited: 25 chronically victimized and 21 non-victimized. During an fMRI scan, participants completed a risk-taking task. Chronic peer victimization was associated with greater risk-taking behavior during the task and higher levels of self-reported antisocial behavior in everyday life. At the neural level, chronically victimized girls showed greater activation in regions involved in affective sensitivity, social cognition, and cognitive control, which significantly mediated victimization group differences in self-reported antisocial behavior.
Keywords: peer victimization, fMRI, risk taking, adolescence
The need for social connection and acceptance is one of the most fundamental and universal human needs (Baumeister & Leary, 1995). When this need is unmet, it poses serious threats to the well-being of individuals, particularly during adolescence, a developmental period marked by an increased orientation towards peer acceptance (Allen, Porter, McFarland, Marsh, & McElhaney, 2005; Guyer, Pine, Choate, & Nelson, 2012). Not surprisingly, childhood peer victimization, which involves repeated exposure to adverse experiences in the peer group that likely threaten youths’ sense of social connection, is associated with a host of negative outcomes, including delinquency, antisocial behavior, truancy, and substance abuse, which tend to increase in severity as youth enter adolescence (Hanish & Guerra, 2002; Rudolph et al., 2014). Moreover, experimental studies in children, adolescents, and adults have shown that experiences of acute exclusion result in impaired self-regulation and greater risk-taking behavior, suggesting a causal link between victimization and risk taking (Baumeister, DeWall, Ciaracco, & Twenge, 2005; Mead, Baumeister, Stillman, Rawn, & Vohs, 2011; DeWall, Baumeister, & Vohs, 2008; Twenge, Catanese, & Baumeister, 2002; Nesdale & Lambert, 2008; Peake, Dishion, Stormshak, Moore, & Pfeifer, 2013). Although this exclusion-risk taking link has been well established, the neural processes by which social exclusion results in greater risk taking and antisocial behavior are not well understood, and it is unclear how a history of chronic victimization intersects with acute exclusion experiences to heighten risk taking and antisocial behavior. In the current study, we employed functional magnetic resonance imaging (fMRI) to examine neural sensitivity during risk taking following an acute episode of exclusion and its link to everyday antisocial behavior in chronically victimized and non-victimized youth.
Social Exclusion, Risk Taking, and Antisocial Behavior
One promising theory for the exclusion-risk taking link is that socially excluded individuals engage in risky and antisocial behavior as a way to conform to peer group norms and regain social stature, which may be in the form of perceived popularity, likeability or approval, social acceptance, or attention from peers (Allen et al., 2005; Dewall & Richman, 2011; Rawn & Vohls, 2011). In particular, adolescents who perceive that their peers value antisocial behavior are more likely to increase their substance use and delinquent behavior, suggesting that youth engage in risk taking to increase their social standing within the peer group (Allen et al., 2005). Experimental work has shown that adults exposed to social exclusion versus acceptance are more likely to express willingness to try illicit drugs only if given the option to try drugs with new friends as opposed to alone (Mead et al., 2011), consistent with the theory that socially excluded individuals may be willing to engage in risky behavior as a way to reestablish their sense of social connection (Dewall & Richman, 2011; Rawn & Vohls, 2011). Thus, the expected value of engaging in risky behavior may become greater due to the increased motivational currency of social approval and connection (Peake et al., 2013).
The Role of Chronic Peer Victimization
Although a few studies have investigated the neural processes involved in heightened risk taking following acute, experimentally manipulated social exclusion (Peake et al., 2013; Falk et al., 2014), no study has examined whether naturally occurring chronic childhood peer victimization sensitizes adolescents to risk taking following social exclusion. This is an important limitation given that the link between childhood peer victimization and later externalizing behaviors is pronounced in youth experiencing chronic compared to little or no victimization (see Dodge & Pettit, 2003). Moreover, early childhood victimization and increasing victimization across childhood independently contribute to antisocial behavior in adolescence, highlighting the enduring effects of early victimization experiences for youth’s well-being (Rudolph et al., 2014). Together, these studies point towards the importance of understanding the legacy of peer victimization experiences for youths’ risk taking and antisocial behavior. Adolescents who are chronically victimized across childhood may be particularly likely to engage in behaviors aimed at restoring their sense of social connection and need to belong following acute exclusion, even if such behaviors are maladaptive or harmful to the self or others.
Neural Systems Involved in Risk Taking and Antisocial Behavior
Recent evidence from developmental neuroimaging research has shown that neural systems important in detecting motivationally and emotionally relevant cues in the environment undergo remodeling during adolescence (see Nelson, Leibenluft, McClure, & Pine, 2005). This research points towards three important neural networks that may be particularly relevant for adolescent social decision making: affective sensitivity, cognitive control, and social cognition. These neural networks are thought to underlie changes in affective and cognitive responses to social experiences at a developmental period when the importance of peers increases (Nelson et al., 2005). Consistent with stress-sensitization models (Monroe & Harkness, 2005; Post, 1992), these neural systems may be altered by chronic early life stressors, such that childhood adversity in the form of peer victimization may enhance the probability of stress-induced alterations of brain function after exposure to subsequent acute stress. As a result, early life stressors may lower the threshold for reactivity to later stress.
Affective network
Upon detecting socially and emotionally relevant cues in one’s environment, the affective network, including the amygdala, ventral striatum, and orbitofrontal cortex (OFC), comes online, particularly among adolescents (Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2012). For example, neural regions involved in affective sensitivity show nonlinear developmental trajectories, peaking in functional reactivity during adolescence in response to emotional arousal, reward anticipation, risk taking, and social influence (Cascio et al., in press; Chein et al., 2011; Ernst et al., 2005; Galvan et al., 2006; Hare et al., 2008; Van Leijenhorst et al., 2010). Moreover, adolescent girls and socially anxious youth show heightened affective sensitivity when anticipating peer approval as well as when receiving peer acceptance versus rejection feedback (Guyer et al., 2009; Guyer et al., 2012; Lau et al., 2012). Finally, adolescents who have experienced high levels of peer conflict show heightened affective sensitivity during risk taking, whereas those who report strong peer support show attenuated affective sensitivity during risk taking (Telzer et al., 2015). Together, these studies provide strong evidence for the role of affective regions in adolescent reward sensitivity and risk taking following social rejection. Because the expected value of high-risk social rewards may increase in chronically victimized youth due to their desire for social connection and stature, this group may show enhanced affective processing during risk taking, which may explain victimized youths’ higher levels of everyday antisocial behavior.
Cognitive control network
Cortical systems comprising neural regions involved in higher-order cognition and impulse control (e.g., ventral and dorsal lateral prefrontal cortices [VLPFC, DLPFC]) gradually mature over adolescence and into adulthood (Gogtay et al., 2004). This gradual development is thought to result in a more flexible cognitive control system, such that PFC activation is sometimes compromised depending on the social and motivational context (Crone & Dahl, 2012). Developmental deficits in cognitive control, coupled with heightened affective sensitivity, are thought to underlie adolescents’ increased risk taking (Casey, Jones, & Hare, 2008). Experimental research has shown that social exclusion is associated with impaired self-regulation, including lower persistence in the face of failure, poorer attentional control, as well as altered PFC activation during risk taking (Baumeister et al., 2005; DeWall, Baumeister, & Vohs, 2008; Oaten, Williams, Jones, & Zadro, 2008; Peake et al., 2013). Thus, adolescents who have experienced chronic peer victimization may have impaired self-regulatory capacities, accounting for their higher risky and antisocial behavior. Reflecting this impairment, chronically victimized youth may evidence less prefrontal activation when making risky choices but more PFC activation when making safe choices (i.e., safe behavior requires more effort). Alternatively, risky behaviors may not occur due to heightened impulsivity or poor self-control, but instead may result from more deliberative decisions to regain social affiliation and acceptance following exclusion (Rawn & Vohs, 2011). Therefore, chronically victimized youth may show heightened PFC activation when making risky choices, indicative of more deliberative decision making during risk taking. Such heightened PFC activation may explain victimized youths’ higher levels of everyday antisocial behavior.
