Abstract
Purpose of review
Hypereosinophilic syndrome (HES) is characterized by persistent hypereosinophilia associated with end-organ damage. As our understanding of the pathogenesis of various forms of HES broadens, so does our ability to tailor steroid-sparing therapies for each subtype. The purpose of this review is to summarize recent literature related to the etiology, diagnosis, and management of HES.
Recent findings
Mutations involved in subsets of HES can guide the choice of tyrosine kinase inhibitors beyond just imatinib. Several biologics that target interleukin-5 or its receptor have shown beneficial and selective eosinophil reducing effects in clinical trials for asthma and other disorders including HES. Early clinical data with emerging therapies such as dexpramipexole and anti-Siglec-8 antibody show promise, but need to be confirmed in randomized trials.
Summary
Several new biologics and tyrosine kinase inhibitors have been shown to lower eosinophil numbers, but more randomized trials are needed to confirm efficacy in HES.
Keywords: Hypereosinophilic syndrome, tyrosine kinase inhibitor, dexpramipexole, biologic, IL-5, Siglec-8
INTRODUCTION
Eosinophils are innate immune cells whose likely primary role is to defend against parasitic infections. However, they contribute to a number of different disorders including atopic and autoimmune diseases (1, 2). Hypereosinophilia (HE) is defined as an elevation of the peripheral blood absolute eosinophil count (AEC) greater than 1,500/μL on at least two separate occasions. This may or may not be associated with tissue eosinophilia as confirmed by biopsy (3). The term hypereosinophilic syndrome (HES) is used to describe conditions in which the HE is associated with end-organ damage, regardless of the etiology. There are several categories of HES (Table 1) that are separated according to etiology. The purpose of this review is to update non-allergists on newer developments in the field of HES and its treatment, including several new and emerging therapies targeting eosinophils.
Table 1. Subtypes of HES and appropriate therapy considerations.
Regardless of etiology, the presence of end-organ damage in HES requires prompt and aggressive initial workup and treatment, typically with glucocorticoids, to reduce eosinophil numbers and prevent further morbidity or mortality. Once work up is completed, steroid-sparing therapy can be further tailored according to HES subtype.
| Category | Variant | Clinical and laboratory characteristics | Pathophysiology | First line therapies | Second line therapies |
|---|---|---|---|---|---|
| Primary | Chronic eosinophilic leukemia, not otherwise specified | Circulating myeloblasts and/or clonal cytogenetic abnormalities, cytopenias, constitutional symptoms, hepatomegaly, splenomegaly, may accelerate or transform into acute myeloid leukemia | Various mutations, including those involving KIT, JAK2 V617F, ETV6-PDGFRB, or ETV6-ABL1 | Imatinib for ETV6-PDGFRB or ETV6-ABL1 mutations; | Chemotherapy; hematopoietic stem cell transplant |
| Myeloid variant (M-HES) | Circulating leukocyte precursors and/or clonal cytogenetic abnormalities, cytopenias, hepatomegaly, splenomegaly, elevated serum B12 and tryptase levels (typically in FIP1L1-PDGFRA fusion only) | FIP1L1-PDGFRA fusion (most frequent genetic abnormality observed in M-HES) | Imatinib, often as little as 100 mg daily or less | Other tyrosine kinase inhibitors | |
| PDGFRA or PDGFRB rearrangement (nearly exclusive to males) | Imatinib | Other tyrosine kinase inhibitors; hydroxyurea | |||
| FGFR1 rearrangement | Ponatinib; Midostaurin | Other tyrosine kinase inhibitors | |||
| JAK2 point mutation or fusion | Ruxolitinib | Other kinase inhibitors | |||
| Familial HES | HE is present at birth. Patients are often asymptomatic, but may rarely progress to end-organ damage | Unknown. Mutations have been mapped to 5q31-33 with autosomal dominant transmission. | Observation | GCs if necessary | |
| Secondary | T cell lymphocytic variant (L-HES) | Typically with pronounced dermatologic findings and abnormal T cell immunophenotyping, may progress to T cell lymphoma | Clonal lymphoproliferative disorder, clones produce excess IL-5 | GCs | IFN-α; JAK inhibitors; immunosuppressive agents; anti-eosinophil biologics |
| Organ-restricted HES (EGID, EGPA, etc) | Symptoms of organ-specific disorders (e.g. gastrointestinal, sinopulmonary involvement) | Eosinophilic expansion in a single organ | Aimed at specific disorders (e.g. topical swallowed GCs for EGID); Cyclophosphamide | Methotrexate; Azathioprine; Rituximab; Mepolizumab (300 mg monthly dosing for EGPA) | |
| Parasitic infection | Exposure history may be suggestive. May have gastrointestinal symptoms, but may also be asymptomatic | Polyclonal eosinophilic expansion in response to parasites | Treat underlying infection | ||
| Idiopathic | n/a | Varied signs and symptoms, may affect any organ system | Unknown | GCs | Hydroxyurea; IFN-α; imatinib; anti-eosinophil biologics, immunosuppressive agents |
Abbreviations: HES: hypereosinophilic syndromes; PDGFRB: platelet-derived growth factor receptor beta; FGFR1: fibroblast growth factor receptor 1; GC: glucocorticoid; IFN-α: interferon alpha; JAK2: Janus kinase 2; FIP1L1: Fip1-like1; PDGFRA: platelet-derived growth factor receptor alpha; L-HES: lymphocytic variant HES; IL-5: interleukin-5
Primary HES
Nearly all of the primary forms of HES are caused by neoplastic disorders that directly cause eosinophil lineage expansion, such as clonal myeloid or eosinophil disorders.
Secondary HES
Secondary HES is often termed “reactive” HES, in that the expansion of eosinophils is driven by cytokines (such as IL-5) produced by other cell types. Eosinophilopoietic cytokines may be produced as a result of adverse drug reactions, parasitic infections, connective tissue diseases, lymphomas, and certain solid tumors. The lymphocytic variant (L-HES) is a subtype of secondary HES in which the IL-5-producing cells are an aberrant clonal population of lymphocytes. It is an important subtype to distinguish from others because the treatment options differ from other forms of HES (see below). Some cases appear to be caused by STAT3 gain of function mutations in T cells (4).
