Abstract
Endocrine disrupting chemicals (EDCs) are compounds that alter the structure and function of the endocrine system and may be contributing to disorders of the reproductive, metabolic, neuroendocrine and other complex systems. Typically, these outcomes cannot be modeled in cell-based or other simple systems necessitating the use of animal testing. Appropriate animal model selection is required to effectively recapitulate the human experience, including relevant dosing and windows of exposure, and ensure translational utility and reproducibility. While classical toxicology heavily relies on inbred rats and mice, and focuses on apical endpoints such as tumor formation or birth defects, EDC researchers have used a greater diversity of species to effectively model more subtle but significant outcomes such as changes in pubertal timing, mammary gland development, and social behaviors. Advances in genomics, neuroimaging and other tools are making a wider range of animal models more widely available to EDC researchers.
Keywords: zebrafish, vole, peromyscus, toxicology, neurosciences, collaborative cross, PFOA
Introduction
An endocrine disrupting chemical is an “exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” [1, 2]. Although classical toxicology heavily relies on rodent models, especially rats, the EDC field owes much of its origins to studies in wild animal species. Its core principles and concepts were derived from studies conducted in a wide range of taxa both terrestrial and aquatic, and species diversity remains a core element of ongoing EDC research [3]. Despite comprehensive experimental and epidemiological data for some of the most notorious chemicals, regulatory action on their manufacture and use has been glacially slow to nonresponsive, leading to frustration among families, communities, activists, and scientists. Difficulty translating effects in animals to humans has been cited as a core obstacle, thus experimental animal model selection is critically important. There is growing consensus that classical, apical endpoint-based toxicity testing is not being conducted at human-relevant doses, during the appropriate life stages, or in appropriately susceptible test models to identify or predict endocrine-related disorders such as endometriosis or premature puberty, or to fully assess complex neurodevelopmental disorders that do not have clear pathology, such as schizophrenia and autism. Thus, although there is growing pressure to move away from animal-based toxicity testing, whole organism-based studies remain a critical tool for EDC research because they allow for the interrogation of chemical influences at the phenotypic, physiological, behavioral, and molecular levels and are particularly useful to assess outcomes that are difficult to model in simpler in vitro or organotypic systems.
EDCs have since garnered considerable attention and rapidly compounding evidence reveals that exposure, particularly during critical windows of organ development, is likely contributing to rising rates of multiple disorders and chronic diseases in humans including premature female puberty, compromised fertility, obesity, cardiovascular disease risk, and disorders of neurodevelopment [1, 2]. The incidence of these diseases/disorders has increased faster than can be explained by genetics alone, and is now thought to be heavily attributable to environmental factors, including EDCs [4]. Although, historically, the field has focused primarily on the estrogen-disrupting effects of EDCs, and effects on reproductive development and function [3], it is now recognized that EDCs can also act via other mechanisms and have impacts on non-reproductive physiology.
Common mechanisms of EDC action include hormone agonism, hormone antagonism, modulation of hormone receptor expression, and disruption of hormone production and/or clearance. While most work still heavily focuses on estrogen, androgen and thyroid disruption via their respective receptors, non-steroidal hormone disruption has repeatedly been shown [5]. For example, studies in multiple species of birds and mammals have revealed that kisspeptin, gonadotropin releasing hormone (GnRH), and oxytocin (OT)/vasopressin (AVP) pathways (vasotocin (AVT) in non-mammalian species) are vulnerable [5–9]. Some of these effects involve steroid hormone receptor dependent mechanisms, but others do not. There is also growing interest in possible epigenetic, and immune mechanisms of disruption [2, 10, 11].
There are an estimated 90,000+ anthropogenic chemicals in the wild and built environment, although an accurate accounting has proved nearly impossible to obtain, even for regulators such as the US Environmental Protection Agency (EPA) charged with monitoring their distribution and potential toxicity (http://cen.acs.org/articles/95/i9/chemicals-use-today.html). The vast majority have not been tested for any form of toxicity at all, let alone endocrine disruption, so information on their potential health risks is patchy and often contested. A subset of at least a few hundred (estimates vary) are categorized as endocrine disrupting compounds (examples shown in Table 1) with dozens in our bodies at any given time [12]. This complex exposure landscape is largely unavoidable illustrating the critically importance of understanding how EDCs affect human health. Although there is considerable interest and pressure to develop high throughput screening assays and other tools which do not use whole animals to more efficiently and rapidly accomplish the goal of “predictive toxicology [13, 14],” the development and acceptance of effective approaches remains controversial and primarily focused on estrogen, androgen and thyroid activity [15–17]. Additionally, the inherent biological complexity of the whole organism has not been adequately replicated or modeled in simpler systems and complex behavioral phenotypes and processes such as pubertal onset, affiliative interactions, and learning can only be observed in whole animals. Animal models, with organ and endocrine systems modeling those in humans, allow for the evaluation of chemical influences at many levels and are particularly useful to investigate mechanisms of action, critical windows of susceptibility, sex and age specific effects, and dose responses.
Table 1.