Social cognition network
Victimized youth are particularly sensitive to social threat, as reflected in an enhanced tendency to attribute hostile intent to peers (Yeung & Leadbeater, 2007), heightened threat appraisals in the context of stress (Taylor, Sullivan, & Kliewer, 2013), and heightened concerns about being negatively socially evaluated (Storch, Nock, Masia-Warner, & Barlas, 2003) and becoming socially isolated (Hunter & Boyle, 2004); this sensitivity may lead them to be more concerned about gaining peer approval and thus more likely to adjust their behavior to conform to group norms, a process that recruits neural regions involved in social cognition (e.g., temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS), medial prefrontal cortex (MPFC), dorsomedial prefrontal cortex (DMPFC), and medial posterior parietal cortex (MPPC)). Indeed, adolescents show increased activation in social cognition regions following an experimental social exclusion manipulation, with such increases in social cognitive neural regions predicting increased risk-taking behavior (Falk et al., 2014; Peake et al., 2013). Moreover, adolescents demonstrate greater activation in social cognition regions during mentalizing tasks relative to adults, indicative of adolescents’ greater focus on social processes more generally (Blakemore, 2010; Burnett, Bird, Moll, Frith, & Blakemore, 2009; Gweon, Dodell-Feder, Bedny, & Saxe, 2012; Wang, Lee, Sigman, & Dapretto, 2006; van den Bos, van Dijk, Westenberg, Rombouts, & Crone, 2011). Together, this prior research provides compelling evidence to suggest that socially excluded youth may show greater social-cognitive neural processing during risk taking due to greater social monitoring and a concern with peers’ evaluations of their risky decisions. Such heightened social-cognitive neural processing may explain victimized youths’ higher levels of everyday antisocial behavior.
Study Overview
Taken together, we hypothesized that (1) chronically victimized youth would show greater risk taking (measured during an experimental risk-taking task) and everyday antisocial behavior (measured via self-report); (2) chronically victimized youth would show altered activation in neural regions involved in affective sensitivity, cognitive control, and social cognition during risk taking; and (3) this heightened neural sensitivity would explain (i.e., mediate) group differences in antisocial behavior. To test this hypothesis, we recruited adolescent girls based on well-documented childhood victimization experiences from the 2nd through 8th grades. We focused on adolescent girls because girls tend to be more sensitive and reactive than boys to interpersonal stress (see Hankin et al., 2007; Natsuaki, Klimes-Dougan, Ge, Shirtcliff, Hastings, & Zahn-Waxler, 2009; Rudolph, Flynn, Abaied, Groot, & Thompson, 2009). Indeed, girls show a steep increase in behavioral (i.e., anxiety about peer acceptance) and neural (i.e., heightened affective processing during peer evaluation) sensitivity to social threat that peaks around age 15–16 years (Guyer et al., 2009, 2012; Kloep, 1999), as well as heightened biological sensitivity (e.g., cortisol peaks) following social rejection challenges (Stroud, Salovey, & Epel, 2002). Moreover, some research suggests that girls evidence more detrimental effects of victimization for both externalizing (Rusby et al., 2005, Khatri, Kupersmidt, & Patterson, 2000) as well as internalizing (Loukas & Pasch, 2013) symptoms (but see Hanish & Guerra, 2002 for boys being more reactive than girls, and Rudolph et al., 2014 for comparable effects across sex).
Methods
Participants
Forty-six 9th grade adolescent girls (Mage=15.3 years, SD=.34, range=14.8–16.1 years) were recruited from a longitudinal study that tracked 636 (337 female) youth from 2nd–8th grade. Each year, youth self-reported on their victimization experiences using the Social Experiences Questionnaire-Revised (Rudolph, Troop-Gordon, Hessel, & Schmidt, 2011; Rudolph et al., 2014), which taps overt and relational forms of peer victimization. From this large sample, we recruited adolescent girls based on their victimization scores across the 7 years, such that we recruited the most victimized and least victimized girls. Of the 636 total participants, 115 (66 chronic victims and 49 non-victims) were identified as eligible to participate based on being female and meeting criteria for low or high peer victimization. Of these, 8 had contraindications for MRI, 6 were not interested in participating, 51 were not recruited because they had either moved out of town, were not reachable, or we reached our target sample size of 50 prior to their recruitment, and 4 completed the scan but did not complete the Stoplight Task. Our final sample included 46 adolescent girls, 25 who were classified as chronically victimized and 21 as non-victimized. Chronically victimized girls scored ≥ .75 SD above the mean on victimization for at least three years (range = 3 to 7 years), with an average of 1.21 SD above the mean across the 7 years (range = .76 to 2.72 SD). Non-victimized girls scored ≤ .75 below the mean on victimization for at least three years (range = 3 to 7 years) with an average of .83 SD below the mean across the 7 years (range = −1.17 to −.61 SD). Participants were ethnically diverse [African American (n=10), European American (n=31), or other (n=5)]. The chronically victimized group had marginally more non-White participants (n=11, 44%) than did the non-victimized group (n=4, 19%), χ2=3.2, p=.072. Parents provided written consent and adolescents provided written assent in accordance with the university’s Institutional Review Board.
Self-Reported Antisocial Behavior
Adolescents completed a 13-item antisocial behavior questionnaire adapted from Nolen-Hoeksema and colleagues (Nolen-Hoeksema, Stice, Wade, & Bohon, 2007). Participants used a 5-point scale (1=not at all, to 5=extremely) to indicate how much each item describes them (e.g., “I stole things,” “I cut classes or skipped school,” “I hung around with kids who get in trouble.”). Participants completed this measure when they were in the sixth grade and again on the day of the brain scan when they were in the 9th grade (αs=.92 and .90, respectively). Sixth grade antisocial behavior was used as a control in mediation analyses examining the neural correlates by which childhood chronic peer victimization experiences are associated with later adolescent antisocial behavior. By controlling for earlier antisocial behavior, we can be relatively more confident of the direction of the effects and that early antisocial behavior does not account for any significant effects.
Risk-Taking Task Procedures
Group Membership Manipulation
In order to increase the salience of risk taking in a social context, participants were randomly assigned to a team, the red team or blue team. Prior to the scan, participants were introduced to everyone on their team and the opposing team by viewing a slideshow of pictures of each participant overlaid on a color background representing team membership. The other players, who were half males and half females, were described as participants who had already come in for the study. Participants’ photo was also taken and included in the slideshow to increase the salience of group belonging. Participants were then given instructions on the risk-taking task and instructed that their performance on the task would determine how many points their team won; the faster they completed the driving course, the more points they would earn for their team. At the end of the study, the team with the most points would win. Unfortunately, their team was currently behind, so they should try to help their team earn more points.
Social Exclusion Experience
Prior to completing the risk-taking task in the scanner, participants were exposed to social exclusion using Cyberball (Williams et al., 2000), which creates a subjective experience of being excluded. Participants were told they would be playing an on-line ball-throwing game with two peers (ostensibly in another room) also completing the same study and connected via the Internet. Importantly, these peers were not part of either team in the group membership manipulation, and participants were not told whether the other participants were part of their team or the other team. Both of the confederates were females.
