Abstract
Human pesticide exposure can occur both occupationally and environmentally during manufacture and after the application of indoor and outdoor pesticides, as well as through consumption via residues in food and water. There is evidence from experimental studies that numerous pesticides, either in isolation or in combination, act as endocrine disruptors, neurodevelopmental toxicants, immunotoxicants, and carcinogens. We reviewed the international literature on this subject for the years between 1990 and 2017. The studies were considered in this review through MEDLINE and WHO resources. Out of the n = 1817 studies identified, n = 94 were reviewed because they fulfilled criteria of validity and addressed associations of interest. Epidemiological studies have provided limited evidence linking pre- and post-natal exposure to pesticides with cancers in childhood, neurological deficits, fetal death, intrauterine growth restriction, preterm birth, and congenital abnormalities (CAs). In this review, the potential association between pesticide exposure and the appearance of some human CAs (including among others musculoskeletal abnormalities; neural tube defects; urogenital and cardiovascular abnormalities) was investigated. A trend towards a positive association between environmental or occupational exposure to some pesticides and some CAs was detected, but this association remains to be substantiated. Main limitations of the review include inadequate exposure assessment and limited sample size. Adequately powered studies with precise exposure assessments such as biomonitoring, are warranted to clarify with certainty the potential association between pesticide exposure and human CAs.
Keywords: Congenital abnormalities, Ecotoxicology, Human, Musculoskeletal abnormalities, Neural tube defects, Pesticides, Urogenital abnormalities
1. Introduction
According to the Food and Agriculture Organization of the United Nations (FAO), a pesticide is a chemical, biological, or mixture of agents used for the prevention, control, or extermination of pests. Pests including human/animal disease vectors and unwanted species of animals/plants (weeds), cause harm during/interfere with the production, processing, storage, transport or food marketing, agricultural commodities, wood products or animal feedstufis, or agents administered to animals for controlling pests in or on their bodies (FAO, 2005). Pesticides are extensively tested chemicals. Yet their widespread use estimated to be 2 × 109 kg worldwide annually, continues to raise significant public concerns regarding safety (Grube et al., 2011; Kiely et al., 2004). Human exposure to pesticides can occur environmentally, through consumption via residues in food and water, as well as occupationally, during or after indoor/outdoor application (van den Berg et al., 2012).
Many pesticides act as endocrine disruptors (EDs), neurodevelopmental toxicants, immunotoxicants and carcinogens in animals and humans (Bahadar et al., 2015; Blair et al., 2015). The nervous system is particularly susceptible to many pesticides of several distinct chemical classes. A number of studies show that prenatal and early childhood exposure to organophosphates (OPs) is associated with neurodevelopmental effects (Munoz-Quezada et al., 2013). A meta-analysis concluded that low-dose exposures to OPs were linked to reduced psychomotor speed, executive function, visuospatial ability as well as work and visual memory (Ross et al., 2013). Other studies have also associated organochlorines (OCs), OPs and other pesticides with dementias such as Alzheimer’s disease, amyotrophic lateral sclerosis, but mainly with Parkinson’s disease (Blair et al., 2015; Mostafalou and Abdollahi, 2013).
Other epidemiological studies have linked pesticide exposure to higher risks for chronic health disorders, including infectious diseases. Pesticides dysregulate and disturb immune responses by causing alterations to the normal structure of the immune system. Contaminated breast milk due to maternal exposure revealed pronounced immunological deficiencies and increased risks of infections, mainly meningitis and inner ear infections (World Resources Institute: Pesticides and the Immune System. The Public Health Risks). Furthermore, epidemiological and experimental studies displayed evidence for carcinogenic effects of exposure to pesticides (Petrakis et al., 2017). Some experimental studies support that there is no evidence for pesticide mutagenicity. However, epigenetic mechanisms underlie its association with cancer. Epidemiological studies reported several sites of cancer which were linked to pesticide exposure, including the lungs, the prostate, and the lymphatic and hematopoietic systems (Bonner et al., 2017). Childhood cancer has also been associated with environmental and parental occupational pesticide exposure (World Resources Institute: Pesticides and the Immune System. The Public Health Risks).
Several classes of pesticides such as 1,2-dibromo-3-chloropropane, vinclozolin, and OPs interfere with normal male reproductive system function (Petrakis et al., 2017), leading to reduction and/or inhibition of spermatogenesis; sperm count, viability, density and motility impairment; abnormal sperm morphology; induction of deoxyribonucleic acid damage; seminiferous tubule degeneration; and reduction of epididymis, prostate or seminal vesicle weight. They may also alter the follicle-stimulating hormone (FSH), the luteinizing hormone (LH), and testosterone levels; lower activity/level of antioxidant enzymes in the testes; and inhibit testicular steroidogenesis. Furthermore, dichlorodiphenyl-trichloroethane (DDT) and its metabolites have estrogenic effects on males (Mehrpour et al., 2014). Pesticides and EDs have several biological adverse effects in females as well (Petrakis et al., 2017). Most of them are related to the development of the reproductive system and are specifically attributed to folliculogenesis (Sifakis et al., 2017). The primordial follicles change to primary, pre-antral and antral follicles. Bisphenol A, methotrexate, 2,3,7,8-Tetrachlorodibenzodioxin and phthalates are examples of EDs that can cause toxic effects on the development of follicles, leading to infertility. Bisphenol A has been highly associated with toxicity in the female reproductive system, polycystic ovary syndrome and endometriosis. Several studies correlated bisphenol A with the female reproductive system intoxication (Caserta et al., 2014; Kandaraki et al., 2011; Souter et al., 2013) as a high bisphenol A concentration in plasma or urine has been associated with lower amounts of antral follicle, decreased number of mature and fertilized oocytes, lower peak E2 in response to hyperstimulation with human chorionic gonadotrophin, and increased probability for implantation failure in women undergoing fertility treatments (Caserta et al., 2013; Ehrlich et al., 2012b; Ehrlich et al., 2012a). The toxic effects caused by pesticides and EDs on the human reproductive system have been associated with the dose, frequency and route of exposure as well as with the genotypic characteristics of the exposed individuals (Hernandez et al., 2013). Additionally, human exposure to pesticides has been associated with genetic/epigenetic modifications and chronic diseases (Mostafalou and Abdollahi, 2013), while epidemiological studies have revealed associations of pre- and post-natal exposure to pesticides with fetal death, neurological deficits, childhood cancers, intrauterine growth restriction, preterm birth and birth defects (Weselak et al., 2007).
Congenital abnormalities (CAs) are structural or functional abnormalities (e.g. metabolic disorders) that occur in utero and can be identified prenatally, at birth, or later in life. They consist of a diverse group of disorders attributed to single gene defects, chromosomal disorders, multifactorial inheritance, environmental teratogens and micronutrient malnutrition (WHO/CDC/ICBDSR, 2014). Although the majority of CAs cannot be linked to a specific cause, prenatal indoor exposure to pesticides (chlorpyrifos, OPs, vinclozolin etc.) and herbicides (triazines, metolachlor etc.) has been suggested to increase teratogenicity risk (Stillerman et al., 2008) due to the high susceptibility of most fetal systems during certain periods of development (Selevan et al., 2000).
2. Objective and methods of the review
This review aimed to elucidate the potential association between exposure to pesticides and development of the most prevalent, among others, human CAs according to the National Birth Defects Prevention Network (Parker et al., 2010); namely musculoskeletal abnormalities (MSAs), neural tube defects (NTDs), urogenital abnormalities (UGAs), cardiovascular abnormalities (CVAs), as well as some gastrointestinal, ocular, and facial CAs. Medline was systematically searched up to June 2017 to detect all publications focusing on the topic “Congenital Abnormalities OR Birth defects AND Pesticides AND Human”. Specific CA categories were also searched, applying the following additional literature search strategies:
1. MSAs: pesticide AND (gastroschisis OR hernia OR syndactyly OR craniosynostosis OR polydactyly OR omphalocele OR (limb AND defect); 2. NTDs: pesticide AND (anencephaly OR spina bifida OR “neural tube defects”); 3. UGAs: pesticide AND (hypospadias OR cryptorchidism OR micropenis) pesticide AND urogenital AND (defect OR anomaly); 4. CVAs: pesticide AND (Fallot OR (heart AND defect) OR (valve AND defect) OR (septal AND defect)); 5. Gastrointestinal abnormalities: pesticide AND ((stomach AND defect) OR (intestinal AND defect) OR (gastrointestinal AND (defect OR anomaly OR malformation))) pesticide AND ((esophageal AND atresia) OR (tracheoesophageal AND fistula) OR (rectal AND (atresia OR stenosis)) OR (intestinal AND atresia))); 6. Ocular abnormalities: pesticide AND (anophthalmia OR microphthalmia); 7. Facial abnormalities: pesticide AND cleft.