Summary of Common EDCs and their Histories, Uses, Sources and Effects*
| EDC | General chemical structure |
Group | Introduction date |
Restrictions | Route of exposure |
Sources | Half-life | Effects/animal model notes |
|---|---|---|---|---|---|---|---|---|
| Atrazine |
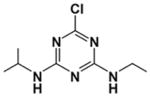
|
Chlorotriazine herbicide | 1959 | European Union ban 2004 | Ingestion, inhalation | Pesticide/herbicide, water and soil contaminant | 10–12 hours | Endocrine, respiratory and nervous system targets, liver damage |
| BPA |
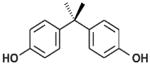
|
Bisphenols | 1960s | Varies by country. In the United States, voluntarily restricted in baby products 2012 | Ingestion, inhalation, dermal absorption | Polycarbonate plastics, epoxy resins, plastic toys and bottles, lining of food cans | 4–5 hours | Estrogenic, obesogenic, neurological effects, reproductive and developmental effects |
| DDT |
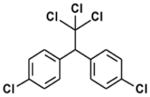
|
Organochloride | 1940s | Widely banned 1972 but still used in some countries. | Ingestion, inhalation, dermal absorption | Contaminated water, soil crops, fish | 6–10 years | Estrogenic, anti-androgenic, reproductive effects, carcinogen, central nervous system, kidney, liver and peripheral nervous system effects |
| DES |
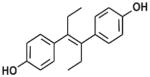
|
Non-steroidal synthetic estrogen | 1941–1947 | Restricted 1971–1975 | Ingestion, injection, vaginal suppository | Pharmaceutical for humans and livestock | 2–3 days | Transplacental carcinogen, teratogen |
| PCBs |
|
Organochloride | 1927 | Banned 1979 | Ingestion, inhalation, dermal absorption | Contaminated air and food, skin contact with old electrical equipment | 12 days to 16 years | Carcinogen, stomach and liver damage, reproductive and nervous system effects including IQ loss, thyroid injury |
| Phthalates |
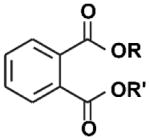
|
Plasticizers | 1920s | Restricted 2009 | Ingestion, inhalation, dermal absorption | Contaminated food, PVC plastics and flooring, personal care products, fragrance, medical devices and tubing | ~12 hours | Antiandrogenic activity, carcinogen, liver damage, reproductive and developmental effects, asthma, obesogen, possible neuroendocrine disruptor; Rat testis is vulnerable, mouse and human fetal testis xenografts are not. |
| PFOA |
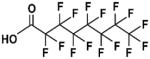
|
Fluorosurfactant | 1940s | United States 2015 voluntary production restriction | Ingestion, inhalation | Contaminated food and water, dust, floor waxes, fire fighting foam, electrical wiring, lining of food wrappers, stain resistant carpeting | 2–4 years | Liver, developmental, and immune system toxicant, carcinogen; Rats an inappropriate model |
| TCDD |
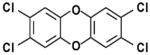
|
Polychlorinated dibenzo-p-dioxin | Synthesized 1872 | Ingestion, inhalation | By-product of chlorinated herbicide production, smelting, chlorine bleaching of paper; can be naturally occurring | 7–11 years | Liver damage, weight loss, atrophy of thymus gland, immunosuppression, reproductive effects and cancer; susceptibility varies widely across species and strains | |
| Vinclozolin |
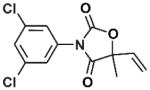
|
Dicarboximide fungicide | 1981 | Ingestion, inhalation, dermal absorption | Diet and occupational | Aerobic soil 28 days, plasma 20 hours | Antiandrogenic activity, male reproductive and neurological effects, transgenerational reproductive effects, potential carcinogen |
BPA=bisphenol A; DDT=dichlorodiphenyltrichloroethane; DES=diethylstilbestrol; MXC=methoxychlor; PCBs=polychlorinated biphenyls; PFOA=perfluorooctanoic acid; TCDD= 2,3,7,8-tetrachlorodibenzodioxin
Adapted from Gore, A. C. et al. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 36, E1-E150, doi:10.1210/er.2015-1010 (2015).
Animal Models in Toxicology: Lessons Learned
Justification and utility of animal-based work rests on several key assumptions, the most fundamental of which is that other organisms can serve as accurate predictive models of toxicity in humans. Thus, selection of an appropriately sensitive animal model is key for accurately guiding health decisions. Animal models are simply that, models, all of which have strengths and weaknesses. Understanding the advantages and limitations of any particular model is essential to maximizing the translational value of the data collected. Although this may seem obvious in principle, there are a number of species and strain-specific limitations that are unique to some toxicants, and can lead to erroneous conclusions about human risk.
Thalidomide
The thalidomide tragedy is a seminal example (reviewed in [18]). Introduced in 1957 by a German pharmaceutical company as a nonaddictive, nonbarbiturate sedative, and then as an anti-emetic for morning sickness in pregnant women, it was widely marketed and distributed in 46 countries until the early 1960s (not the US, despite intense pressure on the FDA to approve its use). Unfortunately, thalidomide caused infant mortality rates as high as 40% and severe birth defects in over 10,000 children including, most commonly, phocomelia, but also malformations of the face, eyes, ears, genitalia, and multiple internal organs, including heart, kidney, and gastrointestinal tract. Although the details remain disputed, the drug appears to have been tested primarily in mice, which was standard practice for the era, but clearly insufficient and later found to have failed for several reasons: (1) species differences in metabolism, (2) species differences in susceptibility, (3) species differences in the nature of the resulting adverse outcomes and, (4) a very short embryonic window of exposure susceptibility. Consequently, the thalidomide tragedy completely revolutionized how drugs were tested for safety and efficacy, including how animal models are selected and used. For the first time, there was recognition that critical species differences exist in drug reaction/response. It is now appreciated that mice, traditionally used to screen for drug action, are less sensitive to thalidomide than other species, and the primary outcome is fetal resorption not phocomelia. This species-specific resilience is not unusual, and differences in sensitivity can also exist across rodent strains. For example, vulnerability to the potent toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) varies considerably across species with some strains being completely resistant to this and other chemical carcinogens, while others succumb to even very low doses [19, 20].
In humans, Thalidomide acts via several mechanisms [21], including interference with the Wnt/beta-catenin pathway, thereby increasing apoptosis in the developing limbs, eyes, brain and other organs. The same occurs in chick and rabbit embryos, but not mice [22]. Mice also metabolize Thalidomide differently and much faster than humans and other rodent species. Notably, the active metabolites are responsible for much of the drug's activity, but these metabolites are species specific, explaining, at least in part, why thalidomide toxicity is species dependent. Additional work over the intervening decades has shown thalidomide damage in non-human primates, rabbits, armadillos, Xenopus, hamster, chicken, zebrafish, marine fish, and even hydra and bacteria. This wide range of vulnerable taxa is a powerful reminder to the biomedical community not to rely so heavily on mice, but also that effects in wildlife and other “non-model organisms” can produce informative, human-relevant data for toxicants.
The thalidomide tragedy also demonstrated the importance of critical windows. The severity and location of the deformities exquisitely depend on when fetal exposure occurs, with the most sensitive window being between 34 and 49 days after the last menstrual period [21]. Exposure on the early end of that critical window results in central brain damage, with risk then shifting to the eyes, ears, face, arms and legs on subsequent days. Critically, teratogenicity is not seen if taken beyond these early days of gestation. Thus, a mouse study with exposure in the later stages of pregnancy would yield the deceptively false conclusion that thalidomide is safe.
Diethylstilbestrol (DES)
A similar story unfolded for the notorious EDC, diethylstilbestrol (DES). Administered to as many as 10 million pregnant women and newborns (to promote weight gain) in the USA between 1938 and 1971, this potent estrogen agonist produced no obvious teratogenic effects in newborns but rather more insidious, and less obvious outcomes which only became apparent years after exposure. An estimated 90–95% of DES daughters suffer from unusual cancers of the reproductive tract, and/or reproductive problems including reproductive tract malformations, endometriosis, infertility, and more complicated and unsuccessful pregnancies [23]. DES exposure is also associated with increased risk of psychiatric disorders including depression, anorexia, phobias and learning disabilities [24]. DES sons also have adverse outcomes including elevated rates of urogenital malformations, undescended testes, urogenital inflammation, low sperm density/quality, and testicular cancer [23]. As with thalidomide, outcome depends on timing of exposure [25, 26]. Most of the reproductive outcomes following fetal exposure to DES were recapitulated in mouse models [27] suggesting that appropriate animal studies could have theoretically caught the effects. At the time, however, the focus was primarily on overtly teratogenic endpoints and not more subtle, long-term outcomes such as compromised fertility and pregnancy complications. With EDCs, latency between exposure and outcome can be years to decades long with prenatal exposure heightening risk to later in life cancers, such as breast or prostate cancer, or fertility-related deficits such as low sperm count or poor implantation.
