Abstract
Recent national cancer plans address high cancer mortality in Latin America, particularly in Andean countries. Little is known about which individual, interpersonal, and institutional facilitators and barriers persist, particularly from the perspective of cancer survivors. We conducted 15 semi-structured interviews with survivors of breast and cervical cancers during and after a Pan American Health Organization-sponsored conference on women’s cancers in Lima, Peru. We analyzed data using an inductive content analysis approach. Patients reported primarily psychosocial barriers and facilitators at individual, interpersonal, and institutional levels. Additionally, survivors provided recommendations to refine existing policy to improve the cancer care experience for patients.
Keywords: Breast cancer, cervical cancer, Andean, psychosocial, barriers
Breast and cervical cancers affect growing numbers of women worldwide. Despite progress in screening and treatment knowledge and technology, incidence and mortality from both cancers continue to increase (Forouzanfar, Foreman, Delossantos, Lozano et al., 2011). With this study, we sought to better understand individual, interpersonal and institutional barriers and facilitators to screening and treatment in Andean countries from the perspective of breast and cervical cancer survivors. Our goal was to provide some individual level insights that may be useful for women’s cancer prevention and treatment research, policy and intervention efforts worldwide, particularly in low and middle income countries. Additionally, we sought to demonstrate for a global audience, the importance of engaging those directly affected by policies and programs in the assessment and improvement of these efforts.
High rates of breast and cervical cancer mortality afflict Latin America as a whole (DeSantis et al., 2015; Justo, Wilking, Jonsson, Luciani, & Cazap, 2013; Shulman, Willett, Sievers, & Knaul, 2010) and Peru in particular (PAHO, 2013; Zelle et al., 2013). The breast cancer mortality-to-incidence ratio is nearly twice as high in South America as North America (DeSantis et al., 2015). Breast and cervical cancers comprised 9% and 11%, respectively, of Peru’s total 2010 cancer mortality among women (PAHO, 2013). Higher mortality partially results from screening underutilization and advanced stage at diagnosis for this population (Huaman, Kamimura–Nishimura, Kanamori, Siu, & Lescano, 2011; Soneji & Fukui, 2013; Zelle et al., 2013). For example, officials from Peru’s primary cancer hospital, the National Institute for Neoplastic Diseases (INEN), reported that between 2007 and 2011, over 50% of breast cancer patients were diagnosed in stages III or IV (“late”) and only 7% in stage I (Zelle et al., 2013). In contrast, only about 10% of Northern Europe’s breast cancer cases are diagnosed late (Justo et al., 2013). These trends are likely due to particularly low screening rates for cervical (Soneji & Fukui, 2013) and breast cancer (Huaman et al., 2011; Zelle et al., 2013) in Peru. Indeed, the majority of researchers working in Peru have highlighted institutional factors as having significant impact on patients’ cancer care, including geographic disparities in care, cost, and bureaucratic delays (Huaman et al., 2011; Paz–Soldán, Bayer, Nussbaum, & Cabrera, 2012; Soneji & Fukui, 2013; Zelle et al., 2013). In response, staff in Ministries of Health in Peru, Bolivia, Colombia, and Ecuador recently developed national cancer policies, strategies, or action plans (“Combatiendo el cáncer a través del Plan Esperanza,” n.d., “WHO | Cancer country profiles 2014,” n.d.).
