Abstract
In recent years, Epstein‐Barr virus (EBV) lytic infection has been shown to significantly contribute to carcinogenesis. Thus, therapies aimed at targeting the EBV lytic cycle have been developed as novel strategies for treatment of EBV‐associated malignancies. In this review, focusing on the viral lytic proteins, we describe recent advances regarding the involvement of the EBV lytic cycle in carcinogenesis. Moreover, we further discuss 2 distinct EBV lytic cycle‐targeted therapeutic strategies against EBV‐induced malignancies. One of the strategies involves inhibition of the EBV lytic cycle by natural compounds known to have anti‐EBV properties; another is to intentionally induce EBV lytic replication in combination with nucleotide analogues. Recent advances in EBV lytic‐based strategies are beginning to show promise in the treatment and/or prevention of EBV‐related tumors.
Keywords: carcinogenesis, EBV lytic replication, lytic induction, natural compound, vaccine
1. INTRODUCTION
Epstein‐Barr virus (EBV) is one of the most common human viruses and infects approximately 95% of the world's population. EBV was the first human tumor virus to be identified, accounting for more than 200 000 cases of cancer each year, and its infection is associated with 1.8% of all cancer deaths.1 Currently, EBV is known to be strongly associated with the development of several human cancers, including nasopharyngeal carcinoma (NPC), Burkitt's lymphoma (BL), Hodgkin's lymphoma, and gastric cancer.2
Similar to all herpesviruses, EBV establishes 2 alternative modes of infection in its host, latent and lytic. During latency, EBV expresses only a limited number of products. Years of research results have shown that latent infection plays an essential role in carcinogenesis. Several latent proteins, such as LMP1, LMP2A, and EBNA1, are considered to show obvious carcinogenic activities.3 Upon reactivation induced by various stimuli, EBV undergoes 3 consecutive lytic stages, including immediate early (IE), early (E), and late (L) stages. The IE proteins, Zta and Rta, activate transcription from the promoters of lytic E genes, which trigger EBV genomic DNA replication from the lytic origin of replication, oriLyt. Then the lytic L genes that encode structural proteins are expressed, followed by viral genome packaging into infectious virion particles. In recent years, the EBV lytic cycle has been reported to contribute to carcinogenesis of several cancer types. In the present study, we highlight recent findings related to the roles of EBV lytic proteins in carcinogenesis. Furthermore, we discuss therapeutic strategies aimed at targeting the viral lytic cycle for patients with EBV‐associated malignancies.
2. EPSTEIN‐BARR VIRUS LYTIC PROTEINS IN CARCINOGENESIS
Accumulating data suggest the contribution of the EBV lytic cycle to carcinogenesis through the induction of oncogenic cytokine secretion and genome instability.4, 5 A small subset of lytically infected cells is commonly detected in clinical tissue samples of EBV‐associated malignancies,6, 7 suggesting a potential role for viral lytic replication in promoting tumor growth in vivo. Fang et al8 first reported that compared to latent infection, the recurrent induction of the EBV lytic cycle contributed more profoundly to NPC carcinogenesis. Also, epidemiological studies have shown that individuals with elevated antibody titers against EBV lytic antigens (viral capsid antibody [VCA] and early antigen [EA] antibody) and increased plasma EBV DNA load have a higher risk of developing NPC.9, 10 Recently, detecting the titers of IgA antibodies against VCA and Zta in serum has been widely studied and even used in clinical practice for NPC diagnosis and screening in high‐risk populations.11, 12
During the lytic cycle, EBV expresses a series of intriguing proteins. These proteins show homology to a wide variety of cytokines and anti‐apoptotic proteins. They can also enhance genomic instability or target tumor suppressors, thereby making a significant contribution to human pathology (Table 1).5, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
Table 1.
Overview of EBV lytic proteins and oncogenic functions
| EBV lytic proteins | Lytic phase | Oncogenic functions | References |
|---|---|---|---|
| Zta | IE | Induces production of oncogenic cytokinesand VEGF | 13, 14, 15, 16, 17 |
| BHRF1 | E | Anti‐apoptotic function | 18, 19 |
| BALF1 | E | Anti‐apoptotic function | 20, 21, 22 |
| BARF1 | E | Malignant transformation and anti‐apoptotic activity | 23, 24, 25 |
| BGLF4 and BGLF5 | E | Leads to genomic instability | 26, 27 |
| BALF3 | E | Induces genomic instability | 5, 28 |
| BCRF1 | E | Homologue of cellular IL‐10 | 29 |
| BILF1 | E | Transforms cells and induces VEGF secretion | 30, 31 |
E, early; EBV, Epstein‐Barr virus; IE, immediate early; IL‐10, interleukin‐10; VEGF, vascular endothelial growth factor.
