Abstract
Tumor‐infiltrating neutrophils (TIN) carry out quite significant but opposite functions in different cancers, and their function in biliary cancer has not been fully characterized. To investigate the prognostic significance of TIN in biliary cancer, a training set (n = 118) and a validation set (n = 127) were involved in this study. TIN were evaluated by immunohistochemical staining of CD66b, and then defined as low (neutrophils <18/high‐power field [HPF]) vs high (neutrophils ≥18/HPF). Kaplan‐Meier curve, Cox proportional hazards models and receiver operating characteristic curve were used to assess the prognostic significance. TIN was identified as an independent prognostic factor for overall survival in the training set (HR: 4.720; 95% CI: 2.623‐8.493; P < .001) which was confirmed in the validation set (HR: 4.993; 95% CI: 2.626‐9.492; P < .001). Notably, among patients with stage III and IV disease, those with low TIN could benefit from adjuvant chemotherapy, with a reduced risk of compromised survival compared with those with high TIN (HR: 0.294; 95% CI: 0.099‐0.873; P = .047 in the training set; and HR: 0.100; 95% CI: 0.022‐0.462; P = .006 in the validation set). In addition, TIN were negatively related to biological pathways as regulation of activated T‐cell proliferation and lymphocyte‐mediated immunity, and showed a negative correlation with CD8 + T cells (r = −.324, P < .001). Taken together, our results implicate TIN as an independent marker of prognosis and indicator of patients who would benefit from adjuvant chemotherapy in biliary cancer.
Keywords: adjuvant chemotherapy, biliary cancer, immunosuppression, prognosis, tumor infiltrating neutrophil
Abbreviations
- ACT
adjuvant chemotherapy
- CI
confidence interval
- GSEA
Gene Set Enrichment Analysis
- HR
hazard ratio
- ICC
intraclass correlation coefficient
- IHC
immunohistochemistry
- OS
overall survival
- TIN
tumor‐infiltrating neutrophil
- TME
tumor microenvironment
1. INTRODUCTION
Biliary cancer, including gallbladder cancer and cholangiocarcinoma,1 is a relatively uncommon and aggressive malignancy, making up about 4% of all malignant digestive system tumors.2 However, the incidence of this disease has gradually risen in the past years.3 Complete surgical resection is the only curative treatment for biliary cancer, but prognosis of patients receiving radical operation is still unsatisfactory with a 5‐year survival rate of 24% to 53%.4 Local recurrence is regarded as a main limitation for postoperative patients with biliary cancer, making ACT extremely critical. Nevertheless, a substantial body of literature provided conflicting evidence regarding the role of ACT for biliary cancer.5, 6 Precise risk stratification for biliary cancer patients that can be used to predict ACT response is urgently needed.
As 1 of the 10 hallmarks of cancer, inflammation is described as a “wound that does not heal”, and is the most prevalent risk factor for biliary cancer, which mainly manifests as cholelithiasis and calcification for gallbladder cancer, and primary sclerosing cholangitis and choledochal cysts for cholangiocarcinoma.7, 8 As indispensable antagonists of microbial infection and facilitators of wound healing, neutrophils play an important role in inflammation. In recent years, the profound influence of TIN throughout each step of carcinogenesis has been seen: from tumor initiation to primary tumor growth to metastasis.9 It was reported that TIN have a protumor role through induction of angiogenesis,10, 11 immunosuppression,12 counteracting senescence,13 stimulating cancer cell migration, and guiding cancer cells into tissue.14, 15 However, some other intriguing literature has reported the antitumor function of TIN through their cytotoxic effects mediated by H2O2 16 or antibody‐dependent cellular cytotoxicity.17 These completely opposite roles of TIN promoted the distinction of antitumor (N1) and protumor (N2) neutrophils.9, 18
Similarly, the prognostic effect of TIN is also multifarious. Positive correlations with patient survival were observed in gastric cancer,19 whereas negative correlations were observed in hepatoma,20 melanoma,21 renal cancer22 and lung cancer.23 As in biliary cancer, the role of TIN has not been investigated extensively.
With regard to the critical value of TIN in the TME, in the present study, we evaluated the level of TIN in biliary cancer, assessed its relationship with clinical outcome, especially with ACT, and explored the effect of TIN on other TME components. These results might illustrate the importance of TIN, develop a promising prognostic system to predict the outcome of patients receiving ACT, and help improve the understanding of TME in biliary cancer.
