Abstract
Synovial sarcoma (SS) is a rare yet refractory soft‐tissue sarcoma that predominantly affects young adults. We show in a mouse model that radioimmunotherapy (RIT) with an α‐particle emitting anti‐Frizzled homolog 10 (FZD10) antibody, synthesized using the α‐emitter radionuclide astatine‐211 (211At‐OTSA101), suppresses the growth of SS xenografts more efficiently than the corresponding β‐particle emitting anti‐FZD10 antibody conjugated with the β‐emitter yettrium‐90 (90Y‐OTSA101). In biodistribution analysis, 211At was increased in the SS xenografts but decreased in other tissues up to 1 day after injection as time proceeded, albeit with a relatively higher uptake in the stomach. Single 211At‐OTSA101 doses of 25 and 50 μCi significantly suppressed SS tumor growth in vivo, whereas a 50‐μCi dose of 90Y‐OTSA101 was needed to achieve this. Importantly, 50 μCi of 211At‐OTSA101 suppressed tumor growth immediately after injection, whereas this effect required several days in the case of 90Y‐OTSA101. Both radiolabeled antibodies at the 50‐μCi dosage level significantly prolonged survival. Histopathologically, severe cellular damage accompanied by massive cell death was evident in the SS xenografts at even 1 day after the 211At‐OTSA101 injection, but these effects were relatively milder with 90Y‐OTSA101 at the same timepoint, even though the absorbed doses were comparable (3.3 and 3.0 Gy, respectively). We conclude that α‐particle RIT with 211At‐OTSA101 is a potential new therapeutic option for SS.
Keywords: α‐particle, β‐particle, frizzled homolog 10, radioimmunotherapy, synovial sarcoma
1. INTRODUCTION
Synovial sarcoma (SS) is rare but a highly aggressive soft‐tissue sarcoma (STS) that can develop at any site in the body but often arises at lower extremities in adolescents and young adults.1, 2 Although cases with a resectable SS have a relatively better prognosis, there remains a high risk of local recurrence and metastasis to the lymph nodes and lung. Patients with metastatic SS have a poor prognosis.3, 4 Although the molecular mechanisms underlying the oncogenesis of SS have remained elusive, a balanced t(X,18; p11,q11) chromosomal translocation is found in virtually all cases, which creates a fusion oncogene, SS18‐SSX, that could be the driver mutation gene for these cancers.5, 6 Surgical therapy, accompanied by radiation and/or chemotherapy, has been shown to be effective for early stage SS but no effective therapies have been established so far for advanced SS. Novel therapeutic strategies for this disease are, thus, highly desired.
Frizzled homolog 10 (FZD10) is a transmembrane protein member of the Frizzled family7 and serves as a putative receptor in the Wnt signal pathway. Previous studies have revealed that FZD10 is highly expressed in SS tumors and cell lines but is absent in most normal tissues, and that immunotherapy using an antibody against FZD10 inhibits the growth of SS xenografts.8, 9 Furthermore, radioimmunotherapy (RIT) using a yttrium‐90 (90Y)‐labeled anti‐FZD10 antibody emitting β‐particle radiation has been reported to be effective against SS in a preclinical mouse model.10, 11 These studies raised the possibility that FZD10 has potential as a therapeutic target for SS.
Recently, α‐particle‐based RIT (α‐RIT) has emerged as a novel antitumor strategy. Astatine‐211 (211At) is one of the more attractive α‐emitters in this regard because of its 7.2‐h half‐life and 100% α‐particle emission during its decay.12 The remarkably high cell killing potency of 211At derives from its high energy transfer within a short range of the α‐particle, which is considered to be stronger than that of a β‐particle.13 We have previously reported, also in a preclinical mouse model, that α‐RIT using 211At‐labeled trastuzumab is highly effective against peritoneal metastases of gastric cancers that are positive for human epidermal growth factor receptor 2 (HER2).14 Thus, we speculated that α‐RIT may be more beneficial than β‐RIT in the treatment of solid tumors, including STS and SS, which are often radio‐resistant or chemo‐resistant. This notion is supported by the findings of several previous studies of solid tumor models.15, 16 However, few reports to date have directly compared the therapeutic efficacy of α‐RIT and β‐RIT against SS in vivo.
In our current study, we conducted comparative analysis of α‐RIT using a 211At‐anti‐FZD10 antibody and β‐RIT using a 90Y‐anti‐FZD10 antibody in a preclinical SS xenograft model and evaluated both their therapeutic efficacy and toxicity against SS.