2.1. Zta
Zta is a transcription factor that binds to and activates the EBV lytic E gene promoters, thereby triggering EBV reactivation from latency. Enhanced Zta expression has been shown to cause lymphomas in vivo.13 Zta contributes to secretion of immunosuppressive cytokines, including interleukin‐10 (IL‐10)14 and IL‐13,15 and also plays an important role in tumor growth through release of vascular endothelial growth factor (VEGF).16 Beyond that, Zta reportedly inhibits tumor necrosis factor alpha (TNFα)‐induced apoptosis by downregulating TNF receptor 1 (TNF‐R1).17
2.2. BHRF1
BHRF1 is an early lytic protein that shows both sequence and functional homology to the human anti‐apoptotic protein Bcl‐2 and inhibits apoptosis of EBV‐positive cancer cells. Recent studies suggest that BHRF1 exerts its anti‐apoptotic activity by interacting with the pro‐apoptotic BH3‐only protein, Bcl‐2 interacting mediator (Bim), and by upregulating Bcl‐2 to amplify the anti‐apoptotic effect.18 Inducible BHRF1 expression protects BL cells from apoptosis, which contributes to EBV‐associated lymphomagenesis in vivo.19
2.3. BALF1
In addition to BHRF1, the EBV genome encodes a second Bcl‐2 homolog, BALF1. In an earlier study, Marshall et al20 showed that BALF1 suppresses apoptosis by associating with 2 cellular pro‐apoptotic proteins, Bax and Bak. Recently, BALF1 was shown to increase cell survival by suppressing serum starvation‐induced apoptosis,21 thereby playing an important role in oncogenesis of EBV‐associated tumors. BALF1 also enhances cellular metastasis and invasion in vitro and in vivo, which suggests a key role for BALF1 in facilitating the potential of increased tumor metastasis.22
2.4. BARF1
BARF1, reported as an early lytic gene,23 is a homologue to the colony‐stimulating factor‐1 (CSF‐1) receptor. Sakka et al24showed that BARF1 is capable of inducing malignant transformation in human B cell lines and rodent fibroblasts. They then found that BARF1 leads to activation of the cell cycle from passage of G1 to S phase in nude mice, suggesting that it could act as a growth factor to induce tumor formation in vivo.24 Moreover, BARF1 promotes cancer cell survival by activating the expression of the anti‐apoptotic Bcl‐2 protein.25
2.5. BGLF4 and BGLF5
The lytic E genes, BGLF4 and BGLF5, encode a Ser/Thr kinase and an alkaline DNase, respectively. Recent studies indicate that both BGLF4 and BGLF5 induce the DNA damage signal that eventually leads to genomic instability, which consequently contributes to the carcinogenesis of human epithelial cells.26, 27
2.6. BALF3
BALF3 is a homologue of the enzyme, terminase, which is involved in viral DNA synthesis and packaging.28 Chiu et al5 provided evidence showing that BALF3 contributes effectively to the induction of genomic instability and mediates NPC relapse by enhancing the expression of the oncogenes, EVI1, FIGF, SOX2 and TP63.
2.7. BCRF1
BCRF1 is a homologue of cellular IL‐10. Recently, Han et al29 provided evidence showing that the distribution of the vIL‐10 variant types is tumor‐specific and is found in lymphoma, NPC, and EBV‐associated gastric carcinoma (EBVaGC), implying a pathogenic role in these diseases.
2.8. BILF1
BILF1 is an EBV‐encoded constitutively active G protein‐coupled receptor (GPCR) that modulates various intracellular signaling pathways, including CRE (cAMP response element) and nuclear factor kappa B (NF‐κB), through Gαi.30 Lyngaa et al31 suggested that BILF1 can transform cells and induce VEGF secretion in a constitutively active way. Moreover, BILF1 promotes tumor formation in 90% of cases in nude mice, suggesting that BILF1 could be involved in the pathogenesis of EBV‐associated malignancies.31
3. INHIBITORY EFFECTS OF SMALL MOLECULES ON EBV LYTIC REPLICATION
By inhibiting the EBV lytic cycle, anti‐EBV agents may block the transmission of the virus from cell to cell and are therefore valuable in chemoprevention or clinical treatment of EBV‐associated malignancies. This leads to the search for new compounds that may prevent EBV lytic replication. Until now, many small molecules have been identified to effectively suppress EBV lytic replication. Earlier studies demonstrated that nucleoside analogs can inhibit EBV lytic replication.32 Daigle et al recently reported that EBV reactivation can be blocked by valproic acid (VPA)33 and its amide derivative valpromide (VPM)34 in B lymphoma cells. The inhibitory effect of the multifunction drug maribavir (MBV) on EBV lytic replication is produced mainly by attenuating viral DNA replication as well as by suppressing EBV lytic gene expression.35 Rapamycin, a specific inhibitor of mTOR activity, was reported to decrease EBV lytic gene expression in B cell lines, but not in epithelial cell lines.36
In contrast to chemicals, compounds from natural sources are more attractive for inhibiting the EBV lytic cycle. Before 2000, only genistein, found in soy, was reported to downregulate Zta expression in Akata cells.37 Currently, a wide variety of active phytochemicals or dietary ingredients have been identified as inhibitors of the EBV lytic cycle (Table 2).7, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50
Table 2.
