Abstract
Objectives—
Polyhydramnios and placentomegaly are commonly observed in nonimmune hydrops fetalis (NIHF); however, whether their ultrasonographic identification is relevant for prognosis is controversial. We evaluated outcomes of fetal or neonatal death and preterm birth (PTB) in cases of NIHF alone and in those with polyhydramnios and/or placentomegaly (P/PM).
Methods—
We conducted a retrospective cohort of singletons with NIHF evaluated between 1994 and 2013. Nonimmune hydrops fetalis was defined as 2 or more abnormal fluid collections, including ascites, pericardial effusion, pleural effusion, and skin edema. Primary outcomes were intrauterine fetal demise (IUFD) and neonatal death. Secondary outcomes were PTB (<37,< 34, and <28 weeks) and spontaneous PTB. Outcomes were compared between cases of NIHF alone and NIHF with P/PM.
Results—
A total of 153 cases were included; 21% (32 of 153) had NIHF alone, and 79% (121 of 153) had NIHF with P/PM. There was no significant difference in neonatal death (38.1% versus 43.0%; P=.809) between the groups. Intrauterine fetal demise was seen more frequently in NIHF alone (34.4% versus 17.4%; P=.049). Nonimmune hydrops fetalis-with-P/PM cases were more likely to deliver before 37 weeks (80.0% versus 57.1%; P=.045) and before 34 weeks (60.0% versus 28.6%; P=.015) and to have spontaneous PTB (64.4% versus 33.3%; P=.042). Adjusted odds ratios accounting for the etiology of NIHF supported these findings, with the exception of IUFD.
Conclusions—
Compared to NIHF alone, pregnancies with NIHF and P/PM had a lower risk of IUFD and were at increased risk of PTB (<37 and <34 weeks) and spontaneous PTB. This information may help providers in counseling patients with NIHF and supports the need for close antenatal surveillance.
Keywords: intrauterine demise, obstetrics, neonatal death, nonimmune hydrops fetalis, placentomegaly, polyhydramnios, preterm birth
Hydrops fetalis is estimated to occur in 1 per every 1700 pregnancies and is traditionally defined as an abnormal accumulation of fluid in 2 or more fetal serous cavities, including ascites, pleural effusion, pericardial effusion, and skin edema.1 Although most hydrops fetalis cases were historically caused by Rhesus isoimmunization, the etiology has shifted to predominantly nonimmune causes after the implementation of Rhesus (D) immune globulin.1,2 The presence of nonimmune hydrops fetalis (NIHF) generally portends a poor prognosis, with substantial risks of intrauterine fetal demise (IUFD), preterm labor, and neonatal morbidity and mortality.3–7 In most cases of NIHF, it is challenging to estimate the postnatal prognosis because of variability in the underlying etiology, perinatal management, and diagnostic criteria.
A few studies have evaluated the importance of the affected fetal cavities in the setting of NIHF and have reported various results. Although some showed that the presence of pleural and pericardial effusions predicted poor survival,4,8,9 others found no difference in the outcome by the affected compartment.10 These discrepant findings may stem from the idea that the underlying etiology of NIHF, rather than the affected fetal cavity, is an important predictor of survival.6,11–13 However, the etiology of NIHF is not always apparent, and the provider is dependent on ultrasonographic findings and available laboratory studies to aid in management of the pregnancy. Even less well understood is the importance of polyhydramnios and placentomegaly when seen in conjunction with NIHF. Although polyhydramnios and placentomegaly are frequently seen in NIHF, they are not formally included in the diagnostic criteria, and data are lacking regarding their implications for the prognosis.1 Virtually no literature has investigated the role of polyhydramnios in cases of NIHF, whereas placentomegaly has been reported with underlying genetic syndromes, particularly Beckwith-Wiedemann syndrome.14,15
We designed a retrospective cohort study to address the importance of polyhydramnios and placentomegaly in the setting of NIHF, with the objective of comparing the outcomes of fetal or neonatal death and preterm birth (PTB) in cases of NIHF alone compared to those with concurrent polyhydramnios and/or placentomegaly (P/PM). We hypothesized that compared to cases with NIHF only, those with P/PM would have an increased risk of IUFD or neonatal death as well as PTB, given the excess amount of abnormal fluid in these cases.