2. MATERIALS AND METHODS
2.1. Cells
The human synovial sarcoma cell line SYO‐1 was kindly provided by Dr A. Kawai (National Cancer Center, Tokyo, Japan).17 Cells were cultured with D‐MEM medium, supplemented with 10% FBS and 1% penicillin‐streptomycin (Wako, Osaka, Japan), and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
2.2. Reagents
A humanized chimeric anti‐FZD10 antibody, OTSA101, was provided by OncoTherapy Science (Kanagawa, Japan). N‐succinimidyl‐3‐(trimethylastannyl) benzoate (m‐MeATE) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and stored at −30°C. N‐chlorosuccinimide (NCS) was purchased from Tokyo Chemical Industry (Tokyo, Japan).
2.3. Radiolabeling of the anti‐FZD10 antibody
Astatine‐211 (211At) was produced by the 209Bi (α, 2n)211At reaction as described previously.18 The 211At labeling of anti‐FZD10 antibody (OTSA101) was also carried out following a previously described procedure.14 Briefly, OTSA101 (5 mg/mL) was conjugated to m‐MeATE (26 mmol/L in DMSO) in a reaction containing 0.2 mol/L sodium carbonate buffer (pH 8.5). The immunoconjugate was subsequently isolated in PBS using a Sephadex G‐50 spin column (GE Healthcare Japan, Tokyo, Japan) and then adjusted to pH 5.5 by adding citric acid prior to 211At labeling. 211At was dissolved using 0.04 mg/mL NCS in methanol with 1% acetic acid for labeling. The OTSA101‐m‐MeATE immunoconjugate (2 mg/mL) was added to 211At and reacted for 1 minute followed by another 1‐minute reaction with 2‐mg/mL NCS. Finally, sodium ascorbate (50 mg/mL) was added to stop the reaction. 211At‐labeled OTSA101 (211At‐OTSA101) was isolated in PBS using a Sephadex G‐50 spin column and verified by high‐performance liquid chromatography (HPLC). Yttrium‐90 (90Y) and indium‐111 (111In) were obtained from Eckert & Ziegler Radiopharma (Berlin, Germany) and Nihon Medi‐Physics (Tokyo, Japan), respectively. Both the111In and 90Y labeling were performed as described previously.19 Radiochemical purities were evaluated by methanol precipitation, HPLC or thin‐layer chromatography.
2.4. Animal experiments
All animal experiments were approved by the Animal Care and Use Committee of the National Institute of Radiological Sciences at the National Institutes for Quantum and Radiological Science and Technology (Chiba, Japan) and were undertaken in compliance with the institutional guidelines regarding animal care and handling.
2.5. Tumor xenograft mouse model
The SS xenograft mouse model was established by subcutaneously implanting SYO‐1 cells (1 × 107 cells in 0.1‐mL PBS) into the flank of BALB/c nude mice (Japan SLC, Hamamatsu, Japan).
2.6. Biodistribution
Biodistribution studies were performed as described previously.14 Briefly, 111In‐labeled OTSA101 (111In‐OTSA101, 1 μCi) or 211At‐OTSA101 (12.5 μCi) was injected into the tail veins of the SS xenograft mice. Tumor and tissues were dissected at 1 hour, 3 hours, 1 day, 2 days and 4 days post‐injection, excluding the 2‐day and 4‐day timepoints for the 211At‐OTSA101 group. The radioactivity in each tissue was measured using a gamma counter (Aloka, Tokyo, Japan) to calculate the percentage of the injected dose per tissue gram (%ID/g).