Anti‐EBV reagents based on natural products
| Natural compounds family | Mechanisms of anti‐EBV action | References | |
|---|---|---|---|
| Polyphenolics | EGCG | Inhibits the MEK/ERK1/2 and PI3‐K/Akt pathways | 7, 38 |
| Curcumin | Inhibits BZLF1 gene transcription | 39 | |
| Resveratrol | Suppresses the activation of the redox‐sensitive transcription factors NF‐κB and AP‐1 | 40 | |
| Sulforaphane | Reduces the transactivation activity of the BRLF1 gene | 41 | |
| Flavonoids | Genistein | Blocks the activation of IgG‐mediated tyrosine kinase | 37 |
| Protoapigenone | Reduces the transcriptional function of Zta | 43 | |
| Luteolin | Represses the promoter activities of the BZLF1 and BRLF1 genes | 44 | |
| Terpenoids | Andrographolide | Inhibits Zta and Rta expression | 45 |
| Moronic acid | Suppresses the transactivation function of Rta | 46 | |
| Lignans | Emodin | Inhibits EBV IE protein expression and DNA replication | 47 |
| Saponins | Glycyrrhizic acid | Inhibits EBV IE protein expression and DNA replication | 48 |
| Adenosine analogues | Cordycepin | Suppresses EBV lytic replication | 49 |
| Antioxidants | Vitamin C | Reduces EBV EA IgG and VCA IgM antibody levels | 50 |
AP‐1, activator protein 1; EA, early antigen; EBV, Epstein‐Barr virus; EGCG, epigallocatechin gallate; IE, immediate early; NF‐κB, nuclear factor kappa B; VCA, viral capsid antigen.
3.1. Polyphenolics
Chang et al38 first reported the inhibitory effect of epigallocatechin gallate (EGCG) on the EBV lytic cycle. Using NPC and lymphoma as models, our group showed that the inhibition of EBV spontaneous lytic replication by EGCG involves suppression of the MEK/ERK1/2 and PI3‐K/Akt pathways.7 Curcumin, the main yellow bioactive component of turmeric, suppresses BZLF1 gene transcription.39 Resveratrol (RV) was shown to inhibit EBV lytic gene expression and production of viral particles by suppressing the activation of redox‐sensitive transcription factors NF‐κB and activator protein 1(AP‐1).40 At the concentrations used for inhibition of EBV reactivation, RV inhibits the proliferation of BL cells without increasing cell death.40 The histone deacetylase (HDAC) inhibitor sulforaphane (SFN) has the potential to be consumed as a dietary compound for prevention of EBV reactivation by reducing the transcription of the BRLF1 gene.41
3.2. Flavonoids
Koyama et al42 first found a correlation between the antioxidant potential of flavonoids and inhibition of EBV lytic replication. Protoapigenone reduces the ability of Zta but not Rta to activate transcription from lytic promoters in B lymphoma cells.43 A recent study indicated that luteolin could inhibit EBV reactivation by decreasing the promoter activities of both BZLF1 and BRLF1 genes, and thus repress NPC tumorigenesis in a mouse model.44
3.3. Terpenoids
Andrographolide is extracted from Andrographis paniculata. Lin and colleagues reported that andrographolide effectively inhibited EBV lytic replication by reducing the expression of Zta and Rta in P3HR1 cells.45 Moronic acid, found in the galls of Rhus chinensis and Brazilian propolis, suppresses the ability of Rta but not Zta to transactivate lytic early promoters.46
3.4. Lignans and saponins
Lignans such as emodin47 and saponins such as glycyrrhizic acid48 were also reported to inhibit the expression of EBV IE proteins and EBV DNA replication.
Additionally, cordyceps has been shown to be a novel chemical suppressor of EBV lytic reactivation.49 A recent clinical study suggests that high‐dose vitamin C results in the reduction of EBV EA IgG and VCA IgM antibody levels in patients with EBV infection.50
4. LYTIC‐INDUCTION THERAPY FOR EBV‐POSITIVE TUMORS
It is already well established that expression of the herpes simplex virus thymidine kinase gene allows tumor cells to be killed by ganciclovir (GCV).51 During the lytic phase of infection, EBV expresses 2 specific kinases, thymidine kinase (TK) and protein kinase (PK). EBV‐PK rather than EBV‐TK phosphorylates and converts the prodrug GCV into its active, cytotoxic form in EBV‐infected cells.52 Active GCV interferes with the viral and host DNA polymerase to lead to early termination of DNA replication and cell death of tumor cells, which enables exposure of EBV to host immune surveillance. Also, phosphorylated GCV can be transferred to adjacent cells, thus inducing “bystander” killing.53 Thus, the intentional induction of the EBV lytic cycle in EBV‐positive tumor cells may be developed as a novel therapy for EBV‐related tumors (Figure 1).
Figure 1.