Policy solutions for alleviating institutional barriers are crucial. Simultaneously, multi-level approaches that address factors at individual, interpersonal, and institutional levels, are increasingly important for cancer prevention and control (Elkan et al., 2007; Yano et al., 2012). At the individual level, international researchers have, over decades, identified numerous barriers, including (but not limited to) lack of information or misinformation (Elkan et al., 2007; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting, 2008; Zapka, Taplin, Ganz, Grunfeld, & Sterba, 2012), cultural beliefs (Dein, 2004; Elkan et al., 2007; Errico & Rowden, 2006; Lannin et al., 1998; Simon, 2006; Zapka et al., 2012), cancer worry/fear(Hay, McCaul, & Magnan, 2006), fatalism(Espinosa de Los Monteros & Gallo, 2011), stigma (Butts Stahly, 1989; Elkan et al., 2007; Holland, Kelly, & Weinberger, 2010; Koller et al., 1996), psychological sequelae (Holland, 2002; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting, 2008) (e.g., co-morbid depression (Fann et al., 2008; Mitchell et al., 2011; Reich, Lesur, & Perdrizet–Chevallier, 2008), symptom-induced self-confidence loss (Dua, Heiland, Kracen, & Deshields, 2015)), and medical mistrust (Bickell, Weidmann, Fei, Lin, & Leventhal, 2009). At the interpersonal level, individuals may experience distress about burdening family, and/or stigma due to their condition (Errico & Rowden, 2006; Mathieson & Stam, 1995; Shepherd & Gerend, 2014; Meacham, Orem, Nakigudde, Zujewski, & Rao, 2016) or due to other marginalized social identities (Elkan et al., 2007; Jacobs et al., 2014). Further, socioeconomic and access barriers are not the only institutional factors affecting breast and cervical cancer mortality (Elkan et al., 2007; Lannin et al., 1998; Soneji & Fukui, 2013). High-quality, coordinated services enable patients to optimize benefits of greater access to care (Paz–Soldán et al., 2012; Yano et al., 2012; Zapka et al., 2012), and conversely, lack of such services adversely affects survivorship. Notably, multi-level approaches are helpful for understanding protective factors and facilitators to care. In a substantial body of literature, researchers describe facilitators at individual levels (e.g., hopefulness (Coughlin, 2008), active coping (Coughlin, 2008), self-efficacy (Coughlin, 2008)), interpersonal levels (e.g., social support from family and peer cancer patients (Bloom & Kessler, 1994; Coughlin, 2008; Dunkel–Schetter, 1984; Nausheen, Gidron, Peveler, & Moss–Morris, 2009)), and institutional levels (e.g., financial support (Justo et al., 2013)).
Despite the emergence of multi-level approaches, relatively few researchers have evaluated which barriers and at what level those countries developing national cancer plans could best address patients’ well-being. Such research must be focused on treatment, in addition to screening/early detection, in populations from low- and middle-income countries (LMICs). Understanding the extent to which, and at what level, barriers persist in the new era of Andean country cancer policies is important for future research, practice, and policy. As well, it is critical to obtain patient perspectives on and recommendations for solutions to address multi-level factors and optimize care. Accordingly, we conducted a pilot study with a purposive sample of survivors at a regional informational conference on women’s cancers. We aimed to examine breast and cervical cancer survivors’ narratives to elucidate multi-level barriers faced during cancer care in Andean countries. Survivors are ideally positioned to provide information on the multi-level factors influencing the cancer care experience because they have: engaged in self-advocacy within their own care (Ashing et al., 2014); served as navigators and examples of success to newly diagnosed and unscreened populations (Coughlin, 2008); and may have experience serving as advocates within research, practice, and policy settings (Ashing et al., 2014; Errico & Rowden, 2006; Molina et al., 2016). With our findings, we invoke women’s experiences on the Andean cancer care continuum, in order to inform policy recommendations for clinics, hospitals, Plan Esperanza, and similar cancer plans.
Methods
Participants and Procedures
We conducted in-depth interviews with 15 survivors at the March 2015 First International Cancer Symposium survivorship conference in Lima, Peru. Using a purposive sampling strategy, we asked female cancer survivors attending the conference – a likely place to find resilient survivors – to participate in our study. Eligibility criteria were: 1) having had a previous breast or cervical cancer diagnosis, and 2) having initiated or completed treatment for breast or cervical cancer. Survivors were from Peru, Colombia, Ecuador, and Bolivia.
Ethical approval was obtained from the University of Washington and the Universidad Peruana Cayetano Heredia institutional review boards. After informed consent was obtained from all participants, four experienced Latina researchers conducted interviews with survivors in private rooms. Each one-hour interview was audio-recorded and transcribed verbatim in Spanish by a professional transcriptionist. Participants were asked open-ended questions following a semi-structured interview guide and received approximately 8 Peruvian Soles ($3) compensation for their time.
Qualitative Data Analysis
Two bilingual research assistants independently open-read and coded all transcripts to identify themes and subthemes that emerged, using inductive content analysis and the principle of constant comparison from grounded theory (Bernard & Ryan, 2010). The research assistants developed a codebook and code definitions, compared the two sets of coded interviews, and then came to a consensus on individual codes for quotations. Data were further organized using ATLAS.ti 7.5.12 software outputs and matrices. Identified quotations were translated into English by both research assistants and compared for accuracy with bilingual study team members.