Materials and Methods
This work was a retrospective cohort study of NIHF cases evaluated at the University of California, San Francisco, Fetal Treatment Center. Institutional Review Board approval was obtained for this study (protocol No. 10–04093). Cases were identified from the Fetal Treatment Center hydrops fetalis data set. This data set includes all hydrops cases evaluated between January 1, 1994, and July 1, 2013, and was used for a previously published study with the objective of identifying prognostic indicators of survival in hydrops cases.10 Specifically, the previous study evaluated the association between fluid distributions, echocardiographic findings, and fetal therapy with survival in immune and nonimmune fetal hydrops as well as twin-twin trans- fusion10 and did not focus on P/PM as a predictors of perinatal outcomes.
For this study, inclusion criteria were cases with NIHF diagnosed at any point during the pregnancy. Criteria used to define hydrops were those published by the Society for Maternal-Fetal Medicine1: 2 or more abnormal fluid collections in fetal serous cavities (ascites, pericardial effusion, pleural effusion, and skin edema with skin thickness <5 mm). The following cases were excluded: multiple gestations and hydrops caused by isoimmunization, owing to the different pathophysiologic mechanism, and pregnancy terminations due to the lack of outcome data for the pregnancy.
The primary outcome examined in this study was IUFD or neonatal death within 30 days of life. Secondary outcomes were any PTB before 37 weeks’ gestation, PTB before 34 weeks, PTB before 28 weeks, and, specifically, spontaneous PTB before 37 weeks. Primary and secondary outcomes were examined among cases of NIHF alone compared to those of NIHF with P/PM. Placentomegaly was defined as placental thickness of 4 cm or greater in second trimester, or 6 cm or greater in the third trimester.1 Polyhydramnios was defined as an amniotic fluid index of 24 cm or greater or a maximum vertical pocket of 8 cm or greater.1
All patients in the cohort underwent a workup for NIHF as considered appropriate by the evaluating physician. This workup typically included an assessment of the maternal serum antibody status, detailed fetal anatomic ultrasonography and echocardiography, infectious studies, and karyotype as well as genetic testing if amniocentesis was performed. For our analyses, NIHF cases were classified into 7 categories based on the known or suspected etiology of the hydrops: (1) chest mass (including congenital pulmonary airway malformation, congenital cystic adenomatoid malformation, and congenital high airway obstruction), (2) aneuploidy, (3) hematologic or lymphatic disease, (4) cardiac disease, (5) primary hydrothorax, (6) congenital diaphragmatic hernia (CDH), and (7) other (including idiopathic, multiple anomalies, genitourinary anomalies, and sacrococcygeal teratoma).
Maternal and neonatal characteristics, ultrasonographic findings, delivery details, and neonatal outcomes were extracted from our Fetal Treatment Center database, which was populated with information from the electronic medical record. Trained research clinicians routinely maintain the information in this database for all patients evaluated at our center. Neonatal outcomes were extracted from the medical records for all cases delivered at our institution. For those delivered at outside institutions, a member of our research team called the family or the delivering institution to obtain details of the neonatal course.
The Fisher exact test was used for categorical variables, and the Wilcoxon rank sum test compared nonparametric continuous variables. Multivariable logistic regression generated odds ratios (ORs), adjusting for clinically relevant potential confounders. Odds of the primary and secondary outcomes were adjusted for the known or suspected category of the NIHF etiology, as the underlying etiology may be related to both NIHF and the primary and secondary outcomes. Analyses were performed with Stata version 14.1 software (StataCorp, College Station, TX), and statistical significance was defined as P < .05 or a 95% confidence interval (CI) not crossing 1.