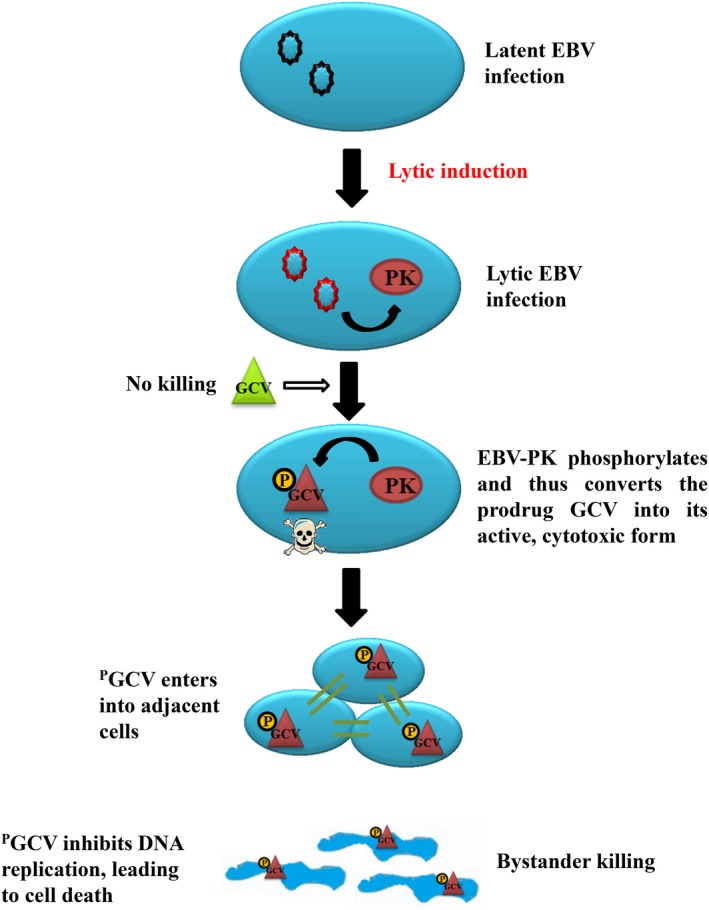
Lytic‐inducing therapy for Epstein‐Barr virus (EBV)‐positive tumors. Upon reactivation by lytic‐inducing agents, EBV enters into the lytic form of infection. During the lytic cycle, EBV expresses EBV‐PK, a Ser/Thr protein kinase, which phosphorylates and thus converts the prodrug, ganciclovir (GCV), into its active, cytotoxic form in EBV‐infected cells. Phosphorylated GCV (PGCV) interferes with host DNA polymerase, leading to early termination of DNA replication and thus cell death. Phosphorylated GCV can be transferred to adjacent cells, thus inducing “bystander” killing. The intentional induction of the EBV lytic cycle can enhance GCV‐induced cytotoxicity, which has been developed as a novel therapy against EBV‐positive tumors
For this purpose, searching for agents that induce EBV reactivation seems imperative. Diverse small molecules have been identified as stimulators of EBV lytic replication (Table 3). For example, TPA, sodium butyrate (SB),54 trichostatin A (TSA),55 and 5‐aza‐2′‐deoxycytidine (5‐AZA)56 serve as common agents to induce EBV lytic gene expression. TPA, an agonist of protein kinase C (PKC), ultimately promotes activation of transcription factors of BZLF1 and BRLF1 genes.57 Epigenetic inhibitors such as SB, TSA, and 5‐AZA can open chromatin at promoters of both genes, accounting for the effective and widely used strategy to induce the EBV lytic cycle by TPA in combination with the epigenetic inhibitors. Recent studies have shown that various specific anti‐tumor agents induce EBV lytic replication and, by doing so, sensitize cancer cells to nucleoside antiviral agents. Suberoylanilide hydroxamic acid (SAHA), a HDAC inhibitor, promotes EBV reactivation by disrupting BZLF1 and BRLF1 gene silencing to facilitate the access of transcription factors, and the addition of GCV enhances the ability to kill EBV‐positive lymphoma cells in vitro and in lymphoma‐bearing nude mice.58, 59 A series of apoptotic inducers, including gemcitabine, doxorubicin, etoposide, and icaritin, have been reported to induce EBV reactivation. Both gemcitabine and doxorubicin activate transcription from promoters of the BZLF1 and BRLF1 genes, and the effects require the early growth response protein 1 (EGR‐1) motif in the BRLF1 promoter and the CRE (ZII) and myocyte enhancer factor 2D (MEF‐2D) (ZI) binding sites in the BZLF1 promoter.60, 61 The p38 MAPK, PI3K, and MAPK/ERK kinase (MEK) signaling pathways are also involved in the induction of EBV lytic replication. It has been demonstrated that etoposide causes EBV reactivation in Akata cells.61 Icaritin induces the expression of EBV lytic genes by inhibiting the LMP1‐mediated STAT3 and Akt pathways.62 Rituximab and dexamethasone synergistically induce lytic EBV infection, rendering EBV‐positive lymphoma cells more susceptible to GCV cytotoxicity in vitro and in vivo.63 Aspirin also plays an important role in inducing high levels of EBV lytic replication. Results from Liu et al64 showed that aspirin upregulates lytic gene expression by suppressing the activity of NF‐κB, a corepressor of Zta. They also showed that treatment with aspirin in combination with GCV amplifies the cytotoxic effect in B95.8 and Raji cells. From 66 840 small molecule compounds, Tikhmyanova and colleagues recently identified 5 tetrahydrocarboline derivatives, referred to as C09, C50, C53, C60, and C67, that efficiently reactivate latent EBV in a variety of cell types. Importantly, these 5 compounds show promise for lytic therapy in combination with GCV.65 Choi et al66 carried out high‐throughput screening of a chemical library of 50 240 small organic compounds to identify the strongest inducers of the EBV lytic cycle in EBV‐positive epithelial malignancies.