2. PATIENTS AND METHODS
2.1. Study design
Two independent sets comprising 245 consecutive patients with biliary cancer undergoing surgical resection with the aim of cure were enrolled into the present study from Shanghai Zhongshan Hospital in China. The training set that comprised 118 consecutive patients was obtained between 2004 and 2007 and the validation set that comprised 127 consecutive patients was obtained between 2008 and 2011 with the same enrolment criteria. This study was approved by the Research Medical Ethics Committee of Fudan University and informed consent was obtained from each patient. Inclusion criteria included confirmed postoperative histopathology diagnosis, accurate tumor stage classification according to 2010 American Joint Committee on Cancer (AJCC) TNM classification, and complete available follow‐up data. Patients who were lost to follow up or who died within the first month after surgery were excluded. The following clinicopathological variables were collected: age at surgery, gender, serum data related to obstructive inflammation (including total bilirubin, alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), tumor location, TNM stage, tumor differentiation, surgical margin and microvascular invasion. Patients were evaluated with physical examination, laboratory studies, chest imaging, and abdominal ultrasound or computed tomography (CT) scan every 6 months in the first 2 years and every 12 months in the subsequent 3 years. OS was calculated from the date of surgery to the day of death or the last follow up.
2.2. Immunohistochemistry and evaluation of immunostaining
Tissue microarray and normal sections were constructed with formalin‐fixed paraffin‐embedded surgical specimens. Tissue blocks were sectioned at 2.0 mm and mounted on glass slides for tissue microarray, whereas normal sections were mounted on glass slides at original tumor size. IHC study was then carried out as described previously24 with appropriate antibodies (anti‐CD66b polyclonal antibody; BD Biosciences, Franklin Lakes, NJ, USA; Clone G10F5, diluted 1:200; anti‐CD8; IR623, ready‐to‐use; DAKO, Glostrup, Denmark). CD66b is known as carcinoembryonic antigen (CEA)‐related cell adhesion molecule 8 (CEACAM8) and is a highly glycosylated CEA family protein encoded by the CGM6 gene, which can be found on both neutrophils and eosinophils. As a marker mainly expressed by neutrophils in TME,25 CD66b can be used to identify neutrophils using high‐throughput screening flow cytometry with a purity of 99%,26 and has been used to identify TIN in many tumors, including renal cell carcinoma,22 hepatocellular carcinoma,27 melanoma,21 gastric cancer28 and cervical cancer.29 Positive stainings were calculated under HPF (400×) using Image Pro plus 6.0 (Media Cybernetics Inc., Bethesda, MD, USA). Intensity of neutrophils or CD8 + T cells in tissue microarray was recorded as the mean number of CD66b or CD8‐positive cells/HPF from 3 randomized fields, whereas it was recorded from 4 randomized fields in tumor core or stroma in the normal section, respectively. Two independent pathologists were blinded to the clinicopathological data and evaluated the staining intensity of CD66b and CD8, and the results were averaged. Median value of tissue microarray was defined as the cutoff value for low and high neutrophil infiltration.
2.3. Gene set enrichment analysis
Gene Set Enrichment Analysis is a robust method for evaluating microarray data at the level of gene sets (defined based on prior biological knowledge, such as published information about biochemical pathways or coexpression in previous experiments) and determining whether the members of the gene set are randomly distributed or primarily found at the top or bottom. Sets related to the phenotypic distinction will tend to show the latter distribution. Herein, a total of 36 random sample permutations of cholangiocarcinoma in The Cancer Genome Atlas (TCGA) database were divided into a low TIN group and a high TIN group by CIBERSORT, and then GSEA was carried out to determine the pathways that showed statistically significant and concordant differences between a low‐TIN state and a high‐TIN state by MSigDB. If the majority of genes included in a gene set are primarily found at the top or the bottom, there will be a positive enrichment score (ES) and a normalized enrichment score (NES), indicating the different expression patterns between the predefined low‐TIN group and the high‐TIN group.
2.4. Statistical analyses
Continuous variables were analyzed by the Student's t test or Wilcoxon rank‐sum test, and categorical variables were calculated by chi‐squared test. ICC was carried out to describe how strongly units in the same group resemble each other. ICC higher than 0.75 means that values from the same group tend to be similar, whereas ICC lower than 0.40 means that there is no tendency for values from the same group to be similar. Survival curves were constructed using Kaplan‐Meier method and log‐rank test. Univariate and multivariate Cox proportional hazards models were carried out to calculate HR and 95% CI, and those parameters significantly associated with OS in the univariate analysis were included in the following multivariate analysis. Prognostic accuracy of the prognostic models was evaluated by receiver operating characteristic (ROC) analysis. Two‐sided P‐value <.05 was considered to be statistically significant. Statistical analysis was done by SPSS 22.0 (IBM Corp, Armonk, NY, USA) and MedCalc 15.2.2 (MedCalc Software bvba, Ostend, Belgium).