Results
In the 19-year study period, a total of 186 cases of NIHF were identified. Thirty-three underwent therapeutic abortion and were excluded from our cohort, with the remaining 153 cases analyzed (Figure 1). Of the 153 cases, 21% (32 of 153) met criteria for NIHF alone, and 79% (121 of 153) had NIHF with P/PM.
Figure 1.
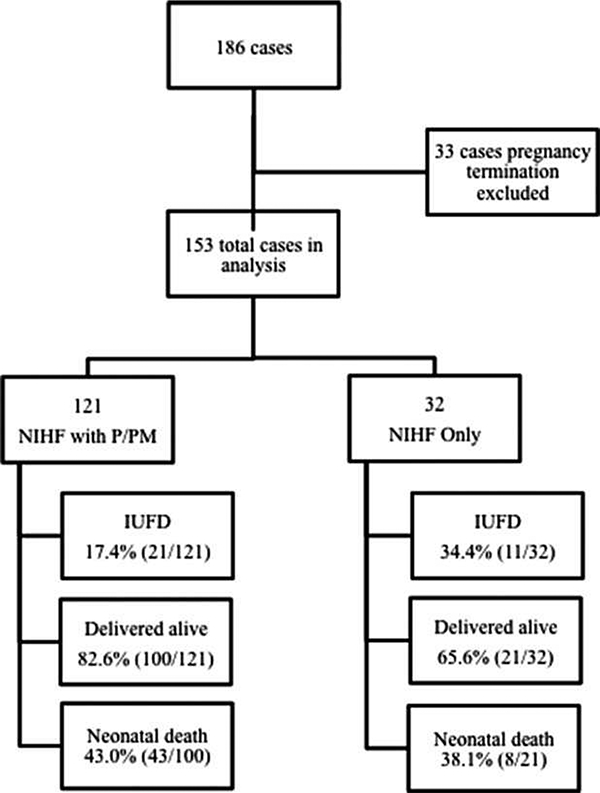
Flow diagram of cases included and outcomes in each group.
The demographics of the cohort are displayed in Table 1. Compared to NIHF alone, those with P/PM were delivered at an earlier gestational age and had a lower median birth weight. There were no significant differences between groups in maternal age, gravity, parity, neonatal sex, 1- or 5-minute Apgar scores, or the length of neonatal intensive care unit admission. Categorizing by the known or suspected etiology of NIHF led to the following proportions: 35.3% chest mass (including congenital pulmonary airway malformation, congenital cystic adenomatoid malformation, and congenital high airway obstruction), 5.8% aneuploidy, 11.1% hematologic or lymphatic disease, 15.7% cardiac disease, 12.4% primary hydrothorax, 7.8% congenital diaphragmatic hernia, and 11.8% other (including idiopathic, multiple anomalies, genitourinary anomalies, and sacrococcygeal teratoma). Stratifying by category type, whereas P/PM was seen more frequently in cases with a chest etiology compared to those with a nonchest etiology (88.89% versus 73.74%; P=.037), there was no association between the presence of P/PM and the other categories.
Table 1.
Cohort Characteristics
| Characteristic | NIHF Alone (n = 32) | NIHF With P/PM (n = 121) | P |
|---|---|---|---|
| Maternal age, y | 28.5 (16–46) | 28.0 (18–45) | .957 |
| Parity > 1, % (n) | 31.2 (10/32) | 35.5 (43/121) | .683 |
| Gestational age at diagnosis, wk | 25.0 (18.1–37.6) | 26.2 (16.7–38.1) | .080 |
| Gestational age at delivery, wk | 36.0 (27.1–39.9) | 32.3 (24.0–41.0) | .001 |
| Male, % (n) | 50.0 (10/20) | 50.5 (49/97) | >.999 |
| Birth weight, g | 2900 (1260–3600) | 2190 (600–5220) | .049 |
| Apgar 1min | 6 (0–9) | 3 (0–9) | .145 |
| Apgar 5 min | 7(0–9) | 5(0–9) | .234 |
| Neonatal intensive care unit stay, d | 39.5 (14–72) | 31.0 (5–180) | .849 |
| Known or suspected etiology of NIHF, % (n) | .084 | ||
| Chest | 18.8 (6/32) | 39.7 (48/121) | |
| Aneuploidy | 3.1 (1/32) | 6.6 (8/121) | |
| Hematologic or lymphatic | 9.4 (3/32) | 11.6 (14/121) | |
| Cardiac | 28.1 (9/32) | 12.4 (15/121) | |
| Primary hydrothorax | 9.4 (3/32) | 13.2 (16/121) | |
| Congenital diaphragmatic hernia | 12.5 (4/32) | 6.6 (8/121) | |
| Other | 18.8 (6/32) | 9.9 (12/121) |
Continuous variables are presented as medians with ranges.