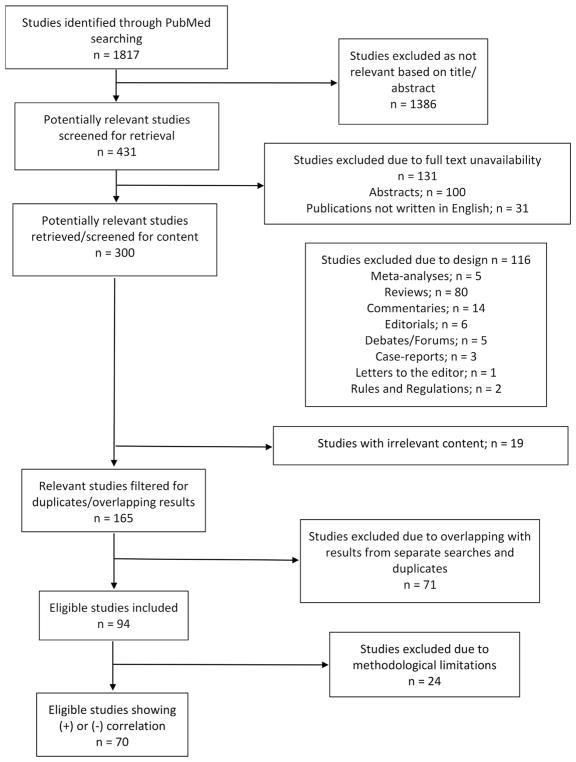
After excluding duplicates, citations in abstract form, and non-English citations, titles and abstracts of full papers were screened for relevance. Reference lists of selected papers were hand-searched to detect potentially relevant studies. For a study to be relevant, a causative link between human prenatal exposure to a specific pesticide group, substance, or pesticides as a whole and structural CAs had to be considered. Functional defects and chromosomal anomalies were not investigated. In vitro and animal studies were excluded, as well as studies, which did not specifically focus on CAs but rather on other outcomes such as birth weight or fetal death. Studies linking pesticide exposure to fetal death due to CAs were also excluded since the prevalence of fetal loss, rather than the prevalence of CAs was investigated. Studies were further filtered by design to include only original research. A total of 94 epidemiological studies, including case-control, nested case-control, prospective and retrospective cohorts, ecological, and cross-sectional studies were finally selected for review following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) methodology (Moher et al., 2009) (Fig. 1).
Fig. 1.
PRISMA flow chart showing the study selection procedure.
Selected studies were classified according to CA category based on the International Statistical Classification of Diseases and Related Health Problems (ICD-10 Version: 2016). Studies linking pesticides with more than one CA category or with CAs in general were grouped into an extra category called “all abnormalities”. Categories yielding limited number of studies (gastrointestinal, ocular, facial) were merged into the “other” category. Most of the studies evaluate parental exposure to pesticides, including maternal/paternal and/or occupational, and/or environmental, and/or home exposure, in relation to the occurrence of CAs of the offspring.
Studies that are based on biomarker-data, are considered the most reliable in comparison to self-reported or other studies, as biomarkers may indicate individual hypersusceptibility to specific chemical exposures and may consequently reveal the appropriate background-knowledge for risk prediction (Grandjean, 1995). Biomarkers refer to events that occur in a biological system, which can be measured and eventually reflect the general state of the organism or severity of influences on it (Grandjean, 1995). It has been previously showed that the self-reported studies or questionnaire-based studies are characterized by measurement errors, which can overshadow potential associations as well as support false-positive correlations. The use of objective bio-markers instead reduced the measurement errors, providing a more accurate approach that enhances reliability (Freedman et al., 2010; Prentice et al., 2009).
3. Pesticides and development of congenital abnormalities
Potential associations of environmental and occupational exposure to pesticides with more than one CA category or with CAs in general (“all abnormalities” category) were investigated in a total of 30 studies. Results on environmental or mixed exposures were inconclusive due to inadequate study design/exposure assessment (Table 1). Most studies evaluating environmental exposure were ecological, assessing pesticide exposure indirectly through national databases of pesticide use, whereas those that were not ecological, used primarily self-report to assess exposure. On the other hand, studies evaluating occupational exposure were case-control with sufficient sample size, thus their design was more reliable. Nevertheless, exposure assessment relied on self-report as well and results should therefore be interpreted with caution. Many studies have reported no increased risk of CAs in offspring residing in areas with pesticide use (de Siqueira et al., 2010; Marshall et al., 1997). Potential association between permethrin/benzyl benzoate lotion during pregnancy for therapeutic reasons (headlice and/or scabies treatment) and CAs was investigated in one study (Kennedy et al., 2005) concluding that no association exists and thus these pesticides are safe. However, the main limitation of this study was the small number of exposed women during the first trimester of pregnancy, when the child is more susceptible to developmental CAs (Kennedy et al., 2005). An exposure-dependent positive link between maternal residence in near toxic waste sites in New York state and CAs and an increased risk for MSAs (OR = 1.2, 95%C = 1.05–1.38) and pesticide exposure was reported (Geschwind et al., 1992) but these results could not be replicated in a following study done in California (Croen et al., 1997). On the other hand, residence in areas with highest wheat acreage increased the risk for circulatory/respiratory and musculoskeletal/integumental abnormalities for combined sexes. A stronger effect was observed for the circulatory subcategory, which excluded heart defects. Infants conceived from April–June had increased chances of having defects from this subcategory (Schreinemachers, 2003). Additional factors contributing to an increase in CAs are maternal pesticide exposure (OR: 2.30, 95% CI: 1.16–4.57) (Yang et al., 2012), exposure to specific pesticides (cyanazine and dicamba (Weselak et al., 2008) or pesticide groups (petroleum derivatives; Anencephaly, hydroxy benzonitrile; Spina Bifida, 2,6-dinitroani-line herbicides, dithiocarbamates-methyl isothiocyanate; Cleft lip) (Yang et al., 2014) and spring conception (Schreinemachers, 2003; Winchester et al., 2009). A case-control study in South Africa (Heeren et al., 2003) linked three exposure types to CAs (plastic containers used for agricultural chemicals storage, particular garden chemicals, and keeping of dipped cattle). Last but not least, an association between per capital consumption of pesticides and CAs was reported in rural but not in urban microregions in Brazil (Cremonese et al., 2014).
Table 1.
Studies associating environmental/mixed pesticide exposures with congenital malformations.
| Studies | Exposure | Pesticides Tested | General Association |
Association | Circulatory- Respiratory/ Association |
UGAs/ Association |
NTDs/ Association |
Clefts/ Association |
Exposure Assessment | Study Type/ Sample size |
Region | Confounding Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Geschwind et al., 1992) | Environmental | Unspecified | + | + | − | − | +/− | +/− | Hazardous Waste Site Inspection Program database/US Census Dual Independent Map Encoding files | Case-control/27,115 | New York State (USA) |
|
| (Croen et al., 1997) | Environmental | Unspecified | − | − | +/− | − | +/− | +/− | Interviews/U.S. and California Environmental Protection Agencies’ listings on hazardous waste sites | Case-control/2119 | California (USA) |
|
| (Marshall et al., 1997) | Environmental | Unspecified | − | +/− | − | − | +/− | − | Geocoding (birth certificate data)/distance from waste sites | Case-control/16,214 | New York State |
|
| (Heeren et al., 2003) | Environmental | OPs, Pesticides, Blue death, Insecticides, Fertilizers | + | + | + | + | + | + | Questionnaires | Case-control/267 | Eastern Cape (South Africa) | Not used (Matching was undertaken with respect to age/occupation/type of live stock owned/location) |
| (Schreinemachers, 2003) | Environmental | Chlorophenoxy pesticides | + | + | + | +/− | +/− | +/− | U.S.D.A. 1992/each county was assigned to either the low-or high-wheat group depending on its percentage of wheat acreage with respect to the median of all selected counties | Ecological | Minnesota/Montana/North Dakota/South Dakota |
|
| (Kennedy et al., 2005) | Therapeutic | Permethrin 1% (for headlice) | − | − | − | +/− | − | − | Questionnaires | Prospective controlled cohort/226 | Toronto (Canada) |
|
| (Weselak et al., 2008) | Occupational/Environmental | All/Unspecified | + | +/− | + | + | + | + | Questionnaires | Case-control/3412 | Ontario (USA) |
|
| (Winchester et al., 2009) | Environmental | Nitrates, atrazine, other unspecified (surface water) | + | + | + | + | + | + | USGS NAWQA database | Ecological | USA |
|
| (de Siqueira et al., 2010) | All | Unspecified | − | − | − | − | − | − | indication of pesticide use by IBGE/Data from National Systematic Survey of Agricultural Production/National Association of Agricultural Defensive Industries | Ecological | Brazil |
|
| (Yang et al., 2012) | All | Unspecified | + | + | + | + | + | + | Questionnaire based interviews | Cross-sectional/16,541 | Shaanxi (China) |
|
| (Yang et al., 2014) | Environmental | 461 individual chemicals | − | − | − | − | + | +/− | CEHTP Geocoding Service/California Department of Pesticide Regulation records/Interviews | Case-control/1375 | San Joaquin Valley (California) |
|
| (Cremonese et al., 2014) | Dietary | Unspecified | + | − | + | − | + | − | Agricultural Census Data | Ecological | Brazil | Not used |
BBL: Benzyl Benzoate Lotion; BMI: Body Mass Index; CA: Congenital Abnormality; CEHTP: California Environmental Health Tracking Program; IBGE: Brazilian Institute for Geography and Statistics; MITC: Methyl Isothiocyanate; MSAs: Musculoskeletal Abnormalities; NAWQA: National Water Quality Assessment; NTDs: Neural Tube Defects; OCs: Organochlorines; OPs: Organophosphates; OR: Odds Ratio; SGA: Small for Gestational Age; UGAs: Urogenital Abnormalities; USDA: United States Department of Agriculture; USGS: United States Geological Survey; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
Studies examining occupational exposure showed a general trend towards a positive association with CAs (Table 2). Maternal and paternal environmental exposure (Crisostomo and Molina, 2002; Garry et al., 1996; Kristensen et al., 1997) as well as maternal (Kristensen et al., 1997) and paternal occupational exposure (Dimich-Ward et al., 1996; El-Helaly et al., 2011; Salazar-Garcia et al., 2004), residence in areas with high pesticide use (Garry et al., 1996; Garry et al., 2002; Shaw et al., 1999), and spring conception (Garry et al., 1996) emerged as risk factors. Nevertheless, other studies challenge these results. Kristensen et al. (1997) and Shaw et al. (1999) detected an increased risk of specific defects with exposure; no association was detected between parental occupational exposure and CAs in general. Only Restrepo et al. reported increased risk for hemangiomas (Restrepo et al., 1990). Garcia et al. detected a positive association of maternal but not of paternal occupational exposure with CAs (OR of 3.16, 95% CI: 1.11–9.01) that has also been shown by Rappazzo et al. (Garcia et al., 1999; Rappazzo et al., 2016).