Perfluorinated Compounds: A Contemporary Example
There are also contemporary examples where metabolism of the EDC varies between rodent species and even between strains within a species. Having some information on the pharmacokinetics in humans of the test chemical is imperative in selection of a model species/strain for further testing. For example, the link between perfluorooctanoic acid (PFOA), an industrial surfactant and common water pollutant, and altered breast development timing and function was first uncovered by studies in animal models. PFOA delivered to pregnant mice caused a lack of normal lactational competency in the exposed dam [28] and led to increased offspring mortality [28, 29]. Gestational PFOA exposure resulted in delays in mammary gland epithelial cell development and ductal elongation, and reductions in ductal branching and terminal end bud appearance in CD-1 female mouse offspring [28, 30]. Subsequent studies using lower doses and exposures spanning full or late gestation to focus on the time of mammary bud development, were sufficient to stunt mouse mammary gland development [31] and alter gene expression required for normal gland development in C57Bl/6 mice [32]. PFOA exposure during the peripubertal period can also induce altered estrous cyclicity, decreased ovarian steroid hormonal synthetic enzymes, and reduced gene expression of steroid-induced mammary growth factors but effects vary significantly by strain [33]. A recent paper highlighting the strain differences in metabolism or excretion of PFOA, reported that when Sv/129 mice received doses 3 to 300 times higher than those that affected CD-1 mice, Sv/129 mice had no mammary effects and lower than expected PFOA blood concentrations [34]. Enhanced excretion of PFOA in C57Bl/6 mice was also the basis for species differences in low dose effects in mammary tissue [32]. Female rats cannot be used to investigate these human relevant PFOA exposures because they exhibit a sex-specific increase in elimination rates compared to male rats; a difference that is not found in mice or humans.
Human Chemical Catastrophe and the Need to Model Mechanisms of Toxicity
Environmental accidents provide unfortunate and tragic evidence of human susceptibility to chemical exposures and critical windows of exposure. The 1976 pesticide plant explosion in Seveso, Italy [35], revealed a relationship between dioxin exposure and significantly increased cancer rates in women [36], increased metabolic disease in women who were 12 or younger at the time of the explosion [37], permanently reduced sperm quality in men who were breastfed as infants just after the explosion [38], and a dose-related association between serum dioxin levels and time to pregnancy and infertility in women [39]. Other pollution events have also been correlated with health effects in large residential cohorts. Specific examples include Agent Orange exposure to servicemen in south Vietnam [40], contamination of drinking water sources with a myriad of volatile organic chemicals at the Camp Lejeune Marine base, Jacksonville, NC, from the 1950s-mid 1980s [41, 42], and widespread PFOA contamination of the Little Hocking River and surrounding areas of Northern Kentucky and Ohio from as early as the 1950s to the present [43]. In all of these instances, exposure was linked to numerous documented health effects, but their mechanisms of action were unclear, making it difficult to predict possible future health effects and predict risk in other exposed populations. When correlations are made between health outcomes in humans and a particular chemical, the confirmation of cause-and-effect and the elucidation of a mechanism or mode of action must be derived from experimental studies, usually animal models.
For any toxicological or EDC study, a number of criteria for choosing the animal model must be met (Table 2). Some (of the many) reasons that a given animal model may be inappropriate include differences in metabolic fate, differences in the critical window(s) of sensitivity, lack of a sensitive or homologous target (the organism fails to model the human-relevant pathway), and the presence of an irrelevant target (the drug acts via a mechanism not relevant to humans). Practicalities such as cost and housing availability may also be important considerations. Housing challenges, for example, have limited use of classic EDC models such as sheep [44] and quail [45, 46]. Other species sometimes used in toxicology such as the mini pig, dogs, ferrets, rabbits, hamsters and non-human primates are rarely used for EDC research for similar reasons. Daphnia Magna have been used for decades by some groups in the context of chemical screening, albeit with some concerns about sensitivity and chemical specificity [47, 48]. Wildlife species including fish, birds, and reptiles such as alligators also remain critically valuable sentinels of organism and ecosystem health but pose their own logistical challenges [3, 49]. For some endpoints, particular model organisms have unique features that make them more advantageous. The guinea pig, for example has a more human-typical placental structure, but are more expensive per unit than rats and mice. A list of some commonly used EDC models with their unique strengths and weaknesses is listed in Table 3.
Table 2.
Considerations in Animal Model Selection and Use for EDC Research
|
|
|
|
|
|
|
|
|
Table 3.
Strengths and Weaknesses of Common EDC Animal Models
| Species | Strain | Unique Characteristics |
|---|---|---|
| Mouse | CD1 | Large litters, excellent maternal behavior, sensitive to estrogens, robust data on historical control disease incidence, outbred strain so higher variability in experiments |
| C57BL/6J | Inbred strain particularly sensitive to immune challenges, good embryo donor and transplant recipient | |
| BTBR | Used as a model of autism but lacks a corpus callosum and has a severely reduced hippocampal commissure. | |
| B6C3F1 | Moderate sized litters, cross of 2 inbred strains, large dataset on controls, longevity | |
| Collaborative Cross | Genetic diversity with reproducibility | |
| Diversity outbred | Genetic diversity with maximum QTL mapping power and resolution | |
| Rat | Wistar Han | Excellent maternal behavior, low spontaneous tumor incidence |
| Long Evans | Outbred strain, not albino, spontaneously displays more ethologically relevant behaviors | |
| Sprague | Vendor strains are not congruous; Harlan or Taconic are | |
| Dawley | preferred source. Background incidence of cardiomyopathy can be as high as 100%, CRL demonstrate high rate of mammary galactocoeles, poor longevity, and low estrogen sensitivity. | |
| F344/N | High spontaneous tumor rate in testes and mammary gland due to prolactin sensitivity | |
| Voles (Microtus) | Some strains are socially monogamous, display paternal care, alloparental care and other pro-social traits; biological basis for pro-sociality well understood | |
| Deer mice (Peromyscus) | Some strains are socially monogamous, display paternal care and other pro-social traits; have been used to characterize “real world” exposures from contaminated sites. | |
| Zebrafish | Transparent; relatively easy and inexpensive to house, breed and maintain; rapid development | |
| Guinea Pig | Human-relevant placental structure | |
| Sheep | Human-relevant placental structure |
Novel Animal Models in EDC Research
Although classic toxicology still heavily relies on inbred lines of rats and mice, powerful new options in rodents and other species created to leverage significant advances in gene editing, genomics, and neuroimaging hold the potential to significantly advance EDC research. Although some fields, particularly genetics and the neurosciences, have made significant discoveries with lower order species such as the nematode Caenorhabditis elegans, and the fruit fly Drosophila melanogaster, there is reticence to use them for toxicological testing because of concerns about data translation to humans. However, a greater diversity of vertebrate models is gaining in popularity including transgenic mouse (and rat) lines, zebrafish Danio rerio, and monogamous rodents such as the prairie vole Microtus ochrogaster. Perhaps most exciting are powerful new mouse lines for interrogating population-level effects, and gene by environment interactions.
