Abstract
Norcantharidin (NCTD) is a promising antitumor drug with low toxicity. It was reported to be able to regulate immunity, but the mechanism is not yet clear. Here we explored whether NCTD could enhance the antitumor immunity induced by prostate cancer cell vaccine. The results of the in vitro study showed that NCTD induced apoptosis and inhibited proliferation of regulatory T cells (Tregs). Mechanistic research showed that NCTD inhibited Akt activation and activated FOXO1 transcription, resulting in a pro‐apoptotic effect. The results of the in vivo study showed that more tumor‐infiltrating Tregs existed within peripheral blood and tumor tissue after treatment with the vaccine. Adding NCTD to vaccine treatment could decrease the number of tumor‐infiltrating Tregs and increase the number of CD4+ and CD8+ T cells. Combination therapy with NCTD and vaccine was more effective in inhibiting tumor growth than the vaccine alone. In general, this is the first report that NCTD could induce apoptosis of Tregs and enhance the vaccine‐induced immunity.
Keywords: cancer immunotherapy, mGM‐CSF vaccine, norcantharidin, prostate cancer, Tregs
Abbreviations
- CTD
cantharidin
- DC
dendritic cell
- FasL
Fas ligand
- FOX
Forkhead box
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- mGM‐CSF
mouse GM‐CSF
- NCTD
norcantharidin
- p‐
phosphorylated
- PCa
prostate cancer
- PE
phycoerythrin
- PI
propidium iodide
- SA
streptavidin
- Tcon
conventional T cell
- Treg
regulatory T cell
1. INTRODUCTION
Prostate cancer is a common type of cancer in men in Europe and the USA.1 Immunotherapy with tumor cell vaccine has been proven to be effective in treating PCa.2 However, suppressive immunity could negatively affect the outcome of tumor cell vaccine treatment. Regulatory T cells are a subpopulation of CD4 T cells expressing CD25 and FoxP3, which mainly confer immunity suppression.3 Several studies had reported that Tregs widely infiltrate into tumor tissues and exist in peripheral blood of patients with malignant cancer.4, 5, 6 Increasing numbers of Tregs negatively correlate with patients’ survival and prognosis.4, 5, 6, 7, 8 As reported by several studies, depleting Tregs by CD25‐specific antibody9 and cytotoxic T‐lymphocyte‐associated protein 4 antibody10 could improve the antitumor immune response. Therefore, eliminating Tregs or blocking their inhibitory functions is essential in cancer immunotherapy.
Norcantharidin, the demethylated analogue of CTD,11 has strong anti‐oxidant, antitumor, and antimetastatic activity,12 but much less toxicity than CTD in the clinical application.13 Published studies have reported that NCTD could significantly induce apoptosis and inhibit proliferation of tumor cells through different signaling pathways.14, 15 Mechanistic studies showed that NCTD‐induced apoptosis is mediated by regulating some transcription factors, including nuclear factor‐κB, activator protein‐1, Forkhead box O, and signal transducer and activator of transcription, which can independently or collaboratively regulate the expression of apoptotic or anti‐apoptotic genes.15, 16 Norcantharidin was reported to have an effect on immune regulation,17 but its effect on Tregs is not yet clear.
In our previous research, a cancer cell vaccine was developed by anchoring streptavidin‐tagged GM‐CSF on the surface of prostate cancer cells.18 We found that the vaccine could enhance antitumor immune responses, but the number of Tregs also increased in the vaccine‐treated mice at the same time. The present study was undertaken to evaluate the effect of NCTD on apoptosis and proliferation of Tregs in vitro and explore the underlying mechanism. A mouse prostate cancer model was used to evaluate the efficacy of a combination therapy with NCTD and cancer cell vaccine.
2. MATERIALS AND METHODS
2.1. Materials
Tumor tissues were obtained from 20 PCa patients and normal tissues were selected from 10 patients who received pathological biopsy of benign disease in Nanfang Hospital (Guangzhou, China). None of these patients had received immune‐regulating drugs or radiation therapy before surgery. The study was approved by the ethics committee and informed consent was obtained from all patients. Norcantharidin, CCK‐8, and DAPI reagents were purchased from Sigma (St. Louis, MO, USA). Streptavidin‐tagged mouse GM‐CSF (SA‐mGM‐CSF) fusion protein was prepared by our laboratory. Western blotting antibodies against p‐ERK1/2, p‐Akt, ERK1/2, Akt, Bcl‐2, FOXO1, FasL, and GAPDH were purchased from Abcam (Cambridge, UK). Phycoerythrin‐labeled anti‐mGM‐CSF, FITC‐labeled anti‐mCD11c, PE‐labeled anti‐mCD80, FITC‐labeled anti‐mCD4, FITC‐labeled anti‐mCD8 and PerCP‐Cyanine 5.5‐labeled anti‐mFoxP3 antibodies were purchased from eBioscience (San Diego, CA, USA). C57BL/6 male mice (6‐8 weeks old) were purchased from the experimental animal center of Southern Medical University (Guangzhou, China). All animal studies were undertaken in accordance with the Southern Medical University guidelines for experimental animals.