Table 3.
Agonists of the EBV lytic cycle
| Agonists category | Mechanisms of lytic‐inducing action | References | |
|---|---|---|---|
| Epigenetic inhibitors | SB, TSA, and 5‐AZA | Opening chromatin at promoters of the BZLF1 and BRLF1 genes | 54, 55, 56 |
| SAHA | Promoting EBV reactivation by disrupting BZLF1 and BRLF1 gene silencing | 58, 59 | |
| Phorbol esters | TPA | An agonist of PKC, ultimately promoting activation of transcription factors of BZLF1 and BRLF1 genes | 57 |
| Apoptotic inducers | Gemcitabine and doxorubicin | Activating transcription of the BZLF1 and BRLF1 genes by upregulating expression of EGR‐1, CRE, and MEF‐2D | 60, 61 |
| Etoposide | Inducing EBV reactivation | 61 | |
| Icaritin | Inducing the expression of EBV lytic genes by inhibiting the LMP1‐mediated STAT3 and Akt pathways | 62 | |
| Rituximab and dexamethasone | Synergistic induction of Zta and EA‐D expressions | 63 | |
| Anti‐inflammatory agent | Aspirin | Upregulating lytic gene expression by suppressing the activity of NF‐κB, a corepressor of Zta | 64 |
| Tetrahydrocarboline derivatives | C09, C50, C53, C60, and C67 | Reactivating EBV lytic markers Zta and EA‐D in all EBV‐positive cell lines | 65 |
| Small organic compounds | E7, E11, C7, C8, and A10 | Induction of lytic proteins involves activation of PKCδ or/and JNK MAPK | 66 |
5‐AZA, 5‐aza‐2′‐deoxycytidine; CRE, cAMP response element; EBV, Epstein‐Barr virus; EGR‐1, early growth response protein 1; MEF‐2D, myocyte enhancer factor 2D; NF‐κB, nuclear factor kappa B; PKC, protein kinase C; SAHA, suberoylanilide hydroxamic acid; SB, sodium butyrate; TSA, trichostatin A.
5. CONCLUSIONS
Because EBV is a common human tumor virus, understanding the function of EBV‐encoded products such as lytic proteins in EBV‐associated malignancies is clearly essential for determining the role of viral lytic infection in carcinogenesis. Hence, the presence of EBV infection in malignancies allows the development of lytic cycle‐targeted therapeutic strategies. Anti‐EBV compounds, especially natural compounds, have been developed to be useful for the prevention of EBV‐induced carcinogenesis. Moreover, intentional induction of lytic replication combined with prodrug treatment, such as GCV, has been regarded as a novel strategy for the treatment of EBV‐positive tumors. Notably, recurrent EBV reactivation through deregulated expression of Zta or Rta might also be a potential therapy against EBV‐related tumors. These strategies show great potential in the prevention and/or treatment of EBV‐associated malignancies in the future.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China/National Institutes of Health pilot project grant (81161120410 to Y.C.), the National Natural Science Foundation of China grant (81672705 to Y.C.), the Innovation Foundation of Central South University grant (2013zzts072 to H.L.), the State Key Laboratory of Medicinal Chemical Biology grant (201611001 to X.L.).