2.7. Radioimmunotherapy
Radioimmunotherapy was performed as described previously.14 Briefly, SS xenograft mice received a single injection of 12.5, 25 or 50 μCi of 211At‐OTSA101 or 90Y‐OTSA101 into the tail vein. Unlabeled and intact OTSA101 were also injected as controls. The protein doses were adjusted to the same amount (50 μg) by adding intact antibody immediately after verification using HPLC. Injection of radiolabeled antibodies was performed within 30 minutes after HPLC verification. Tumor sizes and body weights were monitored continuously to evaluate anti‐tumor effects and toxicity. Mice were killed at the defined study endpoint (ie when the tumor volume reached 1200 mm3 or at 28 days post‐injection). The tumor‐absorbed and tissue‐absorbed doses for radiolabeled antibodies were estimated from the biodistribution data as previously described.19 Briefly, the area under the curve (AUC) was calculated based on the biodistribution data and the absorbed dose was then estimated using the AUC and the mean energy emitted per transition of 90Y, or 211At and a daughter nuclide 211Po with a correction for the branching ratio.20
2.8. Histological analysis and immunohistochemistry
Histological analyses were performed as described previously.19 Briefly, SS xenografts were sampled from the model mice at 1, 3 and 7 days post‐injection with either 211At‐OTSA101 (50 μCi) or 90Y‐OTSA101 (50 μCi) and fixed with 10% (v/v) formalin and embedded in paraffin. Untreated SS xenografts were also sampled. After sectioning, the samples were stained with H&E. Immunohistochemical staining of CD31 was performed according the manufacturer's instruction. Briefly, paraffin sections of tumors were deparaffinized and then heat‐induced antigen retrieval and endogenous peroxidase quenching were performed using Histofine Deparaffinization Antigen Retrieval Solution pH 9 (Nichirei Biosciences, Tokyo, Japan) and Dako REALTM Peroxidase‐Blocking Solution (Dako, Dlostrup, Denmark), respectively. After blocking using Blocking One Histo (Nacalai tesque, Kyoto, Japan), tumor sections were incubated with anti‐CD31 antibody (ab28364, Abcam, Cambridge, UK) overnight at 4°C. SignalStain Boost IHC Detection Regent and DAB Substrate Kit (Cell Signaling Technology, Danvers, MA9) were used to visualize the reaction and hematoxylin was used for counterstaining.
2.9. Statistical analysis
Statcel 3 software (OMS, Tokorozawa, Japan) was used for all statistical analysis. Tumor volumes and survival data were analyzed using 2‐way repeated measures ANOVA and the Kaplan‐Meier method, respectively. A P‐value of <.05 was considered significant.
3. RESULTS
3.1. Radiochemical analyses of radiolabeled OTSA101