2.2. Cell sorting
To obtain Tcons, the spleens were collected from C57/BL6 mice, then ground and isolated by lymphocyte separation liquid (Solarbio). Regulatory T cells were isolated from Tcons according to the procedure of the CD4+ CD25+ Regulatory T Cells Isolation Kit (Miltenyi Biotec, Germany), and the rest of the cells were considered as non‐Tregs. Their purity and the ratio of viability were assessed by flow cytometry (Becton Dickinson, San Jose, CA, USA).
2.3. Cell culture and stimulation
The mouse prostate cancer cell line (RM‐1) was purchased from ATCC (Manassas, VA, USA). The cells were maintained in RPMI‐1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/L streptomycin at 37°C in a humidified atmosphere of 5% CO2. The Tcons, Tregs, and non‐Tregs were cultured in the presence of 1 μg/mL soluble anti‐mouse CD3 and anti‐mouse CD28 (1:1) antibody.
2.4. Cell proliferation assay
The Tcons, Tregs, and non‐Tregs were inoculated into 96‐well plates (1 × 104 cells/well) and then incubated with different concentrations of NCTD (0‐80 μmol/L) for 24 hour or with 20 μmol/L NCTD for 0, 24, 48, or 72 hour. The effect of NCTD on cell proliferation was determined by the CCK‐8 assay. Cell viability was assessed by microplate reader (Bio‐Rad, Hercules, CA, USA).
2.5. Apoptosis assay
The Tcons, Tregs, and non‐Tregs were cultured in 24‐well plates as previously described and treated with various concentrations of NCTD (0‐80 μmol/L) for 24 hours or with 20 μmol/L NCTD for 0, 24, 48, or 72 hours. The cell apoptosis was measured by flow cytometry using an annexin V/FITC and PI apoptosis detection kit (eBioscience). Annexin V(+)/PI(−) and annexin V(+)/PI(+) were defined as early apoptosis and late apoptosis, respectively.
2.6. Western blot analysis
Regulatory T cells were cultured and treated with NCTD as previously described. Cells were collected and dissolved in RIPA buffer. Equal amounts of protein were separated on 10% SDS‐PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA, USA), then the membrane was blocked with 5% milk and incubated with primary antibodies in TBST overnight at 4°C. Primary antibodies against the following proteins were used: ERK1/2, p‐ERK1/2, Akt, p‐Akt, FOXO1, Bcl2, FasL, and GAPDH. The membranes were washed four times for 7 minutes with TBST and then incubated with HRP‐conjugated IgG secondary antibody for 1 hour at room temperature. Protein binding was visualized using an Immobilon Western‐HRP substrate (Millipore, Billerica, MA, USA).
2.7. Real‐time RT‐PCR
Total RNA of Tregs was extracted using RNAiso Plus reagent (Takara) and reversed transcribed using a PrimeScript RT reagent Kit with gDNA Eraser (Takara). For each gene, the mRNA level was normalized against GAPDH expression. The mRNA levels of Fas, FasL, FOXO1, FOXO3a, and FOXO4 were determined using SYBR Premix Ex Taq II (Takara). Primers for PCR were designed as follows: GAPHD, AGGTCGGTGTGAACGGATTTG (forward) and GGGGTCGTTGATGGCAACA (reverse); FOXO1, ATGCTCAATCCAGAGGGAGG (forward) and ACTCGCAGGCCACTTAGAAAA (reverse); FOXO3a, GGGGAACCTGTCCTATGCC (forward) and TCATTCTGAACGCGCATGAAG (reverse); FOXO4, CTTCCTCGACCAGACCTCG (forward) and ACAGGATCGGTTCGGAGTGT (reverse); Fas, TATCAAGGAGGCCCATTTTGC (forward) and TGTTTCCACTTCTAAACCATGCT (reverse); and FasL, CAGCCCATGAATTACCCATGT (forward) and ATTTGTGTTGTGGTCCTTCTTCT (reverse).
2.8. Vaccine preparation
The RM‐1 cells were fixed in 30% ethanol (v/v) for 30 minutes at room temperature. Ethanol‐fixed RM‐1 cells (1 × 107 cells/mL) were incubated with 10 mmol/L fresh EZ‐Link Sulfo‐NHS‐LC‐Biotin (Pierce Biotechnology) for 3 minutes at room temperature, washed with PBS, then incubated with SA‐mGM‐CSF at 200 ng/106 cells for 1 hour and washed with PBS again. The presence of SA‐GM‐CSF on the surface of tumor cells was detected by flow cytometry (Becton Dickinson) using PE‐anti‐mGM‐CSF mAb.
2.9. Efficacy of combination therapy with vaccine and NCTD
To establish the s.c. tumor model, each mouse was injected with 2 × 106 RM‐1 cells in the hind leg of C57BL/6/male mice (6‐8 weeks old). The mice were randomly divided into four groups (PBS, NCTD, Vaccine, and Vaccine + NCTD [combination group]). For the Vaccine group, the mice were injected s.c. into the right hind leg with 1 × 106 the RM‐1 tumor cells vaccine on day 0 (10 days after inoculation of tumor cells). The process was repeated on days 4, 8, and 12. For the NCTD or combination group, the mice were i.p. injected with NCTD beginning at day 10 after inoculation of tumor cells at a dose of 10 mg/kg/day for 14 days.