Table 2.
Studies associating occupational pesticide exposures with congenital malformations.
| Studies | Pesticides Tested | General Association |
MSAs/ Association |
Circulatory- Respiratory/ Association |
UGAs/ Association |
NTDs/ Association |
Clefts/ Association |
Other/ Association |
Exposure Assessment |
Study Type/ Sample size |
Region | Confounding Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Restrepo et al., 1990) | Phenoxy herbicides | − | +/− | +/− | +/− | − | +/− | + | Questionnaire based interviews | Nested case-control/665 | Colombia |
|
| (Dimich-Ward et al., 1996) | Chlorophenate wood preservatives | + | +/− | +/− | + | + | +/− | + | Minnesota Department of Agriculture data | Case-control/19,675 | Minnesota (USA) |
|
| (Garry et al., 1996) | 2,4-D, alachlor, atrazine, bentazon, bromoxynil, cyanazine, dicamba, EPTC, imazethapyr, MCPA, metolachlor, trifluralin | + | + | + | + | + | − | +/− | Cohort of sawmill workers/Expert raters’ estimation | Epidemiological/210,723 | British Columbia |
|
| (Kristensen et al., 1997) | Unspecified | − | + | − | + | + | +/− | − | Agricultural/horticultural census information | Cohort/253,768 | Norway |
|
| (Shaw et al., 1999) | Unspecified | − | +/− | +/− | − | + | +/− | − | Telephone interviews/Exposure assessed by industrial hygienist | Case-control/2,033 | California |
|
| (Garcia et al., 1999) | Unspecified | + | + | + | + | + | + | + | Telephone interviews | Case-control/522 | Spain |
|
| (Garry et al., 2002) | 14 types of pesticides | + | + | + | + | + | − | + | Telephone interviews/Questionnaires | Cross-sectional/1,532 | Minnesota (USA) |
|
| (Salazar-Garcia et al., 2004) | DDT | + | + | + | − | + | + | + | Questionnaire/Exposure assigned by experts | Nested cross-sectional/9,187 | Mexico |
|
CAs: Congenital Abnormalities; EPTC: S-Ethyl dipropylcarbamothioate; MCPA: 2-Methyl-4-Chlorophenoxyacetic Acid; MSAs: Musculoskeletal Abnormalities; NTDs: Neural Tube Defects; OPs: Organophosphates; OR: Odds Ratio; UGAs: Urogenital Abnormalities; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
Pesticide residues are highest in surface and ground water during the growing seasons (spring and first summer months) (Winchester et al., 2009). Spring conception emerged multiple times as a risk factor for CAs (Garry et al., 1996; Schreinemachers, 2003; Waller et al., 2010; Winchester et al., 2009), resulting in more CAs in rural areas since pesticides are widely used during that season. A recent study however, found not only that spring spike is more pronounced in urban non-agricultural than in other types of counties but also that this lasts longer until fall (McKinnish et al., 2014), which could be attributed to agricultural pesticides contaminating urban water supplies or to the commercial and/or residential pesticide use. Nevertheless, it is also possible that the spring spike is a result of seasonal variations of other environmental pollution types, viral infections, or even use of decongestants.
4. Musculoskeletal abnormalities
MSAs include CAs of the skeletal and muscular system with gastroschisis (1 in 2229 births) and reduction defects of the upper limb (1 in 2869) being the most common (Parker et al., 2010). The literature in the field is limited with only six studies available to date, most of which performed in USA. Nevertheless, most of the studies are case-controlled with adequate sample sizes. Most of the studies detected a positive association between occupational and/or environmental exposure and MSAs (Table 3) (Agopian et al., 2013b; Engel et al., 2000; Kielb et al., 2014; Waller et al., 2010), such as gastroschisis and reduction defects of the upper limb (Lin et al., 1994; Shaw et al., 2014). Risk factors include: maternal exposure (Agopian et al., 2013b; Engel et al., 2000; Waller et al., 2010), maternal co-exposure (Kielb et al., 2014), smaller distance from high pesticide use sites (Waller et al., 2010), spring conception (Waller et al., 2010) and increased maternal age (Agopian et al., 2013b; Kielb et al., 2014). Interestingly two studies showed that increased maternal age is a risk factor specifically for gastroschisis (Agopian et al., 2013b; Kielb et al., 2014). On the contrary, other studies reported younger maternal age as a risk factor. Authors argue whether this is an effect of bioaccumulation, resulting from chronic exposure, or whether it is a result of a pesticide, atrazine in this case, acting as an ED, given that older women have lower first trimester estrogen levels than younger women (WHO/CDC/ICBDSR, 2014). None of these studies, assessed pesticide exposure directly. Distance from residence to pesticide use sites as a proxy for exposure was mostly used. To minimize exposure misclassification, statewide databases were merely used (Agopian et al., 2013b; Lin et al., 1994; Shaw et al., 2014; Waller et al., 2010). Although there is evidence of a positive association between pesticide use and MSAs, more studies are needed to link these CAs to directly assessed pesticide exposure.
Table 3.
Studies associating pesticides with musculoskeletal malformations.
| Studies | Association | Pesticide Tested | CA Tested | Exposure Type | Exposure Assessment | Biomarker Available |
Study Type/ Sample size |
Region | Confounding Factors |
|---|---|---|---|---|---|---|---|---|---|
| (Lin et al., 1994) | +/− | Unspecified/All | Limb reduction defects | Occupational/Environmental | Birth Certificates/New York State census of Agriculture data/Industrial hygienist’s assessment | No | Case-control/277 | New York |
|
| (Engel et al., 2000) | + | Unspecified/All | Limb defects | Occupational | Birth Certificates → Maternal occupation | No | Retrospective cohort/33,972 | Washington State |
|
| (Waller et al., 2010) | + | Atrazine/2,4-D (surface waters) | Gastroschisis | Environmental | US Geological Survey Data/zip coding | Yes | Case-control/4421 | Washington State |
|
| (Agopian et al., 2013b) | + | Atrazine (crops) | Gastroschisis | Environmental | Registry/Birth Certificates/US Geological Survey Data | Yes | Case-control/9551 | Texas |
|
| (Kielb et al., 2014) | + | Unspecified/All | Craniosynostosis/Gastroschisis/Diaphragmatic hernia/Transverse limb deficiencies | Occupational | Interviews/Expert raters/Job exposure matrix | No | Case-control/3728 | Arkansas/California/Iowa/Massachusetts/New Jersey/New York/Texas/Atlanta |
|
| (Shaw et al., 2014) | +/− | 22 chemical groups | Gastroschisis | Residential | Interviews/CEHTP Geocoding Service/Public Land Survey Section data/Pesticide Use Reporting records | No | Case-control/931 | San Joaquin Valley of California |
|
BMI: Body Mass Index; CAs: Congenital Abnormalities; CEHTP-California Environmental Health Tracking Program; OR: Odds Ratio; 2,4-D: 2,4-Dichlorophenoxyacetic acid; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
5. Neural tube defects
According to World Health Organization, NTDs affect the brain and spinal cord and are among the most common CAs (WHO/CDC/ICBDSR, 2014). Main risk factors include genetic predisposition, reduced pregnancy folate intake and environmental factors such anticonvulsant drugs, maternal obesity and maternal diabetes (Copp et al., 2013).