Zebrafish
The zebrafish model is rapidly being adopted as an option for EDC research, particularly chemical screening, because of several critical advantages [50]. Compared to mammalian models zebrafish are easy to rear and relatively inexpensive to maintain, even for small labs, and can be bred and studied at high speed, and used in high numbers. Less than a few millimeters in size, the larvae are small enough to be accommodated in multiwell plates, making their large scale use in semi-automated systems feasible [50–52]. The translucent body of zebrafish embryos combined with advances in high dimensional imaging tools facilitates non-invasive visualization of organs and biological processes in vivo, including neurodevelopment and the fate/transport of putative EDCs. The relative ease by which the zebrafish genome can be manipulated is also a unique advantage. The zebrafish genome is comprehensively annotated, a large number of transgenic lines are readily available, and alteration of gene expression is easily achievable using antisense morpholinos or gene-editing techniques, such as TALENs or CRISPR/Cas9. There are also robust genomic tools and consortium-level projects enhancing their utility. Launched in 2011, the Zebrafish Mutation Project (ZMP) is an initiative to create a knockout allele in every protein-coding gene in the zebrafish genome. The data and resources are readily available on the ZMP website and via the Zebrafish International Resource Center (ZIRC). The mammalian and zebrafish genomes display reasonably high homology with an overall conservation of over 70%, and an estimated 80% of human genes expressed in zebrafish [53]. They also effectively model some of the most basic modes of endocrine disruption. Many basic physiological, sensory, and anatomical and signal-transduction mechanisms are also homologous to those of mammals [54].
Significant advances in instrumentation and –omics technology have made it possible to use early life stage zebrafish for EDC screening. For example, several studies have used such systems to screen the EPA’s ToxCast chemicals for endocrine disrupting and other toxicity outcomes, and to evaluate the validity of comparable in vitro and in vivo toxicity data [50, 52, 55]. Zebrafish were also used to identify a critical pathway by which Thalidomide inhibits limb development [56].
As with all model systems, zebrafish have noteworthy limitations. Most obvious is that EDC exposure is typically via absorption from tank water (dermally and, as the gills mature, via circulation), meaning that chemical uptake and metabolism can be quite different from the human experience where exposure is typically oral or inhaled. Tissue concentrations are also poorly understood and rarely reported, which can make it difficult to extrapolate concentration-response results in zebrafish to dose-response studies in mammals. By extension, although zebrafish metabolism is similar to other vertebrates, there are subtle differences that could yield inaccurate conclusions. As with any animal model, some pathway components may not necessarily be highly conserved and some cellular systems may bear little homology. For example zebrafish lack brown adipose fat so exploring pathways activated by non-shivering thermogenesis, such as the β-adrenergic system, will be limited. Finally, as with rodents, inter-laboratory replication difficulties have occurred, most likely due to strain, housing and other differences, but are not well documented or understood. As in mammals, lab-reared colonies are vastly different in many respects from wild-derived strains, and allelic variations between individuals can result in marked differences in chemical susceptibility [57]. Accounting for this in future study designs could enhance an already uniquely advantageous EDC screening tool.
Transgenic Rodents
Developed in the 1970s and now commonly used in virtually every biomedical field, transgenic rodents are surprisingly seldom used in toxicology. Literally thousands of different lines are now available and have proved to be powerful tools for modeling human disease and exploring the roles of specific genes in biological pathways and systems, Although the older, more traditional techniques for generating rodent transgenics only reliably worked in mice, newer tools such as CRISPR/Cas9 are rapidly making it possible to create transgenic lines in rats and other species [58]. Using Cre-lox, Flp-Frt, or similar approaches, it is also now possible to make site-specific conditional transgenics where genes can be manipulated, for example, in specific cell populations, or under certain conditions [59]. Using a combination of approaches transgenics can be used to perform gene targeting experiments to achieve genotypic/phenotypic “rescue,” spatio-temporally inactivate genes in specific cells, and perform cell fate mapping and gene/protein expression profiling. For example, transgenic techniques have been used in combination with viral vectors and other tools to introduce visual (such as GFP) and other reporters to manipulate and visualize entire neural circuits and pathways [60].
In classical toxicology transgenics are grossly underutilized. To date, they have really only been used for hazard characterization of mutagenics, and even then only rarely [61]. They have, however, been used by EDC researchers to probe mode of action and generate humanized mouse models. For example a variety of ER-mutated and knockout mouse lines are now available [62, 63] and have been used to explore non-canonical signaling mechanisms [64] as well as mechanisms of estrogenic endocrine disruption [65, 66]. Examples of models developed to overcome known species differences in toxicological vulnerability and outcomes include mutated and humanized aryl hydrocarbon receptor (AhR) [67] and PPARα-humanized mice [68]. Use of knockout and other transgenic models have also been used to probe EDC conjugation and metabolism [69].
While powerful tools to elucidate mechanisms of endocrine disruption and possible gene by environment interactions, transgenic animals have limitations, most significantly the high cost and time required to generate them. There is also the potential for incomplete knockout of the target gene, off-target expression, biological compensation, and incomplete recapitulation of the expected disease phenotype (many “Alzheimer’s mice” have this problem). Numerous Cre lines have phenotypes that are not obvious or fully characterized, and Cre activity in off-target tissues is proving to be fairly common. In some strains, Cre mosaicism is also a known limitation and Cre recombinase activity can be less robust when paternally inherited. Researchers should be sure proper controls are used to validate and maintain the models.
Population-Based Genetic Resources
Natural genetic variation plays an important role in nearly all biological processes and traits, including response to EDCs [70]. Population-based mouse resources capture individual differences in EDC susceptibility and may better model diverse human populations. Multi-parent populations such as the Collaborative Cross (CC) and Diversity Outbred (DO) mice are designed to maximize genetic variation while controlling the relationships between individuals, so that they are highly amenable to genetic analysis such as quantitative trait locus (QTL) mapping [71–73]. The CC population consists of 83 inbred mouse strains that are all descended from eight existing strains. These eight founder strains selected are a combination of common strains that were historically important in biomedical research and wild-derived strains that were included to maximize genetic diversity. The recombinant inbred line breeding design means that each strain is equally related to the others, and eliminates population structure that can limit QTL mapping ability or create false positive QTL. Each CC line is an inbred mouse strain, and can be reproduced at will in multiple environmental contexts. DO mice are descended from the same eight founder strains as the CC, and therefore both populations have the same sets of alleles. In contrast to the CC, DO mice are outbred and each individual is unique; hundreds of individual DO mice can be used in a single mapping study. These large populations combined with the outbred genomes make the DO uniquely powerful for detecting genetic associations. While each DO population has similar properties in terms of genotype frequencies, individual DO mice are not reproducible.