Familial HES
Familial eosinophilia is a rare autosomal dominant disorder defined as the presence of HE from birth without any apparent etiology. Oddly, these patients rarely have symptoms, and even more rarely progress to HES, likely due to a lack of eosinophil activation (5). A recent study has identified IL-5 and IL-5Rα dysregulation together as causes of familial eosinophilia in two family cohorts, which may re-categorize familial eosinophilia as a subtype of secondary cause if this dysregulation is confirmed in other families as well (6). Though most cases of familial eosinophilia had previously been mapped to chromosome 5q31-q33, the causative mutation for the IL-5 dysregulation is still unknown (7). A completely different family cohort with uniquely severe HES and immune dysfunction was found to have gain-of-function mutations in JAK1, which had previously been found only as a somatic mutation in various malignancies (8).
Idiopathic HES
In some cases, a thorough workup may not reveal a cause of eosinophilia, and a patient is diagnosed with idiopathic HES. It should be noted that several studies have shown that a significant proportion of patients whom are thought to have idiopathic HES will have either mutations in genes involved in cellular function and signaling (especially ASXL1, TET2, EZH2, SETBP1, CBL, and NOTCH1) and/or abnormal genetic or morphological findings on bone marrow biopsy, and the presence of either of these findings reduces overall 5-year survival (9–11). As many of these genes are known to be mutated in the context of several solid and/or myeloid cancers, these findings suggest that some cases of ‘idiopathic’ HES are in fact due to a limited number of driver mutations. A small portion of these patients eventually develop clonal disorders; however, it is unclear if these patients are simply at higher risk for later developing a clonal disorder, or if they in fact already have an undetected clonal lymphocyte population at the time of ‘idiopathic’ HES diagnosis which was then allowed to expand after administration of eosinophil-depleting therapies (12–14). In the end, as many as 80 percent of HE cases may not have an identifiable cause, in which case treatment is targeted towards preventing end-organ damage (15).
WORKUP
It is not sufficient to attribute persistent HE simply to atopy, but disorders such as drug reactions with eosinophilia and systemic symptoms (DRESS), eosinophilic gastrointestinal disease (EGID), allergic bronchopulmonary aspergillosis, eosinophilic granulomatosis with polyangiitis (EGPA) and a few others can present with HE and may be part of the differential diagnosis based on history and presenting signs and symptoms (16, 17). To determine the etiology of HE and potentially arrive at a diagnosis of HES, standard workup for all patients should include complete blood count with differential, immunophenotyping by flow cytometry to detect abnormal T lymphocyte clones (e.g., CD3-CD4+), serum vitamin B12 and tryptase levels (often elevated in myeloid forms of HES), and examination of a peripheral blood smear for atypical cells. All patients should undergo serologic testing for strongyloides, even those without a suspicious clinical or travel history for it, as strongyloides infection can be acquired in parts of North America and must be treated before administration of glucocorticoids (GCs) to avoid a potentially life-threatening disseminated infection. Based on exposure history, testing for other parasitic infections may be sent (stool ova and parasites and serologies for filaria, schistosomes, trypanosomes, and trichinella). To detect possible end-organ involvement, serum kidney and liver function tests and chest radiography should be performed. In patients with suspected cardiac involvement, serum troponin, magnetic resonance imaging, and endomyocardial biopsy may be superior to echocardiography for detecting myocardial damage (18). Targeted sequencing for mutations (BCR-ABL1, PDGFRB, KIT, JAK2) may be considered to establish clonality; at minimum, testing of blood for the FIP1L1-PDGFRA fusion should be performed given this subtype’s excellent response to imatinib therapy (see section on Tyrosine Kinase Inhibitors below) (10). Bone marrow biopsy should also be considered for persistent and/or severe cases of HES to rule out a clonal disorder that is not otherwise detected by sequencing of blood cells (9). HE patients with suggestive family histories should have their family members screened as well to rule out familial causes.
Large retrospective studies have shown that about 5% of all patients with HE (regardless of etiology) will eventually develop a hematologic malignancy (13, 14). The onset of malignancy can be months to years after the diagnosis (median time is 30 months); therefore; all patients with HE should be monitored for potential symptoms and laboratory evidence of malignancy with regular clinical exams, complete blood count with differential, and other tests based on evolution of signs or symptoms.
MANAGEMENT
Peripheral eosinophilia is not in itself harmful unless it causes leukostasis, which only ever results from extremely high counts. Instead, tissue eosinophilia is potentially damaging and/or life threatening. Almost any organ can be affected, but the most common targets for eosinophils include the skin, lung, heart, and gastrointestinal tract (15). When possible, therapy is directed at the underlying etiology, but even in the absence of a known cause, HES must be treated promptly and aggressively to reduce potential morbidity and mortality that can result from organ damage. In the absence of overt HES, severe HE may be treated as well on a case-by-case basis.
Corticosteroids
Eosinophils readily undergo apoptosis in response to corticosteroids; thus, GCs are the mainstay for slowing and preventing end-organ damage caused by HES until workup is completed and alterative agents can be initiated (19) (Figure 1). GCs may be utilized as a first line stabilizing therapy for all types of HES, though recent research has shown that the clinical response to GCs is largely dependent on HES subtype, with the myeloid and lymphocytic variants being the least responsive (20).
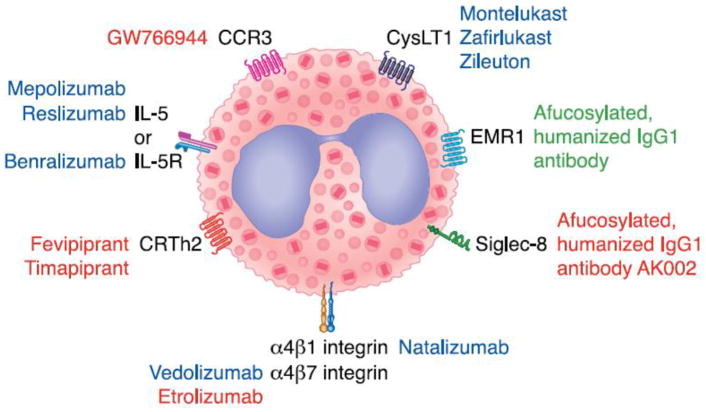
Figure 1. Eosinophil-selective therapeutic targets.