Throughout the game, the ball was thrown back and forth among the three players, with the participant choosing the recipient of her own throws, and the throws of the other two ‘players’ determined by the pre-set program. Participants could see the photograph of the other two players on a computer screen as well as their own ‘hand’ that they controlled using a button-box. Participants completed two rounds of Cyberball, one during which they were equally included, and a second during which the participant was excluded after 10 throws. Providing validity for the exclusion experience, chronically victimized girls reported feeling significantly more rejected following Cyberball (M=3.25, SD=.68) than did non-victimized girls (M=2.66, SD=.51, t(44)=2.92, p<.005), as measured by the Need-Threat Scale (Williams et al., 2000), a 12-item self-report measure that assesses participants’ feelings of self-esteem, belongingness, and social control during the Cyberball game (e.g., “I felt rejected, ” “I felt disconnected”).
Risk-Taking Task
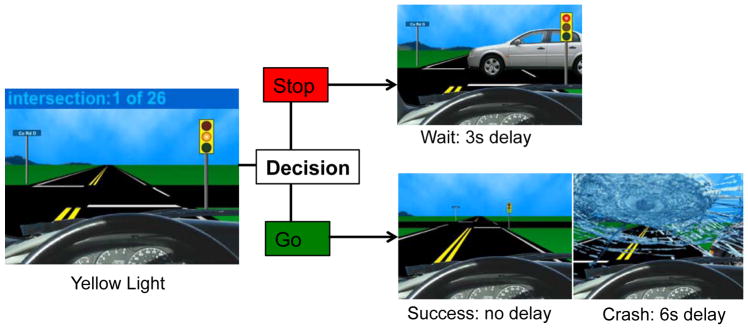
Adolescents completed the Stoplight Task, which is a well-validated risk-taking game that has shown robust behavioral and neural effects in adolescents (Gardner and Steinberg, 2005; Chein et al., 2011; Peake et al., 2013; Telzer et al., 2015). During the task, participants complete a simulated driving course in which they encounter a number of yellow stoplights and must decide whether to “stop” or “go” at each intersection (see Figure 1). Participants were instructed that a decision to “go” through the intersection is the fastest option (no delay), but they risk the possibility of crashing, which causes a long delay (6 seconds). If they choose to “stop,” participants do not risk crashing, but it results in a short delay (3 seconds). Participants were told that the goal is to get through the driving course in as short a time as possible, and the faster they complete the game, the more points they will earn for their team. Participants completed 26 intersections; 8 intersections out of the 26 total intersections had cars approaching on the cross street, resulting in a crash if the participant made a “go” decision, but this was not explicitly revealed to participants. The timing of traffic signals and the presence of a car on the cross street varied so as to be unpredictable by the participant and to introduce variable inter-trial-intervals (ITIs).
Figure 1.
Stoplight Task.
Participants played the Stoplight Task twice. Prior to the scan, participants completed one entire round of the task. This round was used as a baseline to test whether victimized and non-victimized participants would show different risk-taking behaviors before the group membership manipulation and prior to the social exclusion task. During the scan, directly following the social exclusion task, participants completed a second round of the Stoplight Task that was identical in timing and number of intersections as the first round.
fMRI Data Acquisition and Analysis
fMRI data acquisition
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The Stoplight task included T2*-weighted echoplanar images (EPI) [slice thickness=3 mm; 38 slices; TR=2sec; TE=25msec; matrix=92x92; FOV=230 mm; voxel size 2.5x2.5x3mm3]. Structural scans consisted of a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR=4sec; TE=64msec; FOV=230; matrix=192x192; slice thickness=3mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR=1.9sec; TE=2.3msec; FOV=230; matrix=256x256; sagittal plane; slice thickness=1mm; 192 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
fMRI Data Preprocessing and Analysis
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant’s images included spatial realignment to correct for head motion (no participant exceeded 2mm of maximum image-to-image motion in any direction). The realigned functional data were coregistered to the high resolution MPRAGE, which was then segmented into cerebrospinal fluid, grey matter, and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and T2 structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8mm Gaussian kernel, full-width-at-half maximum, to increase the signal-to-noise ratio. Statistical analyses were performed using the general linear model in SPM8. Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128 seconds was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1.
In each participant’s fixed-effects analysis, a general linear model (GLM) was created with four regressors of interest, modeled as events: two decision regressors (Stop and Go; i.e., Safe and Risky) and two outcome regressors (Crash and Pass). In addition, the wait time after safe decisions was modeled as well as the final “Game Over” period, in order to remove these from the implicit baseline. Because the task was self-paced, the duration of the decision trials (stop or go) represented the time from which the traffic light appeared until the participant made a response, and the duration for the outcome (pass or crash) was 1s. The onset of the crash event corresponded to another car crashing into the participant’s car. The pass and wait events had no specific onset time. However, because the crash events happened at most 2s after the yellow light, we modeled the pass and wait events as being 2s after the yellow light, the point at which the outcome of the risky decision was clear. Each was modeled with a 1s duration. Null events, consisting of the jittered inter-trial intervals, were not explicitly modeled and therefore constituted an implicit baseline. The parameter estimates resulting from the GLM were used to create linear contrast images comparing each of four event conditions (decisions: Risky, Safe; outcomes: Crash, Pass). Random effects, group-level analyses were performed on all individual subject contrasts using GLMFlex. GLMFlex corrects for variance-covariance inequality, partitions error terms, removes outliers and sudden activation changes in the brain, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex).
We conducted independent samples t-tests at the group level to examine differences in neural activation during risk taking. Our main analyses focus on “go” decisions, as these trials encompass adolescents’ risky choices, which are the focus of the current study. However, we also examined group differences in neural activation during safe decisions (i.e., “stop”) as well as when receiving a positive outcome (i.e., successfully passing through the intersection following a risky choice). We did not model negative outcomes (i.e., crashes), as we did not have enough trials in this condition to model.
To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (Ward, 2000). We used our group-level brain mask, which included only gray matter. Results of the simulation indicated a voxel-wise threshold of p<.005 combined with a minimum cluster size of 42 voxels for the whole brain, corresponding to p<.05, False Wise Error (FWE) corrected. We used the MarsBaR toolbox to extract parameter estimates from significant clusters in the group-level analyses.
Results
Behavioral Results
Group Differences in Risk-Taking and Antisocial Behavior
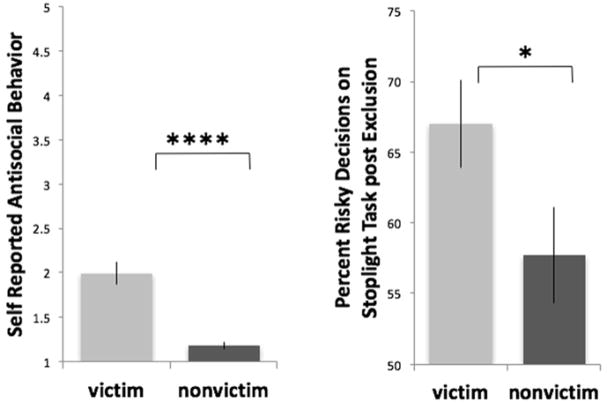
In terms of risk-taking behavior on the Stoplight Task, we found a significant group (victim, non-victim) x time (pre-exclusion, post-exclusion) interaction (F(1,44)=4.95, p<.05, η2=.10). Prior to being socially excluded, and when the goal of the task was not yet described as a team game, chronically victimized (63.1% of intersections) and non-victimized (63.3% of intersections) youth did not differ in the number of risky choices (i.e., “go”; t(44)=.07, ns, Cohen’s d=.018), whereas after the social exclusion experience, chronically victimized girls made significantly more risky choices than non-victimized girls (t(44)=2.11, p<.05, Cohen’s d=.62; Figure 2), an effect that remained significant when controlling for ethnicity.
Figure 2.