Both of these mouse resources allow replicable populations to be studied across a range of environmental contexts [74] and reveal gene by environment (GxE) interactions. Population studies can both measure the range of response to a specific toxicant and to identify specific genes driving susceptibility. In one example, DO mice exposed to benzene showed an order of magnitude more variation in chromosomal damage than isogenic B6C3F1 mice. QTL mapping in the population revealed a novel role for the sulfotransferase Sult3a1 in benzene response [75, 76]. A separate study found that trichloroethylene metabolism varied in CC populations due to multiple interacting factors, and individual CC strains showed distinct dose-response trajectories [77]. Other studies using these resources with environmental factors have found new candidate genes involved in the asthma response [78, 79], differential toxicity to chemotherapeutics [80, 81], and sensitivity to a popular food additive [82]. Thoughtful application of these resources to EDC research will directly inform human studies regarding population-level variation and mechanisms of susceptibility.
Monogamous Rodents
Rapidly and inexplicably rising rates of autism spectrum disorder (ASD), attention deficit activity disorder (ADHD) and other developmental disorders of non-reproductive behaviors including social interaction have spurred widespread interest in understanding how EDCs might impact the social brain. Genetic factors contribute, at best, only an estimated 30–40% of ASD heritability [83, 84], indicating that it and other disorders of sociality have a significant environmental component. Although it is widely speculated that chemical exposures are contributory, there is actually very little direct evidence linking any specific chemical, including EDCs, to adverse effects on the social brain or social impairments in humans [84–89]. Additionally, relatively little is known about the mechanisms by which the social brain could be vulnerable to chemical exposures, or which chemical classes are most likely to be detrimental [90–92].
Because classical rodent models are limited in terms of what human-relevant social behaviors they can recapitulate, animal model suitability has been cited as a significant experimental barrier. For example, common ASD mouse models often have some sort of neuropathology not seen in human patients (ex. the BTBR mouse completely lacks a corpus callosum and has a severely reduced hippocampal commissure) and none display partner attachment or paternal care; defining elements of human sociality. In that regard, monogamous rodent species could prove uniquely valuable. Although no animal model can fully recapitulate the sophisticated complexity of human social behavior, the neuroendocrine pathways coordinating social traits are highly conserved [93, 94] including the sexually differentiating influence of steroid hormones, and coordinating roles of the neuropeptides vasopressin (AVP) and oxytocin (OT).
Much of what is known about the evolution and manifestation of pro-social traits came from groundbreaking work in microtine voles. Prairie and pine voles (Microtus ochrogaster and Microtus pinetorum respectively) are socially monogamous while montane and meadow voles (Microtus montanus and Microtus pennsylvanicus respectively) are socially promiscuous and solitary providing an ideal comparative system for exploring species-level differences in social brain structure and development. Decades of transformative work in these and other microtine species has linked pro-social traits to the OT/AVP system and its interactions with mesolimbic dopamine pathways [95–97]. The translational importance of the vole model has now been demonstrated in humans (reviewed in [97]). Most significantly, intranasal OT administration is being used therapeutically for ASDs. Because OT/AVP and the downstream dopaminergic pathways they project to are heavily influenced by sex steroids across the lifespan [98–101], it is highly plausible that their sexually dimorphic ontogeny and function may be susceptible to endocrine disruption. With the prairie vole genome now sequenced, it is feasible to do the types of gene by environment experiments needed to identify possible mechanisms by which EDCs might contribute to social decrements relevant to ASD and other behavioral disorders [96, 102].
Only a handful of studies have used voles to explore endocrine disruption of the social brain. The first linked perinatal methoxychlor exposure to reduced affiliative behavior by exposed females, and reduced oxytocin receptor binding in the cingulate [103], a region thought to play a role in stress responses and emotional processing [104]. More recent studies have reported that BPA can alter aspects of anxiety-related behaviors, eliminate or reverse sexually dimorphic social behaviors, alter OT/AVP and dopaminergic neuron numbers in multiple brain regions critical for sociality, and disrupt microglial colonization [105, 106].
Similar pro-social models include Peromyscus mice (often collectively referred to as deer mice). As in Microtus, some species are socially monogamous (most notably Peromyscus californicus but also Peromyscus polionotus) while others are not. The mechanisms underlying monogamous behavior in this genus, however, may differ from Microtus, and are not as well understood [107]. As with zebrafish, Peromyscus are a well-established model for studying the evolution and genomics of complex traits and have been backed by consortium-level projects to advance their use. Most significantly, various “wild type” and mutant lines have been created and maintained by the Peromyscus Genetic Stock Center at the University of South Carolina.
Like Microtus they can be more challenging to breed and maintain the laboratory environment than rats or mice, but have key advantages including their genetic diversity and the spontaneous display of human-relevant social traits such as pair bonding and paternal care. Notably, deer mice have been utilized to monitor real world exposures by comparing exposed wild animals with laboratory-bred controls [108, 109]. Peromyscus have also been used to explore the possible effects of BPA on exploratory and socio-sexual behaviors [110, 111]. Because of their limited sources, the cost may be higher than other rodent models, but their environmental relevance may outweigh the disadvantages for some studies.
Summary
In conclusion, numerous considerations must be made when designing studies to address disease conditions that occur in human populations as the result of EDC exposure. Although cell models may be useful in identifying some components of signaling pathways, they lack feedback loops, endocrine systems, and initiation and progression of disease processes that allow for translation of EDC effects. They are also incapable of modeling or recapitulating complex behavior. Rodent and other relevant animal models are critical in testing the effects of EDCs on developmental exposures, systems biology, immunity, reproductive and neurobehavioral outcomes. Emerging models such as zebrafish, monogamous rodents, and population-based mouse resources hold tremendous promise for addressing key challenges in EDC research including differences in individual and population-level susceptibility, effects on complex behaviors, including social behaviors, the capacity for rapid EDC screening, and the ability to explore gene by environment interactions.
Practice Points.
EDCs are widespread and likely contributing to a myriad of human diseases, thus greater physician and patient awareness of these chemicals and their sources is needed.
Human exposure is typically low, life long, and to complex mixtures with exposures highest in children.
Some exposures can be mitigated by simple lifestyle changes such as using unscented products and glass instead of plastic.
Research Agenda.
Research has typically focused on reproductive and thyroid targets but exciting new research is identifying immune, cardiovascular, metabolic, epigenetic and other endpoints.