Perinatal death, including IUFD and neonatal demise within 30 days, was observed in 54.3% (83 of 153) of the entire cohort (Figure 1). Of these, 38.6% (32 of 83) resulted in an IUFD, and 61.4% (51 of 83) had a postnatal demise within 30 days. Comparing NIHF alone to NIHF with P/PM (Table 2), there was no significant difference in neonatal death: 38.1% (8 of 21) versus 43.0% (43 of 100), respectively (P=.809). However, IUFD was observed more frequently in cases of NIHF alone compared to cases of NIHF with P/PM: 34.4% (11 of 32) versus 17.4% (21 of 121; P=.049).
Table 2.
Outcomes by Hydrops Group
| Outcome | NIHF Alone (n = 32) | NIHF With P/PM (n = 121) | P |
|---|---|---|---|
| IUFD, % (n) | 34.4 (11/32) | 17.4 (21/121) | .049 |
| Neonatal death, % (n) | 38.1 (8/21) | 43.0 (43/100) | .809 |
| PTB < 37 wk, % (n) | 57.1 (12/21) | 80.0 (80/100) | .045 |
| PTB < 34 wk, % (n) | 28.6 (6/21) | 60.0 (60/100) | .015 |
| PTB < 28 wk, % (n) | 4.8 (1/21) | 15.0 (15/100) | .300 |
| Spontaneous PTB, % (n)a | 33.3 (5/15) | 64.4 (47/73) | .042 |
Sufficient information to confirm spontaneous versus iatrogenic PTB was available for 15 NIHF-alone cases and 73 NIHF with P/PM cases.
With regard to PTB, cases of NIHF with P/PM were significantly more likely than those with NIHF alone to be delivered preterm. Furthermore, cases of NIHF with P/PM were significantly more likely to have a spontaneous PTB rather than iatrogenic (Table 2). Considering all PTB before 37 weeks’ gestation, delivery occurred in this range for 80.0% (80 of 100) of NIHF-with-P/PM cases compared to 57.1% (12 of 21) of NIHF alone cases (P=.045). For PTB before 34 weeks, 60.0% (60 of 100) of NIHF-with-P/PM cases were delivered in this range compared to 28.6% (6 of 21) of NIHF-alone cases (P=.015). No significant difference, however, was seen in PTB before 28 weeks between the groups. Among the cases with sufficient information to confirm spontaneous versus iatrogenic PTB, 64.4% (47 of 73) of NIHF cases with P/PM had spontaneous PTB compared to 33.3% (5 of 15) of NIHF-alone cases (P=.042). Finally, stratifying by category type, NIHF cases with a chest etiology were more likely to deliver before 34 weeks if they had P/PM (72.09% versus 0.00%; P=.01), but no significant association was seen between PTB (<37, < 34, and <32 weeks) and the presence of P/PM in any of the other categories.
Significantly decreased odds of IUFD were seen for the NIHF-with-P/PM group compared to the NIHF alone group in the unadjusted model (OR, 0.40; 95% CI, 0.17–0.96; P=.039; Table 3). However, after adjusting for known or suspected NIHF categories, the odds of IUFD were no longer significantly decreased for NIHF with P/PM (adjusted OR, 0.42; 95% CI, 0.17–1.09; P=.075). The odds of neonatal death were not significantly different for the NIHF-with-P/PM group relative to the NIHF-alone group in either the unadjusted or adjusted model. Similar to our univariate analyses, the odds of PTB before 37 and 34 weeks, as well as the odds of spontaneous PTB, were significantly higher for the NIHF-with-P/PM group.