Victimization group differences in self-reported antisocial behavior (left) and risky decisions on the Stoplight Task following social exclusion (right).
In terms of self-reported antisocial behavior, chronically victimized youth reported significantly greater antisocial behavior than non-victimized youth in the 9th grade (t(44)=5.50, p<.0001, Cohen’s d=1.70; Figure 2). These effects remained significant when controlling for 6th grade antisocial behavior as well as ethnicity. Ethnicity was not associated with risk-taking behavior on the task or with antisocial behavior.
fMRI Results
Group Differences in Neural Reactivity during Risky Decisions
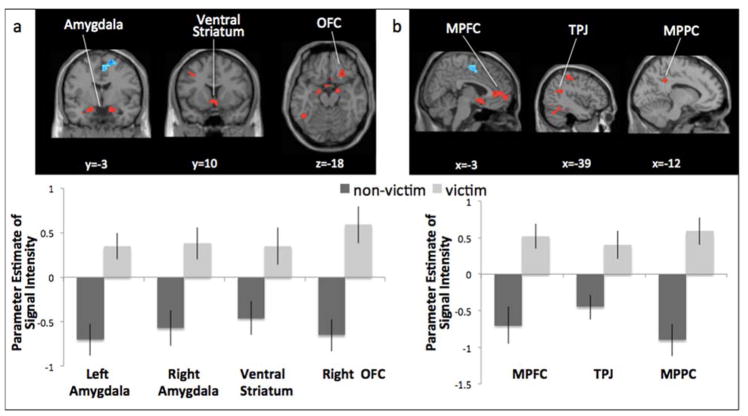
We conducted whole-brain, independent samples t-tests to examine group differences in neural reactivity when making risky decisions (i.e., “go” through yellow light; see Table 1). Victimized girls showed greater reactivity in several brain regions involved in affective sensitivity, including the bilateral amygdala, ventral striatum, and OFC (Figure 3a), as well as regions involved in social cognition, including the MPFC, TPJ, and MPPC (Figure 3b). For descriptive purposes, we extracted parameter estimates of signal intensity from each significant cluster from the contrast Risky Decisions relative to baseline. We plotted the parameter estimates for victimized and non-victimized girls separately (Figure 3). Because the baseline represents the majority of the task (e.g., driving, time between stoplights, time immediately before making a decision), it is not an ideal control and is therefore used only for descriptive purposes. Thus, the relative activation in each group compared to baseline should be interpreted with caution. Non-victimized girls showed greater activation in the supplementary motor area (Table 1).
Table 1.
Group differences in neural reactivity
| Anatomical Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Risky Decisions, victim>non-victim | ||||||
| R Ventral Striatum | 3 | 8 | −11 | 3.38 | 71 | |
| R Amygdala | 24 | −5 | −22 | 4.62 | 48 | |
| L Amygdala | −16 | −3 | −20 | 3.92 | 44 | |
| R OFC | 11 | 27 | 29 | −17 | 3.88 | 42 |
| MPFC | 10/32 | 0 | 47 | 7 | 4.35 | 185 |
| L MPPC | −12 | −43 | 34 | 3.49 | 46 | |
| L TPJ | −39 | −46 | 22 | 4.62 | 46 | |
| L pSTS | −54 | −46 | 1 | 3.88 | 42 | |
| L STS | −57 | −43 | 4 | 3.22 | 49 | |
| L Fusiform gyrus | −45 | −52 | −14 | 3.84 | 137 | |
| R Middle temporal gyrus | 57 | −58 | 1 | 3.57 | 43 | |
| L Postcentral gyrus | −39 | −28 | 52 | 3.15 | 69 | |
| Risky Decisions, non-victim>victim | ||||||
| R SMA | 4 | −4 | 53 | 4.15 | 99 | |
| Safe Decisions, victim>non-victim | ||||||
| R MPFC | 10 | 9 | 65 | −2 | 5.07 | 66 |
| R MPFC | 10/32 | 9 | 47 | 7 | 3.56 | 89 |
| L Middle temporal gyrus | 18/19 | −46 | −76 | 5 | 4.5 | 499 |
| L Fusiform | −42 | −44 | −20 | 4.96 | 320 | |
| R Fusiform | 32 | −36 | −18 | 3.92 | 69 | |
| R MPPC | 12 | −43 | 16 | 3.18 | 52 | |
| L TPJ/STS | −45 | −52 | 13 | 3.48 | 71 | |
| L Postcentral gyrus | −48 | −10 | 28 | 4.22 | 142 | |
| R Postcentral gyrus | 54 | −4 | 23 | 3.77 | 104 | |
| L VLPFC | 10 | −47 | 40 | 6 | 4.22 | 53 |
| R DLPFC | 46 | 48 | 42 | 12 | 3.27 | 46 |
| R DMPFC | 8 | 46 | 36 | 5.80 | 795 | |
| R DMPFC | 16 | 60 | 23 | 3.89 | 83 | |
| Pass Outcome, victim>non-victim | ||||||
| R Ventral striatum | 15 | 20 | 7 | 4.01 | 40 | |
| Pass Outcome, non-victim> victim | ||||||
| L Insula | 22 | −51 | −4 | −2 | 3.21 | 101 |
| R Insula | 22 | 48 | 3 | −2 | 3.50 | 56 |
| L Postcentral gyrus | −36 | −25 | 49 | 4.20 | 46 | |
Note. BA refers to putative Broadman’s areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. R and L refer to right and left hemisphere respectively. MPFC=medial prefrontal cortex, VLPFC=ventrolateral prefrontal cortex, DLPFC=dorsolateral prefrontal cortex, DMPFC=dorsomedial prefrontal cortex, SMA=supplementary motor area, TPJ=temporoparietal junction, STS=superior temporal sulcus, MPPC=medial posterior parietal cortex, OFC=orbital frontal cortex.
Figure 3.
Victimization group differences in neural activation when making risky decisions on the Stoplight Task. Victimized adolescents showed significantly greater activation than non-victimized adolescents in neural regions involved in a) affective sensitivity and b) social cognition.
Group Differences in Neural Reactivity during Safe Decisions
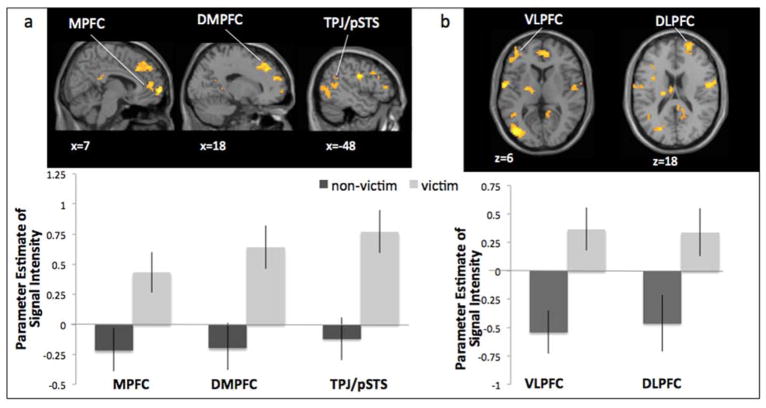
When making safe decisions (i.e., “stop” at yellow light), victimized girls showed greater activation in brain regions involved in social cognition, including the MPFC, DMPFC, TPJ, MPPC, and STS (Figure 4a), as well as regions involved in cognitive control, including the VLPFC and DLPFC (Figure 4b; Table 1). For descriptive purposes, we extracted parameter estimates of signal intensity from each significant cluster from the contrast Safe Decisions relative to baseline. We plotted the parameter estimates for victimized and non-victimized girls separately (Figure 4). Non-victimized girls did not show greater activation in any brain regions during safe decisions.