Several receptors unique to eosinophils or with limited expression on other cell types are attractive targets for depleting eosinophils. Examples of therapies are shown with their corresponding receptor targets. Therapies in blue font are FDA approved, those in red font are in clinical trials, and those in green are in preclinical stage. Note that dexpramipexole is not shown because its target of action remains unknown. Modified with permission from Wechsler ME, Fulkerson PC, Bochner BS, Gauvreau GM, Gleich GJ, Henkel T, et al. Novel targeted therapies for eosinophilic disorders. J. Allergy Clin. Immunol. 2012;130(3):563–71
Abbreviations: CRTh2, also known as the prostaglandin D2 receptor 2; CysLT1, cysteinyl leukotriene receptor 1; IL-5, interleukin 5; IL-5R, interleukin 5 receptor; EMR1, EGF-like module containing mucin-like hormone receptor; Siglec-8, Sialic acid-binding immunoglobulin-like lectin 8
Biologics
Several FDA-approved biologics have shown potential benefit in reducing or depleting circulating eosinophils, either by targeting eosinophilopoietic cytokines, actively depleting eosinophils via antibody-dependent cellular cytotoxicity or more indirectly by reducing Th2-mediated inflammation. These include monoclonal antibodies against IL-5 (mepolizumab and reslizumab) or its receptor (benralizumab), IgE (omalizumab), and the IL-4α receptor subunit (dupilumab) (Figure 1). Other emerging biologics of interest are also discussed.
Mepolizumab
Mepolizumab is a humanized anti-IL-5 antibody that is FDA approved for severe eosinophilic asthma (at a dose of 100 mg sq every 4 weeks) and EGPA (at a dose of 300 mg sq every 4 weeks). Clinical trials for severe asthma and EoE demonstrated its ability to reduce AECs and tissue eosinophils in these patients. In a randomized, placebo-controlled trial in adults with FIP1L1-PDGFRA fusion gene negative HES, 95% of patients receiving mepolizumab (750 mg IV monthly) achieved AEC counts of 600/μL or less for at least eight consecutive weeks, compared to 45% of those receiving placebo (21). Additionally, mepolizumab demonstrated a significant steroid-sparing effect in the treatment group, and an open label extension showed that long-term treatment of HES was effective and well tolerated with few serious side effects (mean treatment time: 251 weeks) (22). Another trial explored efficacy in 13 adults with L-HES; those receiving mepolizumab were significantly more likely to reduce their oral GC dose to below 10 mg daily over a span of 24 weeks compared to the placebo group (23). However, two of the five subjects in the treatment group did not achieve AECs below 600/μL, suggesting that mepolizumab is more effective in GC-sensitive subtypes of HES. Encouragingly, there have also been reports showing successful use of mepolizumab in two pediatric cases of HES, one with FIP1L1/PDGFRA fusion-positive HES refractory to imatinib, and the other with L-HES refractory to prednisolone, azathioprine, and methotrexate (24), though no placebo-controlled trials have been done in children. Unfortunately, the effective doses of mepolizumab in HES trials (750 mg IV every 4 weeks) are substantially higher than the FDA approved doses for asthma or EGPA (100–300 mg sq every 4 weeks), though two case reports have shown efficacy of the 100 mg monthly dose for idiopathic HES (25). A larger placebo-controlled trial of mepolizumab 300 mg monthly is currently actively recruiting subjects (NCT02836496) and should help to further delineate the minimum effective dose and safety in HES.
Reslizumab
Another anti-IL-5 monoclonal antibody, reslizumab (3 mg/kg IV every 4 weeks), has shown similar clinical efficacy as mepolizumab in subjects with severe eosinophilic asthma and EoE (26). An open label extension of these trails demonstrated sustained suppression of AECs throughout reslizumab therapy, and that AECs began to recover within four months of subjects’ last dose (27). Unfortunately, trials in HES patients have been limited. A small pilot study in patients with HES either refractory to or intolerant of GCs showed mixed results, with only two of four patients treated with reslizumab achieving rapid reduction in AEC and significant improvement in associated symptoms (28). Notably, these subjects were not screened for the FIP1L1/PDGFRA fusion gene, because at the time it was not yet known to be associated with HES. A recent trial in asthma using a subcutaneous formulation of a lower dose failed to reach target endpoints (29), so the future of a subcutaneous formulation may be in jeopardy.
Benralizumab
Benralizumab is a humanized anti-IL-5Rα monoclonal antibody which was FDA approved in November 2017 for the treatment of severe asthma in patients age 12 and up. Its advantage over the anti-IL-5 antibodies is that it is afucosylated; therefore, upon binding to IL-5R, the eosinophil (and to a lesser degree the basophil, which also expresses IL-5R) becomes a target for destruction by natural killer cells via antibody-dependent cellular cytotoxicity and virtually eliminates eosinophils (30). It also has a long functional half-life, so after a few initial monthly doses it is given every other month. While its benefits in asthma outcomes have been remarkable, its eosinophil-depleting effects in clinical trials have also raised hopes for effectiveness in HES. In the Phase 3 SIROCCO trial, subjects with severe eosinophilic asthma in the benralizumab groups (30 mg sq every four weeks and 30 mg sq every 4 weeks for three doses, then every 8 weeks) had drastically reduced AECs from a median of 450/μL (range 300–720) and 440/μL (range 280–291) at baseline to 0/μL by week 4 of treatment, whereas AECs in the placebo group did not change (31). The CALIMA trial showed similar results over 56 weeks of therapy (32). The latest phase 3 trial (the ZONDA trial) demonstrated comparable results in patients with severe steroid-dependent asthma, with subjects in the benralizumab treatment groups showing dramatically reduced AECs from a median of 462/μL (range 0–560) and 467/μL (range 0–440) at baseline to 0/μL within 12 weeks of initiating treatment, though time points prior to 12 weeks were not studied (33). Subjects in the benralizumab arms also achieved a median reduction in their oral GC doses by 75% compared to baseline, compared to a 25% reduction in the placebo group (33). All of the Phase 3 trials further stratified subjects’ clinical response according to baseline AEC; as might be expected given its mechanism of action, benralizumab showed the greatest benefit in patients with higher AECs (over 300 cells/μL) compared to those with lower AECs. Thus far, there has only been one trial using benralizumab in 20 adults with HES and HE (NCT02130882). Though full results are only published in abstract form, a subgroup analysis showed that all 7 of the subjects with HES with gastrointestinal involvement achieved a reduction in AEC to 0 cells/μL in addition to complete or near complete elimination of eosinophils on endoscopic biopsies after 24 weeks of benralizumab therapy (34).