Table 3.
Odds of Primary and Secondary Outcomes in NIHF With P/PM Compared to NIHF Alone
| Outcome | Unadjusted OR | 95% CI | P | Adjusted ORa | 95% CI | P |
|---|---|---|---|---|---|---|
| IUFD | 0.40 | 0.17–0.96 | .039 | 0.42 | 0.17–1.09 | .075 |
| Neonatal death | 1.22 | 0.47–3.22 | .679 | 1.52 | 0.53–4.35 | .433 |
| PTB < 37 wk | 3.00 | 1.11–8.10 | .030 | 3.35 | 1.04–10.69 | .042 |
| PTB < 34 wk | 3.75 | 1.34–10.48 | .012 | 4.71 | 1.46–15.21 | .010 |
| PTB < 28 wk | 3.52 | 0.44–28.30 | .235 | 3.86 | 0.42–35.24 | .232 |
| Spontaneous PTB | 3.62 | 1.11–11.71 | .032 | 3.96 | 1.02–15.34 | .046 |
Adjusted for category of anomaly.
Discussion
In our study, most cases had NIHF with P/PM, with the remainder meeting criteria for NIHF alone. Intrauterine fetal demise was observed less frequently among the cases of NIHF with P/PM. This relationship did not persist in the adjusted multivariable analyses, likely because of limitations in power, and may be largely explained by the earlier gestational age at delivery in this group. Furthermore, the odds of PTB before 37 and 34 weeks’ gestation, as well as spontaneous PTB, were significantly higher for the NIHF group with P/PM relative to NIHF alone.
Although we hypothesized that that the presence of P/PM would portend an increased risk of IUFD, cases with P/PM were associated with 60% decreased odds of this outcome. Although the implications of P/PM have not been previously reported in the context of NIHF, our findings of a decreased IUFD risk in the NIHF- with-P/PM cases are contrary to literature suggesting an increased risk of IUFD with polyhydramnios in anomalous and nonanomalous fetuses.16 These studies were not specific to cases with NIHF, however, and did not consider the importance of placentomegaly. Furthermore, the increased risk of IUFD with polyhydramnios has been demonstrated most drastically at term.17 As only 20% of our NIHF-with-P/PM cases delivered after 37 weeks, it is likely that most delivered before the increased risk attributable to polyhydramnios became substantial. Therefore, we suspect that the decreased IUFD rate seen in hydropic fetuses with P/PM was driven by the higher rate of PTB in this group, hence allowing less time for the demise to occur. Considering neonatal mortality, we found no difference in this outcome between cases with NIHF only and those with P/PM. Similar to our findings, Yeom et al18 reported no difference in the incidence of polyhydramnios or placental thickness between cases of neonatal survival and those with neonatal death but included immune causes of hydrops and twin-twin transfusion cases in their cohort. A few studies have suggested that the presence of fluid in more than 2 compartments worsens survival of the hydropic fetus, although they did not consider P/PM specifically.18–20 It is likely that the underlying etiology of the NIHF is a more influential predictor of postnatal survival.12,20
With regard to PTB, we found that NIHF-with-P/PM cases were more likely to deliver preterm before 37 and 34 weeks compared to NIHF-alone cases. It is possible that NIHF cases with P/PM showed evidence of a worsening clinical status or concerning findings on antenatal testing that our study did not capture, leading to an earlier iatrogenic delivery. However, NIHF cases with P/PM had nearly 4-fold greater adjusted odds of spontaneous PTB compared to those with NIHF alone. It may be that the myometrial stretch in the setting of larger amniotic fluid volumes predisposes to uterine activity, thus increasing the risk of preterm premature rupture of membranes and preterm labor. There may also be inherent genetic differences between hydropic fetuses who are predisposed some to develop P/PM, as has been suggested in cases of Beckwith-Wiedemann syndrome and Niemann-Pick disease,14,15,21 and these underlying genetic differences may directly increase the risk of PTB and influence the prognosis. Our finding of an increased risk of spontaneous PTB among NIHF cases with P/PM contributes to the scant literature addressing this subject and illustrates that this population may require closer antenatal surveillance for PTB.