Figure 4.
Victimization group differences in neural activation when making safe decisions on the Stoplight Task. Victimized adolescents showed significantly greater activation than non-victimized adolescents in neural regions involved in a) social cognition and b) cognitive control
Group Differences in Neural Reactivity during Pass Outcomes
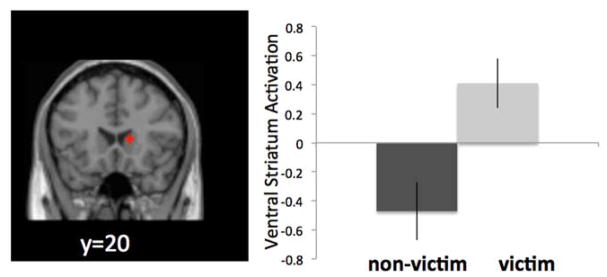
When adolescents successfully passed through an intersection without crashing following a risky decision, victimized girls showed greater activation than non-victimized girls in the striatum (Figure 5; Table 1). For descriptive purposes, we extracted parameter estimates of signal intensity from each significant cluster from the contrast Pass Outcomes relative to baseline. We plotted the parameter estimates for victimized and non-victimized girls separately (Figure 5). Non-victimized girls showed greater activation in the bilateral insula (Table 1). Due to the low frequency of crash events (i.e., there were only 8 possible crash events, with most participants having only 1–5 total crashes), we did not have enough trials to run analyses for the Crash Outcome. 1
Figure 5.
Victimization group differences in neural activation following risky decisions on the Stoplight Task (i.e., pass outcome). Victimized adolescents showed significantly greater activation than non-victimized adolescents in the ventral striatum.
Association between Neural Reactivity during Risky Decisions and Antisocial Behavior
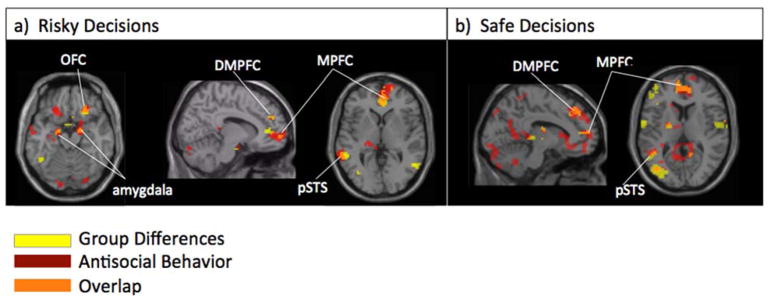
We examined how adolescents’ neural reactivity during risky decisions was associated with self-reported antisocial behavior. In whole-brain regression analyses, we regressed antisocial behavior on neural reactivity when making risky decisions (i.e., “go” through yellow light). Adolescents reporting higher antisocial behavior showed greater activation in several brain regions involved in affective sensitivity, including the bilateral amygdala and OFC, as well as regions involved in social cognition, including the MPFC, DMPFC, and pSTS (Table 2). Notably, many of these regions overlap with those that demonstrated group differences during risk taking (bilateral amygdala, OFC, MPFC, pSTS; see Figure 6a).
Table 2.
Activation in neural regions during risk taking correlated with antisocial behavior
| Anatomical Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Risky Decisions, positive correlation | ||||||
| R DMPFC* | 18 | 44 | 22 | 5.35 | 52 | |
| R Amygdala* | 18 | −7 | −20 | 5.10 | 61 | |
| L Amygdala* | −18 | −7 | −20 | 3.77 | 161 | |
| R MPFC* | 10 | 9 | 56 | −5 | 4.77 | 238 |
| L pSTS* | 22 | −63 | −43 | 1 | 4.53 | 54 |
| L OFC | −21 | 29 | −17 | 4.47 | 47 | |
| R OFC* | 27 | 29 | −17 | 4.40 | 42 | |
| R Cerebellum | 3 | −82 | −29 | 4.22 | 92 | |
| L Cerebellum | −36 | −67 | −26 | 4.12 | 123 | |
| L Middle Temporal Gyrus | 21 | −57 | −10 | −11 | 3.69 | 63 |
| Risky Decisions, negative correlation | ||||||
| R SMA | 12 | 5 | 61 | 4.78 | 471 | |
| Safe Decisions, positive correlation | ||||||
| L DMPFC* | −12 | 35 | 40 | 4.97 | 664 | |
| L Precuneus | 3 | −76 | 49 | 4.99 | 160 | |
| R VLPFC | 47 | 18 | 11 | −20 | 4.95 | 58 |
| R MPFC* | 10 | 12 | 62 | 1 | 4.57 | 882a |
| R OFC | 3 | 35 | −26 | 3.32 | 882a | |
| R DLPFC | 9 | 36 | 44 | 32 | 4.10 | 882a |
| R MPPC | 15 | −40 | 4 | 4.08 | 1096b | |
| L pSTS | −57 | −49 | 1 | 3.93 | 1096b | |
| L TPJ | −48 | −73 | 28 | 3.75 | 1096b | |
| L MPPC | −9 | −55 | 7 | 3.70 | 1096b | |
| R Cerebellum | 9 | −91 | −23 | 4.72 | 1000 | |
| L VLPFC | −54 | 44 | −8 | 4.63 | 85 | |
| L Inferior Temporal Gyrus | 37 | −45 | −43 | −20 | 4.51 | 57 |
| L STS* | 21 | −54 | −13 | −8 | 4.35 | 182 |
| R Fusiform Gyrus | 39 | −34 | −14 | 4.26 | 44 | |
| L Caudate | −12 | 26 | 1 | 4.09 | 233c | |
| R OFC | 32 | −11 | −6 | 3.41 | 233c | |
| L VLPFC | 11/47 | −33 | 32 | −17 | 3.97 | 233c |
| R Cerebellum | 9 | −52 | −47 | 3.68 | 48 | |
| L Cerebellum | −9 | −52 | −35 | 3.38 | 46 | |
| L Caudate | −12 | 26 | 1 | 4.09 | 233 | |
| Safe Decisions, negative correlation | ||||||
| R SMA | 3 | −13 | 55 | 3.65 | 66 | |
| L Precuneus | −15 | −49 | 61 | 4.13 | 42 | |
| R Precuneus | 18 | −48 | 61 | 3.68 | 116 | |
| Pass Outcomes, positive correlation | ||||||
| - | ||||||
| Pass Outcomes, negative correlation | ||||||
| R pSTS/TPJ | 40/22 | 69 | −19 | 10 | 5.38 | 671 |
| R VLPFC | 47 | 36 | 23 | −14 | 4.28 | 158d |
| R Amygdala | 30 | 2 | −17 | 3.28 | 158d | |
| R Cuneus | 15 | −61 | 25 | 4.04 | 426e | |
| L MPPC | −9 | −46 | 19 | 3.20 | 426e | |
| L pSTS/TPJ | 40/22 | −45 | −34 | 19 | 4.17 | 296 |
| L STS | 22 | −45 | −16 | −8 | 4.16 | 216 |
| ACC | 32 | 0 | 41 | 13 | 4.14 | 226 |
| R Fusiform | 12 | −58 | −5 | 3.99 | 132 | |
| R pSTS/TPJ | 45 | −52 | 13 | 3.75 | 152 | |
| L Hippocampus | −24 | −25 | −5 | 3.81 | 292f | |
| L Pallidum | −15 | −4 | 4 | 3.52 | 292f | |
| L VLPFC | 47/13 | −27 | 17 | −11 | 3.65 | 53 |
| R Ventral Striatum | 9 | −1 | −2 | 3.65 | 43 | |
| L Fusiform | −12 | −58 | −5 | 3.53 | 73 | |
| L Precentral Gyrus | −6 | −19 | 67 | 3.51 | 70 | |
Note. BA refers to putative Broadman’s areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. R and L refer to right and left hemisphere respectively. MPFC=medial prefrontal cortex, VLPFC=ventrolateral prefrontal cortex, DLPFC=dorsolateral prefrontal cortex, DMPFC=dorsomedial prefrontal cortex, SMA=supplementary motor area, TPJ=temporoparietal junction, STS=superior temporal sulcus, MPPC=medial posterior parietal cortex, ACC=anterior cingulate cortex, OFC=orbitofrontal cortex.
indicates that the cluster of activation overlaps with that found for the group differences analyses (Table 1) and was used in mediation analyses. Clusters which share the same superscript are part of the same cluster of activation.