Omalizumab
Initially FDA approved for asthma and subsequently for chronic spontaneous urticaria, omalizumab is an anti-IgE monoclonal antibody that has shown promise in some eosinophilic disorders. Pooled data from five of the omalizumab trials demonstrated significant reductions in AEC for patients on omalizumab compared to placebo, albeit not as impressive or as consistent as see with anti-IL-5 or anti-IL-5R antibodies (35). Moreover, several studies have determined that omalizumab is most efficacious in patients with baseline AECs of 300/μL or higher (36, 37). Though two open label trials in EoE and EGID patients showed reduction in symptoms and AEC counts with omalizumab treatment (38, 39), these results were not replicated in a placebo-controlled trial in EoE (40). It is likely that omalizumab affects AECs indirectly in the context of allergic diseases such as asthma, but overall these trials suggest that it would be an inferior choice for treating primary eosinophilic disorders.
Dupilumab
Dupilumab binds to the IL-4α receptor subunit, thus blocking both IL-4 and IL-13 receptor signaling. FDA approved for the treatment of atopic dermatitis, there are limited published data on dupilumab’s effects in targeting eosinophils. It has shown clinical efficacy in treating eosinophilic disorders such as asthma, perennial allergic rhinitis, and chronic rhinosinusitis with nasal polyposis, though interestingly, clinical response was not correlated with baseline AEC (41, 42). A recent placebo-controlled trial with dupilumab in 47 adults with EoE has completed enrollment (NCT02379052), though preliminary results are only available in abstract form (43). In some of these studies, initiation of dupilumab treatment is associated with a modest transient increase, rather than a decrease, in AECs, that has been attributed to inhibition of eosinophil extravasation.
Alemtuzumab
Alemtuzumab is monoclonal antibody developed for chronic lymphocytic leukemia that targets CD52, which is found on the surface of eosinophils, lymphocytes, monocytes, macrophages, and dendritic cells. Two separate case reports demonstrated successful treatment of HES with alemtuzumab (44, 45), prompting a small clinical trial wherein 10 of 11 patients with advanced HES or eosinophilic leukemia achieved remission with alemtuzumab therapy (46). However, 7 of these 10 relapsed after cessation of treatment, and long term use of alemtuzumab has a very poor side effect profile including frequent infusion reactions and induction of new autoimmune disorders.
Tezepelumab
Tezepelumab is a novel biologic that targets thymic stromal lymphopoietin (TSLP), a cytokine involved in type 2 inflammation. In a recent Phase 2 trial in moderate to severe asthma, the low and medium dose tezepelumab treatment groups showed a mean decrease in AEC of about 150/μL compared to baseline (mean 361/μL), whereas high dose tezepelumab showed an average drop in AEC of about 200/μL, again representing only a modest effect compared to the IL-5 and IL-5R biologics mentioned above (47). Interestingly, clinical response in terms of asthma exacerbation rates and lung function were similar in all patients within each treatment group, despite blood eosinophil count at baseline.
Tyrosine kinase inhibitors
The use and efficacy of tyrosine kinase inhibitors for the treatment of hypereosinophilic disorders began empirically based on shared features between HES and myeloid leukemias, and early use showed rather spectacular results in small numbers of patients (48, 49). Since the paradigm-changing report by Cools et al. of the FIP1L1-PDGFRA fusion gene as a cause of one HES subtype (50), much has been learned about other rearrangements associated with myeloid forms of HES (e.g., ETV6-PDGFRB on chromosome 5, ETV6-ABL1 on chromosome 9, ETV6-FLT3 on chromosome 13, ZMTM2-FGFR1 on chromosome 8, and PCM1-JAK2 on chromosome 9) as well as the use of various tyrosine kinase inhibitors in certain forms of HES (51).
Imatinib and related drugs
Imatinib is currently the only FDA approved medication for the treatment of HES. It has become the drug of choice for myeloid forms of HES driven by PDGFRA, estimated to account for about 10% of all forms of HES (52, 53). The exquisite sensitivity of this deletion fusion to imatinib commonly allows much lower doses to induce remission, sometimes as low as 100 mg daily or even less (54). Studies have documented complete and durable genetic remission during treatment (55, 56), although a risk of relapse after discontinuation of imatinib remains real (57–59). While nearly universally effective in FIP1L1-PDGFRA disease, imatinib is also often effective in HES presenting with myeloid features but without any identifiable kinase mutations (60, 61). Despite its effectiveness, imatinib sometimes fails to work in PDGFRA-driven disease, and this resistance is typically due to a second mutation such as T674I, which may still respond to other tyrosine kinase inhibitors such as nilotinib or sorafinib (62).
When neoplastic eosinophilia results from other kinase abnormalities besides those involving PDGFRA, imatinib may still remain effective, for example in disease involving ETV6-PDGFRB or ETV6-ABL1, but is not likely to be effective in others. Instead, drugs such as ponatinib, midostaurin, sorafinib, sunitinib, nilotinib or dasatinib may be tried (51). Finally, ‘chronic eosinophilic leukemia-not otherwise specified’ is a form of hypereosinophilia associated with myeloblasts or clonal cytogenetic abnormalities involving genes other than tyrosine kinases. When explored using next-generation sequencing techniques, many such patients are found to have unique patterns of single or multiple mutations (10). Because these forms of myeloproliferative HES do not involve PDGFRA, PDGFRB or related tyrosine kinases, imatinib is not effective (63).