3. RESULTS
3.1. Immunohistochemical findings
Tumor‐infiltrating neutrophils were evaluated by immunohistochemical staining of CD66b. Neutrophils infiltrating tumor tissues varied greatly in different specimens of biliary cancer (Figure 1A) and ranged from 0 to 226 cells/HPF. The median value (18/HPF) was set as the cutoff value which determined high and low TIN in the training set, and was then applied to the validation set. To estimate the heterogeneity of TME, we explored the distribution of CD66‐positive cells using 20 cases of normal sections. As shown in Figure S1, ICC is 0.849, which means that the intensity of CD66‐positive cells in a randomized field could be an ideal representation of that in the normal section. In other words, the intensity of CD66‐positive cells in TMA could also be an ideal representation of that in the normal section.
Figure 1.
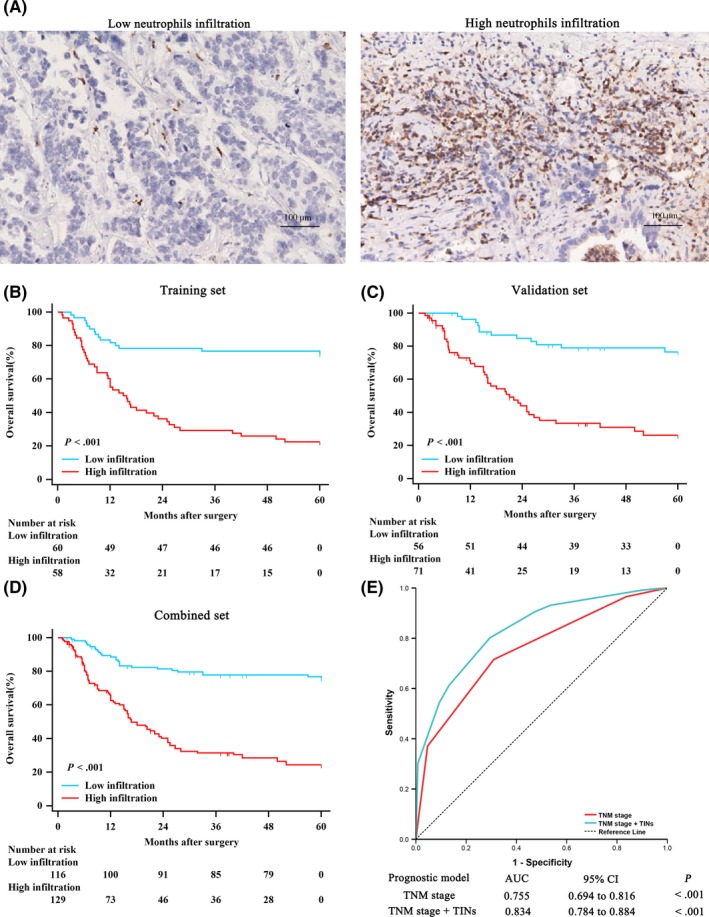
Tumor‐infiltrating neutrophils (TIN) represent an independent prognostic factor in biliary cancer and improve the prognostic accuracy of the TNM system. A, Representative example of CD66b staining with low level of neutrophil infiltration (left) and high level of neutrophil infiltration (right). Scale bars, 100 μm; magnification, 200×. B‐D, Kaplan‐Meier analysis of overall survival (OS) in biliary cancer according to TIN in the B, training set (n = 118, P < .001); C, validation set (n = 127, P < .001); and D, combined set (n = 245, P < .001). E, Receiver operating characteristic of TNM stage (area under the curve [AUC] = 0.755) vs TNM stage +TIN (AUC = 0.834)
3.2. Patient characteristics
Baseline characteristics of patients are described in Tables 1, S1, and S2. In total, 118 patients (56 men [47.5%] and 62 women [52.5%]) were included in the training set, and 127 patients (47 men [37.0%] and 80 women [63.0%]) were included in the validation set. Considering the limitation of patient number, we united the training and the validation sets to the combined set. No clinicopathological characteristic was significantly associated with TIN except for TNM stage. In addition, we explored the association between TIN and obstructive inflammation in the bile duct, and results showed insignificant association between TIN and serum data, including total bilirubin, ALT and AST (Table S2).
Table 1.