Figure 6.
Neural regions identified in whole brain analyses comparing group differences (i.e., victim>non-victim; depicted in yellow) and antisocial behavior (depicted in red) during risk taking. Regions showing overlap between the two independent analyses are depicted in orange.
Association between Neural Reactivity during Safe Decisions and Antisocial Behavior
When making safe decisions (i.e., “stop” at yellow light), antisocial behavior was associated with greater activation in brain regions involved in social cognition, including the MPFC, DMPFC, TPJ, pSTS, MPPC, as well as regions involved in cognitive control, including the VLPFC and DLPFC (Table 2). Notably, many of these regions overlap with those that demonstrated group differences during safe decisions (MPFC, DMPFC, pSTS).
Association between Neural Reactivity during Pass Outcomes and Antisocial Behavior
When adolescents successfully passed through an intersection without crashing following a risky decision, greater antisocial behavior was associated with less activation in brain regions involved in affective sensitivity, including the bilateral amygdala, and brain regions involved in social cognition, including the TPJ, STS, MPFC, and DMPFC (Table 2). Greater antisocial behavior was not associated with heightened activation in any brain region.
Neural Reactivity as a Mediator of Group Differences in Antisocial Behavior
Given that similar neural patterns were found when examining group differences in neural activation and associations between neural activation and antisocial behavior, we examined whether group differences in neural reactivity explained chronically victimized girls’ greater antisocial behavior. To this end, we extracted parameter estimates of signal intensity from the ROIs that showed overlap in the two sets of independent analyses (see Figure 6 and Table 2, note clusters with an asterisk are those which showed overlap). We calculated the magnitude and the significance of the indirect effects of group status (victim=1, non-victim=0) on antisocial behavior through neural reactivity using the procedures described by Preacher and Hayes (2008), in which bootstrapping was performed with 1,000 samples and a bias-corrected confidence interval (CI) was created for the indirect effect. Sixth grade antisocial behavior was used a control variable in all mediation models. By controlling for earlier antisocial behavior, we can be more confident of the direction of the effects such that early antisocial behavior does not account for any significant effects. For risky decisions, the indirect effect of victimization on antisocial behavior through adolescents’ neural sensitivity was significant (i.e., the 95% CI did not include 0) for the bilateral amygdala, DMPFC, and pSTS (see Table 3). For safe decisions, the indirect effect of victimization on antisocial behavior through adolescents’ neural sensitivity was significant for the DMPFC and pSTS (see Table 3).
Table 3.
Indirect effect of mediation models for group differences (victim vs. non-victim) on antisocial behavior via neural activation
| Effect | SE | 95% CI | |
|---|---|---|---|
| Risky Decisions | |||
| Bilateral Amygdala | .159 | .076 | [.044, .362] |
| OFC | .044 | .062 | [−.029, .230] |
| DMPFC | .167 | .093 | [.014, .379] |
| MPFC | .095 | .070 | [−.016, .264] |
| pSTS | .135 | .078 | [.024, .355] |
| Safe Decisions | |||
| DMPFC | .100 | .057 | [.007, .239] |
| MPFC | .055 | .064 | [−.020, .274] |
| pSTS | .169 | .081 | [.027, .350] |
| VLPFC | −.036 | .083 | [−.268, .106] |
| DLPFC | −.062 | .055 | [−.240, .003] |
Discussion
Peer victimization is a salient and distressing experience, conferring significant risk for poor adjustment outcomes including risky and antisocial behavior (Hanish & Guerra, 2002; Laird et al., 2001; Parker & Asher, 1987; Prinstein & La Greca, 2004; Rudolph et al., 2014). Although significant research reveals a prospective association between exposure to peer victimization and antisocial behavior, research has not explored the neural processes underlying this link. Drawing from experimental research documenting the effect of acute social exclusion on risk taking and associated neural activity (Peake et al., 2013), the present study examined whether chronically victimized girls show differences in neural processing following exclusion and whether these differences help to account for the association between chronic victimization and subsequent antisocial behavior in adolescence. At the behavioral level, we confirmed that chronic peer victimization was significantly associated with more risky behavior following acute exclusion measured via an experimental task and with more antisocial behavior in everyday life measured via self-report. At the neural level, we found group differences in neural regions involved in affective sensitivity, social cognition, and cognitive control during the risk-taking task; moreover, several of these group differences mediated the association between chronic victimization and antisocial behavior.
Although prior research has examined risk-taking following acute, experimentally manipulated social exclusion (e.g., Peake et al., 2013; Falk et al., 2014), and prior studies have shown that chronically rejected and victimized youth show heightened neural sensitivity to social threat (Will et al., 2016; Rudolph et al., 2016), our findings are the first to show that the neural correlates of risk taking differ in youth with versus without a history of chronic peer victimization. Not all youth respond to social exclusion similarly, and it is important to understand the processes by which those with a history of chronic victimization react following such experiences. Importantly, we show that risk-taking behavior prior to a social exclusion manipulation did not differ in victimized and non-victimized girls. However, following the exclusion experience, and when the goal of the risk-taking task was described as a means to gain points for one’s collective team, chronically victimized and non-victimized girls showed distinct patterns of behavior, such that the victimized youth showed greater risk taking than non-victimized youth. These findings provide further evidence that the potential goals of engaging in risk taking are different among adolescents who have a history of victimization experiences, and suggest that acute instances of social exclusion may trigger heightened sensitivity and an orientation to group belonging via risk taking in this group. These findings are also consistent with experimental work in adults, which shows that being exposed to acute social exclusion increases one’s willingness to try illicit drugs only if given the option to try drugs with new friends as opposed to alone, consistent with the theory that socially excluded individuals may be willing to engage in risky behavior as a way to reestablish their sense of social connection (Mead et al., 2011).
At the neural level, we found that chronically victimized and non-victimized adolescent girls showed differing patterns of activation during the risk-taking task. In particular, victimized adolescents showed significantly greater activation in the ventral striatum, amygdala, and OFC when making risky choices. Moreover, heightened amygdala activation mediated victimization group differences in self-reported antisocial behavior. Because the ventral striatum, amygdala, and OFC are involved in emotional arousal, reward anticipation, and social influence, particularly during adolescence (Cascio et al., in press; Chein et al., 2011; Ernst et al., 2005; Galvan et al., 2006; Hare et al., 2008; Van Leijenhorst et al., 2010), our findings suggest that the expected value of high-risk rewards is heightened in youth who have a history of peer victimization. Our risk-taking task manipulation had participants complete the task in order to gain points for their collective team. Heightened affective sensitivity is consistent with the idea that victimized girls have a strong need for approval (i.e. they are sensitive to receiving positive judgments from peers). Thus, chronically victimized youth may find risky choices more appealing as they provide a sense of expected social reward or social connection, consistent with theory and research suggesting that increases in risk taking following social exclusion occur as a means to regain social status (Dewall & Richman, 2011; Rawn & Vohls, 2011). Therefore, the expected value of high-risk rewards may become greater due to the increased motivational currency of social approval and connection (Peake et al., 2013). Unfortunately, although the ultimate aim may be the restoration of the victim’s status, increased risky behavior may actually serve to perpetuate their social standing in the peer group (Nesdale & Lambert, 2008). Thus, rejected individuals may engage in risk taking behaviors as a way to gain immediate rewards rather than focusing on the more abstract and long-term consequences of their behavior and making efforts to develop their relationships in more adaptive ways. An alternative explanation is that this heightened affective sensitivity reflects greater sensitivity to negative social feedback (i.e., chronically victimized youth were more worried about disappointing teammates). Thus, heightened amygdala activation in particular may represent avoidance emotions.