Ruxolitinib and tofacitinib
Enhanced activity of the JAK and STAT signaling cascade contributes to many forms of cancer. In recent years, some FDA approved JAK inhibitors have been tried in HES, especially cases associated with gain-of-function mutations involving JAKs or STATs (64). Ruxolitinib, a JAK1 and JAK2 inhibitor, has been used to successfully treat a form of familial HES with immune dysregulation caused by a gain of function JAK1 mutation (8). Another case report describes a patient who presented with hypereosinophilia due to a BCR-JAK2 fusion gene positive myeloid neoplasm; remission was achieved with ruxolitinib, although the patient relapsed within 2 years of starting treatment (65). This is in contrast to a report involving a more durable remission in two subjects receiving this same drug for treatment of PCM1-JAK2 fusion gene-driven disease (66, 67). Additionally, five patients with idiopathic HES or L-HES (the latter with documented STAT mutation) with skin involvement were treated in an open-label study with either tofacitinib (a JAK1 and JAK3 inhibitor) 5 mg twice daily or ruxolitinib 25 mg in the morning and 10 mg at night, with resulting steroid-sparing activity and normalization or near normalization of their skin findings and blood eosinophilia (68). These promising outcomes require further validation in larger numbers of patients.
Chemotherapy and stem cell transplant
Chemotherapy and/or autologous stem cell transplant are generally reserved for malignant neoplasms such as eosinophilic leukemia or T cell lymphomas. They may also be used as a last resort for other types of primary HES that are refractory to treatment with tyrosine kinase inhibitors or other therapies (69, 70).
Emerging therapies with anti-eosinophil properties
Several therapies have shown encouraging eosinophil-depleting results in small pilot or preclinical studies. Larger, placebo controlled trials are needed to determine their clinical efficacy for HES.
Dexpramipexole
Dexpramipexole is the enantiomer of pramipexole, a dopamine agonist used orally for the treatment of Parkinson’s disease. It was initially studied as a possible treatment for amyotrophic lateral sclerosis (ALS), and during its Phase 2 clinical trial researchers noted that patients receiving dexpramipexole unexpectedly showed markedly reduced AECs compared to placebo (71, 72). Unfortunately, dexpramipexole was not effective for ALS, but there is interest in using it as a possible therapy for eosinophil-related diseases. Data from multiple ALS trials as well as an open label trial in patients with chronic rhinosinusitis with polyposis showed marked reductions in circulating eosinophils and basophils, but only slightly reduced circulating neutrophils, monocytes, and lymphocytes (72, 73). Effects were seen in as little as one month of treatment, though it took three to four months to gain maximum eosinophil-lowering impact. After cessation of treatment, AECs recovered gradually at roughly the same rate as the onset of action. It should be noted that the patients in these trials typically had AECs within the normal range, and additional studies are needed to determine dexpramipexole’s efficacy in the context of much higher AECs. Indeed, a clinical trial is currently in progress investigating its effects on both peripheral and tissue eosinophilia in patients with HES (Clinical Trial NCT02101138).
Anti-Siglec-8
Sialic acid-binding immunoglobulin-like lectins (or Siglecs) are surface receptors found on leukocytes. Siglec-8 is expressed on the surface of eosinophils, mast cells, and basophils, making it an attractive target for treating eosinophil-related disorders, including those in which mast cells may also play a pathogenic role (Figure 1). Studies have shown that engagement of Siglec-8 with antibody or glycan ligand induces apoptosis in IL-5-primed eosinophils in vitro (74), though depending on the priming status of the cell, Siglec-8 can also act as an activating receptor (75). One anti-Siglec-8 targeting antibody, an afucosylated humanized IgG1 monoclonal called AK002, is in development. In a mouse model of EGID employing a novel strain of mice engineered to express human Siglec-8, intraperitoneal dosing of an anti-Siglec-8 mAb significantly reduced eosinophilic and mast cell infiltration into the gut as well as peripheral eosinophil counts (76). A Phase 1 trial in 51 healthy subjects showed complete depletion of circulating eosinophils within one hour after a single dose of AK002. At doses approaching 1 mg/kg, this effect was sustained for a maximum of 84 days with low rates of adverse effects (77). Additional Phase 1 trials exploring the safety and efficacy of AK002 in chronic urticaria, indolent systemic mastocytosis and atopic keratoconjunctivitis are underway (NCT03436797, NCT02808793 and NCT03379311, respectively).
Anti-EMR1
EGF-like module containing mucin-like hormone receptor (EMR1) is a receptor that is highly specific to eosinophils (78). Preclinical data has been encouraging in that a study using an anti-EMR1 monoclonal antibody in non-human primates showed a rapid (within eight hours) and sustained depletion of peripheral blood eosinophils after a single intravenous dose (79).
Therapies unlikely to be effective in HES
Though several of the therapies below had previously seemed promising in preclinical or early clinical stages, recent clinical trials have demonstrated lack of efficacy for reducing peripheral eosinophils, and they are unlikely to be utilized to treat HES in the future.
Fevipiprant and timapiprant
Fevipiprant and timapiprant are orally-dosed small molecule antagonist of the prostaglandin D2 receptor (also known as CRTh2). Fevipiprant showed promising results in a Phase 2 clinical trial in patients with moderate to severe asthma. After 12 weeks of treatment with twice daily oral dosing, fevipiprant significantly reduced mean sputum eosinophil percentage (from 5.4 to 1.1%) as well as eosinophil numbers on bronchial biopsy (80). The treatment was well tolerated and showed a good safety profile. Unfortunately, in this small study it did not have any significant effect on blood AECs. Timapiprant has shown similar results, and is currently in Phase 3 studies in eosinophilic asthma (81, 82).