All studies reported a positive association in newborns. The general trend refers merely to occupational rather than to environmental exposure that was not often assessed (Table 4). Main risk factors include parental occupational exposure to pesticides, with agricultural workers/people living in farms been significantly exposed (Blatter and Roeleveld, 1996; Blatter et al., 2000; Fear et al., 2007; Lacasana et al., 2006; Makelarski et al., 2014). The significance of maternal or paternal environmental/occupational exposure was debated. Maternal exposure to OCs such as endosulfan, DDT and dichlorodiphenyldichloroethylene (DDE) was linked to fetal NTDs (Kalra et al., 2016), with mothers delivering affected neonates reported to have 11.3 times greater chances of been exposed to DDE levels above median concentration of controls. Other risk factors are maternal residential proximity to pesticide application, parental pesticide exposure prior/during a periconceptual period of three months and co-exposure (Brender et al., 2010; Makelarski et al., 2014; Wang et al., 2014).
Table 4.
Studies associating pesticides with neural tube defects.
| Studies | Association | Pesticide Tested | CAs Tested | Exposure Type | Exposure Assessment | Study Type/ Sample Size |
Region | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| (Blatter and Roeleveld, 1996) | +/− | Unspecified | Spina bifida | Occupational | Questionnaires/Interviews | Case Control/1446 | Sweden |
|
| (Blatter et al., 2000) | +/− | Unspecified | Spina Bifida | Occupational | Questionnaires/Interviews | Case Control/4966 | Sweden, Spain, and Hungary |
|
| (Lacasana et al., 2006) | + | Unspecified/All | Anencephaly | Occupational | Questionnaires | Case Control/302 | State of Mexico, Puebla, Guerrero |
|
| (Fear et al., 2007) | + | Unspecified/All | All | Occupational | Hospital records | Case Control/1388 | England |
|
| (Brender et al., 2010) | + | Unspecified/All | Anencephaly/Spina bifida | Environmental, Occupational | Interviews/Questionnaires | Case Control/409 | Texas-Mexico border |
|
| (Makelarski et al., 2014) | − | Insecticides/Fungicides/Herbicides | Unspecified/All | Occupational | Self-reported occupation/Job exposure matrix | Case Control/3452 | Arkansas, California, Iowa, Massachusetts, New Jersey, New York, Texas, Atlanta NBDPS sites (USA) |
|
| (Wang et al., 2014) | +/− | 25 OCs | Anencephaly/Spina bifida/Encephalocele | Unspecified | Interview/Questionnaire/Maternal serum samples | Case Control/238 | Shanxi Province of China |
|
ARP: Acute Risk Period; BMI: Body Mass Index; CAs: Congenital Abnormalities; DDT: Dichlorodiphenyltrichloroethane; HCH: hexachlorocyclohexane; NTDs: Neural Tube Defects; OCs: Organochlorines; OR: Odds Ratio; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
The main limitation in these studies is that they are mostly based on self-report (questionnaires and interviews). Consequently, mis–/partial lack of information on parental exposure is quite likely. As a result, this positive trend should be interpreted with caution, since more studies with better exposure assessment are needed.
6. Urogenital abnormalities
Cryptorchidism and hypospadias are the commonest CAs in human males. Based on epidemiological, clinical, biological and experimental evidence, it has been hypothesized that cryptorchidism, hypospadias, testicular cancer, and poor spermatogenesis are signs of a sole developmental disturbance, named testicular dysgenesis syndrome (Skakkebaek et al., 2001). This syndrome is considered the result of embryonal programming/gonadal development disruption during fetal life and may be increasingly common due to adverse environmental influences, mainly exposure to EDs (Skakkebaek et al., 2001; Virtanen et al., 2005). Seasonal trends in the prevalence of hypospadias and cryptorchidism may also support such a link (Mamoulakis et al., 2002; Mamoulakis et al., 2017). A great amount of research has therefore been focused on the effect of environmental factors among others, on male reproductive parameters (Sharpe, 2003). A growing body of toxicology data on animals suggests that exposure to EDs are linked to male reproductive system disorders (Petrakis et al., 2017). Human appears to be less susceptible to many compounds compared to other species but the issue of mixed exposures remains an unresolved problem; each ED may be present in modest concentration but total effect may be additive/multiplicative. Furthermore, the effect of environmental toxicants may be modified by genetic susceptibility.
The relationship between pesticides-UGAs appears to be the most thoroughly explored, with search yielding over 30 studies. The most commonly UGA investigated was hypospadias. It is believed that the “estrogen hypothesis” is crucial to this (Sharpe, 2003). According to this hypothesis, the increase in human male reproductive developmental disorders may have occurred due to increased estrogen exposure in utero (Sharpe and Skakkebaek, 1993). The action of several pesticides as EDs added to the increased interest in this category of CAs (Svechnikov et al., 2010).
No firm conclusions can be generally drawn. It should be stressed that this CA category, apart from being more frequently investigated, it is also the only one extensively explored using biomarkers. This method of exposure assessment, as stated above, is the most reliable since it measures pesticides or metabolites directly in tissues/secretions such as maternal serum (Carmichael et al., 2010; Fernandez et al., 2007; Giordano et al., 2010; Longnecker et al., 2002; Pierik et al., 2007), breast milk (Brucker-Davis et al., 2008; Damgaard et al., 2006), hair (Michalakis et al., 2014), urine (Chevrier et al., 2011), cord blood (Brucker-Davis et al., 2008), and placenta (Fernandez et al., 2007).
Most largest ecological studies did detect a positive association. Nevertheless, it should be noted that such studies are most useful for generating hypotheses rather than assessing true cause-effect relationships at an individual level (Agopian et al., 2013a; Carmichael et al., 2013; Giordano et al., 2010). This limitation was apparent when the positive results of an ecological study in Sicily could not be replicated by a following case-control study conducted by the same authors on the same population (Carbone et al., 2007). Consequently, although a positive trend was found in ecological studies, results were ambiguous in observational studies, which have an inherently more reliable design for assessing environmental and occupational exposures and no conclusions could be drawn in studies with better exposure assessment using biomarkers or with a larger exposure (occupational exposure).
Concerning environmental or mixed exposures (i.e., exposure to more than one pesticide or unspecified exposure) using biomarkers (Table 5), a positive trend towards an association was observed, largely supported by ecological studies reviewed (Bianca et al., 2003; Garcia-Rodriguez et al., 1996). Ecological studies aside, results were ambiguous; with three studies supporting a positive association (Agopian et al., 2013a; Carmichael et al., 2013; Giordano et al., 2010) and four studies negating it (Brouwers et al., 2007; Carbone et al., 2007; Dugas et al., 2010; Meyer et al., 2006). The primary risk factor was maternal exposure (Agopian et al., 2013a; Giordano et al., 2010). A large case-control study reported increased risk for mothers having a medium-low/medium exposure but no association was detected in the highly exposed group (Agopian et al., 2013a). There are studies, however, supporting that parental and paternal exposure do not increase the risk of UGAs (Brouwers et al., 2007; Carbone et al., 2007). Meyer et al. examined 38 different compounds, finding a positive association only for diclofopmethyl (Meyer et al., 2006), while Carmichael et al. examined 292 chemicals finding a positive association for only a few of them (Carmichael et al., 2014). Last but not least, a French cohort study of 300 children focusing on hypospadias showed that fetal exposure to pesticides in pregnancies resulting in hypospadiac births was about 9% with 78% of exposures occurring around the period of genital differentiation during the first trimester of pregnancy (Kalfa et al., 2015).
Table 5.