Demographics and clinicopathological characteristics of patients with biliary cancer
| Characteristic | Training set | Validation set | Combined set | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| All patients | 118 | 100 | 127 | 100 | 245 | 100 |
| Age at surgery, yearsa | ||||||
| Mean ± SD | 62.5 ± 10.5 | 61.7 ± 10.5 | 62.1 ± 10.5 | |||
| Gender | ||||||
| Female | 62 | 52.5 | 80 | 63.0 | 142 | 58.0 |
| Male | 56 | 47.5 | 47 | 37.0 | 103 | 42.0 |
| Tumor location | ||||||
| Gallbladder | 66 | 55.9 | 108 | 85.0 | 174 | 71.0 |
| Bile duct | 52 | 44.1 | 19 | 15.0 | 71 | 29.0 |
| TNM stage | ||||||
| I | 9 | 7.6 | 16 | 12.6 | 25 | 10.2 |
| II | 34 | 28.8 | 63 | 49.6 | 97 | 39.6 |
| III | 41 | 34.8 | 33 | 26.0 | 74 | 30.2 |
| IV | 34 | 28.8 | 15 | 11.8 | 49 | 20.0 |
| pT stage | ||||||
| T1 | 6 | 5.1 | 19 | 15.0 | 25 | 10.2 |
| T2 | 39 | 33.1 | 61 | 48.0 | 100 | 40.8 |
| T3 | 47 | 39.8 | 31 | 24.4 | 78 | 31.8 |
| T4 | 26 | 22.0 | 16 | 12.6 | 42 | 17.1 |
| pN stage | ||||||
| N0 | 76 | 64.4 | 96 | 75.6 | 172 | 70.2 |
| N1 + N2 | 42 | 35.6 | 31 | 24.4 | 73 | 29.8 |
| Tumor differentiation | ||||||
| Well‐moderate | 74 | 62.7 | 59 | 46.5 | 133 | 54.3 |
| Poor | 44 | 37.3 | 68 | 53.5 | 112 | 45.7 |
| Surgical margin | ||||||
| Negative | 106 | 89.8 | 115 | 90.6 | 221 | 90.2 |
| Positive | 12 | 10.2 | 12 | 9.4 | 24 | 9.8 |
| Microvascular invasion | ||||||
| Absent | 66 | 55.9 | 83 | 65.4 | 149 | 60.8 |
| Present | 52 | 44.1 | 44 | 34.6 | 96 | 39.2 |
| Adjuvant chemotherapy | ||||||
| Absent | 84 | 71.2 | 67 | 52.8 | 151 | 61.6 |
| Present | 34 | 28.8 | 60 | 47.2 | 94 | 38.4 |
Results of continuous variables are presented as mean ± SD.
Combined set was generated by combing the training and validation set together.
3.3. Correlations between TIN and OS
To identify the prognostic value of TIN, we compared the OS between different groups according to TIN level by Kaplan‐Meier survival analysis. Sixty and 56 patients died before the last follow up in the training set and validation set, respectively. As shown in Figure 1B,C and D, patients with high‐TIN disease showed compromised OS compared to those with low‐TIN disease in the training (P < .001), validation (P < .001) and combined sets (P < .001).
3.4. Univariate and multivariate regression analyses
Univariate analysis was carried out for OS to further identify the clinical significance of TIN. As shown in Table 2, a high level of TIN proved to be a significant negative prognostic indicator (HR: 4.720; 95% CI: 2.623‐8.493; P < .001 in the training set; HR: 4.993; 95% CI: 2.626‐9.492; P < .001 in the validation set; and HR: 4.688; 95% CI: 3.050‐7.206; P < .001 in the combined set; Table 2, respectively). In addition, TNM stage was also identified to be significantly associated with OS in all 3 sets (P < .001). Moreover, tumor location (P = .028) was identified to be significantly associated with OS in the training set, as was differentiation in the validation set (P = .029) and surgical margin and microvascular invasion in the combined set (P = .038 and P = .011, respectively). These factors were then entered into the multivariate Cox proportional hazards model, and we found that only TIN (HR: 4.213; 95% CI: 2.320‐7.650; P < .001; HR: 4.010; 95% CI: 2.217‐7.253; P < .001; and HR: 4.133; 95% CI: 2.660‐6.423; P < .001; Table 2, respectively) and TNM stage (P < .001) were independent prognostic indicators for OS.
Table 2.