2.10. Analysis of leukocytes in the blood by flow cytometry
Blood samples (100 μL) were collected from each group on day 30 after inoculation of tumor cells, and the red blood cells were then lysed by ACK lysing buffer (Solarbio). To detect DCs, cells were stained with FITC‐labeled anti‐mCD11c and PE‐labeled anti‐mCD80 antibody for 30 minutes. To qualify the CD4+/CD8+ T populations, cells were stained with FITC‐labeled anti‐mCD4 or FITC‐labeled anti‐mCD8 antibody for 30 minutes. To detect the Treg populations in the blood of each experimental group, the cells were incubated with FITC‐labeled anti‐mCD4 for 30 minutes, then the cells were stained with PerCP‐Cyanine 5.5‐labeled anti‐mFoxP3 antibodies for 30 minutes after fixed and permeabilized using the Foxp3 staining kit (eBioscience). T‐cell subsets were measured by flow cytometry (Becton Dickinson).
2.11. Immunohistochemical analysis
A total of 20 tumor tissues and 10 normal tissues were obtained from patients in Nanfang Hospital. Subcutaneous tumor tissues of mice were collected from each group on day 30 after inoculation of tumor cells. Tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Paraffin sections (5 μm) were deparaffinized and rehydrated and treated with hydrogen peroxide and then carried out with antigen retrieval. The sections of patients were blocked for 10 minutes and then were incubated with anti‐FoxP3 antibody (1:3000; Abcam) for 1 hour, the sections of mouse tissues were incubated with anti‐mCD4 (1:700; Abcam), and anti‐mCD8 (1:1000; eBioscience) antibody according to the operation manual of rabbit‐specific HRP/DAB (ABC) Detection IHC Kit (Abcam). All tissues were counterstained with H&E.
2.12. Immunofluorescent analysis
Tissue samples were fixed with 4% paraformaldehyde and embedded in paraffin. Paraffin sections (4‐5 μm) were deparaffinized, rehydrated, and antigen retrieval was carried out. The sections were then incubated with rat anti‐mouse FoxP3 (eBioscience) antibodies after blocking. The Alexa Fluor 647‐labeled goat anti‐rat (Cell Signaling Technology) for FoxP3 as secondary antibodies and then were detected by the fluorescence microscope (Nikon Eclipse Ti‐SR) under 200× magnification.
2.13. Statistics
The results show the most typical of three independent experiments. Data are shown as mean ± SD and were analyzed with IBM spss version 20.0. Differences between groups were analyzed using the repeated measures or one‐way anova, or t test, with significant difference defined as P < .05.
3. RESULTS
3.1. Tumor‐infiltrating Tregs in human prostate cancer
Immunohistochemistry was carried out to detect the infiltration of Tregs in human prostate cancer tissue. The results indicated that the quantity of Tregs in tumor tissues was significantly higher than that in normal tissues (P < .05; Figure 1A).
Figure 1.
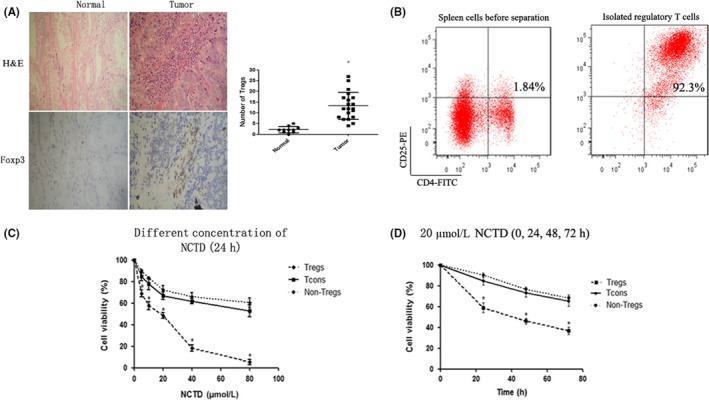
Tumor‐infiltrating regulatory T cells (Tregs) in human prostate cancer and the effect of norcantharidin (NCTD) on the proliferation of Tregs. A, Twenty tumor tissues and 10 normal tissues were collected from patients and analyzed by immunohistochemistry. Sections are representative of the total vital tumor tissue (magnification, ×200). B, Tregs were isolated from spleens by magnetic selection and the purity of Tregs was measured by flow cytometry. Conventional T cells (Tcons), non‐Tregs, and Tregs were treated with different concentrations of NCTD (0‐80 μmol/L) for 24 h or with 20 μmol/L NCTD for 0, 24, 48, or 72 h. Cell viability was determined by the CCK‐8 assay. C, NCTD (0‐80 μmol/L) for 24 h. D, 20 μmol/L NCTD for 0, 24, 48, and 72 h. All experiments were repeated at least three times. *P < .05. PE, phycoerythrin
3.2. Effect of NCTD on proliferation and apoptosis of Tregs
Magnetic cell sorting collected a high purity of Tregs from the spleens of mice (>90%; Figure 1B). To investigate the effect of NCTD on the proliferation and apoptosis of Tregs, non‐Tregs, and Tcons, cells were incubated with different concentrations of NCTD (0‐80 μmol/L) for 24 hours, or with 20 μmol/L NCTD for 0, 24, 48, or 72 hours. The results of the CCK‐8 analysis showed that NCTD could dramatically inhibit the proliferation of Tregs compared with Tcons (P < .05; Figure 1C,D), but there was no difference between Tcons and non‐Tregs. In addition, NCTD dramatically induced the apoptosis of Tregs (P < .05; Figure 2). All effects were in a dose‐ and time‐dependent manner.