Studies associating environmental/mixed pesticide exposure with urogenital abnormalities.
| Studies | Association | Pesticide Tested | CAs Tested | Exposure Type | Exposure Assessment | Study Type/Sample Size | Confounding Factors |
|---|---|---|---|---|---|---|---|
| (Sharpe and Skakkebaek, 1993) | +/− | Diethylstilbestrol (DES) | Cryptorchidism Hypospadias | Environmental Pharmacological | Unspecified | Collection of Epidemiological Studies |
|
| (Garcia-Rodriguez et al., 1996) | + | Unspecified/All | Cryptorchidism | Environmental | Use of 4-point scale in each municipality, according to total surface area under cultivation and predominant crops. | Ecological |
|
| (Skakkebaek et al., 2001) | +/− | Unspecified/All | Testicular dysgenesis syndrome | Environmental/Occupational | Unspecified | Collection of Epidemiological Studies |
|
| (Mamoulakis et al., 2002) | + | Unspecified/All | Cryptorchidism | Environmental | Three major pediatric center databases in Athens (Aghia Sophia Children’s Hospital, Aglaia Kyriakou Children’s Hospital and Pentelis Children’s Hospital | Retrospective epidemiological study/208,912 live-born boys |
|
| (Bianca et al., 2003) | + | Unspecified/All | Hypospadias | Unspecified/All | Towns with intense industrial (Augusta) and agricultural (Vittoria) activity compared to a commercial one (Catania) | Ecological |
|
| (Sharpe, 2003) | + | Oestrogens and oestrogenic chemicals | Testis cancer, cryptorchidism, hypospadias, low sperm counts | Environmental Pharmacological | Unspecified Interviews/Questionnaire | Collection of Epidemiological Studies |
|
| (Virtanen et al., 2005) | + | 9 compounds | Testicular dysgenesis syndrome | Environmental/Occupational | The Turku University Central Hospital data GLOBOCAN 2000 | Collection of clinical and epidemiological findings and biological and experimental studies |
|
| (Meyer et al., 2006) | +/− | 38 compounds | Hypospadias | Environmental | Interviews/NASS data/Arkansas agricultural databases/usage guidelines published by University of Arkansas Cooperative Extension Service and the Southern Integrated Pest Management Center | Case-control/1081 |
|
| (Carbone et al., 2007) | +/− | Unspecified/All | Hypospadias/Cryptorchidism | Environmental/Occupational | Interviews/Job exposure matrix | Case-control/293 |
|
| (Brouwers et al., 2007) | +/− | Unspecified/All | Hypospadias | Unspecified/All | Mailed questionnaires | Case-control/834 |
|
| (Dugas et al., 2010) | +/− | Unspecified/All | Hypospadias | Environmental | Questionnaire-based telephone interviews | Case-control/961 |
|
| (Giordano et al., 2010) | + | HCB/DDE | Hypospadias | Occupational/Dietary | Interviews/Job exposure matrix/Questionnaires | Case-control/58 |
|
| (Carmichael et al., 2013) | + | 292 chemicals | Hypospadias | Environmental | Residence geocoding/Pesticide Use Reporting records | Case-control/2885 |
|
| (Agopian et al., 2013a) | + | Atrazine | All | Residential | US Geological Survey Data/AgroTrak surveys/Census of Agriculture and National Agriculture Statistics Service | Case-control/32,866 |
|
| (Kalfa et al., 2015) | + | All EDCs | Hypospadias | Environmental/Occupational | Job history questionnaire completed by mothers. | Case-control/219 newborns |
|
| (Mamoulakis et al., 2017) | + | Unspecified/All | Hypospadias | Environmental | Data on the population at risk were collected from the Hellenic Statistical Authority | Case-control/542 |
|
CAs: Congenital Abnormalities; DDE: Dichlorodiphenyldichloroethylene; DDT: p,p′-Dichlorodiphenyltrichloroethane; EDs: Endocrine Disruptors; HCB: Hexachlorobenzene; HCH: Hexachlorocyclohexane; NASS: National Agricultural Statistics Service; OPs: Organophosphates; OR: Odds Ratio; RR: Relative Risk; VSDs: Ventricular Septal Defects; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
Studies assessing individual-level exposure using pesticide bio-markers (Bhatia et al., 2005; Brucker-Davis et al., 2008; Carmichael et al., 2010; Chevrier et al., 2011; Damgaard et al., 2006; Fernandez et al., 2007; Giordano et al., 2010; Longnecker et al., 2002; Michalakis et al., 2014; Pierik et al., 2007; Trabert et al., 2012) yielded conflicting results (Table 6). Most of them failed to detect an association (Bhatia et al., 2005; Brucker-Davis et al., 2008; Carmichael et al., 2010; Chevrier et al., 2011; Longnecker et al., 2002; Pierik et al., 2007; Toft et al., 2016; Trabert et al., 2012) while some of them directly linked UGAs risk with DDE and/or DDT (Bhatia et al., 2005; Brucker-Davis et al., 2008; Longnecker et al., 2002). Some others detected just a positive association (Damgaard et al., 2006; Fernandez et al., 2007; Giordano et al., 2010; Michalakis et al., 2014). Four studies linked hypospadias/cryptorchidism with specific pesticides including mirex/lindane (Fernandez et al., 2007), hexachlorobenzene (Giordano et al., 2010), DDT and its metabolites (Longnecker et al., 2002), as well as OPs/OCs (Michalakis et al., 2014). Increased concentrations of eight substances (p,p′-DDE, p,p′-DDT, β-HCH, hexachlorobenzene (HCB), α-endosulfan, cis-heptachloroepoxide, dieldrin, oxychlordane) were measured in cases of cryptorchid boys in another study (Damgaard et al., 2006). Finally, significantly increased levels of OCs and OPs in cryptorchid boys and their parents compared to occupationally exposed adults were reported (Damgaard et al., 2006).
Table 6.
Studies associating pesticides with the use of biomarkers with urogenital abnormalities.
| Studies | Association | Pesticide Tested | CAs Tested | Exposure Type | Biomarker Assessment |
Study Type/Sample Size |
Confounding Factors Examined |
|---|---|---|---|---|---|---|---|
| (Longnecker et al., 2002) | +/− | DDE | Hypospadias/Cryptorchidism/Polythelia | Environmental | Maternal serum | Nested case-control/1137 |
|
| (Bhatia et al., 2005) | +/− | DDT/DDE | Hypospadias/Cryptorchidism | Environmental | Maternal serum | Nested case-control/428 |
|
| (Damgaard et al., 2006) | + | 27 OCs | Cryptorchidism | Environmental | Breast milk | Case-control/130 | Unspecified/Not used (Lipid content in milk, maternal age, maternal BMI, parity, and smoking habits were matched between the 2 groups) |
| (Fernandez et al., 2007) | + | 16 OCs | Hypospadias/Cryptorchidism | Unspecified/All | Placenta tissue | Nested case-control/164 |
|
| (Pierik et al., 2007) | +/− | HCE/HCB/β-HCCH | Cryptorchidism | Unspecified/All | Maternal serum | Nested case-control/783 |
|
| (Brucker-Davis et al., 2008) | +/− | DDE | Cryptorchidism | Unspecified/All | Cord blood/Colostrum | Case-control/378 |
|
| (Carmichael et al., 2010) | +/− | 9 persistent pesticides | Hypospadias | Environmental | Maternal serum | Nested case-control/48 |
|
| (Giordano et al., 2010) | + | HCB/DDE | Hypospadias | Occupational/Dietary | Maternal serum | Case-control/58 |
|
| (Chevrier et al., 2011) | − | Triazine and chloroacetanilide herbicides | Hypospadias/Undescended testis/Micropenis | Environmental | Maternal urine | Nested case-control/715 (malformation and control subgroups) |
|
| (Trabert et al., 2012) | +/− | Trans-nonachlor, Oxychlordane | Hypospadias/Cryptorchidism | Environmental | Maternal serum | Nested case-control/971 |
|
| (Michalakis et al., 2014) | + | OCs/OPs | Hypospadias | Environmental | Parental & Offspring Hair and Blood | Retrospective cohort/29 (children) + 49 (parents) | Unspecified/Not used |
AFP: a-Fetoprotein, BMI: Body Mass Index; DDE: Dichlorodiphenyldichloroethylene; DDT: p,p′-dichlorodiphenyltrichloroethane; HCB: Hexachlorobenzene; HCH: Hexachlorocyclohexane; HCE: Heptachlor Epoxide; OCs: Organochlorines; OPs: Organophosphates; OR: Odds Ratio; PCBs: Polychlorinated Biphenyls; UGBD− urogenital birth defects, β-HCCH− β-hexachlorocyclohexane; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
Occupational exposure results assessed without using biomarkers differed (Table 7). More than half of the studies reported maternal occupational exposure as a risk factor for UGAs (Andersen et al., 2008; Gaspari et al., 2011; Jorgensen et al., 2014; Weidner et al., 1998). It should be noted, that one study negating positive association with mild hypospadiac cases included only low exposure cases (Rocheleau et al., 2011), possibly compromising final outcome. Paternal was not as strongly associated as maternal occupational exposure with UGAs.
Table 7.