Lebrikizumab, tralokinumab, and dectrekumab
Despite the mechanistic rationale behind targeting IL-13 in allergic disorders, anti-IL-13 biologics have not shown efficacy in reducing peripheral eosinophilia, nor have they achieved consistent clinical improvement in asthma trials. Interestingly, asthma trials have actually shown that lebrikizumab causes mild transient increases in peripheral eosinophil counts compared to placebo (55/μL higher after a treatment period of 12 weeks) (83, 84). This effect, like that seen with dupilumab, is thought to be due to inhibition of eosinophil emigration into tissues. This, combined with the lack of clinical benefit in asthma, suggests that lebrikizumab would have limited efficacy in HES. Tralokinumab failed to reach primary endpoints in two separate Phase 3 trials (STRATOS and TROPOS), and dectrekumab was abandoned in Phase 2 after it failed to show benefit in atopic dermatitis.
Anti-GM-CSF and anti-CCR3
A Phase 2 trial with KB003, an anti-GM-CSF monoclonal antibody, failed to show clinical benefit or reduction in AECs in subjects with asthma (85). GW766994 is an orally-dosed small molecule antagonist of CCR3, a chemokine receptor for eotaxins on eosinophils. Unfortunately, a clinical trial showed only minimal clinical benefit and no reduction in AECs, sputum eosinophils or eosinophil progenitors in patients with asthma and eosinophilic bronchitis (86).
SUMMARY AND CONCLUSIONS
Several FDA approved biologics and tyrosine kinase inhibitors, as well as a few emerging therapies, show great potential in being efficacious in HES. A thorough workup for HES will aid the practitioner in choosing an appropriate management strategy for each patient. Future studies will help to guide the choice of optimal therapy according to HES subtype.
Abbreviations
- AEC
absolute eosinophil count
- ASXL1
putative polycomb group protein ASXL1
- CBL
Cbl ubiquitin-protein ligase
- CD
cluster of differentiation
- EGID
eosinophilic gastrointestinal disease
- EGPA
eosinophilic granulomatosis with polyangiitis
- EMR1
EGF-like module containing mucin-like hormone receptor
- EoE
EGF-like module containing mucin-like hormone receptor
- EZH2
enhancer of zeste homolog 2
- GC
glucocorticoid
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HE
hypereosinophilia
- HES
hypereosinophilic syndrome
- IL
interleukin
- JAK
Janus kinase
- NOTCH1
notch homolog 1, translocation-associated
- STAT
signal transducer and activator of transcription
- SETBP1
SET binding protein 1
- sq
subcutaneous
- TET2
tet methylcytosine dioxygenase 2
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of Interest
Dr. Bochner has current or recent consulting or scientific advisory board arrangements with or has received honoraria from Sanofi-Aventis, TEVA, GlaxoSmithKline, AstraZeneca, and Allakos, and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate™ and is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University on the potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
Melanie C. Dispenza declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bochner B. The eosinophil: for better or worse, in sickness and in health. Ann Allergy Asthma Immunol. 2018 doi: 10.1016/j.anai.2018.02.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diny NL, Rose NR, Cihakova D. Eosinophils in Autoimmune Diseases. Front Immunol. 2017;8:484. doi: 10.3389/fimmu.2017.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5(2):157–76. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker S, Wang C, Walradt T, Hong BS, Tanner JR, Levinsohn JL, et al. Identification of a gain-of-function STAT3 mutation (p.Y640F) in lymphocytic variant hypereosinophilic syndrome. Blood. 2016;127(7):948–51. doi: 10.1182/blood-2015-06-654277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin AY, Nutman TB, Kaslow D, Mulvihill JJ, Fontaine L, White BJ, et al. Familial eosinophilia: clinical and laboratory results on a U.S. kindred. Am J Med Genet. 1998;76(3):229–37. [PubMed] [Google Scholar]
- 6*.Prakash Babu S, Chen YK, Bonne-Annee S, Yang J, Maric I, Myers TG, et al. Dysregulation of interleukin 5 expression in familial eosinophilia. Allergy. 2017;72(9):1338–45. doi: 10.1111/all.13146. This study showed that IL-5 and IL-5R dysregulation are involved in the pathogenesis of familial eosinophilia in one family cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rioux JD, Stone VA, Daly MJ, Cargill M, Green T, Nguyen H, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63(4):1086–94. doi: 10.1086/302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol. 2017;139(6):2016–20. doi: 10.1016/j.jaci.2016.12.957. [DOI] [PubMed] [Google Scholar]
- 9.Wang SA, Hasserjian RP, Tam W, Tsai AG, Geyer JT, George TI, et al. Bone marrow morphology is a strong discriminator between chronic eosinophilic leukemia, not otherwise specified and reactive idiopathic hypereosinophilic syndrome. Haematologica. 2017;102(8):1352–60. doi: 10.3324/haematol.2017.165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Wang SA, Tam W, Tsai AG, Arber DA, Hasserjian RP, Geyer JT, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854–64. doi: 10.1038/modpathol.2016.75. This study showed that a significant proportion of patients diagnosed with “idiopathic” HES in fact have mutations in various housekeeping and signaling genes that are known to be associated with cancers. [DOI] [PubMed] [Google Scholar]
- 11.Pardanani A, Lasho T, Wassie E, Finke C, Zblewski D, Hanson CA, et al. Predictors of survival in WHO-defined hypereosinophilic syndrome and idiopathic hypereosinophilia and the role of next-generation sequencing. Leukemia. 2016;30(9):1924–6. doi: 10.1038/leu.2016.73. [DOI] [PubMed] [Google Scholar]
- 12.Harfi I, Schandene L, Dremier S, Roufosse F. Eosinophils affect functions of in vitro-activated human CD3-CD4+ T cells. J Transl Med. 2013;11:112. doi: 10.1186/1479-5876-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin JJ, Butterfield JH, Weiler CR. Hematologic Malignancies Identified in Patients with Hypereosinophilia and Hypereosinophilic Syndromes. J Allergy Clin Immunol Pract. 2015;3(6):920–5. doi: 10.1016/j.jaip.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Andersen CL, Siersma VD, Hasselbalch HC, Lindegaard H, Vestergaard H, Felding P, et al. Eosinophilia in routine blood samples and the subsequent risk of hematological malignancies and death. Am J Hematol. 2013;88(10):843–7. doi: 10.1002/ajh.23515. [DOI] [PubMed] [Google Scholar]