The labeling yields of 211At, 111In and 90Y to OTSA101 were 49.0 ± 4.2, 86.2 ± 2.1 and 98.1%, respectively. The radiochemical purities of the radiolabeled OTSA101 molecules were consistently above 95%.
3.2. Biodistribution of 211At‐OTSA101 and 111In‐OTSA101
We investigated the biodistribution of 211At‐OTSA101 and 111In‐OTSA101 in the SS xenograft mouse model (Figure 1). The biodistribution of 211At‐OTSA101 was very similar to that of 111In‐OTSA101 within 1 day of injection other than the stomach where relatively higher radioactivity was observed only for 211At‐OTSA101 at approximately a 6‐fold higher level than 111In‐OTSA101 (Figure 1A,B).
Figure 1.
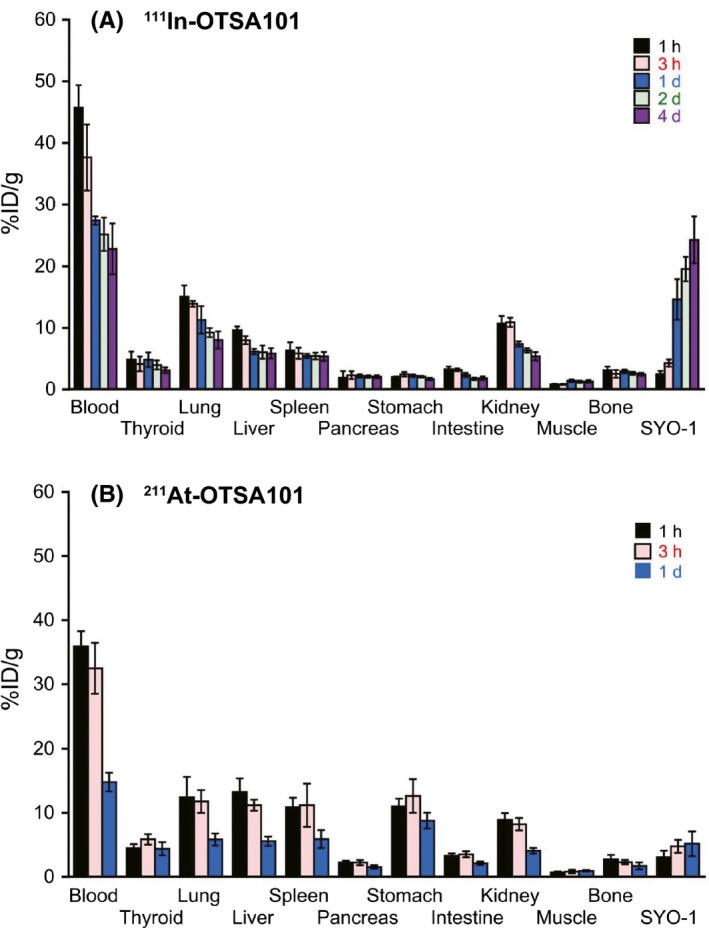
Biodistribution of 111In‐OTSA101 and 211At‐OTSA101 in the SS subcutaneous xenograft mouse model. Uptakes (%ID/g) of 111In in the tumor and other organs at 1 h, 3 h, 1 d, 2 d and 4 d after injection of 111In‐OTSA101 (A) and 211At at 1 h, 3 h and 1 d after injection of 211At‐OTSA101 (B). Five mice were used at each timepoint in both groups. All data represent the mean ± SD
We observed increased tumor uptake for both of the radiolabeled OTSA101 molecules over time but to different maximum levels of 5.1 ± 1.9%ID/g up to 1‐day post‐injection for 211At‐OTSA101 compared with 14.6 ± 3.3%ID/g for 111In‐OTSA101 at the same timepoint. The maximum tumor uptake of 111In‐OTSA101 was 24.2 ± 3.8%ID/g at 4 days post‐injection. The maximum blood uptake of 211At‐OTSA101 was considerably lower (35.9 ± 2.3%ID/g vs 45.7 ± 3.7%ID/g) and showed a faster decline (14.8 ± 1.5%ID/g vs 27.4 ± 0.7%ID/g at 1 day post‐injection) compared to that of 111In‐OTSA101. There was no apparent difference in the maximum uptake at the thyroid where halogens preferentially bind (5.9 ± 0.8%ID/g for 211At‐OTSA101 vs 4.9 ± 1.3%ID/g for 111In‐OTSA101).
3.3. Therapeutic efficacy of 211At‐OTSA101 and 90Y‐OTSA101 against synovial sarcoma tumors in the mouse
Figure 2 shows the measured therapeutic efficacy (ie tumor suppression and survival time) of RIT using 211At‐OTSA101 and 90Y‐OTSA101. A single injection of 25 and 50 μCi of 211At‐OTSA101 and of 50 μCi of 90Y‐OTSA101 significantly suppressed the growth of SYO‐1 xenografts compared to treatment with intact OTSA101 in our SS mouse model. Twenty‐five μCi of 90Y‐OTSA101 and 12.5 μCi each of both types of radiolabeled OTSA101 moderately suppressed the SS tumor growth but not to a significant level. Notably, SYO‐1 tumors treated with a 50 μCi dose of 211At‐OTSA101 suppressed tumor growth immediately after injection, whereas tumors continued to grow after a 50‐μCi injection of 90Y‐OTSA101 and only started to shrink from several days after this treatment. Tumor regrowth occurred from 17 to 20 days post‐injection in both treatment groups (Figure 2A,B).
Figure 2.