Studies associating occupational pesticide exposure with urogenital abnormalities.
| Studies | Association | Pesticide Tested | CAs Tested | Exposure Assessment | Study Type/Sample Size | Confounding Factors |
|---|---|---|---|---|---|---|
| (Weidner et al., 1998) | + | Unspecified/All | Hypospadias/Cryptorchidism | Tax authority information sheets (indicating employee’s working place) | Register-based case-control/30,795 |
|
| (Andersen et al., 2008) | + | 200 compounds | All | Questionnaire-assisted interview/governmental risk assessment/employer’s interview | Cohort/113 | Birth weight/birth length/gestational age/age at examination/IUGR/smoking during pregnancy |
| (Gaspari et al., 2011) | + | Unspecified/All | Hypospadias/Cryptorchidism/Micropenis/Disorders of sexual differentiation | Questionnaire | Nested case-control/115 |
|
| (Rocheleau et al., 2011) | − | Unspecified/All | Hypospadias | Interview/Job exposure matrix (only low exposures assessed) | Case-control/2143 |
|
| (Jorgensen et al., 2014) | + | Unspecified/All | Cryptorchidism | Employment Classification Module from Statistics Denmark | Cohort/ > 600,000 |
|
BMI: Body Mass Index; CAs: Congenital Abnormalities; HR: Hazard Ratio; OR: Odds Ratio; UGAs: Urogenital Abnormalities; 95% CI: Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
7. Cardiovascular abnormalities
CVAs constitute a major proportion of clinically significant CAs and are an important component of pediatric cardiovascular disease, with an estimated prevalence of 6 to 9 per 1000 live births (Botto et al., 2001; Hoffman et al., 2004), and with VSD being the most common (Bjornard et al., 2013; Botto et al., 2001). During the first year of life, CVAs are the leading cause of death from CAs (Yang et al., 2006). The prevalence of some CVAs, especially mild types, is increasing, while the prevalence of other types has remained stable (Botto et al., 2001; Yang et al., 2006). Despite the frequency of these CAs, the literature on their association with pesticides is limited. Only four studies were identified from 1990 till mid-2015 (Agopian et al., 2013b; Carmichael et al., 2014; Loffredo et al., 2001; Rocheleau et al., 2015). They all presented adequate study designs and moderate sample sizes. However, their main limitation was that exposure assessment was based on self-report in all cases.
A general positive association between CVAs (heart abnormalities only) and pesticide use was observed (Table 8) (Carmichael et al., 2014; Loffredo et al., 2001; Rocheleau et al., 2015). Residential proximity to specific chemicals (Carmichael et al., 2014) and exposure to certain types of pesticides (Loffredo et al., 2001; Rocheleau et al., 2015) were found to be associated with specific CVAs. A dose-response relationship was detected between one-time exposure, monthly exposure, and no exposure but not for once a week and a few times per week exposure (Loffredo et al., 2001).
Table 8.
Studies associating pesticides with heart and other malformations.
| Studies | Association | Pesticide Tested | CAs Tested | Exposure Type | Exposure Assessment | Study Type/ Sample Size |
Region | Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| (Loffredo et al., 2001) | + and +/− | Unspecified | Great Arteries Transposition/Laterality and looping defects/Non-TGA cardiac outflow tract anomalies/Endocardial cushion defects/Left-sided obstructive lesions/Pulmonic stenosis/VSDs/Atrial septal defects | Environmental | Interviews, Questionnaires | Case Control/837 | Maryland/District of Columbia/adjacent counties of northern Virginia |
|
| (Agopian et al., 2013c) | + | Atrazine (crops) | Choanal atresia/stenosis | Environmental | Registry/US Geological Survey Data | Case-control/4000 | Texas |
|
| (Carmichael et al., 2014) | + and +/− | 461 individual chemicals and 62 physicochemical groupings | Heterotaxia/Tetralogy of Fallot/D-transposition of the great arteries/Hypoplastic left heart syndrome/Coarctation of the aorta/Pulmonary valve stenosis/Perimembranous VSD/Atrial septal defect secundum | Environmental | Interviews/Questionnaires/Pesticide use report records/Geocoding | Case-control/1354 | San Joaquin Valley (California) |
|
| (Rocheleau et al., 2015) | + and +/− | Fungicides/Insecticides/Herbicides | Congenital heart defects | Occupational | Expert-guided task-exposure matrix/self-reported job history details | Case-control/6316 | USA |
|
EUROCAT: European Surveillance of Congenital Anomalies; TGA: Transposition of Great Arteries; CVAs: Cardiovascular Abnormalities; 95% CI: 95% Confidence Interval; +: association detected; −: no association detected; +/−: trend towards association (not statistically significant).
A positive association between transposition of great arteries and herbicides/rodenticides (Loffredo et al., 2001), other CVAs and insecticides, herbicides and fungicides (Rocheleau et al., 2015) as well as with specific chemicals rather than chemical groups was reported (Carmichael et al., 2014). Such associations include pulmonary valve stenosis-bipyridylium/organophosphorus, perimembranous ventricular septal defects (VSD)-avermectin, coarctation of the aorta-pyridazinone, atrial septal defect secundum-dichlorophenoxy acid or ester and Fallot’s tetralogy/hypoplastic left heart syndrome-neonicotinoids.
8. Other defects
The remaining studies were classified into this category. Overall, the results of these studies were inadequate to draw conclusions for these categories of CAs and thus they were not further analyzed.
9. Conclusions
The association between CAs and pesticides remains uncertain, regardless of the type. A trend suggestive of a positive association was detected for MSAs, NTDs, and CVAs, but no firm conclusions could be drawn. Pesticides are a very diverse group of compounds with multiple modes of action. Assuming that few selective active ingredients contribute to CAs, their actions may be masked in studies examining them in conjunction with other innocuous chemicals, resulting in an ambiguous picture. The main limitation of the studies was poor exposure assessment, since many of them relied solely on self-report and only studies associating pesticides with UGAs utilized biomarkers extensively. Use of specific biomarkers of exposure in mothers may be preferable for detecting such associations between exposure and possible teratogenic effects; an issue that should be addressed in future studies. Investigation of potential associations between specific CAs with specific active ingredients of occupational or daily used chemicals might prove to be more promising in the long run.
Abbreviations
- CAs
Congenital abnormalities
- CVAs
Cardiovascular abnormalities
- DDE
Dichlorodiphenyldichloroethylene
- DDT
Dichlorodiphenyltrichloro ethane
- EDs
Endocrine disruptors
- FSH
Follicle-stimulating hormone
- HCB
Hexachlorobenzene
- HCH
beta-hexachlorocyclohexane
- LH
Luteinizing hormone
- MSAs
Musculoskeletal abnormalities
- NTDs
Neural tube defects
- OCs
Organochlorines
- OPs
Organophosphates
- UGAs
Urogenital abnormalities
- VSD
Ventricular septal defect
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest from any funding sources.
References
- Agopian AJ, Lupo PJ, Canfield MA, Langlois PH. Case-control study of maternal residential atrazine exposure and male genital malformations. Am J Med Genet A. 2013a;161A:977–982. doi: 10.1002/ajmg.a.35815. [DOI] [PubMed] [Google Scholar]
- Agopian AJ, Langlois PH, Cai Y, Canfield MA, Lupo PJ. Maternal residential atrazine exposure and gastroschisis by maternal age. Matern Child Health J. 2013b;17:1768–1775. doi: 10.1007/s10995-012-1196-3. [DOI] [PubMed] [Google Scholar]
- Agopian AJ, Cai Y, Langlois PH, Canfield MA, Lupo PJ. Maternal residential atrazine exposure and risk for choanal atresia and stenosis in offspring. J Pediatr. 2013c;162:581–586. doi: 10.1016/j.jpeds.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jorgensen E, Kjaerstad MB, et al. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect. 2008;116:566–572. doi: 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadar H, Abdollahi M, Maqbool F, Baeeri M, Niaz K. Mechanistic overview of immune modulatory effects of environmental toxicants. Inflamm Allergy Drug Targets. 2015;13:382–386. doi: 10.2174/1871528114666150529103003. [DOI] [PubMed] [Google Scholar]
- van den Berg H, Zaim M, Yadav RS, Soares A, Ameneshewa B, Mnzava A, et al. Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect. 2012;120:577–582. doi: 10.1289/ehp.1104340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect. 2005;113:220–224. doi: 10.1289/ehp.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianca S, Li Volti G, Caruso-Nicoletti M, Ettore G, Barone P, Lupo L, et al. Elevated incidence of hypospadias in two sicilian towns where exposure to industrial and agricultural pollutants is high. Reprod Toxicol. 2003;17:539–545. doi: 10.1016/s0890-6238(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Bjornard K, Riehle-Colarusso T, Gilboa SM, Correa A. Patterns in the prevalence of congenital heart defects, metropolitan Atlanta, 1978 to 2005. Birth Defects Res A Clin Mol Teratol. 2013;97:87–94. doi: 10.1002/bdra.23111. [DOI] [PubMed] [Google Scholar]
- Blair A, Ritz B, Wesseling C, Freeman LB. Pesticides and human health. Occup Environ Med. 2015;72:81–82. doi: 10.1136/oemed-2014-102454. [DOI] [PubMed] [Google Scholar]
- Blatter BM, Roeleveld N. Spina bifida and parental occupation in a Swedish register-based study. Scand J Work Environ Health. 1996;22:433–437. doi: 10.5271/sjweh.164. [DOI] [PubMed] [Google Scholar]