Li H, Hu J, Luo X, Bode AM, Dong Z, Cao Y. Therapies based on targeting Epstein‐Barr virus lytic replication for EBV‐associated malignancies. Cancer Sci. 2018;109:2101–2108. https://doi.org/10.1111/cas.13634
Funding information
Collaborative Innovation Center for Chemistry and Molecular Medicine of Hunan Province, China, State Key Laboratory of Medicinal Chemical Biology (201611001), National Natural Science Foundation of China/National Institutes of Health Pilot Project (81161120410), National Natural Science Foundation of China (81672705), Innovation Foundation of Central South University (2013zzts072)
REFERENCES
- 1. Khan G, Hashim MJ. Global burden of deaths from Epstein‐Barr virus attributable malignancies 1990‐2010. Infect Agents Cancer. 2014;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young LS, Rickinson AB. Epstein‐Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757‐768. [DOI] [PubMed] [Google Scholar]
- 3. Lee DY, Sugden B. The latent membrane protein 1 oncogene modifies B‐cell physiology by regulating autophagy. Oncogene. 2008;27:2833‐2842. [DOI] [PubMed] [Google Scholar]
- 4. Fang CY, Huang SY, Wu CC, et al. The synergistic effect of chemical carcinogens enhances Epstein‐Barr virus reactivation and tumor progression of nasopharyngeal carcinoma cells. PLoS ONE. 2012;7:e44810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu SH, Wu CC, Fang CY, et al. Epstein‐Barr virus BALF3 mediates genomic instability and progressive malignancy in nasopharyngeal carcinoma. Oncotarget. 2014;5:8583‐8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng XH, Lu LX, Li XZ, Jia WH. Quantification of Epstein‐Barr virus DNA load in nasopharyngeal brushing samples in the diagnosis of nasopharyngeal carcinoma in southern China. Cancer Sci. 2015;106:1196‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu S, Li H, Chen L, et al. (‐)‐Epigallocatechin‐3‐gallate inhibition of Epstein‐Barr virus spontaneous lytic infection involves ERK1/2 and PI3‐K/Akt signaling in EBV‐positive cells. Carcinogenesis. 2013;34:627‐637. [DOI] [PubMed] [Google Scholar]
- 8. Fang CY, Lee CH, Wu CC, et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer. 2009;124:2016‐2025. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Huang Q, Liu W, et al. Establishment of VCA and EBNA1 IgA‐based combination by enzyme‐linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two‐stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. 2012;131:406‐416. [DOI] [PubMed] [Google Scholar]
- 10. Jia WH, Qin HD. Non‐viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22:117‐126. [DOI] [PubMed] [Google Scholar]
- 11. Xu FH, Xiong D, Xu YF, et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein‐Barr virus activation. J Natl Cancer Inst. 2012;104:1396‐1410. [DOI] [PubMed] [Google Scholar]
- 12. Hsu WL, Chen JY, Chien YC, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20‐year follow‐up study on 9622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18:1218‐1226. [DOI] [PubMed] [Google Scholar]
- 13. Ma SD, Yu X, Mertz JE, et al. An Epstein‐Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol. 2012;86:7976‐7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CH, Yeh TH, Lai HC, et al. Epstein‐Barr virus Zta‐induced immunomodulators from nasopharyngeal carcinoma cells upregulate interleukin‐10 production from monocytes. J Virol. 2011;85:7333‐7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsai SC, Lin SJ, Chen PW, et al. EBV Zta protein induces the expression of interleukin‐13, promoting the proliferation of EBV‐infected B cells and lymphoblastoid cell lines. Blood. 2009;114:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong GK, Kumar P, Wang L, et al. Epstein‐Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J Virol. 2005;79:13984‐13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morrison TE, Mauser A, Klingelhutz A, Kenney SC. Epstein‐Barr virus immediate‐early protein BZLF1 inhibits tumor necrosis factor alpha‐induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J Virol. 2004;78:544‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milian E, Prats E, Cairo JJ, Godia F, Vives J. BHRF1 exerts an antiapoptotic effect and cell cycle arrest via Bcl‐2 in murine hybridomas. J Biotechnol. 2015;209:58‐67. [DOI] [PubMed] [Google Scholar]
- 19. Kelly GL, Long HM, Stylianou J, et al. An Epstein‐Barr virus anti‐apoptotic protein constitutively expressed in transformed cells and implicated in burkitt lymphomagenesis: the Wp/BHRF1 link. PLoS Pathog. 2009;5:e1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marshall WL, Yim C, Gustafson E, et al. Epstein‐Barr virus encodes a novel homolog of the bcl‐2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol. 1999;73:5181‐5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cabras G, Decaussin G, Zeng Y, et al. Epstein‐Barr virus encoded BALF1 gene is transcribed in Burkitt's lymphoma cell lines and in nasopharyngeal carcinoma's biopsies. J Clin Virol. 2005;34:26‐34. [DOI] [PubMed] [Google Scholar]