Figure 2.
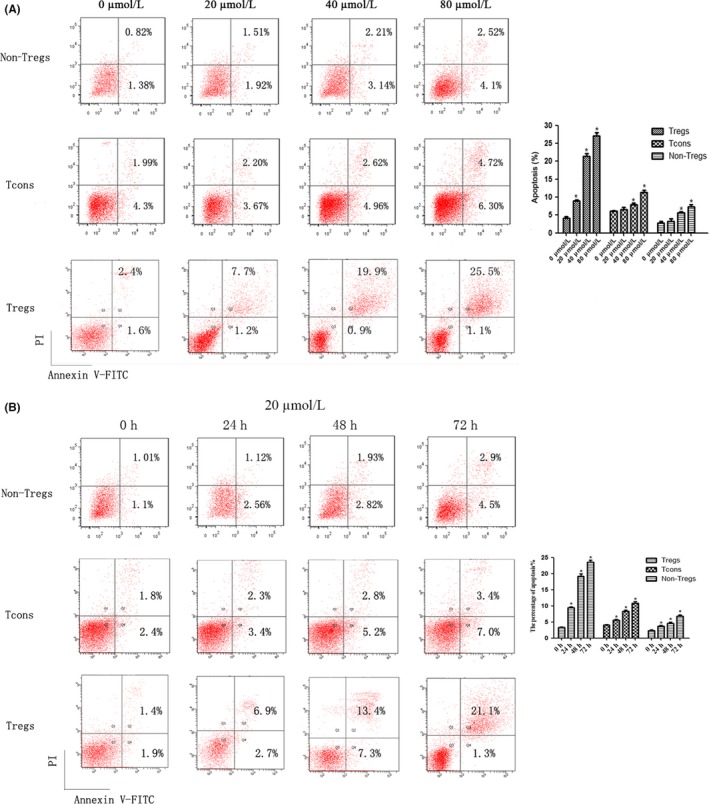
Effect of norcantharidin (NCTD) on the apoptosis of regulatory T cells (Tregs), non‐Tregs, and conventional T cells (Tcons). Tcons, non‐Tregs, and Tregs were treated with different concentrations of NCTD (0‐80 μmol/L) for 24 h or with 20 μmol/L NCTD for 0, 24, 48, or 72 h. A, NCTD (0‐80 μmol/L) for 24 h. B, 20 μmol/L NCTD for 0, 24, 48, and 72 h. All experiments were repeated at least three times. *P < .05. PI, propidium iodide
3.3. Norcantharidin downregulated p‐AKT and activated FOXO1
Western blot analysis was used to explore the associated mechanism regarding apoptosis of Tregs. The results showed that NCTD downregulated p‐Akt, whereas the total Akt protein level unchanged. Treatment with NCTD increased the levels of FOXO1 and FasL protein and decreased the level of Bcl2 protein (P < .05; Figure 3A), but there is no obvious change in the level of ERK or p‐ERK. Real‐time PCR analysis showed that mRNA levels of Fas, FasL, FOXO3a, FOXO4, and FOXO1 were significantly upregulated in NCTD‐treated Tregs (P < .05; Figure 3B). It is suggested that NCTD might induce apoptosis through inhibiting AKT and hence activating the FOXO1–FasL axis.
Figure 3.