Univariate and multivariate Cox regression analyses of overall survival
| Characteristic | Training set | Validation set | Combined set | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Univariate | ||||||
| Age at surgery, years: >60 vs <60 | 1.349(0.814‐2.235) | .248 | 1.118(0.660‐1.895) | .679 | 1.225(0.851‐1764) | .278 |
| Gender: male vs female | 0.638(0.381‐1.070) | .089 | 0.821(0.472‐1.427) | .486 | 0.726(0.498‐1.057) | .097 |
| Tumor location: bile duct vs gallbladder | 0.621(0.407‐0.948) | .028 | 0.917(0.550‐1.528) | .739 | 0.752(0.545‐1.036) | .081 |
| TNM stage: III + IV vs I + II | 4.232(2.148‐8.336) | <.001 | 1.945(1.481‐2.555) | <.001 | 1.976(1.584‐2.463) | <.001 |
| Differentiation: poor vs well‐moderate | 0.892(0.683‐1.165) | .405 | 1.352(1.032‐1.770) | .029 | 1.074(0.895‐1.290) | .444 |
| Surgical margin: positive vs negative | 1.679(0.800‐3.538) | .173 | 1.907(0.902‐4.032) | .093 | 1.750(1.031‐2.970) | .038 |
| Microvascular invasion: present vs absent | 1.571(0.948‐2.605) | .081 | 1.646(0.968‐2.800) | .067 | 1.610(1.118‐2.320) | .011 |
| CD66b expression: high vs low | 4.720(2.623‐8.493) | <.001 | 4.993(2.626‐9.492) | <.001 | 4.688(3.050‐7.206) | <.001 |
| Multivariate | ||||||
| Tumor location: bile duct vs gallbladder | 0.869(0.522‐1.447) | .591 | ||||
| TNM stage: III + IV vs I + II | 3.253(1.537‐6.885) | <.001 | 1.588(1.193‐2.113) | <.001 | 1.640(1.311‐2.051) | <.001 |
| Differentiation: poor vs well‐moderate | 1.252(0.948‐1.653) | .115 | ||||
| Surgical margin: positive vs negative | 1.446(0.821‐2.545) | .204 | ||||
| Microvascular invasion: present vs absent | 1.319(0.892‐1.949) | .167 | ||||
| CD66b expression: high vs low | 4.213(2.320‐7.650) | <.001 | 4.010(2.217‐7.253) | <.001 | 4.133(2.660‐6.423) | <.001 |
P < .05 is considered statistically significant (bold).
Combined set was generated by combing the training and validation sets together (bold).
3.5. Extension of prognostic models with TIN
In view of the distinctly prognostic value, we integrated TIN into the TNM staging system to explore the practical application of TIN. ROC analysis was carried out to assess the prognostic accuracy. As shown in Figure 1E, the area under the curve (AUC) of TNM stage alone was 0.755 (95% CI: 0.694‐0.816), and was elevated to 0.834 (95% CI: 0.784‐0.884) when TIN were added.
3.6. Correlations between TIN and ACT
According to patient characteristics, we evaluated the association between ACT and OS. In patients with stage III and IV disease, those with high TIN could not benefit from ACT in training, validation and combined sets (P = .415, P = .735, P = .556, Figure 2A,C,E, respectively). However, those patients with low TIN could benefit greatly from ACT (P = .047, P = .006, P = .002, Figure 2B,D,F, respectively). Treatment with ACT was correlated with reduced risk of compromised survival in the low‐TIN patient subgroup in all sets (HR: 0.294; 95% CI: 0.099‐0.873; P = .047 in the training set; HR: 0.100; 95% CI: 0.022‐0.462; P = .006 in the validation set; and HR: 0.216; 95% CI: 0.090‐0.520; P = .002 in the combined set; Table 3, respectively), whereas such risk reduction was not observed in patients with high TIN (Table 3) and in patients with stage II disease (Figure S2).
Figure 2.
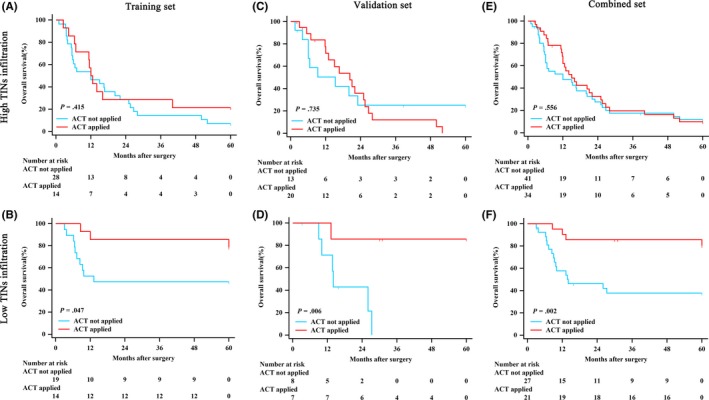
Relationship between tumor‐infiltrating neutrophils (TIN) and benefit from adjuvant chemotherapy (ACT) in patents with stage III and IV disease. Patients with high TIN in the A, training set; C, validation set; and E, combined set. Patients with low TIN in the B, training set; D, validation set; and F, combined set
Table 3.
Hazard ratios for overall survival in stage III and IV biliary cancer patients receiving ACT or not according to TIN
| Patient cohort | Factor | Patients | ACT (yes vs no) | ||
|---|---|---|---|---|---|
| Number | % | Hazard ratio (95% CI) | P‐value | ||
| Training set | TIN | 75 | 0.480(0.275‐0.839) | .016 | |
| Low TIN | 33 | 44.0 | 0.294(0.099‐0.873) | .047 | |
| High TIN | 42 | 56.0 | 0.749(0.383‐1.466) | .415 | |
| Validation set | TIN | 48 | 0.646(0.316‐1.322) | .205 | |
| Low TIN | 15 | 31.3 | 0.100(0.022‐0.462) | .006 | |
| High TIN | 33 | 68.8 | 1.148(0.519‐2.536) | 0.735 | |
| Combined set | TIN | 123 | 0.574(0.373‐0.883) | .012 | |
| Low TIN | 48 | 39.0 | 0.216(0.090‐0.520) | .002 | |
| High TIN | 75 | 61.0 | 0.863(0.527‐1.415) | .556 | |
P < .05 is considered statistically significant (bold).