In addition to heightened affective sensitivity when making risky choices, we found greater ventral striatum activation among chronically victimized youth during successful passes (i.e., successfully passing without a crash following a risky decision). Because the ventral striatum is involved in reward processing and is associated with greater risk-taking behavior during adolescence (Galvan et al., 2006; Galvan et al., 2007; Qu et al., 2013; Telzer et al., 2015), our findings suggest that in addition to anticipatory activation (i.e., when making the decision to go), the outcome itself is a potentially more rewarding event for chronically victimized adolescents. Because both real and anticipated rewards reinforce behavior (Knutson & Greer, 2008), this heightened affective activation during reward anticipation (i.e., go decisions) and reward outcome (successful passes) may be increasing chronically victimized girls’ sensitivity to risk taking.
Adolescents who have experienced chronic levels of peer victimization may also have impaired self-regulatory capacities. Although we did not find altered PFC activation during risky choices, we found that when making safe decisions, chronically victimized youth showed heightened activation in the VLPFC and DLPFC. These regions are involved in the ability to regulate and control one’s prepotent thoughts and behaviors as well as emotion regulation (Baker, Frith, & Dolan, 1997; Gray, Braver, & Raichle, 2002) and are relatively late developing structures of the brain (Gogtay et al., 2004). Particularly in the context of heightened affective sensitivity, cognitive resources may become overwhelmed or taxed, resulting in impaired cognitive control (Hare et al., 2008; Casey et al., 2008). Because victimized girls made significantly more risky decisions during the Stoplight Task, their prepotent choice may be to be risky. Thus, victimized youth may necessitate greater cognitive control to make safe choices, requiring them to recruit the PFC to a greater extent than non-victimized youth. These findings are consistent with prior research showing that adolescents who experience high levels of peer conflict show heightened VLPFC and DLPFC activation during risk taking (Telzer et al., 2015), and adolescents who have experienced acute social exclusion show greater DLPFC activation when making safe decisions during the same task as the current study (Peake et al., 2013). However, the mediation analyses did not identify altered PFC activatuin as a mechanism by which victimization is associated with greater antisocial behavior.
We also found significant group differences in neural regions involved in social cognition. During both safe (i.e., “stop”) and risky (i.e., “go”) decisions, chronically victimized adolescents showed heightened activation in the MPFC, DMPFC, TPJ/pSTS, and MPPC. These findings are consistent with prior research, which has shown that youth experiencing acute, experimentally manipulated social exclusion show heightened mentalizing activation during risky decision making (Peake et al., 2013). This heightened mentalizing may reflect social monitoring and a concern with peers’ evaluations of one’s own risky decisions. Therefore, when considering what decision to make, either safe or risky, chronically victimized youth may be preemptively adjusting their behavior to fit the expected group norms, a process involving mentalizing. Indeed, adolescents who perceive that their peers value antisocial behavior are most likely to adjust their own behavior to fit the expected group norms (Allen et al., 2005). This heightened social-cognitive processing does not imply better perspective taking abilities, but, rather, a greater focus on peer approval, as highlighted in prior experimental studies (Falk et al., 2014; Peake et al., 2013). While we suggest that these regions are representing neural processing associated with social monitoring and a concern with peers’ evaluations, social-cognitive neural regions have multiple, complex roles, and heightened activation in these regions during risk taking has also been linked to more optimal behaviors and less risk taking. For instance, in the presence of their mother, adolescents make fewer risky decisions than when alone, which is associated with heightened MPFC activation in the presence of their mother (Telzer et al., 2015). Therefore, greater social-cognitive neural processing is not necessarily a vulnerability for poor decision making, nor is it indicative of better perspective taking abilities, but instead represents a neurobiological signal for thinking about others’ mental states. The meaning of this activation will depend on the social and motivational context.
In addition to social-cognition, the MPFC and MPPC have been linked to self-referential processing (D’Argembeau, Collette, Van der Linden, Laureys, Del Fiore, Degueldre, et al., 2005; Gusnard, Akbudak, Shulman, & Raichle, 2001; Mitchell, Banaji, & Macrae, 2005; Pfeifer, Lieberman, & Dapretto, 2007). Therefore, group differences in these neural regions may also be related to self-referential processing, including self-reflection. It may be that chronically victimized adolescents view their performance on this task as more relevant to their self-worth and engage in more internal, self-related thinking when being both risky and safe.
Our findings provide novel evidence for the role of affective sensitivity, cognitive control, and social cognition in experiences of risk taking following social rejection. Future research should examine whether the patterns found in the current study are similar or different in males given that adolescent females show heightened social sensitivity relative to males (Guyer et al., 2009, 2012; Kloep, 1999). In addition, we only had scan data available for the risk-taking task following the social exclusion experience, so we were unable to examine changes in neural activation before and after the exclusion. Prior research has found important differences in neural processing during risk taking pre- and post- social exclusion (Peake et al., 2013). Additionally, although our study was longitudinal in nature, our effects are correlational and we therefore cannot be certain about the causal direction of effects. It is possible, for example, that youth already evidence altered neural processing in childhood prior to their chronic victimization experiences. Therefore, future research should examine how neural responses change across development as a function of victimization. Finally, adolescents in this study were a community sample, which is a strength of the study allowing for greater generalizability in the effects. Nonetheless, our antisocial behavior scores were negatively skewed. Future studies should recruit higher risk adolescents who are engaging in high rates of antisocial behavior. Further, while our measure of antisocial behavior captures several aspects of risk taking (e.g., cutting school, rule breaking), future studies should examine more specific risk-taking outcomes, such as substance use and risky sexual behaviors.
In conclusion, our results highlight the neural processes by which a legacy of peer victimization gets under the skin. Our findings show that chronic peer victimization may sensitize adolescents to risk-taking behavior following acute exclusion, perhaps as a means to regain social status and connect with the peer group. These findings have important implications for interventions designed to reduce antisocial behavior in at-risk populations. Given the fundamental need to belong (Baumeister & Leary, 1995), teachers, parents, and policy-makers can focus on finding ways for victimized youth to attain a sense of social connection in more adaptive ways that do not entail risky behavior or deviant peer affiliation.
Acknowledgments
We would like to thank the families and schools who participated in this study. We are grateful to Jamie Abaied, Monica Agoston, Samirah Ali, Suravi Changlani, Megan Flynn, Inge Karosevica, Nicole Llewellyn, Jennifer Monti, Heather Ross and Niwako Sugimura for their assistance in data collection and management. This work was supported by a University of Illinois Research Board Award and a National Institute of Mental Health Grant (MH68444) awarded to K.D.R. and a National Institute of Mental Health Grant (MH105655) awarded to K.D.R. and E.H.T.
Footnotes
We ran follow-up whole brain regression analyses in which we entered group (victim/non-victim), 6th grade antisocial behavior, and risk-taking behavior on the Stoplight (percent Go decisions). While group status was significantly associated with all the neural regions outlined in the results (while controlling for prior antisocial behavior and task behavior), 6th grade antisocial behavior and risk-taking behavior on the Stoplight were not associated with activation in these regions. These findings suggest that differences in neural activation patterns are related to victimization experiences and are not accounted for by prior antisocial behavior or behavior on the Stoplight Task.