An overreliance on one or only a few strains of mice can limit the type of risk and biology that can be effectively modeled.
Zebrafish are emerging as cost effective tool for EDC screening and should be incorporated into regulatory testing strategies to improve screening capacity and accuracy.
New rodent models including a wide range of transgenic strains, monogamous species, and those specifically designed to examine population-level effects are rapidly emerging to address significant limitations in our current capacity to probe complex issues like inter-individual variability and gene by environment interactions.
Acknowledgments
Funding: This work was supported by NIEHS grant P30ES025128
Footnotes
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solecki R, Kortenkamp A, Bergman A, et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch Toxicol. 2017;91(2):1001–1006. doi: 10.1007/s00204-016-1866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore AC, Chappell VA, Fenton SE, et al. Edc-2: The endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillette LJ, Jr, Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122(6):857–864. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- 4.Barouki R, Gluckman PD, Grandjean P, et al. Developmental origins of non-communicable disease: Implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frye CA, Bo E, Calamandrei G, et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol. 2012;24(1):144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patisaul HB. Endocrine disruption of vasopressin systems and related behaviors. Front Endocrinol (Lausanne) 2017;8:134. doi: 10.3389/fendo.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patisaul HB. Effects of environmental endocrine disruptors and phytoestrogens on the kisspeptin system. Adv Exp Med Biol. 2013;784:455–479. doi: 10.1007/978-1-4614-6199-9_21. [DOI] [PubMed] [Google Scholar]
- *8.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottinger MA, Lavoie E, Thompson N, et al. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev. 2008;57(2):376–385. doi: 10.1016/j.brainresrev.2007.08.011. [DOI] [PubMed] [Google Scholar]
- *10.Bilbo SD, Block CL, Bolton JL, et al. Beyond infection - maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2017 doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol a exposure. Proc Natl Acad Sci U S A. 2013;110(24):9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundy WR, Padilla S, Breier JM, et al. Expanding the test set: Chemicals with potential to disrupt mammalian brain development. Neurotoxicol Teratol. 2015;52(Pt A):25–35. doi: 10.1016/j.ntt.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council (U.S.). Committee on Toxicity Testing and Assessment of Environmental Agents. Toxicity testing in the 21st century : A vision and a strategy. Washington, DC: National Academies Press; 2007. p. xvii.p. 196. [Google Scholar]
- *14.National Academies of Sciences Engineering and Medicine (U.S.). Committee on Incorporating 21st Century Science into Risk-Based Evaluations. Using 21st century science to improve risk-related evaluations. xvi:182. [Google Scholar]
- 15.Filer D, Patisaul HB, Schug T, et al. Test driving toxcast: Endocrine profiling for 1858 chemicals included in phase ii. Current opinion in pharmacology. 2014;19:145–152. doi: 10.1016/j.coph.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McPartland J, Dantzker HC, Portier CJ. Building a robust 21st century chemical testing program at the u.S. Environmental protection agency: Recommendations for strengthening scientific engagement. Environ Health Perspect. 2015;123(1):1–5. doi: 10.1289/ehp.1408601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotroff DM, Dix DJ, Houck KA, et al. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ Health Perspect. 2013;121(1):7–14. doi: 10.1289/ehp.1205065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehman W, Arfons LM, Lazarus HM. The rise, fall and subsequent triumph of thalidomide: Lessons learned in drug development. Ther Adv Hematol. 2011;2(5):291–308. doi: 10.1177/2040620711413165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirai T, Nakamura A, Fukushima S, et al. Different carcinogenic responses in a variety of organs, including the prostate, of five different rat strains given 3,2'-dimethyl-4-aminobiphenyl. Carcinogenesis. 1990;11(5):793–797. doi: 10.1093/carcin/11.5.793. [DOI] [PubMed] [Google Scholar]
- 20.Pohjanvirta R, Viluksela M, Tuomisto JT, et al. Physicochemical differences in the ah receptors of the most tcdd-susceptible and the most tcdd-resistant rat strains. Toxicol Appl Pharmacol. 1999;155(1):82–95. doi: 10.1006/taap.1998.8565. [DOI] [PubMed] [Google Scholar]
- 21.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38(3):203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 22.Vargesson N. Thalidomide-induced limb defects: Resolving a 50-year-old puzzle. Bioessays. 2009;31(12):1327–1336. doi: 10.1002/bies.200900103. [DOI] [PubMed] [Google Scholar]
- 23.Harris RM, Waring RH. Diethylstilboestrol--a long-term legacy. Maturitas. 2012;72(2):108–112. doi: 10.1016/j.maturitas.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Vessey MP, Fairweather DV, Norman-Smith B, et al. A randomized double-blind controlled trial of the value of stilboestrol therapy in pregnancy: Long-term follow-up of mothers and their offspring. Br J Obstet Gynaecol. 1983;90(11):1007–1017. doi: 10.1111/j.1471-0528.1983.tb06438.x. [DOI] [PubMed] [Google Scholar]
- 25.Mahalingaiah S, Hart JE, Wise LA, et al. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the nurses' health study ii. Am J Epidemiol. 2014;179(2):186–191. doi: 10.1093/aje/kwt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L. Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol Metab. 2009;20(7):357–363. doi: 10.1016/j.tem.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199(2):142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 28.White SS, Calafat AM, Kuklenyik Z, et al. Gestational pfoa exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 29.Lau C, Thibodeaux JR, Hanson RG, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 30.White SS, Kato K, Jia LT, et al. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol. 2009;27(3–4):289–298. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macon MB, Villanueva LR, Tatum-Gibbs K, et al. Prenatal perfluorooctanoic acid exposure in cd-1 mice: Low-dose developmental effects and internal dosimetry. Toxicol Sci. 2011;122(1):134–145. doi: 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Tucker DK, Macon MB, Strynar MJ, et al. The mammary gland is a sensitive pubertal target in cd-1 and c57bl/6 mice following perinatal perfluorooctanoic acid (pfoa) exposure. Reprod Toxicol. 2015;54:26–36. doi: 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Tan YS, Strynar MJ, et al. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in balb/c and c57bl/6 mice. Reprod Toxicol. 2012;33(4):563–576. doi: 10.1016/j.reprotox.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Albrecht PP, Torsell NE, Krishnan P, et al. A species difference in the peroxisome proliferator-activated receptor alpha-dependent response to the developmental effects of perfluorooctanoic acid. Toxicol Sci. 2013;131(2):568–582. doi: 10.1093/toxsci/kfs318. [DOI] [PubMed] [Google Scholar]
- 35.Mocarelli P, Marocchi A, Brambilla P, et al. Clinical laboratory manifestations of exposure to dioxin in children. A six-year study of the effects of an environmental disaster near seveso, italy. JAMA. 1986;256(19):2687–2695. [PubMed] [Google Scholar]