Combined set was generated by combing the training and validation sets together.
ACT, adjuvant chemotherapy; TIN, tumor infiltrating neutrophil.
3.7. Interaction between TIN and other TME components
Given the interaction above and the ability of TIN to influence CD8 + T cells suggested previously,30 we evaluated the relationship between TINs and CD8 + T cells in biliary tumor tissue (Figure 3A‐D) and found that TIN were negatively correlated with CD8 + T cells (r = −.324, P < .001) (Figure 3E). We then carried out GSEA to determine and verify the pertinent biological pathway and found that regulation of activated T‐cell proliferation and lymphocyte‐mediated immunity were enriched in the low‐TIN group (Figure 3F,G), which indicated that gene expression related to T‐cell proliferation and lymphocyte‐mediated immunity was more activated in patients with low TIN. This result implied the inhibiting effect of TIN to effector T cells and the potential mechanism as to why patients with low TIN had longer OS and much more ACT benefit. In addition, there are other enriched immune‐related pathways, such as lymphocyte differentiation, regulation of T‐cell cytokine production and so on (Table S3). Moreover, infiltration of CD8 + T cells was related to favorable OS (Figure 3H), and the combination of TIN and CD8 + T cells indicated that patients with high‐TIN and low‐CD8 + T‐cell disease experienced the shortest OS compared with any other subgroup (Figure 3I). These results indicated that high TIN was correlated with the state of immunosuppression in the TME of biliary cancer.
Figure 3.
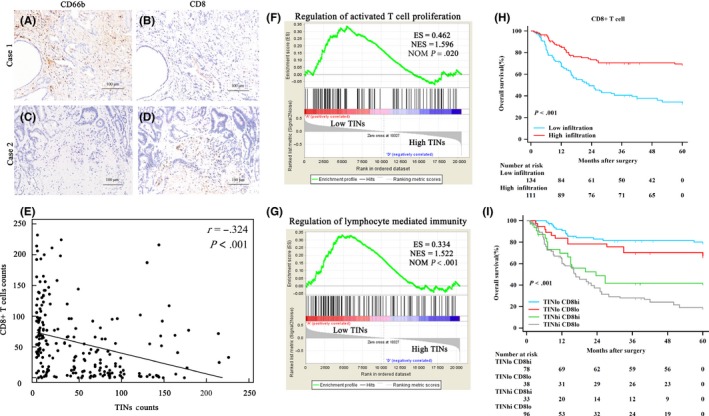
Association between tumor‐infiltrating neutrophils (TIN) and CD8 + T cells. A‐D, Continuous sections from biliary cancer samples immunohistochemically stained for CD66b and CD8. Scale bars, 100 μm; magnification 200×. E, Spearman's correlation analysis for TIN counts and CD8 + T‐cell counts. F, Gene Set Enrichment Analysis (GSEA) of regulation of activated T‐cell proliferation between the low‐TIN group and the high‐TIN group. ES, enriched score; NES, normalized enriched score; NOM, P‐value, normalized P‐value. G, GSEA of regulation of lymphocyte‐mediated immunity between the low‐TIN group and the high‐TIN group. H, CD8 + T‐cell infiltration in biliary cancer is associated with favorable prognosis. Kaplan‐Meier curves illustrating overall survival (OS) probability according to CD8 + T‐cell density. I, Combination of TIN and CD8 + T cells indicated that patients with high TIN and low CD8 + T cells experienced the shortest OS
4. DISCUSSION
As the most common prognostic model for patients with biliary cancer, the TNM staging system focuses only on cancer‐cell‐centered biological phenotypes and ignores the enormous effects of TME, which lead to obvious reduction of prognostic accuracy. That is the reason why so many early‐stage patients progress rapidly, whereas some advanced‐stage patients maintain a relatively stable state for years. In the present study, we proved the prognostic significance of TIN, identified it as an independent prognostic marker for OS in patients with biliary cancer and integrated it into the TNM staging system to elevate its prognostic value. Furthermore, TIN might be a potential predictive marker for the response of ACT in biliary cancer.