Results
Table 1 depicts participants’ demographic and clinical characteristics. Themes emerged in this study in alignment with the social ecological framework, on the individual, interpersonal, and institutional levels (Figure 1). Below, we describe different barriers and facilitators that emerged within these levels. Finally, we report survivors’ recommendations for how to improve existing cancer policy.
Table 1.
Socio-Demographic Information
| Peruvian | Non-Peruvian | |
|---|---|---|
| Total Sample | 12 | 3 |
| Mean Age (Range) | 56 (39–67) | 49 (38–60) |
| Education Completed | Primary or Less: 2 High School: 4 Superior/Technical: 7 University or More: 2 |
Primary or Less: 0 High school: 1 University: 1 Graduate: 1 |
| N nulliparous | 6 | 1 |
| Stage at diagnosis | I: 3 II: 4 III: 5 |
I: 2 II: 0 III: 1 |
| Mean years since cancer diagnosis (Range) | 4.0 (0.8–9.3) | 8.1 (2.4–13.8) |
Figure 1.
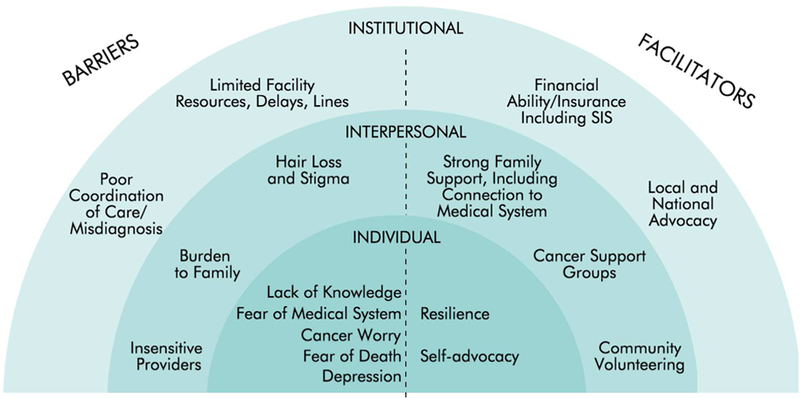
Barriers and Facilitators to Survivorship.
Barriers
Individual.
Lack of knowledge exacerbated all barriers these survivors faced, from symptom identification, screening, diagnosis, and treatment, to recovery and involvement in advocacy. Although a few breast cancer survivors had obtained yearly mammograms, attended screening campaigns, or done self-exams, other participants had postponed screening due to unfamiliarity with cancer’s symptoms, urgency, and relevance. Participants emphasized the importance of risk awareness and screening information:
“Here we need information. I was scared to death because I did not have information. If I had had the information, I would have gone immediately to the doctor.”
(age 51)
About half of participants discussed some fear of the medical system. For example, one participant feared being a medical “guinea pig” or being killed by the treatment. Another explained her fear of contagious fatalism in the cancer hospital, which she considered a product of ignorance. Participants described other women’s fears of painful mammography or “uninhibited” male doctors, which two women identified as a cultural problem.
“I think that many people, out of fear, don’t go to the hospitals. I think that the doctors should go to the communities, especially to the low-income communities. Sometimes, due to our ignorance, we don’t know what prevention is. In my case, no one ever talked to me about it.”
(age 63)
Cancer worry emerged as another major barrier. Many women postpone visiting oncologists, even for free screenings, because they fear a life-changing diagnosis. One participant affirmed this fear of both doctors and the diagnosis as the reasons she and her friends all delay screening. After diagnosis, the majority felt death was imminent. Multiple women worried about leaving children or parents without caregivers. Not surprisingly, many participants cited depression in themselves or others. Several declared that depression, (which can cause patients to skip appointments) not cancer, is what kills cancer patients.
“I am a person who is always informed. I always read a lot and I thought I knew everything about cancer. And then when I got it, the first thing I thought was ‘well, I’m going to die.’”
(age 39)
Interpersonal.
Hair loss emerged as a significant trauma associated with interpersonal interactions and as an indicator of women’s stigmatized identities as cancer patients:
Survivor: It’s a stereotype among women, that a woman without hair is not sexy, beautiful… it [hair] is a feminine feature.
Interviewer: And your breast?