- 36.Warner M, Mocarelli P, Samuels S, et al. Dioxin exposure and cancer risk in the seveso women's health study. Environ Health Perspect. 2011;119(12):1700–1705. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: The seveso women's health study. Environ Health Perspect. 2013;121(8):906–911. doi: 10.1289/ehp.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mocarelli P, Gerthoux PM, Needham LL, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119(5):713–718. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eskenazi B, Warner M, Marks AR, et al. Serum dioxin concentrations and time to pregnancy. Epidemiology. 2010;21(2):224–231. doi: 10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JS, Lim HS, Cho SI, et al. Impact of agent orange exposure among korean vietnam veterans. Ind Health. 2003;41(3):149–157. doi: 10.2486/indhealth.41.149. [DOI] [PubMed] [Google Scholar]
- 41.Ruckart PZ, Bove FJ, Maslia M. Evaluation of contaminated drinking water and preterm birth, small for gestational age, and birth weight at marine corps base camp lejeune, north carolina: A cross-sectional study. Environ Health. 2014;13:99. doi: 10.1186/1476-069X-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bove FJ, Ruckart PZ, Maslia M, et al. Mortality study of civilian employees exposed to contaminated drinking water at usmc base camp lejeune: A retrospective cohort study. Environ Health. 2014;13:68. doi: 10.1186/1476-069X-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (pfoa) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11–12):1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahoney MM, Padmanabhan V. Developmental programming: Impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mrna in sheep hypothalamus. Toxicol Appl Pharmacol. 2010;247(2):98–104. doi: 10.1016/j.taap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viglietti-Panzica C, Mura E, Panzica G. Effects of early embryonic exposure to genistein on male copulatory behavior and vasotocin system of japanese quail. Horm Behav. 2007;51(3):355–363. doi: 10.1016/j.yhbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Ottinger MA, Lavoie ET, Abdelnabi M, et al. An overview of dioxin-like compounds, pcb, and pesticide exposures associated with sexual differentiation of neuroendocrine systems, fluctuating asymmetry, and behavioral effects in birds. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(4):286–300. doi: 10.1080/10590500903310229. [DOI] [PubMed] [Google Scholar]
- 47.Kang Y, Yan X, Li L, et al. Daphnia magna may serve as a powerful tool in screening endocrine disruption chemicals (edcs) Environ Sci Technol. 2014;48(2):881–882. doi: 10.1021/es405379p. [DOI] [PubMed] [Google Scholar]
- 48.Dang Z, Cheng Y, Chen HM, et al. Evaluation of the daphnia magna reproduction test for detecting endocrine disruptors. Chemosphere. 2012;88(4):514–523. doi: 10.1016/j.chemosphere.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss AK, Rider CV, Blystone CR, et al. Fifteen years after "wingspread"--environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol Sci. 2008;105(2):235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reif DM, Truong L, Mandrell D, et al. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2015 doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Current opinion in chemical biology. 2015;24:58–70. doi: 10.1016/j.cbpa.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Truong L, Reif DM, St Mary L, et al. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 2014;137(1):212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dooley K, Zon LI. Zebrafish: A model system for the study of human disease. Curr Opin Genet Dev. 2000;10(3):252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 55.Padilla S, Corum D, Padnos B, et al. Zebrafish developmental screening of the toxcast phase i chemical library. Reproductive toxicology. 2012;33(2):174–187. doi: 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 57.van den Bos R, Mes W, Galligani P, et al. Further characterisation of differences between tl and ab zebrafish (danio rerio): Gene expression, physiology and behaviour at day 5 of the larval stage. PLoS One. 2017;12(4):e0175420. doi: 10.1371/journal.pone.0175420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Rocha-Martins M, Cavalheiro GR, Matos-Rodrigues GE, et al. From gene targeting to genome editing: Transgenic animals applications and beyond. An Acad Bras Cienc. 2015;87(2 Suppl):1323–1348. doi: 10.1590/0001-3765201520140710. [DOI] [PubMed] [Google Scholar]
- 59.Dubois SL, Acosta-Martinez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. doi: 10.1210/en.2014-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wouterlood FG, Bloem B, Mansvelder HD, et al. A fourth generation of neuroanatomical tracing techniques: Exploiting the offspring of genetic engineering. J Neurosci Methods. 2014;235:331–348. doi: 10.1016/j.jneumeth.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Boverhof DR, Chamberlain MP, Elcombe CR, et al. Transgenic animal models in toxicology: Historical perspectives and future outlook. Toxicol Sci. 2011;121(2):207–233. doi: 10.1093/toxsci/kfr075. [DOI] [PubMed] [Google Scholar]
- 62.McDevitt MA, Glidewell-Kenney C, Jimenez MA, et al. New insights into the classical and non-classical actions of estrogen: Evidence from estrogen receptor knockout and knock-in mice. Mol Cell Endocrinol. 2008;290(1–2):24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Stefkovich ML, Arao Y, Hamilton KJ, et al. Experimental models for evaluating non-genomic estrogen signaling. Steroids. 2017 doi: 10.1016/j.steroids.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambliss KL, Wu Q, Oltmann S, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacob DA, Temple JL, Patisaul HB, et al. Coumestrol antagonizes neuroendocrine actions of estrogen via the estrogen receptor α. Proc Soc Exp Biol Med. 2001;226(4):301–306. doi: 10.1177/153537020122600406. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Hamilton KJ, Lai AY, et al. Diethylstilbestrol (des)-stimulated hormonal toxicity is mediated by eralpha alteration of target gene methylation patterns and epigenetic modifiers (dnmt3a, mbd2, and hdac2) in the mouse seminal vesicle. Environ Health Perspect. 2014;122(3):262–268. doi: 10.1289/ehp.1307351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135(1):1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Q, Nagano T, Shah Y, et al. The ppar alpha-humanized mouse: A model to investigate species differences in liver toxicity mediated by ppar alpha. Toxicol Sci. 2008;101(1):132–139. doi: 10.1093/toxsci/kfm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fay MJ, Nguyen MT, Snouwaert JN, et al. Xenobiotic metabolism in mice lacking the udp-glucuronosyltransferase 2 family. Drug Metab Dispos. 2015;43(12):1838–1846. doi: 10.1124/dmd.115.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.La Merrill M, Kuruvilla BS, Pomp D, et al. Dietary fat alters body composition, mammary development, and cytochrome p450 induction after maternal tcdd exposure in dba/2j mice with low-responsive aryl hydrocarbon receptors. Environ Health Perspect. 2009;117(9):1414–1419. doi: 10.1289/ehp.0800530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aylor DL, Valdar W, Foulds-Mathes W, et al. Genetic analysis of complex traits in the emerging collaborative cross. Genome Res. 2011 doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Threadgill DW, Churchill GA. Ten years of the collaborative cross. Genetics. 2012;190(2):291–294. doi: 10.1534/genetics.111.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Threadgill DW, Hunter KW, Williams RW. Genetic dissection of complex and quantitative traits: From fantasy to reality via a community effort. Mamm Genome. 2002;13(4):175–178. doi: 10.1007/s00335-001-4001-Y. [DOI] [PubMed] [Google Scholar]