It was reported that neutrophils can survive longer in tumor tissue than in spleen even for several days,31, 32 which means TIN might have more far‐reaching effects on TME than we have previously realized. TIN can be attracted by granulocyte colony‐stimulating factor (G‐CSF), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), chemokine (C‐X‐C motif) ligand (CXCL)1, CXCL2, CXCL5 and CXCL8 secreted by cancer cells, endothelial cells or fibroblasts.33, 34, 35, 36, 37, 38, 39, 40, 41 Simultaneously, G‐CSF and transforming growth factor beta (TGF‐β) can control the immunosuppressive function of N2 by regulating transcription factors inhibitor of DNA binding 1 (ID1), retinoblastoma 1 (RB1) and interferon regulatory factor 8 (IRF8), whereas IFN‐β can regulate the antitumor phenotype of N1.9, 28, 42 In our study, low TIN predict a favorable survival for biliary cancer. Interestingly, neutrophil to lymphocyte ratios (NLR), another attractive biomarker for risk stratification of cancer patients related to neutrophils, is generally considered a negative factor and taken as evidence of protumor function of neutrophils. However, the different functional roles of neutrophils in solid tumor tissue and in peripheral blood, and the precise roles and corresponding mechanisms of TIN continue to be obscure.
Adjuvant chemotherapy‐induced neutropenia involved the outcomes of patients with non‐small‐cell lung, breast, gastric or colorectal cancer, and lack of neutropenia may indicate insufficient dosing and inadequate killing.9, 43, 44, 45 However, in the present study, low neutrophil infiltration indicated better ACT efficacy. In biliary cancer patients with stage III and IV disease, patients with low TIN might benefit from ACT; that is, ACT for patients with high TIN should be carefully reconsidered. This result will be helpful for better selection of patients receiving ACT and prevention of excessive toxicities and unnecessary resource consumption. In addition, the unfavorable role played by neutrophils in biliary cancer suggest possibilities for new cancer immunotherapies directed at TIN depletion to enhance the sensitivity and response to ACT. This method may improve survival of biliary cancer patients with high TIN, which is in line with findings that neutrophil depletion by C‐X‐C chemokine receptor type 2 (CXCR2) blockage can improve tumor inhibition of chemotherapy.13, 46, 47
As reported previously, neutrophils can suppress CD8 + T cell‐mediated antitumor immune response by inducible nitric oxide synthase (iNOS) and arginase 1 (ARG1).30 In the present study, we found that TIN in biliary cancer were negatively correlated with CD8 + T cells which is an important preserver of antitumor response48 and has already proven to be a favorable marker as mentioned earlier. Also, we verified that high TIN was related to inhibition of antitumor immune effector through GSEA. These results indicate that high TIN was correlated with immunosuppression in TME of biliary cancer and may be the reason for poor prognosis and ACT efficacy.
Limitations of our study are the retrospective nature, relatively small number of patients, as well as the unavailability of full chemotherapy details for the entire cohorts. In addition, although CD66b have a high purity to identify TIN in TME, it is not an extremely specific marker for neutrophils. Therefore, these results need to be validated by a future prospective, multicenter randomized trial with a larger population.
In conclusion, TIN could be used as an independent prognostic factor for prognosis and ACT benefit in biliary cancer patients, especially for patients with stage III and IV disease.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
This study was funded by grants from National Natural Science Foundation of China (81471621, 81472227, 81502004, 81600630, 81671628 and 31770851) and Shanghai Municipal Natural Science Foundation (16411952000). All these study sponsors have no roles in the study design, collection, analysis, and interpretation of data. All authors have contributed significantly to the content of the manuscript.
Wang J, Bo X, Suo T, et al. Tumor‐infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018;109:2266–2274. https://doi.org/10.1111/cas.13627
Funding information
Natural Science Foundation of Shanghai (Grant/Award Number: ‘16411952000‘), National Natural Science Foundation of China (Grant/Award Numbers: ‘31770851‘, ‘81471621‘, ‘81472227‘, ‘81502004‘, ‘81600630‘, ‘81671628‘)
Wang and Bo contributed equally to this work.
Contributor Information
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
Houbao Liu, Email: houbaoliu@aliyun.com.
Yueqi Wang, Email: yueqiwang@fudan.edu.cn.