Survivor: No, that is not visible, but hair is, then everyone sees you. (age 38)
Almost all participants worried about burdening their families throughout screening, diagnosis, and treatment, which occasionally affected disclosure. A few participants waited to tell family until they received a definitive diagnosis, to avoid triggering stress. Some participants witnessed the toll their diagnosis took on their family members, some of whom developed physical, emotional, and psychological problems after finding out. Thus during treatment, many participants hid their discomfort so that family members would not suffer.
“I think the ones who suffer most are the family, more than the individual, and you suffer because you see them suffering. … Because you get used to the pain and all that, you get used to them poking you here and doing something to you there. But the problem is that your mother and siblings are sad and suffer; that’s the problem.”
(age 39)
Although most participants eventually found supportive health providers, about half referenced some insensitive treatment by providers. Multiple participants perceived doctors delivering their diagnosis to be brusque or inappropriate, and lacking encouragement. This discouraged some women, promoting fear and mistrust. Other participants described nurses making offensive statements to them or other cancer patients, such as this 46-year-old woman who fought back verbally when nurses yelled at elders or Quechua-speakers:
“People from the hospital should not treat others that way, especially not cancer patients. Because we do not have a cold or cough; we have cancer, and the behavior should be a little nicer.”
Institutional.
Participants experienced barriers related to limited facility resources, including paperwork, bureaucracy, and insurance access issues, such as being unaware of SIS (Seguro Integral de Salud - Peruvian public insurance) eligibility and enrollment processes. Multiple women referenced long waits while attending public hospitals:
“Sometimes there are problems of limited time; [INEN] is a very small hospital for the number of patients who go because people come from all over Peru. Maybe you just have to have some patience, because they make you wait. Many times I have gone at 6am and I have left the hospital at 6, 7 at night.”
(age 46)
Waits also involved longer-term bureaucratic delays: one woman caught her early-stage cancer in a yearly mammogram, but her diagnostic resolution was delayed 8 months by insurance and biopsy referral issues.
Participants further reported poor coordination of care, including medical misdiagnosis. Despite their fortunate status as survivors, six participants received one or more misdiagnoses. Misread tests and unclear or missing instructions caused stress, out-of-pocket costs, complications, and delays, usually for months, and in one case for more than a year, which sometimes led to increased cancer staging:
“I felt a very small lump. I remember I went to get it checked out. They did an ultrasound but said that it was nothing serious, that it did not require a biopsy or anything. So then a year went by.”
(age 39)
After multiple tests and months of delays, two misdiagnosed women who had the financial ability to do so, traveled long distances for competent cancer care.
Facilitators
Individual.
Participants told their cancer stories with remarkable resilience. While all portrayed cancer as difficult, many survivors experienced post-traumatic growth from cancer, reportedly becoming closer to God or family, or learning valuable lessons, including this 60-year-old woman:
“Now, I think [cancer] should not be synonymous with death. Cancer should be synonymous with life, because it gives you an opportunity to figure out what you have in life that’s worth fighting for.”
Many participants used self-advocacy, determination, and protests to obtain necessary resources for screening and treatment. This included asking questions at appointments, following up after misdiagnosis, rejecting misinformation and stigma, and making decisions independently, such as this 46-year-old participant whose doctor suggested she consult with family before a mastectomy:
“I told him, ‘Here, there is no need to consult my family. The disease is mine, the body is mine, I decide.’”
Four participants actively addressed institutional delays with verbal confrontations, including two threats to call the press, and thus successfully reduced long waits at public hospitals.
Interpersonal.
Strong family support helped women survive in myriad ways. Most participants initially accessed treatment because they knew someone familiar with the medical system. Seven participants received family assistance in making these connections, including siblings who knew an oncologist, or relatives who were medical professionals. For example:
“I remember that an aunt told me, ‘Don’t wait a month; I have a friend, an oncologist who works at INEN, and they can check you over. You have nothing to lose.’”
(age 39)
Almost all participants said family provided emotional, informational, or economic support throughout their disease. Despite worrying, participants quickly confided in a sister, daughter, or other family member about their symptoms and received prompt recommendations to see a doctor or visit INEN. Family and friends often accompanied participants to appointments. Some relatives lent money or provided advice about SIS. Family also provided essential encouragement, which often motivated decision-making:
“I truly learned how to confront [the fear]… my son told me, ‘Mom, don’t be afraid. If the doctor says they should take out a breast, let them take it out, even if it’s both of them.’ So, I faced it with courage.”