Strengths of our study include the relatively large cohort size of NIHF cases, particularly compared to the existing literature, which is mostly composed of case reports, when P/PM is considered. Our study fills a gap in the literature regarding the importance of P/PM when NIHF is present, particularly with respect to perinatal survival and PTB. However, this study was not without limitations. Inherent to the retrospective design, some clinical data were unavailable and could not be included in the analyses, such as clear information on spontaneous versus iatrogenic PTB in all cases. Although carefully reviewed, the extracted data may have been prone to errors, which are difficult to detect. A selection bias may have existed in our cohort, as cases were referred to our Fetal Treatment Center, and many cases included structural anomalies. Some of the cases in our study underwent in utero interventions, which may increase the risk of spontaneous PTB. However, the proportion of NIHF cases that undergo in utero intervention is quite low, and we do not expect that this aspect would have altered our results. Finally, as described previously, the underlying etiology of NIHF may be an important factor influencing the outcomes of IUFD, PTB, and neonatal survival, and we were limited in our ability to account for the effects of this etiology on our findings. Further research will be necessary to clarify the extent to which the underlying NIHF etiology influences perinatal survival as well as other obstetric and neonatal outcomes.
In conclusion, we found that compared to pregnancies with NIHF alone, those with NIHF and P/PM were at an increased risk of PTB before 37 and 34 weeks, as well as spontaneous rather than iatrogenic PTB. Additionally, the risk of IUFD was lower in cases of NIHF with P/PM, a finding that was likely attributable to the earlier gestational age at delivery. Although the etiology of the NIHF is likely an important predictor of outcomes, our study demonstrates that the presence of P/PM may provide further prognostic information irrespective of the NIHF etiology, which is frequently unknown. These findings contribute important information to assist providers in counseling patients with NIHF on in utero as well as postnatal risks, taking P/PM into account. Furthermore, pregnancies with NIHF and P/PM may represent a more tenuous cohort and warrant closer antepartum surveillance for spontaneous PTB. Future studies with other cohorts are needed to substantiate the findings of our study, as well as to further explore reasons for the lower IUFD risk when P/PM is seen, to better understand reasons for the greater risk of spontaneous PTB in this population, and to investigate any differences in subsequent neonatal morbidity when P/PM is present.
Abbreviations
- CI
confidence interval
- IUFD
intrauterine fetal demise
- NIHF
nonimmune hydrops fetalis
- OR
odds ratio
- P/PM
polyhydramnios and/or placentomegaly
- PTB
preterm birth
References
- 1.Society for Maternal-Fetal Medicine; Norton ME, Chauhan SP, Dashe JS. Society for Maternal-Fetal Medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol 2015; 212:127–139. [DOI] [PubMed] [Google Scholar]
- 2.Bellini C, Donarini G, Paladini D, et al. Etiology of non-immune hydrops fetalis: an update. Am J Med Genet A 2015; 167A:1082–1088. [DOI] [PubMed] [Google Scholar]
- 3.Santo S, Mansour S, Thilaganathan B, et al. Prenatal diagnosis of nonimmune hydrops fetalis: what do we tell the parents? Prenat Diagn 2011;31:186–195. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama H, Kukita J, Hikino S, Nakano H, Hara T. Long-term outcome of 51 liveborn neonates with non-immune hydrops fetalis. Acta Paediatr 1999; 88:24–28. [DOI] [PubMed] [Google Scholar]