- 22. Hsu WL, Chung PJ, Tsai MH, Chang CL, Liang CL. A role for Epstein‐Barr viral BALF1 in facilitating tumor formation and metastasis potential. Virus Res. 2012;163:617‐627. [DOI] [PubMed] [Google Scholar]
- 23. Sun L, Che K, Zhao Z, Liu S, Xing X, Luo B. Sequence analysis of Epstein‐Barr virus (EBV) early genes BARF1 and BHRF1 in NK/T cell lymphoma from Northern China. Virol J. 2015;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakka E, Zur Hausen A, Houali K, Liu H, Fiorini S, Ooka T. Cellular localization of BARF1 oncoprotein and its cell stimulating activity in human epithelial cell. Virus Res. 2013;174:8‐17. [DOI] [PubMed] [Google Scholar]
- 25. Wang Q, Tsao SW, Ooka T, et al. Anti‐apoptotic role of BARF1 in gastric cancer cells. Cancer Lett. 2006;238:90‐103. [DOI] [PubMed] [Google Scholar]
- 26. Wu CC, Liu MT, Chang YT, et al. Epstein‐Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010;38:1932‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang YH, Lee CP, Su MT, et al. Epstein‐Barr virus BGLF4 kinase retards cellular S‐phase progression and induces chromosomal abnormality. PLoS ONE. 2012;7:e39217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiu SH, Wu MC, Wu CC, et al. Epstein‐Barr virus BALF3 has nuclease activity and mediates mature virion production during the lytic cycle. J Virol. 2014;88:4962‐4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han L, Sun L, Zhao Z, et al. Sequence variation of Epstein‐Barr virus (EBV) BCRF1 in lymphomas in non‐endemic areas of nasopharyngeal carcinoma. Arch Virol. 2015;160:441‐445. [DOI] [PubMed] [Google Scholar]
- 30. Paulsen SJ, Rosenkilde MM, Eugen‐Olsen J, Kledal TN. Epstein‐Barr virus‐encoded BILF1 is a constitutively active G protein‐coupled receptor. J Virol. 2005;79:536‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyngaa R, Norregaard K, Kristensen M, Kubale V, Rosenkilde MM, Kledal TN. Cell transformation mediated by the Epstein‐Barr virus G protein‐coupled receptor BILF1 is dependent on constitutive signaling. Oncogene. 2010;29:4388‐4398. [DOI] [PubMed] [Google Scholar]
- 32. Meerbach A, Holy A, Wutzler P, De Clercq E, Neyts J. Inhibitory effects of novel nucleoside and nucleotide analogues on Epstein‐Barr virus replication. Antivir Chem Chemoth. 1998;9:275‐282. [DOI] [PubMed] [Google Scholar]
- 33. Daigle D, Gradoville L, Tuck D, et al. Valproic acid antagonizes the capacity of other histone deacetylase inhibitors to activate the Epstein‐barr virus lytic cycle. J Virol. 2011;85:5628‐5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gorres KL, Daigle D, Mohanram S, McInerney GE, Lyons DE, Miller G. Valpromide inhibits lytic cycle reactivation of Epstein‐Barr virus. MBio. 2016;7:e00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitehurst CB, Sanders MK, Law M, et al. Maribavir inhibits Epstein‐Barr virus transcription through the EBV protein kinase. J Virol. 2013;87:5311‐5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adamson AL, Le BT, Siedenburg BD. Inhibition of mTORC1 inhibits lytic replication of Epstein‐Barr virus in a cell‐type specific manner. Virol J. 2014;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daibata M, Mellinghoff I, Takagi S, Humphreys RE, Sairenji T. Effect of genistein, a tyrosine kinase inhibitor, on latent EBV activation induced by cross‐linkage of membrane IgG in Akata B cells. J Immunol. 1991;147:292‐297. [PubMed] [Google Scholar]
- 38. Chang LK, Wei TT, Chiu YF, et al. Inhibition of Epstein‐Barr virus lytic cycle by (‐)‐epigallocatechin gallate. Biochem Biophys Res Commun. 2003;301:1062‐1068. [DOI] [PubMed] [Google Scholar]
- 39. Hergenhahn M, Soto U, Weninger A, et al. The chemopreventive compound curcumin is an efficient inhibitor of Epstein‐Barr virus BZLF1 transcription in Raji DR‐LUC cells. Mol Carcinogen. 2002;33:137‐145. [DOI] [PubMed] [Google Scholar]
- 40. De Leo A, Arena G, Lacanna E, Oliviero G, Colavita F, Mattia E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt's lymphoma cells by affecting multiple molecular targets. Antivir Res. 2012;96:196‐202. [DOI] [PubMed] [Google Scholar]
- 41. Wu CC, Chuang HY, Lin CY, et al. Inhibition of Epstein‐Barr virus reactivation in nasopharyngeal carcinoma cells by dietary sulforaphane. Mol Carcinogen. 2013;52:946‐958. [DOI] [PubMed] [Google Scholar]
- 42. Koyama J, Morita I, Kobayashi N, et al. Correlation between oxidation potentials and inhibitory effects on Epstein‐Barr virus activation of flavonoids. Cancer Lett. 2008;263:61‐66. [DOI] [PubMed] [Google Scholar]
- 43. Tung CP, Chang FR, Wu YC, Chuang DW, Hunyadi A, Liu ST. Inhibition of the Epstein‐Barr virus lytic cycle by protoapigenone. J Gen Virol. 2011;92:1760‐1768. [DOI] [PubMed] [Google Scholar]