- *74.Harrill AH, McAllister KA. New rodent population models may inform human health risk assessment and identification of genetic susceptibility to environmental exposures. Environ Health Perspect. 2017;125(8):086002. doi: 10.1289/EHP1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.French JE, Gatti DM, Morgan DL, et al. Diversity outbred mice identify population-based exposure thresholds and genetic factors that influence benzene-induced genotoxicity. Environ Health Perspect. 2015;123(3):237–245. doi: 10.1289/ehp.1408202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatti D, French JE, Schughart K. Qtl mapping and identification of candidate genes in do mice: A use case model derived from a benzene toxicity experiment. Methods Mol Biol. 2017;1488:265–281. doi: 10.1007/978-1-4939-6427-7_12. [DOI] [PubMed] [Google Scholar]
- 77.Venkatratnam A, Furuya S, Kosyk O, et al. Editor's highlight: Collaborative cross mouse population enables refinements to characterization of the variability in toxicokinetics of trichloroethylene and provides genetic evidence for the role of ppar pathway in its oxidative metabolism. Toxicol Sci. 2017;158(1):48–62. doi: 10.1093/toxsci/kfx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelada SN, Carpenter DE, Aylor DL, et al. Integrative genetic analysis of allergic inflammation in the murine lung. Am J Respir Cell Mol Biol. 2014;51(3):436–445. doi: 10.1165/rcmb.2013-0501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutledge H, Aylor DL, Carpenter DE, et al. Genetic regulation of zfp30, cxcl1, and neutrophilic inflammation in murine lung. Genetics. 2014;198(2):735–745. doi: 10.1534/genetics.114.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gatti DM, Weber SN, Goodwin NC, et al. Genetic background influences susceptibility to chemotherapy-induced hematotoxicity. Pharmacogenomics J. 2017 doi: 10.1038/tpj.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrill AH, Ross PK, Gatti DM, et al. Population-based discovery of toxicogenomics biomarkers for hepatotoxicity using a laboratory strain diversity panel. Toxicological Sciences. 2009;110(1):235–243. doi: 10.1093/toxsci/kfp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Church RJ, Gatti DM, Urban TJ, et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem Toxicol. 2015;76:19–26. doi: 10.1016/j.fct.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. JAMA. 2014;311(17):1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258–260. doi: 10.1289/ehp.1104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: A review of the epidemiological evidence. Current problems in pediatric and adolescent health care. 2014;44(10):277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet neurology. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujiwara T, Morisaki N, Honda Y, et al. Chemicals, nutrition, and autism spectrum disorder: A mini-review. Front Neurosci. 2016;10:174. doi: 10.3389/fnins.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aguiar A, Eubig PA, Schantz SL. Attention deficit/hyperactivity disorder: A focused overview for children's environmental health researchers. Environ Health Perspect. 2010;118(12):1646–1653. doi: 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eubig PA, Aguiar A, Schantz SL. Lead and pcbs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect. 2010;118(12):1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rauh VA, Margolis AE. Research review: Environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry. 2016;57(7):775–793. doi: 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearson BL, Simon JM, McCoy ES, et al. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun. 2016;7:11173. doi: 10.1038/ncomms11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gore AC, Martien KM, Gagnidze K, et al. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35(6):961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adkins-Regan E. Neuroendocrinology of social behavior. ILAR J. 2009;50(1):5–14. doi: 10.1093/ilar.50.1.5. [DOI] [PubMed] [Google Scholar]
- 94.Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Young LJ, Lim MM, Gingrich B, et al. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 96.McGraw LA, Young LJ. The prairie vole: An emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Hormones and Behavior. 2012;61(3):340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simerly RB, Swanson LW, Handa RJ, et al. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 99.Cushing BS, Kramer KM. Mechanisms underlying epigenetic effects of early social experience: The role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29(7):1089–1105. doi: 10.1016/j.neubiorev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Cavanaugh BL, Lonstein JS. Androgenic and oestrogenic influences on tyrosine hydroxylase-immunoreactive cells of the prairie vole medial amygdala and bed nucleus of the stria terminalis. J Neuroendocrinol. 2010;22(4):217–225. doi: 10.1111/j.1365-2826.2010.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cushing BS, Perry A, Musatov S, et al. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci. 2008;28(41):10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGraw LA, Davis JK, Lowman JJ, et al. Development of genomic resources for the prairie vole (microtus ochrogaster): Construction of a bac library and vole-mouse comparative cytogenetic map. BMC Genomics. 2010;11:70. doi: 10.1186/1471-2164-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Engell MD, Godwin J, Young LJ, et al. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol. 2006;28(1):103–110. doi: 10.1016/j.ntt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Singewald GM, Rjabokon A, Singewald N, et al. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(4):793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *105.Sullivan AW, Beach EC, Stetzik LA, et al. A novel model for neuroendocrine toxicology: Neurobehavioral effects of bpa exposure in a prosocial species, the prairie vole (microtus ochrogaster) Endocrinology. 2014;155(10):3867–3881. doi: 10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rebuli ME, Gibson P, Rhodes CL, et al. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen Comp Endocrinol. 2016;238:39–46. doi: 10.1016/j.ygcen.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner LM, Young AR, Rompler H, et al. Monogamy evolves through multiple mechanisms: Evidence from v1ar in deer mice. Mol Biol Evol. 2010;27(6):1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- 108.Kucera E. Effect of smelter emissions on the hemogram of the deer mouse (peromyscus maniculatus) Environ Pollut. 1988;55(3):173–177. doi: 10.1016/0269-7491(88)90150-9. [DOI] [PubMed] [Google Scholar]
- 109.Porter WP, Jaeger JW, Carlson IH. Endocrine, immune, and behavioral effects of aldicarb (carbamate), atrazine (triazine) and nitrate (fertilizer) mixtures at groundwater concentrations. Toxicol Ind Health. 1999;15(1–2):133–150. doi: 10.1177/074823379901500111. [DOI] [PubMed] [Google Scholar]
- 110.Jasarevic E, Sieli PT, Twellman EE, et al. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol a. Proc Natl Acad Sci U S A. 2011;108(28):11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jasarevic E, Williams SA, Vandas GM, et al. Sex and dose-dependent effects of developmental exposure to bisphenol a on anxiety and spatial learning in deer mice (peromyscus maniculatus bairdii) offspring. Horm Behav. 2013;63(1):180–189. doi: 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