- 15.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–25. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92–7. doi: 10.1182/asheducation-2015.1.92. [DOI] [PubMed] [Google Scholar]
- 17.Katre RS, Sunnapwar A, Restrepo CS, Katabathina VS, Mumbower A, Baxi A, et al. Cardiopulmonary and gastrointestinal manifestations of eosinophil-associated diseases and idiopathic hypereosinophilic syndromes: Multimodality imaging approach. Radiographics. 2016;36(2):433–51. doi: 10.1148/rg.2016150145. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield JH, Kane GC, Weiler CR. Hypereosinophilic syndrome: endomyocardial biopsy versus echocardiography to diagnose cardiac involvement. Postgrad Med. 2017;129(5):517–23. doi: 10.1080/00325481.2017.1317215. [DOI] [PubMed] [Google Scholar]
- 19.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126(9):1069–77. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Khoury P, Abiodun AO, Holland-Thomas N, Fay MP, Klion AD. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol Pract. 2018;6(1):190–5. doi: 10.1016/j.jaip.2017.06.006. This study demonstrated that the myeloid and lymphocytic variants of HES are the least-responsive to treatment with glucocorticoids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 22.Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131(2):461–7. doi: 10.1016/j.jaci.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roufosse F, de Lavareille A, Schandene L, Cogan E, Georgelas A, Wagner L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(4):828–35. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz C, Muller T, Lau S, Parasher K, Staab D, Wahn U. Mepolizumab-a novel option for the treatment of hypereosinophilic syndrome in childhood. Pediatr Allergy Immunol. 2018;29(1):28–33. doi: 10.1111/pai.12809. [DOI] [PubMed] [Google Scholar]
- 25.Guo C, BSB Mepolizumab as a successful steroid-sparing agent in two patients with idiopathic hypereosinophilic syndrome (iHES) J Allergy Clin Immunol. 2018;141(2):AB27. [Google Scholar]
- 26.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63. 63. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and ffficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. 2017;5(6):1572–81. doi: 10.1016/j.jaip.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103(8):2939–41. doi: 10.1182/blood-2003-10-3620. [DOI] [PubMed] [Google Scholar]
- 29.Teva Announces Top-Line Results from Phase III Studies of Subcutaneously Administered Reslizumab in Patients with Severe Eosinophilic Asthma. http://www.tevapharm.com/news/teva_announces_top_line_results_from_phase_iii_studies_of_subcutaneously_administered_reslizumab_in_patients_with_severe_eosinophilic_asthma_01_1.8.aspx.
- 30.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 32.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 33*.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. doi: 10.1056/NEJMoa1703501. This is the most recently published Phase 3 trial for benralizumab, confirming findings from the previous trials demonstrating benralizumab’s excellent eosinophil-depleting effects in severe eosinophilic asthma. [DOI] [PubMed] [Google Scholar]
- 34.Kuang FL, Alao H, Kumar S, Powers A, Quezado M, Wang Z, et al. Benralizumab (anti-IL5Rα) depletes gut tissue eosinophilia and improves symptoms in hypereosinophilic syndrome with gastrointestinal involvement. J Allergy Clin Immunol. 2018;141(2):AB196. [Google Scholar]
- 35.Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010;104(2):188–96. doi: 10.1016/j.rmed.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6. doi: 10.1016/j.jaci.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 38.Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120(3):594–601. doi: 10.1016/j.jaci.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10(3):e0113483. doi: 10.1371/journal.pone.0113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein SF, Katial R, Jayawardena S, Pirozzi G, Staudinger H, Eckert L, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2017.11.051. in press. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 43.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Dupilumab efficacy and safety in adult patients with active eosinophilic esophagitis: a randomized double-blind placebo-controlled phase 2 trial. United Eur Gastro J. 2017;5(Suppl 1) [Google Scholar]
- 44.Pitini V, Teti D, Arrigo C, Righi M. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome with abnormal T cells: a case report. Br J Haematol. 2004;127(5):477. doi: 10.1111/j.1365-2141.2004.05206.x. [DOI] [PubMed] [Google Scholar]
- 45.Wagner LA, Speckart S, Cutter B, Gleich GJ. Treatment of FIP1L1/PDGFRA-negative hypereosinophilic syndrome with alemtuzumab, an anti-CD52 antibody. J Allergy Clin Immunol. 2009;123(6):1407–8. doi: 10.1016/j.jaci.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 46.Verstovsek S, Tefferi A, Kantarjian H, Manshouri T, Luthra R, Pardanani A, et al. Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clin Cancer Res. 2009;15(1):368–73. doi: 10.1158/1078-0432.CCR-08-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 48.Gleich GJ, Leiferman KM, Pardanani A, Tefferi A, Butterfield JH. Treatment of hypereosinophilic syndrome with imatinib mesylate. Lancet. 2002;359(9317):1577–8. doi: 10.1016/S0140-6736(02)08505-7. [DOI] [PubMed] [Google Scholar]
- 49.Ault P, Cortes J, Koller C, Kaled ES, Kantarjian H. Response of idiopathic hypereosinophilic syndrome to treatment with imatinib mesylate. Leuk Res. 2002;26(9):881–4. doi: 10.1016/s0145-2126(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 50.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 51*.Gotlib J. Tyrosine Kinase Inhibitors in the Treatment of Eosinophilic Neoplasms and Systemic Mastocytosis. Hematol Oncol Clin North Am. 2017;31(4):643–61. doi: 10.1016/j.hoc.2017.04.009. An excellent and thorough review of the field. [DOI] [PubMed] [Google Scholar]
- 52.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–25. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helbig G. Imatinib for the treatment of hypereosinophilic syndromes. Expert Rev Clin Immunol. 2018;14(2):163–70. doi: 10.1080/1744666X.2018.1425142. [DOI] [PubMed] [Google Scholar]