- 44. Wu CC, Fang CY, Hsu HY, et al. EBV reactivation as a target of luteolin to repress NPC tumorigenesis. Oncotarget. 2016;7:18999‐19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin TP, Chen SY, Duh PD, Chang LK, Liu YN. Inhibition of the epstein‐barr virus lytic cycle by andrographolide. Biol Pharm Bull. 2008;31:2018‐2023. [DOI] [PubMed] [Google Scholar]
- 46. Chang FR, Hsieh YC, Chang YF, Lee KH, Wu YC, Chang LK. Inhibition of the Epstein‐Barr virus lytic cycle by moronic acid. Antivir Res. 2010;85:490‐495. [DOI] [PubMed] [Google Scholar]
- 47. Yiu CY, Chen SY, Yang TH, et al. Inhibition of Epstein‐Barr virus lytic cycle by an ethyl acetate subfraction separated from Polygonum cuspidatum root and its major component, emodin. Molecules. 2014;19:1258‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin JC. Mechanism of action of glycyrrhizic acid in inhibition of Epstein‐Barr virus replication in vitro. Antivir Res. 2003;59:41‐47. [DOI] [PubMed] [Google Scholar]
- 49. Ryu E, Son M, Lee M, et al. Cordycepin is a novel chemical suppressor of Epstein‐Barr virus replication. Oncoscience. 2014;1:866‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mikirova N, Hunninghake R. Effect of high dose vitamin C on Epstein‐Barr viral infection. Med Sci Monitor. 2014;20:725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wildner O, Blaese RM, Candotti F. Enzyme prodrug gene therapy: synergistic use of the herpes simplex virus‐cellular thymidine kinase/ganciclovir system and thymidylate synthase inhibitors for the treatment of colon cancer. Cancer Res. 1999;59:5233‐5238. [PubMed] [Google Scholar]
- 52. Meng Q, Hagemeier SR, Fingeroth JD, Gershburg E, Pagano JS, Kenney SC. The Epstein‐Barr virus (EBV)‐encoded protein kinase, EBV‐PK, but not the thymidine kinase (EBV‐TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J Virol. 2010;84:4534‐4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goodenough DA, Paul DL. Gap junctions. CSH Perspect Biol. 2009;1:a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Westphal EM, Blackstock W, Feng W, Israel B, Kenney SC. Activation of lytic Epstein‐Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV‐positive malignancies. Cancer Res. 2000;60:5781‐5788. [PubMed] [Google Scholar]
- 55. Chang LK, Liu ST. Activation of the BRLF1 promoter and lytic cycle of Epstein‐Barr virus by histone acetylation. Nucleic Acids Res. 2000;28:3918‐3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ben‐Sasson SA, Klein G. Activation of the Epstein‐Barr virus genome by 5‐aza‐cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28:131‐135. [DOI] [PubMed] [Google Scholar]
- 57. Cen H, McKnight JL. EBV‐immortalized isogenic human B‐cell clones exhibit differences in DNA‐protein complex formation on the BZLF1 and BRLF1 promoter regions among latent, lytic and TPA‐activated cell lines. Virus Res. 1994;31:89‐107. [DOI] [PubMed] [Google Scholar]
- 58. Hui KF, Chiang AK. Suberoylanilide hydroxamic acid induces viral lytic cycle in Epstein‐Barr virus‐positive epithelial malignancies and mediates enhanced cell death. Int J Cancer. 2010;126:2479‐2489. [DOI] [PubMed] [Google Scholar]
- 59. Hui KF, Ho DN, Tsang CM, Middeldorp JM, Tsao GS, Chiang AK. Activation of lytic cycle of Epstein‐Barr virus by suberoylanilide hydroxamic acid leads to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int J Cancer. 2012;131:1930‐1940. [DOI] [PubMed] [Google Scholar]
- 60. Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein‐Barr virus‐positive B‐cell lymphomas. J Virol. 2004;78:1893‐1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lima RT, Seca H, Bras S, Nascimento MS, Vasconcelos MH. Treatment of Akata EBV‐positive cells with doxorubicin causes more EBV reactivation than treatment with etoposide. Chemotherapy. 2011;57:195‐203. [DOI] [PubMed] [Google Scholar]
- 62. Wu T, Wang S, Wu J, et al. Icaritin induces lytic cytotoxicity in extranodal NK/T‐cell lymphoma. J Exp Clin Cancer Res. 2015;34:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daibata M, Bandobashi K, Kuroda M, Imai S, Miyoshi I, Taguchi H. Induction of lytic Epstein‐Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV‐positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J Virol. 2005;79:5875‐5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu SF, Wang H, Li ZJ, et al. Aspirin induces lytic cytotoxicity in Epstein‐Barr virus‐positive cells. Eur J Pharmacol. 2008;589:8‐13. [DOI] [PubMed] [Google Scholar]
- 65. Tikhmyanova N, Schultz DC, Lee T, Salvino JM, Lieberman PM. Identification of a new class of small molecules that efficiently reactivate latent Epstein‐Barr Virus. ACS Chem Biol. 2014;9:785‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choi CK, Ho DN, Hui KF, Kao RY, Chiang AK. Identification of novel small organic compounds with diverse structures for the induction of Epstein‐Barr Virus (EBV) lytic cycle in EBV‐positive epithelial malignancies. PLoS ONE. 2015;10:e0145994. [DOI] [PMC free article] [PubMed] [Google Scholar]