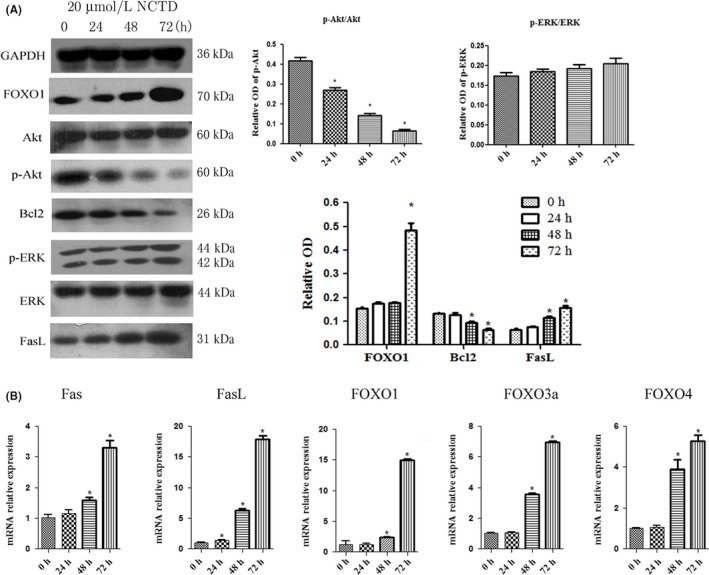
Norcantharidin (NCTD) induces apoptosis of regulatory T cells (Tregs) by regulating phosphorylated (p‐)Akt, Forkhead box (FOX)O1, and Fas ligand (FasL) expression. Tregs were treated with 20 μmol/L NCTD for 0, 24, 48, or 72 h. A, ERK1/2, p‐ERK1/2, Akt, p‐Akt, FOXO1, Bcl2, and FasL levels were measured by Western blot analysis, with GAPDH as control. B, FOXO1, FOXO3a, Fas, FasL, and FOXO4 mRNA levels were determined by RT‐PCR. *P < .05. OD, optical density
3.4. Antitumor efficacy of vaccine therapy combined with NCTD
Flow cytometry revealed that SA‐mGM‐CSF could be efficiently anchored on the surface of biotinylated RM‐1 cells (Figure 4A). The RM‐1 s.c. mouse model was used to evaluate the antitumor efficacy of the vaccine combined with NCTD. Our results showed that the RM‐1 cell vaccine modestly inhibited tumor growth. The addition of NCTD to the vaccine treatment was more effective in restraining tumor growth than vaccine alone (P < .05; Figure 4B). These results suggest that NCTD could promote the antitumor immunity of tumor cell vaccines.
Figure 4.
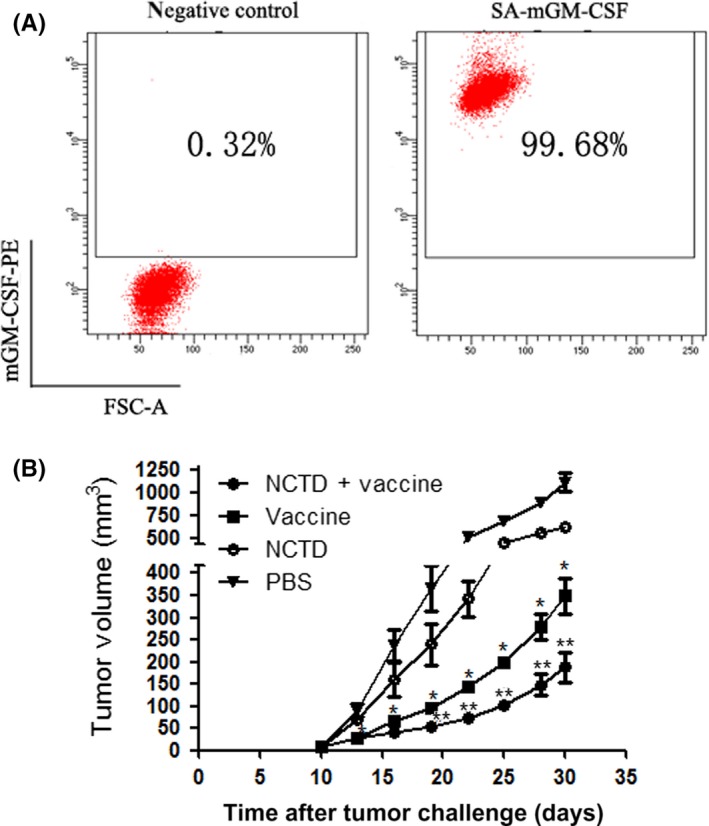
Efficacy of combination therapy with the vaccine and norcantharidin (NCTD) in prostate cancer. A, Presentation of streptavidin‐tagged mouse granulocyte macrophage colony‐stimulating factor (SA‐mGM‐CSF) on the surface of RM‐1 cells was measured by flow cytometry, with unbiotinylated RM‐1 cells as a negative control. B, All mice were injected s.c. with 2 × 106 RM‐1 cells (right hind leg), and the tumor volume was recorded. The tumor size in the vaccine group was much smaller than that in the control group; the tumor size in the combination group was smallest among the four groups. *P < .05 vs PBS; # P < .05 vs Vaccine. FSC‐A, forward scatter area; PE, phycoerythrin
3.5. Flow cytometry analysis of DCs in blood
To evaluate the effect of the GM‐CSF surface‐modified tumor cell vaccine on mature DC cells, cells were collected from the blood and analyzed by flow cytometry. The results showed that mature DCs were distinctly increased after treatment with GM‐CSF surface‐modified vaccine. Treatment with NCTD alone had no significant effect on DC maturation (P < .05; Figure 5A).
Figure 5.