- Blatter BM, Roeleveld N, Bermejo E, Martinez-Frias ML, Siffel C, Czeizel AE. Spina bifida and parental occupation: results from three malformation monitoring programs in Europe. Eur J Epidemiol. 2000;16:343–351. doi: 10.1023/a:1007679525757. [DOI] [PubMed] [Google Scholar]
- Bonner MR, Freeman LE, Hoppin JA, Koutros S, Sandler DP, Lynch CF, et al. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ Health Perspect. 2017;125:544–551. doi: 10.1289/EHP456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- Brender JD, Felkner M, Suarez L, Canfield MA, Henry JP. Maternal pesticide exposure and neural tube defects in Mexican Americans. Ann Epidemiol. 2010;20:16–22. doi: 10.1016/j.annepidem.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr. 2007;166:671–678. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, et al. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum Reprod. 2008;23:1708–1718. doi: 10.1093/humrep/den186. [DOI] [PubMed] [Google Scholar]
- Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: a population-based case-control study in rural Sicily. Int J Androl. 2007;30:3–13. doi: 10.1111/j.1365-2605.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Herring AH, Sjodin A, Jones R, Needham L, Ma C, et al. Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere. 2010;80:641–646. doi: 10.1016/j.chemosphere.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Roberts EM, Kegley SE, Wolff C, Guo L, et al. Hypospadias and residential proximity to pesticide applications. Pediatrics. 2013;132:e1216–1226. doi: 10.1542/peds.2013-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Roberts E, Kegley SE, Padula AM, English PB, et al. Residential agricultural pesticide exposures and risk of selected congenital heart defects among offspring in the San Joaquin Valley of California. Environ Res. 2014;135:133–138. doi: 10.1016/j.envres.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Bordi G, Ciardo F, Marci R, La Rocca C, Tait S, et al. The influence of endocrine disruptors in a selected population of infertile women. Gynecol Endocrinol. 2013;29:444–447. doi: 10.3109/09513590.2012.758702. [DOI] [PubMed] [Google Scholar]
- Caserta D, Di Segni N, Mallozzi M, Giovanale V, Mantovani A, Marci R, et al. Bisphenol A and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol. 2014;12:37. doi: 10.1186/1477-7827-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier C, Limon G, Monfort C, Rouget F, Garlantezec R, Petit C, et al. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ Health Perspect. 2011;119:1034–1041. doi: 10.1289/ehp.1002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Greene ND. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremonese C, Freire C, De Camargo AM, De Lima JS, Koifman S, Meyer A. Pesticide consumption, central nervous system and cardiovascular congenital malformations in the South and Southeast region of Brazil. Int J Occup Med Environ Health. 2014;27:474–486. doi: 10.2478/s13382-014-0269-5. [DOI] [PubMed] [Google Scholar]
- Crisostomo L, Molina VV. Pregnancy outcomes among farming households of Nueva Ecija with conventional pesticide use versus integrated pest management. Int J Occup Environ Health. 2002;8:232–242. doi: 10.1179/107735202800338812. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Sanbonmatsu L, Selvin S, Buffler PA. Maternal residential proximity to hazardous waste sites and risk for selected congenital malformations. Epidemiology. 1997;8:347–354. doi: 10.1097/00001648-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Damgaard IN, Skakkebaek NE, Toppari J, Virtanen HE, Shen H, Schramm KW, et al. Persistent pesticides in human breast milk and cryptorchidism. Environ Health Perspect. 2006;114:1133–1138. doi: 10.1289/ehp.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimich-Ward H, Hertzman C, Teschke K, Hershler R, Marion SA, Ostry A, et al. Reproductive effects of paternal exposure to chlorophenate wood preservatives in the sawmill industry. Scand J Work Environ Health. 1996;22:267–273. doi: 10.5271/sjweh.141. [DOI] [PubMed] [Google Scholar]
- Dugas J, Nieuwenhuijsen MJ, Martinez D, Iszatt N, Nelson P, Elliott P. Use of biocides and insect repellents and risk of hypospadias. Occup Environ Med. 2010;67:196–200. doi: 10.1136/oem.2009.047373. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol a concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012a;27:3583–3592. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012b;120:978–983. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Helaly M, Abdel-Elah K, Haussein A, Shalaby H. Paternal occupational exposures and the risk of congenital malformations—a case-control study. Int J Occup Med Environ Health. 2011;24:218–227. doi: 10.2478/s13382-011-0019-x. [DOI] [PubMed] [Google Scholar]
- Engel LS, O’Meara ES, Schwartz SM. Maternal occupation in agriculture and risk of limb defects in Washington State, 1980–1993. Scand J Work Environ Health. 2000;26:193–198. doi: 10.5271/sjweh.531. [DOI] [PubMed] [Google Scholar]
- FAO. International Code of Conduct on the Distribution and Use of Pesticides. Rome: 2005. [Google Scholar]
- Fear NT, Hey K, Vincent T, Murphy M. Paternal occupation and neural tube defects: a case-control study based on the Oxford Record Linkage Study register. Paediatr Perinat Epidemiol. 2007;21:163–168. doi: 10.1111/j.1365-3016.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Olmos B, Granada A, Lopez-Espinosa MJ, Molina-Molina JM, Fernandez JM, et al. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ Health Perspect. 2007;115(Suppl 1):8–14. doi: 10.1289/ehp.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiol Perspect Innov. 2010;7:2. doi: 10.1186/1742-5573-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Fletcher T, Benavides FG, Orts E. Parental agricultural work and selected congenital malformations. Am J Epidemiol. 1999;149:64–74. doi: 10.1093/oxfordjournals.aje.a009729. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez J, Garcia-Martin M, Nogueras-Ocana M, de Dios Luna-del-Castillo J, Espigares Garcia M, Olea N, et al. Exposure to pesticides and cryptorchidism: geographical evidence of a possible association. Environ Health Perspect. 1996;104:1090–1095. doi: 10.1289/ehp.104-1469503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry VF, Schreinemachers D, Harkins ME, Griffth J. Pesticide appliers, biocides, and birth defects in rural Minnesota. Environ Health Perspect. 1996;104:394–399. doi: 10.1289/ehp.96104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE, Burroughs BL. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect. 2002;110(Suppl 3):441–449. doi: 10.1289/ehp.02110s3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari L, Paris F, Jandel C, Kalfa N, Orsini M, Daures JP, et al. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Hum Reprod. 2011;26:3155–3162. doi: 10.1093/humrep/der283. [DOI] [PubMed] [Google Scholar]
- Geschwind SA, Stolwijk JA, Bracken M, Fitzgerald E, Stark A, Olsen C, et al. Risk of congenital malformations associated with proximity to hazardous waste sites. Am J Epidemiol. 1992;135:1197–1207. doi: 10.1093/oxfordjournals.aje.a116226. [DOI] [PubMed] [Google Scholar]
- Giordano F, Abballe A, De Felip E, di Domenico A, Ferro F, Grammatico P, et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol. 2010;88:241–250. doi: 10.1002/bdra.20657. [DOI] [PubMed] [Google Scholar]
- Grandjean P. Biomarkers in epidemiology. Clin Chem. 1995;41:1800–1803. [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates. Washington, DC: 2011. [Google Scholar]
- Heeren GA, Tyler J, Mandeya A. Agricultural chemical exposures and birth defects in the Eastern Cape Province, South Africa: a case-control study. Environ Health. 2003;2:11. doi: 10.1186/1476-069X-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AF, Parron T, Tsatsakis AM, Requena M, Alarcon R, Lopez-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Jorgensen KT, Jensen MS, Toft GV, Larsen AD, Bonde JP, Hougaard KS. Risk of cryptorchidism among sons of horticultural workers and farmers in Denmark. Scand J Work Environ Health. 2014;40:323–330. doi: 10.5271/sjweh.3399. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Paris F, Philibert P, Orsini M, Broussous S, Fauconnet-Servant N, et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur Urol. 2015;68:1023–1030. doi: 10.1016/j.eururo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Kalra S, Dewan P, Batra P, Sharma T, Tyagi V, Banerjee BD. Organochlorine pesticide exposure in mothers and neural tube defects in offsprings. Reprod Toxicol. 2016;66:56–60. doi: 10.1016/j.reprotox.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480–484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Hurst V, Konradsdottir E, Einarson A. Pregnancy outcome following exposure to permethrin and use of teratogen information. Am J Perinatol. 2005;22:87–90. doi: 10.1055/s-2005-837736. [DOI] [PubMed] [Google Scholar]
- Kielb C, Lin S, Herdt-Losavio M, Bell E, Chapman B, Rocheleau CM, et al. Maternal periconceptional occupational exposure to pesticides and selected musculoskeletal birth defects. Int J Hyg Environ Health. 2014;217:248–254. doi: 10.1016/j.ijheh.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube A. Pesticide Industry Sales and Usage: 2000 and 2001 Market Estimates. Washington, DC: 2004. [Google Scholar]
- Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology. 1997;8:537–544. doi: 10.1097/00001648-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Lacasana M, Vazquez-Grameix H, Borja-Aburto VH, Blanco-Munoz J, Romieu I, Aguilar-Garduno C, et al. Maternal and paternal occupational exposure to agricultural work and the risk of anencephaly. Occup Environ Med. 2006;63:649–656. doi: 10.1136/oem.2005.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Marshall EG, Davidson GK. Potential parental exposure to pesticides and limb reduction defects. Scand J Work Environ Health. 1994;20:166–179. doi: 10.5271/sjweh.1412. [DOI] [PubMed] [Google Scholar]