REFERENCES
- 1. Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v28‐v37. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 4. Murakami Y, Uemura K, Sudo T, et al. Adjuvant gemcitabine plus S‐1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250:950‐956. [DOI] [PubMed] [Google Scholar]
- 5. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689‐1700. [DOI] [PubMed] [Google Scholar]
- 6. Glazer ES, Liu P, Abdalla EK, Vauthey JN, Curley SA. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg. 2012;16:1666‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95:1402‐1410. [DOI] [PubMed] [Google Scholar]
- 8. Tazuma S, Kajiyama G. Carcinogenesis of malignant lesions of the gall bladder. The impact of chronic inflammation and gallstones. Langenbecks Arch Surg. 2001;386:224‐229. [DOI] [PubMed] [Google Scholar]
- 9. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431‐446. [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Pang Y, Gara SK, et al. Gr‐1 + CD11b+ cells are responsible for tumor promoting effect of TGF‐beta in breast cancer progression. Int J Cancer. 2012;131:2584‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benevides L, da Fonseca DM, Donate PB, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75:3788‐3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Mitri D, Toso A, Chen JJ, et al. Tumour‐infiltrating Gr‐1 + myeloid cells antagonize senescence in cancer. Nature. 2014;515:134‐137. [DOI] [PubMed] [Google Scholar]
- 14. Spicer JD, McDonald B, Cools‐Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac‐1‐mediated interactions with circulating tumor cells. Cancer Res. 2012;72:3919‐3927. [DOI] [PubMed] [Google Scholar]
- 15. Cools‐Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Egmond M, Bakema JE. Neutrophils as effector cells for antibody‐based immunotherapy of cancer. Semin Cancer Biol. 2013;23:190‐199. [DOI] [PubMed] [Google Scholar]
- 18. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high‐risk area in northern Italy. Mod Pathol. 2002;15:831‐837. [DOI] [PubMed] [Google Scholar]
- 20. Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497‐505. [DOI] [PubMed] [Google Scholar]
- 21. Jensen TO, Schmidt H, Moller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476‐2485. [DOI] [PubMed] [Google Scholar]
- 22. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709‐4717. [DOI] [PubMed] [Google Scholar]
- 23. Wislez M, Rabbe N, Marchal J, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405‐1412. [PubMed] [Google Scholar]
- 24. Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony‐stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707‐2716. [DOI] [PubMed] [Google Scholar]
- 25. Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev Cancer. 2008;8:618‐631. [DOI] [PubMed] [Google Scholar]
- 26. Lakschevitz FS, Hassanpour S, Rubin A, Fine N, Sun C, Glogauer M. Identification of neutrophil surface marker changes in health and inflammation using high‐throughput screening flow cytometry. Exp Cell Res. 2016;342:200‐209. [DOI] [PubMed] [Google Scholar]
- 27. Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor‐associated neutrophils recruit macrophages and T‐Regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646‐1658. e17. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Liu H, Shen Z, et al. Tumor‐infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267:311‐318. [DOI] [PubMed] [Google Scholar]
- 29. Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour‐associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116‐2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coffelt SB, Kersten K, Doornebal CW, et al. IL‐17‐producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood. 2006;108:2821‐2826. [DOI] [PubMed] [Google Scholar]
- 32. Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine‐mediated rapid turnover of myeloid‐derived suppressor cells in tumor‐bearing mice. Blood. 2008;111:5457‐5466. [DOI] [PubMed] [Google Scholar]
- 33. Casbon AJ, Reynaud D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112:E566‐E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis‐initiating breast cancer cells. Nature. 2015;528:413‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bayne LJ, Beatty GL, Jhala N, et al. Tumor‐derived granulocyte‐macrophage colony‐stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pylayeva‐Gupta Y, Lee KE, Hajdu CH, Miller G, Bar‐Sagi D. Oncogenic Kras‐induced GM‐CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohler A, De Filippo K, Hasenberg M, et al. G‐CSF‐mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117:4349‐4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jamieson T, Clarke M, Steele CW, et al. Inhibition of CXCR2 profoundly suppresses inflammation‐driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127‐3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2‐expressing myeloid‐derived suppressor cells are essential to promote colitis‐associated tumorigenesis. Cancer Cell. 2013;24:631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blaisdell A, Crequer A, Columbus D, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28:785‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang G, Lu X, Dey P, et al. Targeting YAP‐dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou L, Xu L, Chen L, et al. Tumor‐infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology. 2017;6:e1293211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Predictive value of chemotherapy‐induced neutropenia for the efficacy of oral fluoropyrimidine S‐1 in advanced gastric carcinoma. Br J Cancer. 2007;97:37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shitara K, Matsuo K, Takahari D, et al. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first‐line FOLFOX. Eur J Cancer. 2009;45:1757‐1763. [DOI] [PubMed] [Google Scholar]
- 45. Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X. Prognostic value of chemotherapy‐induced neutropenia in early‐stage breast cancer. Breast Cancer Res Treat. 2012;131:483‐490. [DOI] [PubMed] [Google Scholar]
- 46. Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma Y, Adjemian S, Mattarollo SR, et al. Anticancer chemotherapy‐induced intratumoral recruitment and differentiation of antigen‐presenting cells. Immunity. 2013;38:729‐741. [DOI] [PubMed] [Google Scholar]
- 48. Betts BC, Veerapathran A, Pidala J, et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med. 2017;9:eaai8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