(age 67)
Many participants shared how cancer support groups, often sponsored by hospitals and doctors, provided significant psychosocial and informational support:
“[The support group] also helps me a lot. It’s like, I don’t need a psychiatrist or psychologist or anything. It has helped me a lot because it makes me feel good, it makes me feel relaxed, happy. Like I say, I think in those moments I am happier than I was before, when supposedly I was well.”
(age 39)
The majority of participants engaged in community volunteering with support groups during and after treatment. Most participants and their peers offered one-on-one encouragement and information in their community or while waiting at hospitals:
“Because we have time to speak with other patients, I always tell them to not be daunted, to not be afraid, to have courage. Sometimes they are recently diagnosed, they cry. ‘Me too’, I say, ‘I went through the same, I also lost weight, I also got depressed, but look how I am now, I am well, I am upright. You will be like this too, you have to have patience, lots of faith in God, and faith in the doctors.’”
(age 46)
Institutional.
Financial ability and access to insurance facilitated these participants’ survival. Those with private insurance usually received faster diagnosis and treatment and experienced fewer institutional barriers than participants with public or no insurance. Low-income Peruvian participants afforded care through Plan Esperanza.
“In my case, I did not encounter barriers because of the insurance policy I have. I did not have to struggle against the health system. They gave me everything easily… Surgery could have very well been delayed—if it had been through the regular system—between 2 or 3 months, without reconstruction. So, I think that among [my country’s] population, women have a very hard time, they have to struggle a lot.”
(age 38)
In addition to interpersonal advocacy for others, some survivors participated in local and national advocacy. They joined awareness campaigns to share their stories, including speaking on TV or radio:
“I think that, based on my experience, we survivors have a very important role, because fear decreases upon seeing that one can survive.”
(age 56)
Recommendations
To improve cancer care, participants recommended providing destigmatizing (“cancer is survivable if detected early”) and instrumental (“don’t stop treatment”) information to all. Principally, preventive care and screening knowledge must be promoted. Participants recommended widespread education and suggested focusing on empowering women and helping them understand how to care for their bodies. Publicizing cancer’s symptoms and treatment through radio and school forums was proposed, with additional screening events. To address lack of knowledge, improve morale, and reduce stigma, participants recommended that newly diagnosed patients interact more with survivors:
“I tell you truly that the best person who can help a person with cancer is someone who went through it.”
(age 60)
Some participants expressed that after diagnosis cancer patients need more psychological support at the institutional level, including sensitivity training for doctors and more visibility of complementary services such as nutrition counseling and support groups. Several survivors recommended greater availability of high-quality cancer detection and treatment nationwide and within the national hospitals:
“…There should be, I reiterate, more places, more hospitals, and improvement in the scarcity of chemotherapy rooms. At INEN for example, there are 120 patients daily, but more patients [come]. Sometimes there are no spots and then they don’t give it [treatment] to them, they prolong their chemotherapies. And the more days go by, the cancer keeps advancing.”
(age 46)
Conclusion
In this exploratory study, we assessed how systemic solutions to cancer control may be improved through obtaining patient perspectives on multi-level issues faced during cancer care. Understanding what individual, interpersonal, and institutional resources these participants used to overcome barriers can further enhance cancer policies within hospitals, nationwide, and globally.
Notably, most barriers and facilitators we identified have been well-studied in other contexts. Consistent with recent qualitative studies with breast cancer survivors in LMIC and low-income settings (Errico & Rowden, 2006; Meacham et al., 2016), fear of the medical system, fatalism, cancer worry, and lack of knowledge about cancer and its treatment are prominent individual barriers while common interpersonal facilitators included importance of family support and interactions with survivors. Aligning with other researchers (Coughlin, 2008), we suggest that engagement in cancer support groups provides patients with crucial social support and psychological health benefits, including resilience. Local and national advocacy likely facilitates social support via fostering connections among survivors (Molina et al., 2016). Our documentation of these barriers and facilitators provides necessary data for multi-level policy change in this specific context.