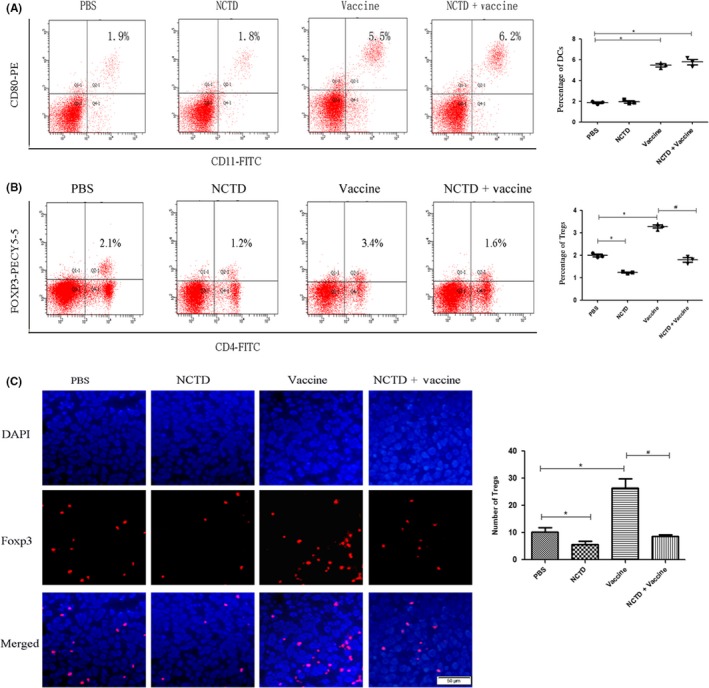
Proportion of mature dendritic cells (DCs) and regulatory T cells (Tregs) in mice after combination therapy with norcantharidin (NCTD) and vaccine. A, To detect the mature DCs, blood samples were collected from each treatment group on day 30 after tumor cell injection. Cells were stained with FITC‐labeled anti‐mCD11c and phycoerythrin (PE)‐labeled anti‐mCD80 antibodies, and measured by flow cytometry. B, Blood samples were collected from mice on day 30 after tumor cell injection and stained with FITC‐labeled anti‐mCD4 and Pecy5.5‐labeled anti‐mFoxp3 antibodies. C, Tumor tissues were collected from each group. Tregs were detected by immunofluorescence (red) (magnification, ×200). All experiments were repeated at least three times. *P < .05 vs PBS; # P < .05 vs Vaccine
3.6. Norcantharidin therapy decreased Treg populations in vivo
Flow cytometry was used to investigate the quantity of Tregs in blood. As anticipated, the frequency of Tregs was increased in the Vaccine group and decreased in the NCTD group compared to the PBS group. Importantly, the frequency was much lower in the Vaccine + NCTD group compared to the Vaccine group (P < .05; Figure 5B).
The proportions of Tregs in tumor tissues were detected using immunofluorescence. The results showed that more Tregs were infiltrated into the tumor tissues after the vaccine therapy. In the combination group, the number of Tregs was less than that in the Vaccine group (P < .05; Figure 5C). These results indicated that NCTD could decrease the number of infiltrating Tregs in vaccine treatment.
3.7. Flow cytometry analysis of CD4+ and CD8+ T lymphocytes in blood after vaccine treatment
The rates of CD4+ and CD8+ T lymphocytes in peripheral blood of mice were analyzed by flow cytometry. Results showed that the proportions of CD4+ and CD8+ T cells were increased after treatment with vaccine or NCTD (P < .05; Figure 6A). The proportions of CD4+ and CD8+ T cells in the combination group were the highest among the four groups (P < .05; Figure 6A).
Figure 6.
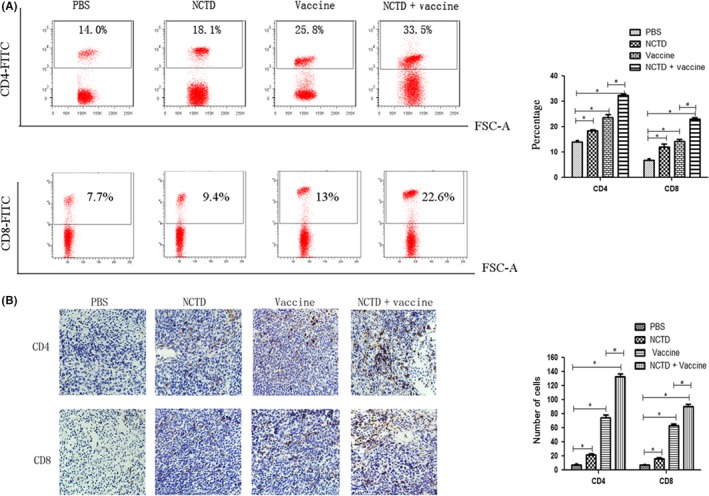
Analysis of T lymphocytes after combination therapy with vaccine and norcantharidin (NCTD). A, Blood samples were collected from each group on day 30 after tumor cell injection. Cells were stained with FITC‐labeled anti‐mCD4 and FITC‐labeled anti‐mCD8 antibodies and analyzed by flow cytometry. B, Tumor tissues were collected from each group on day 30 after tumor cell injection. Tumor‐infiltrating CD4+ and CD8+ T lymphocytes were detected by immunohistochemistry. Sections are representative of the total vital tumor tissue (magnification, ×200). All experiments were repeated at least three times. *P < .05 vs PBS; #P < .05 vs Vaccine
The numbers of CD4+ and CD8+ T cells in the tumor tissues were detected by immunohistochemistry assay. The results revealed that the trend in the tumor tissue was the same as that in blood. Tumor‐infiltrating T lymphocytes were increased after vaccine or NCTD treatment, and combination therapy induced more T‐lymphocyte infiltration compared with vaccine or NCTD alone (P < .05; Figure 6B). These results indicated that NCTD could enhance the vaccine‐induced immune responses.