References
- Allen JP, Porter MR, McFarland FC, Marsh P, McElhaney KB. The two faces of adolescents’ success with peers: Adolescent popularity, social adaptation, and deviant behavior. Child Development. 2005;76(3):747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Frith CD, Dolan RJ. The interaction between mood and cognitive function studied with PET. Psychological Medicine. 1997;27(03):565–578. doi: 10.1017/S0033291797004856. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88(4):589. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The developing social brain: Implications for education. Neuron. 2010;65(6):744–747. doi: 10.1016/j.neuron.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience. 2009;21(9):1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CN, O’Donnell MB, Bayer J, Tinney FJ, Jr, Falk EB. Neural correlates of susceptibility to group opinions in online word-of-mouth recommendations. Journal of Marketing Research in press. [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: A PET study. Neuroimage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Baumeister RF, Vohs KD. Satiated with belongingness? Effects of acceptance, rejection, and task framing on self-regulatory performance. Journal of Personality and Social Psychology. 2008;95(6):1367. doi: 10.1037/a0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Richman SB. Social exclusion and the desire to reconnect. Social and Personality Psychology Compass. 2011;5(11):919–932. doi: 10.1111/j.1751-9004.2011.00383.x. [DOI] [Google Scholar]
- Dodge KA, Pettit GS. A biopsychosocial model of the development of chronic conduct problems in adolescence. Developmental Psychology. 2003;39(2):349. doi: 10.1037/0012-1649.39.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Falk EB, Cascio CN, O’Donnell MB, Carp J, Tinney FJ, Bingham CR, … Simons-Morton BG. Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health. 2014;54(5):S22–S31. doi: 10.1016/j.jadohealth.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M, Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Development. 2012;83(6):1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish LD, Guerra NG. A longitudinal analysis of patterns of adjustment following peer victimization. Development and Psychopathology. 2002;14(01):69–89. doi: 10.1017/S0954579402001049. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity. Child Development. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SC, Boyle JM. Appraisal and coping strategy use in victims of school bullying. British Journal of Educational Psychology. 2004;74(1):83–107. doi: 10.1348/000709904322848833. [DOI] [PubMed] [Google Scholar]
- Khatri P, Kupersmidt JB, Patterson C. Aggression and peer victimization as predictors of self-reported behavioral and emotional adjustment. Aggressive Behavior. 2000;26(5):345–358. [Google Scholar]
- Kloep M. Love is all you need? Focusing on adolescents’ life concerns from an ecological point of view. Journal of Adolescence. 1999;22(1):49–63. doi: 10.1006/jado.1998.0200. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird RD, Jordan KY, Dodge KA, Pettit GS, Bates JE. Peer rejection in childhood, involvement with antisocial peers in early adolescence, and the development of externalizing behavior problems. Development and Psychopathology. 2001;13(02):337–354. doi: 10.1017/S0954579401002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Guyer A, Tone EB, Jenness J, Parrish JM, Pine DS, Nelson EE. Neural responses to peer rejection in anxious adolescents: Contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development. 2011;36(1):36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Pasch KE. Does school connectedness buffer the impact of peer victimization on early adolescents’ subsequent adjustment problems? The Journal of Early Adolescence. 2013;33:245–266. [Google Scholar]
- Mead NL, Baumeister RF, Stillman TF, Rawn CD, Vohs KD. Social exclusion causes people to spend and consume strategically in the service of affiliation. Journal of Consumer Research. 2011;37(5):902–919. doi: 10.1086/656667. [DOI] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child & Adolescent Psychology. 2009;38(4):513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(02):163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nesdale D, Lambert A. Effects of experimentally induced peer-group rejection on children’s risk-taking behaviour. European Journal of Developmental Psychology. 2008;5(1):19–38. doi: 10.1080/17405620600717581. [DOI] [Google Scholar]
- Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in adolescent females. Journal of Abnormal Psychology. 2007;116:198–207. doi: 10.1037/0021-843X.116.1.198. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. Journal of Social and Clinical Psychology. 2008;27(5):471–504. doi: 10.1521/jscp.2008.27.5.471. [DOI] [Google Scholar]
- Parker JG, Asher SR. Peer relations and later personal adjustment: Are low-accepted children at risk? Psychological Bulletin. 1987;102(3):357. doi: 10.1037/0033-2909.102.3.357. [DOI] [PubMed] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. Neuroimage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman M, Dapretto M. “I know you are but what am I?!”: Neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, La Greca AM. Childhood peer rejection and aggression as predictors of adolescent girls’ externalizing and health risk behaviors: a 6-year longitudinal study. Journal of Consulting and Clinical Psychology. 2004;72(1):103. doi: 10.1037/0022-006X.72.1.103. [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience. 2015;35:11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn CD, Vohs KD. People use self-control to risk personal harm: An intra-interpersonal dilemma. Personality and Social Psychology Review. 2011;15(3):267–289. doi: 10.1177/1088868310381084. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M, Abaied JL, Groot A, Thompson R. Why is past depression the best predictor of future depression? Stress generation as a mechanism of depression continuity in girls. Journal of Clinical Child & Adolescent Psychology. 2009;38(4):473–485. doi: 10.1080/15374410902976296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Lansford JE, Agoston AM, Sugimura N, Schwartz D, Dodge KA, … Bates JE. Peer victimization and social alienation: predicting deviant peer affiliation in middle school. Child Development. 2014;85(1):124–139. doi: 10.1111/cdev.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Hessel ET, Schmidt JD. A latent growth curve analysis of early and increasing peer victimization as predictors of mental health across elementary school. Journal of Clinical Child & Adolescent Psychology. 2011;40:111–122. doi: 10.1080/15374416.2011.533413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K, Miernicki ME, Troop-Gordon W, Davis M, Telzer EH. Adding insult to injury: Neural sensitivity to social exclusion is associated with depression in chronically peer-victimized girls. Social Cognitive Affective Neuroscience. doi: 10.1093/scan/nsw021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusby JC, Forrester KK, Biglan A, Metzler CW. Relationships between peer harassment and adolescent problem behaviors. The Journal of Early Adolescence. 2005;25(4):453–477. [Google Scholar]
- Storch EA, Nock MK, Masia-Warner C, Barlas ME. Peer victimization and social-psychological adjustment in Hispanic and African-American children. Journal of Child and Family Studies. 2003;12(4):439–452. [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Sullivan TN, Kliewer W. A longitudinal path analysis of peer victimization, threat appraisals to the self, and aggression, anxiety, and depression among urban African American adolescents. Journal of youth and adolescence. 2013;42(2):178–189. doi: 10.1007/s10964-012-9821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galván A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NI, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity to promote safe behavior during risk taking. Social Cognitive Affective Neuroscience. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Catanese KR, Baumeister RF. Social exclusion causes self-defeating behavior. Journal of Personality and Social Psychology. 2002;83(3):606. doi: 10.1037/0022-3514.83.3.606. [DOI] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. Changing brains, changing perspectives the neurocognitive development of reciprocity. Psychological Science. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Social Cognitive and Affective Neuroscience. 2006;1(2):107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf.
- Will GJ, van Lier PA, Crone EA, Güroğlu B. Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of abnormal child psychology. 2016;44(1):43–55. doi: 10.1007/s10802-015-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79(5):748. doi: 10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Leadbeater BJ. Does hostile attributional bias for relational provocations mediate the short-term association between relational victimization and aggression in preadolescence? Journal of Youth and Adolescence. 2007;36(8):973–983. [Google Scholar]