With this study we begin to fill a gap in research by identifying what barriers persist in the era of Andean country national cancer plans such as Peru’s Plan Esperanza (“Combatiendo el cáncer a través del Plan Esperanza,” n.d.), through the use of important, underused stakeholders – the survivors themselves. Survivors can play pivotal roles in developing and executing these curricula via advocacy and policy involvement (Ashing et al., 2014). By engaging survivors and incorporating their experiences, future researchers who develop psychosocial and multi-level interventions can address stigma barriers among cancer patients and within the community, while simultaneously confronting lack of awareness about prevention, insurance, and timely cancer screening.
Implications
Based on these women’s accounts, as well as reports from other Peruvian key informants (Paz–Soldán et al., 2012), persistent institutional barriers (e.g., miscoordination of care) could be reduced through policy change and enforcement (Zapka et al., 2012). Whenever possible, institutional leadership should incorporate family education and involvement (Zapka et al., 2012) and essential support group activities in patients’ recovery, and provide avenues for patient concerns to be addressed promptly (Errico & Rowden, 2006). Provider sensitivity and communication trainings, as recommended by our participants, can reduce distress, misinformation, and stigma among patients (Holland et al., 2010; Zapka et al., 2012). These survivors are adamant that future cancer patients not suffer as they have, and therefore nationwide education efforts must promote cultures of prevention, as supported by cancer literature in LMICs (Shulman et al., 2010) and elsewhere (Zapka et al., 2012).
This study has two principal limitations. First, our findings may not be generalizable. Although citizens of other Andean countries were included in the study, most of our participants were Peruvian, likely due to the conference’s location in Lima. Second, our study focused on patient perspectives, but additional perspectives are also warranted to develop policies responsive to all stakeholders’ needs. Assessing other key informant perspectives, such as family members and hospital staff, would enhance knowledge about cancer survivorship in Andean countries.
Despite limitations, these survivors contribute richness of experience across socioeconomic strata, geographic regions, and levels of prior knowledge about cancer and the medical system. Our results may not be generalizable to all survivors in Andean countries but nevertheless our study provides insight into some of the struggle the patients undergo to reach survivorship, mainly via utilization of individual, interpersonal, and institutional resources to overcome barriers and cultivate resilience. Researchers conducting follow-up studies should explore barriers and facilitators to engagement and retention in care among less-privileged Andean survivors of women’s cancers, including indigenous and rural survivors.
Acknowledgements:
We acknowledge JoAnne Zujewski for assistance organizing the conference and study logistics. We thank our participants for sharing their deeply personal stories. This project was supported by US NIH grant number R25TW009710 supplement; the National Cancer Institute’s Center for Global Health and under grant numbers R25CA92408, U54CA202997, and U54CA203000; and Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, R24 HD042828, to the University of Washington Center for Studies in Demography & Ecology. YM was also supported by the University of Illinois at Chicago Cancer Center and the Center for Research on Women and Gender.
References
- Ashing KT, Miller AM, Mitchell E, Martin V, McDowell K, Santifer RH, … Carrington A. (2014). Nurturing advocacy inclusion to bring health equity in breast cancer among African–American women. Breast Cancer Management, 3(6), 487–495. https://doi.org/10.2217/bmt.14.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard HR, & Ryan GW (2010). Analyzing qualitative data: systematic approaches. Los Angeles [Calif: ]: SAGE. [Google Scholar]
- Bickell NA, Weidmann J, Fei K, Lin JJ, & Leventhal H (2009). Underuse of Breast Cancer Adjuvant Treatment: Patient Knowledge, Beliefs, and Medical Mistrust. Journal of Clinical Oncology, 27(31), 5160–5167. https://doi.org/10.1200/JCO.2009.22.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JR, & Kessler L (1994). Emotional support following cancer: a test of the stigma and social activity hypotheses. Journal of Health and Social Behavior, 35(2), 118–133. [PubMed] [Google Scholar]