4. DISCUSSION
In this study, we showed that Tregs widely existed in human prostate cancer tissue. Norcantharidin could induce apoptosis and inhibit proliferation of Tregs and its mechanism is probably associated with the Akt–FOXO1–FasL pathway. Moreover, our results indicated that the SA‐mGM‐CSF vaccine could effectively induce antitumor immune response. However, more Tregs existed in the blood and infiltrated into the tumor tissues after vaccine therapy. The addition of NCTD could decrease the number of Tregs and significantly enhance the vaccine‐induced immunity. This is the first report that NCTD could induce apoptosis of Tregs. Norcantharidin therapy might overcome the vaccine‐induced immunosuppression to enhance the vaccine‐induced antitumor immune response in mouse prostate cancer.
Vaccine therapy is an important and effective approach in treating many malignancies.19, 20 Several published studies have shown that GM‐CSF‐modified cell vaccines could induce effective antitumor immunity.19, 21 Granulocyte macrophage colony‐stimulating factor plays a major role in maturation of DCs and activation of CD4+ and CD8+ T cells. In this research, the numbers of mature DCs and activated T cells were increased after vaccine treatment. However, more immune‐suppressive Tregs infiltrated into the tumor tissue and blood after vaccine therapy, and NCTD therapy could deplete the infiltrated Tregs. Therefore, it seems that NCTD could decrease Tregs and enhanced the vaccine‐induced immunity in vivo.
FoxP3‐expressing Tregs are an important subset of CD4 T cells, which suppress the effective immune response.22 Many studies have reported that the infiltration of a large number of Tregs into tumor tissues is closely related to poor prognosis.7, 23 Consistent with previous research, our results also showed that there were abundant tumor‐infiltrated Tregs in human prostate cancer tissue.8 Previous studies have shown that depletion of tumor‐infiltrating Tregs is able to enhance the antitumor immune response.24, 25 Therefore, it is essential to reduce Tregs to enhance antitumor immunity.
Norcantharidin, a demethylated form of CTD, has less toxicity and higher antitumor ability than CTD,26 and could increase the number of white blood cells by stimulating the bone marrow.27 Our results also indicated that NCTD could increase the number of leukocytes. A previous study indicated that NCTD inhibited the proliferation and promoted apoptosis of cancer cells,14 but its effect on Tregs is not yet clear. This study revealed that NCTD could inhibit proliferation and enhance apoptosis of Tregs in a dose‐ and time‐dependent manner. These findings suggest that NCTD might be an effective drug to decrease Tregs in vitro.
It has been shown that NCTD could regulate the activity of FOXO transcription factors,28 which play an important role in regulating diverse immune functions such as proliferation, differentiation, apoptosis, DNA repair, longevity, and stress resistance.29, 30 FOXO families, especially FOXO1 and FOXO3a, promote cell apoptosis through upregulating FasL transcription.31 However, PI3K/AKT could negatively regulate FOXO1 and lead to apoptosis.32 Previous observations suggested that FOXO1 is a pivotal regulator of Treg cell function,33 and negatively regulates Treg differentiation.34 In this study, we analyzed the Akt–FOXO1–FasL pathway in NCTD‐induced apoptosis. The results showed that the level of p‐Akt was decreased, which was closely associated with increased FOXO1 transcription in NCTD‐treated Tregs. Furthermore, the expressions of apoptosis‐related genes, including Fas and FasL, were increased in the experimental group compared with the control group. Therefore, the mechanism of NCTD‐induced apoptosis of Tregs might be related to the inhibition of p‐Akt and activation of FOXO1 transcription protein.
This is the first study to report that NCTD could induce apoptosis of Tregs and enhance the antitumor immunity of tumor cell vaccines. The molecular mechanism of NCTD‐induced apoptosis of Tregs might be through the inhibition of AKT and activation of FOXO1–FasL. We speculate that NCTD could represent a new method of eliminating Tregs. This study provides valuable information in cancer immunotherapy.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81373122 to Z.M. and 81672915 to J.L.).
Mo L, Zhang X, Shi X, et al. Norcantharidin enhances antitumor immunity of GM‐CSF prostate cancer cells vaccine by inducing apoptosis of regulatory T cells. Cancer Sci. 2018;109:2109–2118. https://doi.org/10.1111/cas.13639
Lijun Mo and Xinji Zhang contributed equally to this work.
Contributor Information
Jinlong Li, Email: lijinlong@smu.edu.cn.