- 54.Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007;109(11):4635–40. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 55.Klion AD, Robyn J, Akin C, Noel P, Brown M, Law M, et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103(2):473–8. doi: 10.1182/blood-2003-08-2798. [DOI] [PubMed] [Google Scholar]
- 56.Helbig G, Moskwa A, Hus M, Piszcz J, Swiderska A, Urbanowicz A, et al. Durable remission after treatment with very low doses of imatinib for FIP1L1-PDGFRalpha-positive chronic eosinophilic leukaemia. Cancer Chemother Pharmacol. 2011;67(4):967–9. doi: 10.1007/s00280-011-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klion AD, Robyn J, Maric I, Fu W, Schmid L, Lemery S, et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia: implications for optimal dosing. Blood. 2007;110(10):3552–6. doi: 10.1182/blood-2007-07-100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helbig G, Kyrcz-Krzemien S. Cessation of imatinib mesylate may lead to sustained hematologic and molecular remission in FIP1L1-PDGFRA-mutated hypereosinophilic syndrome. Am J Hematol. 2014;89(1):115. doi: 10.1002/ajh.23588. [DOI] [PubMed] [Google Scholar]
- 59.Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, Cayuela JM, et al. The spectrum of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia: New insights based on a survey of 44 cases. Medicine (Baltimore) 2013;92(5):e1–e9. doi: 10.1097/MD.0b013e3182a71eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helbig G. Imatinib mesylate for unmutated hypereosinophilic syndromes: Does it work? Eur J Intern Med. 2016;32:19–20. doi: 10.1016/j.ejim.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Khoury P, Desmond R, Pabon A, Holland-Thomas N, Ware JM, Arthur DC, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71(6):803–10. doi: 10.1111/all.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lierman E, Cools J. Recent breakthroughs in the understanding and management of chronic eosinophilic leukemia. Expert Rev Anticancer Ther. 2009;9(9):1295–304. doi: 10.1586/era.09.82. [DOI] [PubMed] [Google Scholar]
- 63.Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15. doi: 10.1038/s41408-018-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018 doi: 10.1038/nrclinonc.2018.8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwaab J, Knut M, Haferlach C, Metzgeroth G, Horny HP, Chase A, et al. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol. 2015;94(2):233–8. doi: 10.1007/s00277-014-2221-y. [DOI] [PubMed] [Google Scholar]
- 66.Rumi E, Milosevic JD, Casetti I, Dambruoso I, Pietra D, Boveri E, et al. Efficacy of ruxolitinib in chronic eosinophilic leukemia associated with a PCM1-JAK2 fusion gene. J Clin Oncol. 2013;31(17):269–71. doi: 10.1200/JCO.2012.46.4370. [DOI] [PubMed] [Google Scholar]
- 67.Rumi E, Milosevic JD, Selleslag D, Casetti I, Lierman E, Pietra D, et al. Efficacy of ruxolitinib in myeloid neoplasms with PCM1-JAK2 fusion gene. Ann Hematol. 2015;94(11):1927–8. doi: 10.1007/s00277-015-2451-7. [DOI] [PubMed] [Google Scholar]
- 68*.King B, Lee AI, Choi J. Treatment of Hypereosinophilic Syndrome with Cutaneous Involvement with the JAK Inhibitors Tofacitinib and Ruxolitinib. J Invest Dermatol. 2017;137(4):951–4. doi: 10.1016/j.jid.2016.10.044. This study demonstrated that JAK inhibitors can be effective for the treatment of certain forms of HES. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roufosse F. Management of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2015;35(3):561–75. doi: 10.1016/j.iac.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 70.Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(11):1243–59. doi: 10.1002/ajh.24880. [DOI] [PubMed] [Google Scholar]
- 71.Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med. 2011;17(12):1652–6. doi: 10.1038/nm.2579. [DOI] [PubMed] [Google Scholar]
- 72*.Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME. The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017;63:62–5. doi: 10.1016/j.bcmd.2017.01.008. This study makes the point that an oral agent, dexpramipexole, has a unique and remarkable ability to reduce eosinophil numbers. [DOI] [PubMed] [Google Scholar]
- 73.Prussin C, Laidlaw TM, Panettieri RA, Ferguson BJ, Adappa ND, Lane AP, et al. Dexpramipexole effectively lowers blood and tissue eosinophils in subjects with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2017 Feb;139(Suppl):AB64. [Google Scholar]
- 74.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 75.Carroll DJ, O’Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. Siglec-8 is an activating receptor mediating beta2 integrin-dependent function in human eosinophils. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2017.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Youngblood BA, Brock EC, Leung J, Bebbington C, Tomasevic N. Treatment with an anti-Siglec-8 antibody reduces eosinophilic gastrointestinal inflammation in mice. Gordon Research Conference on Food Allergy; 2018; Ventura, CA. [Google Scholar]
- 77.Rasmussen HS, Chang AT, Tomasevic N, Bebbington C. Phase 1 double-blind, placebo-controlled, ascending dose study of Siglec-8 selective mAb AK002 in healthy subjects. J Allergy Clin Immunol. 2018;141(2):AB403. [Google Scholar]
- 78.Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37(10):2797–802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 79.Legrand F, Tomasevic N, Simakova O, Lee CC, Wang Z, Raffeld M, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol. 2014;133(5):1439–47. doi: 10.1016/j.jaci.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MFM, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 81.Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–85. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 82.Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69(9):1223–32. doi: 10.1111/all.12451. [DOI] [PubMed] [Google Scholar]
- 83.Korenblat P, Kerwin E, Leshchenko I, Yen K, Holweg CTJ, Anzures-Cabrera J, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med. 2018;134:143–9. doi: 10.1016/j.rmed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 85.Molfino NA, Kuna P, Leff JA, Oh CK, Singh D, Chernow M, et al. Phase 2, randomised placebo-controlled trial to evaluate the efficacy and safety of an anti-GM-CSF antibody (KB003) in patients with inadequately controlled asthma. BMJ Open. 2016;6(1):007709. doi: 10.1136/bmjopen-2015-007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, Sousa AR, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014;44(4):508–16. doi: 10.1111/cea.12244. [DOI] [PubMed] [Google Scholar]