- Loffredo CA, Silbergeld EK, Ferencz C, Zhang J. Association of transposition of the great arteries in infants with maternal exposures to herbicides and rodenticides. Am J Epidemiol. 2001;153:529–536. doi: 10.1093/aje/153.6.529. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol. 2002;155:313–322. doi: 10.1093/aje/155.4.313. [DOI] [PubMed] [Google Scholar]
- Makelarski JA, Romitti PA, Rocheleau CM, Burns TL, Stewart PA, Waters MA, et al. Maternal periconceptional occupational pesticide exposure and neural tube defects. Birth Defects Res A Clin Mol Teratol. 2014;100:877–886. doi: 10.1002/bdra.23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoulakis C, Antypas S, Stamatiadou A, Demetriadis D, Kanakas N, Loutradis D, et al. Cryptorchidism: seasonal variations in Greece do not support the theory of light. Andrologia. 2002;34:194–203. doi: 10.1046/j.1439-0272.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- Mamoulakis C, Avgenakis G, Gkatzoudi C, Duyker G, Zisis IE, Heretis I, et al. Seasonal trends in the prevalence of hypospadias: Aetiological implications. Exp Ther Med. 2017;13:2960–2968. doi: 10.3892/etm.2017.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EG, Gensburg LJ, Deres DA, Geary NS, Cayo MR. Maternal residential exposure to hazardous wastes and risk of central nervous system and musculoskeletal birth defects. Arch Environ Health. 1997;52:416–425. doi: 10.1080/00039899709602220. [DOI] [PubMed] [Google Scholar]
- McKinnish T, Rees DI, Langlois PH. Seasonality in birth defects, agricultural production and urban location. Econ Hum Biol. 2014;15:120–128. doi: 10.1016/j.ehb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett. 2014;230:146–156. doi: 10.1016/j.toxlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Meyer KJ, Reif JS, Veeramachaneni DN, Luben TJ, Mosley BS, Nuckols JR. Agricultural pesticide use and hypospadias in eastern Arkansas. Environ Health Perspect. 2006;114:1589–1595. doi: 10.1289/ehp.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis M, Tzatzarakis MN, Kovatsi L, Alegakis AK, Tsakalof AK, Heretis I, et al. Hypospadias in offspring is associated with chronic exposure of parents to organophosphate and organochlorine pesticides. Toxicol Lett. 2014;230:139–145. doi: 10.1016/j.toxlet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009:e1000097, 6. [PMC free article] [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Munoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology. 2013;39:158–168. doi: 10.1016/j.neuro.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Petrakis D, Vassilopoulou L, Mamoulakis C, Psycharakis C, Anifantaki A, Sifakis S, et al. Endocrine disruptors leading to obesity and related diseases. Int J Environ Res Public Health. 2017:14. doi: 10.3390/ijerph14101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik FH, Klebanoff MA, Brock JW, Longnecker MP. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and beta-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environ Res. 2007;105:364–369. doi: 10.1016/j.envres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL, Huang Y, Tinker LF, Beresford SA, Lampe JW, Neuhouser ML. Statistical aspects of the use of biomarkers in nutritional epidemiology research. Stat Biosci. 2009;1:112–123. doi: 10.1007/s12561-009-9003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo KM, Warren JL, Meyer RE, Herring AH, Sanders AP, Brownstein NC, et al. Maternal residential exposure to agricultural pesticides and birth defects in a 2003 to 2005 North Carolina birth cohort. Birth Defects Res A Clin Mol Teratol. 2016;106:240–249. doi: 10.1002/bdra.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo M, Munoz N, Day N, Parra JE, Hernandez C, Blettner M, et al. Birth defects among children born to a population occupationally exposed to pesticides in Colombia. Scand J Work Environ Health. 1990;16:239–246. doi: 10.5271/sjweh.1789. [DOI] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, Sanderson WT, Sun L, Lawson CC, Waters MA, et al. Maternal occupational pesticide exposure and risk of hypospadias in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2011;91:927–936. doi: 10.1002/bdra.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CM, Bertke SJ, Lawson CC, Romitti PA, Sanderson WT, Malik S, et al. Maternal occupational pesticide exposure and risk of congenital heart defects in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2015;103:823–833. doi: 10.1002/bdra.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013;43:21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Salazar-Garcia F, Gallardo-Diaz E, Ceron-Mireles P, Loomis D, Borja-Aburto VH. Reproductive effects of occupational DDT exposure among male malaria control workers. Environ Health Perspect. 2004;112:542–547. doi: 10.1289/ehp.112-1241918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreinemachers DM. Birth malformations and other adverse perinatal outcomes in four U.S. Wheat-producing states. Environ Health Perspect. 2003;111:1259–1264. doi: 10.1289/ehp.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. The ‘oestrogen hypothesis’- where do we stand now? Int J Androl. 2003;26:2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Wasserman CR, O’Malley CD, Nelson V, Jackson RJ. Maternal pesticide exposure from multiple sources and selected congenital anomalies. Epidemiology. 1999;10:60–66. [PubMed] [Google Scholar]
- Shaw GM, Yang W, Roberts E, Kegley SE, Padula A, English PB, et al. Early pregnancy agricultural pesticide exposures and risk of gastroschisis among offspring in the San Joaquin Valley of California. Birth Defects Res A Clin Mol Teratol. 2014;100:686–694. doi: 10.1002/bdra.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- de Siqueira MT, Braga C, Cabral-Filho JE, Augusto LG, Figueiroa JN, Souza AI. Correlation between pesticide use in agriculture and adverse birth outcomes in Brazil: an ecological study. Bull Environ Contam Toxicol. 2010;84:647–651. doi: 10.1007/s00128-010-0027-8. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. 2013;42:224–231. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15:631–650. doi: 10.1177/1933719108322436. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Izzo G, Landreh L, Weisser J, Soder O. Endocrine disruptors and Leydig cell function. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/684504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Jonsson BA, Bonde JP, Norgaard-Pedersen B, Hougaard DM, Cohen A, et al. Perfluorooctane sulfonate concentrations in amniotic fluid, biomarkers of fetal Leydig cell function, and cryptorchidism and hypospadias in Danish boys (1980–1996) Environ Health Perspect. 2016;124:151–156. doi: 10.1289/ehp.1409288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environ Health Perspect. 2012;120:478–482. doi: 10.1289/ehp.1103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen HE, Rajpert-De Meyts E, Main KM, Skakkebaek NE, Toppari J. Testicular dysgenesis syndrome and the development and occurrence of male reproductive disorders. Toxicol Appl Pharmacol. 2005;207:501–505. doi: 10.1016/j.taap.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Waller SA, Paul K, Peterson SE, Hitti JE. Agricultural-related chemical exposures, season of conception, and risk of gastroschisis in Washington State. Am J Obstet Gynecol. 2010;202(241):e241–246. doi: 10.1016/j.ajog.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Wang B, Yi D, Jin L, Li Z, Liu J, Zhang Y, et al. Organochlorine pesticide levels in maternal serum and risk of neural tube defects in offspring in Shanxi Province, China: a case-control study. Sci Total Environ. 2014;490:1037–1043. doi: 10.1016/j.scitotenv.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner IS, Moller H, Jensen TK, Skakkebaek NE. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Perspect. 1998;106:793–796. doi: 10.1289/ehp.98106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselak M, Arbuckle TE, Foster W. Pesticide exposures and developmental outcomes: the epidemiological evidence. J Toxicol Environ Health B Crit Rev. 2007;10:41–80. doi: 10.1080/10937400601034571. [DOI] [PubMed] [Google Scholar]
- Weselak M, Arbuckle TE, Wigle DT, Walker MC, Krewski D. Pre- and post-conception pesticide exposure and the risk of birth defects in an Ontario farm population. Reprod Toxicol. 2008;25:472–480. doi: 10.1016/j.reprotox.2008.05.060. [DOI] [PubMed] [Google Scholar]
- WHO/CDC/ICBDSR. Birth Defects Surveillance: Atlas of Selected Congenital Anomalies. Geneva: 2014. [Google Scholar]
- Winchester PD, Huskins J, Ying J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009;98:664–669. doi: 10.1111/j.1651-2227.2008.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Resources Institute Pesticides and the Immune System. [Accessed date: 31 January 2018];The Public Health Risks. Available online: http://www.wri.org/publication/pesticides-and-immune-system.
- Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76:706–713. doi: 10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- Yang W, Zeng L, Cheng Y, Chen Z, Wang X, Li X, et al. The effects of periconceptional risk factor exposure and micronutrient supplementation on birth defects in Shaanxi Province in Western China. PLoS One. 2012;7:e53429. doi: 10.1371/journal.pone.0053429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB, et al. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am J Epidemiol. 2014;179:740–748. doi: 10.1093/aje/kwt324. [DOI] [PMC free article] [PubMed] [Google Scholar]