Zhiming Hu, Email: hzm@smu.edu.cn.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Obara W, Sato F, Takeda K, et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Can Sci. 2017;108:1452‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakaguchi S. Regulatory T cells: key controllers of immunologic self‐tolerance. Cell. 2000;101:455‐458. [DOI] [PubMed] [Google Scholar]
- 4. Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4 + CD25 + regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756‐2761. [DOI] [PubMed] [Google Scholar]
- 6. Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Can Res. 2005;65:2457‐2464. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller AM, Lundberg K, Ozenci V, et al. CD4 + CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398‐7405. [DOI] [PubMed] [Google Scholar]
- 9. Powell DJ Jr, Felipe‐Silva A, Merino MJ, et al. Administration of a CD25‐directed immunotoxin, LMB‐2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919‐4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte‐associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712‐4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kok SH, Cheng SJ, Hong CY, et al. Norcantharidin‐induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett. 2005;217:43‐52. [DOI] [PubMed] [Google Scholar]
- 12. Shen B, He PJ, Shao CL. Norcantharidin induced DU145 cell apoptosis through ROS‐mediated mitochondrial dysfunction and energy depletion. PLoS One. 2013;8:e84610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karras DJ, Farrell SE, Harrigan RA, Henretig FM, Gealt L. Poisoning from “Spanish fly” (cantharidin). Am J Emerg Med. 1996;14:478‐483. [DOI] [PubMed] [Google Scholar]
- 14. Peng F, Wei YQ, Tian L, et al. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002;128:223‐230. [DOI] [PubMed] [Google Scholar]
- 15. Yeh CH, Yang YY, Huang YF, Chow KC, Chen MF. Induction of apoptosis in human Hep3B hepatoma cells by norcantharidin through a p53 independent pathway via TRAIL/DR5 signal transduction. Chin J Integr Med. 2012;18:676‐682. [DOI] [PubMed] [Google Scholar]
- 16. Kops GJ, Medema RH, Glassford J, et al. Control of cell cycle exit and entry by protein kinase B‐regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan MS, Xiue S, Wei LX, Piao XH. The preliminary observation on immunosuppressive effect of norcantharidin in mice. Immunopharmacol Immunotoxicol. 1993;15:79‐85. [DOI] [PubMed] [Google Scholar]
- 18. Yin W, He Q, Hu Z, et al. A novel therapeutic vaccine of GM‐CSF/TNFα surface‐modified RM‐1 cells against the orthotopic prostatic cancer. Vaccine. 2010;28:4937‐4944. [DOI] [PubMed] [Google Scholar]
- 19. Hu Z, Chen J, Zhou S, et al. Mouse IP‐10 gene delivered by folate‐modified chitosan nanoparticles and dendritic/tumor cells fusion vaccine effectively inhibit the growth of hepatocellular carcinoma in mice. Theranostics. 2017;7:1942‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito H, Kitagawa K, Yoneda T, et al. Combination of p53‐DC vaccine and rAd‐p53 gene therapy induced CTLs cytotoxic against p53‐deleted human prostate cancer cells in vitro. Cancer Gene Ther. 2017;24:289‐296. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Shi X, Li J, et al. A novel therapeutic vaccine of mouse GM‐CSF surface modified MB49 cells against metastatic bladder cancer. J Urol. 2012;187:1071‐1079. [DOI] [PubMed] [Google Scholar]
- 22. Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804‐811. [DOI] [PubMed] [Google Scholar]
- 23. Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3 + regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Can Res. 2006;12:5423‐5434. [DOI] [PubMed] [Google Scholar]
- 24. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti‐CD25 (interleukin‐2 receptor alpha) monoclonal antibody. Can Res. 1999;59:3128‐3133. [PubMed] [Google Scholar]
- 25. Motoyoshi Y, Kaminoda K, Saitoh O, et al. Different mechanisms for anti‐tumor effects of low‐ and high‐dose cyclophosphamide. Oncol Rep. 2006;16:141‐146. [PubMed] [Google Scholar]
- 26. Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26:147‐162. [DOI] [PubMed] [Google Scholar]
- 27. Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin CL, Chen CM, Lin CL, Cheng CW, Lee CH, Hsieh YH. Norcantharidin induces mitochondrial‐dependent apoptosis through Mcl‐1 inhibition in human prostate cancer cells. Biochem Biophys Acta. 2017;1864:1867‐1876. [DOI] [PubMed] [Google Scholar]
- 29. Furukawa‐Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell‐cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752‐760. [DOI] [PubMed] [Google Scholar]
- 30. McCormick M, Chen K, Ramaswamy P, Kenyon C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell. 2012;11:192‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lou K, Chen N, Li Z, et al. MicroRNA‐142‐5p overexpression inhibits cell growth and induces apoptosis by regulating FOXO in hepatocellular carcinoma cells. Oncol Res. 2017;25:65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Cai Z, Liu H, Wu X. Forkhead‐box transcription factor 1 affects the apoptosis of natural regulatory T cells by controlling Aven expression. BMC Immunol. 2017;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ouyang W, Liao W, Luo CT, et al. Novel Foxo1‐dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurebayashi Y, Baba Y, Minowa A, et al. TGF‐beta‐induced phosphorylation of Akt and Foxo transcription factors negatively regulates induced regulatory T cell differentiation. Biochem Biophys Res Comm. 2016;480:114‐119. [DOI] [PubMed] [Google Scholar]