- 5.Moreno CA, Kanazawa T, Barini R, et al. Non-immune hydrops fetalis: a prospective study of 53 cases. Am J Med Genet A 2013; 161A: 3078–3086. [DOI] [PubMed] [Google Scholar]
- 6.Ota S, Sahara J, Mabuchi A, Yamamoto R, Ishii K, Mitsuda N. Perinatal and one-year outcomes of non-immune hydrops fetalis by etiology and age at diagnosis. J Obstet Gynaecol Res 2016; 42:385–391. [DOI] [PubMed] [Google Scholar]
- 7.Turgal M, Ozyuncu O, Boyraz G, Yazicioglu A, Sinan Beksac M. Non-immune hydrops fetalis as a diagnostic and survival problems: what do we tell the parents? J Perinat Med 2015; 43:353–358. [DOI] [PubMed] [Google Scholar]
- 8.Smoleniec J, James D. Predictive value of pleural effusions in fetal hydrops. Fetal Diagn Ther 1995; 10:95–100. [DOI] [PubMed] [Google Scholar]
- 9.Liu CA, Huang HC, Chou YY. Retrospective analysis of 17 liveborn neonates with hydrops fetalis. Chang Gung Med J 2002; 25:826–831. [PubMed] [Google Scholar]
- 10.Derderian SC, Jeanty C, Fleck SR, et al. The many faces of hydrops. J Pediatr Surg 2015; 50:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutall A, Urban MF, Stewart C. Diagnosis, etiology, and outcome of fetal ascites in a South African hospital. Int J Gynaecol Obstet 2011; 115:148–152. [DOI] [PubMed] [Google Scholar]
- 12.Abrams ME, Meredith KS, Kinnard P, Clark RH. Hydrops fetalis: a retrospective review of cases reported to a large national database and identification of risk factors associated with death. Pediatrics 2007; 120:84–89. [DOI] [PubMed] [Google Scholar]
- 13.Whybra C, Mengel E, Russo A, et al. Lysosomal storage disorder in non-immunological hydrops fetalis (NIHF): more common than assumed? Report of four cases with transient NIHF and a review of the literature. Orphanet J Rare Dis 2012; 7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drut RM, Drut R. Nonimmune fetal hydrops and placentomegaly: diagnosis of familial Wiedemann-Beckwith syndrome with trisomy 11p15 using FISH. Am J Med Genet 1996; 62:145–149. [DOI] [PubMed] [Google Scholar]
- 15.Lage JM Placentomegaly with massive hydrops of placental stem villi, diploid DNA content, and fetal omphaloceles: possible association with Beckwith-Wiedemann syndrome. Hum Pathol 1991; 22: 591–597. [DOI] [PubMed] [Google Scholar]
- 16.Golan A, Wolman I, Sagi J, Yovel I, David MP. Persistence of polyhydramnios during pregnancy: its significance and correlation with maternal and fetal complications. Gynecol Obstet Invest 1994; 37: 18–20. [DOI] [PubMed] [Google Scholar]
- 17.Pilliod RA, Page JM, Burwick RM, Kaimal AJ, Cheng YW, Caughey AB. The risk of fetal death in nonanomalous pregnancies affected by polyhydramnios. Am J Obstet Gynecol 2015; 213: 410.e1–410.e6. [DOI] [PubMed] [Google Scholar]
- 18.Yeom W, Paik ES, An JJ, et al. Clinical characteristics and perinatal outcome of fetal hydrops. Obstet Gynecol Sci 2015; 58:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wafelman LS, Pollock BH, Kreutzer J, Richards DS, Hutchison AA Nonimmune hydrops fetalis: fetal and neonatal outcome during 1983–1992. Biol Neonate 1999; 75:73–81. [DOI] [PubMed] [Google Scholar]
- 20.Wy CA, Sajous CH, Loberiza F, Weiss MG. Outcome of infants with a diagnosis of hydrops fetalis in the 1990s. Am J Perinatol 1999; 16: 561–567. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel R, Raas-Rothschild A, Reish O, et al. The clinical spectrum of fetal Niemann-Pick type C. Am J Med Genet A 2009; 149A:446–450. [DOI] [PubMed] [Google Scholar]