- Butts Stahly G (1989). Psychosocial Aspects of the Stigma of Cancer: An Overview. Journal of Psychosocial Oncology, 6(3–4), 3–27. https://doi.org/10.1300/J077v06n03_02 [Google Scholar]
- Combatiendo el cáncer a través del Plan Esperanza. (n.d.). Retrieved June 6, 2016, from https://peruprogresoparatodos.com/notas/combatiendo-el-cancer-a-traves-del-plan-esperanza/
- Coughlin SS (2008). Surviving Cancer or Other Serious Illness: A Review of Individual and Community Resources. CA: A Cancer Journal for Clinicians, 58(1), 60–64. https://doi.org/10.3322/CA.2007.0001 [DOI] [PubMed] [Google Scholar]
- Dein S (2004). Explanatory models of and attitudes towards cancer in different cultures. The Lancet Oncology, 5(2), 119–124. https://doi.org/10.1016/S1470-2045(04)01386-5 [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, & Jemal A (2015). International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiology Biomarkers & Prevention, 24(10), 1495–1506. https://doi.org/10.1158/1055-9965.EPI-15-0535 [DOI] [PubMed] [Google Scholar]
- Dua P, Heiland MF, Kracen AC, & Deshields TL (2015). Cancer-related hair loss: a selective review of the alopecia research literature. Psycho-Oncology. https://doi.org/10.1002/pon.4039 [DOI] [PubMed] [Google Scholar]
- Dunkel-Schetter C (1984). Social Support and Cancer: Findings Based on Patient Interviews and Their Implications. Journal of Social Issues, 40(4), 77–98. https://doi.org/10.1111/j.1540-4560.1984.tb01108.x [Google Scholar]
- Elkan R, Avis M, Cox K, Wilson E, Patel S, Miller S, Kai J (2007). The reported views and experiences of cancer service users from minority ethnic groups: a critical review of the literature. European Journal of Cancer Care, 16(2), 109–121. https://doi.org/10.1111/j.1365-2354.2006.00726.x [DOI] [PubMed] [Google Scholar]
- Errico KM, & Rowden D (2006). Experiences of Breast Cancer Survivor-Advocates and Advocates in Countries with Limited Resources: A Shared Journey in Breast Cancer Advocacy. The Breast Journal, 12, S111–S116. https://doi.org/10.1111/j.1075-122X.2006.00208.x [DOI] [PubMed] [Google Scholar]
- Espinosa de Los Monteros K., & Gallo LC (2011). The relevance of fatalism in the study of Latinas’ cancer screening behavior: a systematic review of the literature. International Journal of Behavioral Medicine, 18(4), 310–318. https://doi.org/10.1007/s12529-010-9119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, & Gralow J (2008). Major depression after breast cancer: a review of epidemiology and treatment. General Hospital Psychiatry, 30(2), 112–126. https://doi.org/10.1016/j.genhosppsych.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011; 378: 1461–84. [DOI] [PubMed] [Google Scholar]
- Hay JL, McCaul KD, & Magnan RE (2006). Does worry about breast cancer predict screening behaviors? A meta-analysis of the prospective evidence. Preventive Medicine, 42(6), 401–408. https://doi.org/10.1016/j.ypmed.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Holland JC (2002). History of psycho-oncology: overcoming attitudinal and conceptual barriers. Psychosomatic Medicine, 64(2), 206–221. [DOI] [PubMed] [Google Scholar]
- Holland JC, Kelly BJ, & Weinberger MI (2010). Why psychosocial care is difficult to integrate into routine cancer care: stigma is the elephant in the room. Journal of the National Comprehensive Cancer Network, 8(4), 362–366. [DOI] [PubMed] [Google Scholar]
- Huaman MA, Kamimura-Nishimura KI, Kanamori M, Siu A, & Lescano AG (2011). Validation of a susceptibility, benefits, and barrier scale for mammography screening among Peruvian women: a cross-sectional study. BMC Women’s Health, 11, 54 https://doi.org/10.1186/1472-6874-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. (2008). Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. (Adler NE & Page AE, Eds.). Washington (DC): National Academies Press (US) Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK4015/ [PubMed] [Google Scholar]
- Jacobs EA, Rathouz PJ, Karavolos K, Everson-Rose SA, Janssen I, Kravitz HM, … Powell LH (2014). Perceived Discrimination Is Associated with Reduced Breast and Cervical Cancer Screening: The Study of Women’s Health Across the Nation (SWAN). Journal of Women’s Health, 23(2), 138–145. https://doi.org/10.1089/jwh.2013.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justo N, Wilking N, Jonsson B, Luciani S, & Cazap E (2013). A Review of Breast Cancer Care and Outcomes in Latin America. The Oncologist, 18(3), 248–256. https://doi.org/10.1634/theoncologist.2012-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M, Kussman J, Lorenz W, Jenkins M, Voss M, Arens E, … Rothmund M. (1996). Symptom reporting in cancer patients: the role of negative affect and experienced social stigma. Cancer, 77(5), 983–995. [DOI] [PubMed] [Google Scholar]
- Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, & Edwards MS (1998). Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA, 279(22), 1801–1807. [DOI] [PubMed] [Google Scholar]
- Mathieson CM, & Stam HJ (1995). Renegotiating identity: cancer narratives. Sociology of Health and Illness, 17(3), 283–306. https://doi.org/10.1111/1467-9566.ep10933316 [Google Scholar]
- Meacham E, Orem J, Nakigudde G, Zujewski JA, & Rao D (2016). Exploring Stigma as a Barrier to Cancer Service Engagement with Breast Cancer Survivors in Kampala, Uganda: Exploring Stigma and Cancer Service Engagement in Kampala, Uganda. Psycho-Oncology. https://doi.org/10.1002/pon.4215 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, & Meader N (2011). Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. The Lancet Oncology, 12(2), 160–174. https://doi.org/10.1016/S1470-2045(11)70002-X [DOI] [PubMed] [Google Scholar]
- Molina Y, Scherman A, Constant TH, Hempstead B, Thompson-Dodd J, Richardson S, … Ceballos RM (2016). Medical advocacy among African-American women diagnosed with breast cancer: from recipient to resource. Supportive Care in Cancer. https://doi.org/10.1007/s00520-016-3123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausheen B, Gidron Y, Peveler R, & Moss-Morris R (2009). Social support and cancer progression: A systematic review. Journal of Psychosomatic Research, 67(5), 403–415. https://doi.org/10.1016/j.jpsychores.2008.12.012 [DOI] [PubMed] [Google Scholar]
- PAHO. (2013). Peru Cancer Profile: Pan American Health Organization. Retrieved from http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=23456&Itemid=
- Paz-Soldán VA, Bayer AM, Nussbaum L, & Cabrera L (2012). Structural barriers to screening for and treatment of cervical cancer in Peru. Reproductive Health Matters, 20(40), 49–58. https://doi.org/10.1016/S0968-8080(12)40680-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M, Lesur A, & Perdrizet-Chevallier C (2008). Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Research and Treatment, 110(1), 9–17. https://doi.org/10.1007/s10549-007-9706-5 [DOI] [PubMed] [Google Scholar]
- Shepherd MA, & Gerend MA (2014). The blame game: Cervical cancer, knowledge of its link to human papillomavirus and stigma. Psychology & Health, 29(1), 94–109. https://doi.org/10.1080/08870446.2013.834057 [DOI] [PubMed] [Google Scholar]
- Shulman LN, Willett W, Sievers A, & Knaul FM (2010). Breast Cancer in Developing Countries: Opportunities for Improved Survival. Journal of Oncology, 2010, 1–6. https://doi.org/10.1155/2010/595167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CE (2006). Breast cancer screening: cultural beliefs and diverse populations. Health & Social Work, 31(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Soneji S, & Fukui N (2013). Socioeconomic determinants of cervical cancer screening in Latin America. Revista Panamericana de Salud Publica = Pan American Journal of Public Health, 33(3), 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | Cancer country profiles 2014. (n.d.). Retrieved July 26, 2016, from http://www.who.int/cancer/country-profiles/en/
- Yano EM, Green LW, Glanz K, Ayanian JZ, Mittman BS, Chollette V, & Rubenstein LV (2012). Implementation and Spread of Interventions Into the Multilevel Context of Routine Practice and Policy: Implications for the Cancer Care Continuum. JNCI Monographs, 2012(44), 86–99. https://doi.org/10.1093/jncimonographs/lgs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapka J, Taplin SH, Ganz P, Grunfeld E, & Sterba K (2012). Multilevel Factors Affecting Quality: Examples From the Cancer Care Continuum. JNCI Monographs, 2012(44), 11–19. https://doi.org/10.1093/jncimonographs/lgs005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle SG, Vidaurre T, Abugattas JE, Manrique JE, Sarria G, Jeronimo J, … Baltussen R. (2013). Cost-Effectiveness Analysis of Breast Cancer Control Interventions in Peru. PLoS ONE, 8(12). https://doi.org/10.1371/journal.pone.0082575 [DOI] [PMC free article] [PubMed] [Google Scholar]