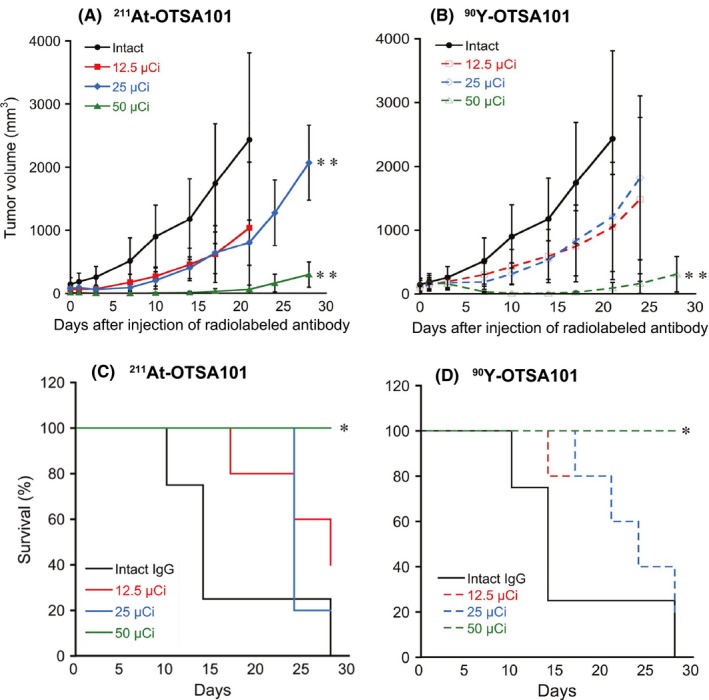
Therapeutic results of radioimmunotherapy (RIT) with 211At‐OTSA101 and 90Y‐OTSA101 in the synovial sarcoma (SS) subcutaneous xenograft mouse model. Changes in tumor volumes in the model mice after RIT with 211At‐OTSA101 (A) and 90Y‐OTSA101 (B) are shown. Plots were interrupted if the animal reached the defined endpoint. Kaplan‐Meier survival curves of mice after RIT with 211At‐OTSA101 (C) and 90Y‐OTSA101 (D). Five mice were enrolled in each of the treatment groups, except for the intact IgG control (4 mice). *P < .05, **P < .01, vs intact IgG control
Survival was significantly prolonged in the SYO‐1 xenograft mice by RIT with 50 μCi of either radiolabeled antibody compared to treatment with intact OTSA101. The mean survival outcomes were 14 days with intact OTSA101 and 24 days for all of the SS model mice treated with 211At‐OTSA101 (25 μCi) or 90Y‐OTSA101 (12.5 or 25 μCi) except for the 12.5‐μCi 211At‐OTSA101 group that showed a 28‐day mean survival. None of the mice reached the study endpoint when treated with a 50‐μCi dose of either radiolabeled antibody during the observation period (30 days) (Figure 2C,D).
We next measured the animal body weights to assess the toxicity of both radiolabeled antibodies (Figure 3). Although these weights tended to be lower in the mice treated with the 50‐μCi doses, no apparent severe body weight loss was observed in any of the experimental animals (Figure 3A,B).
Figure 3.
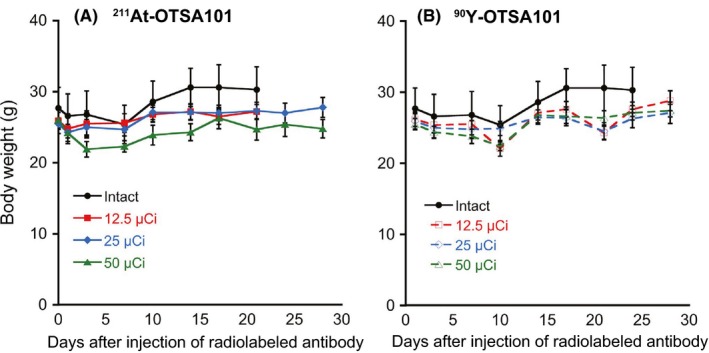
Body weights of the mice after treatment with 211At‐OTSA101 (A) or 90Y‐OTSA101 (B). Plots were interrupted if the animal reached the defined endpoint. Data represent the mean ± SD
3.4. Absorbed dose by the tumor following radioimmunotherapy with 211At‐OTSA101 and 90Y‐OTSA101
The absorbed doses of the radiolabeled antibodies by each mouse tissue were calculated using biodistribution data (Table 1). The biodistribution data for 111In‐OTSA101 were used in the calculations for 90Y‐OTSA101 as they had almost the same biodistribution pattern. The tumor absorbed doses up to 1‐day post‐injection were almost equivalent for 211At‐OTSA101 and 90Y‐OTSA101 at 3.3 and 3.0 Gy, respectively. For 90Y‐OTSA101, this level reached 9.3 Gy at 4 days.
Table 1.
Absorbed doses (Gy) by each mouse tissue following radioimmunotherapy using 211At‐OTSA101 (50 μCi) or 90Y‐OTSA101 (50 μCi)
| Tissue | 211At‐OTSA101 1 d | 90Y‐OTSA101 1 d | 90Y‐OTSA101 4 d |
|---|---|---|---|
| Blood | 24.2 | 9.6 | 16.9 |
| Thyroid | 4.1 | 1.4 | 2.5 |
| Lung | 8.7 | 3.7 | 6.4 |
| Liver | 8.4 | 2.1 | 3.9 |
| Spleen | 8.2 | 1.7 | 3.3 |
| Pancreas | 1.6 | 0.7 | 1.3 |
| Stomach | 9.1 | 0.7 | 1.3 |
| Intestine | 2.6 | 0.8 | 1.3 |
| Kidney | 6.1 | 2.6 | 4.5 |
| Muscle | 0.6 | 0.3 | 0.7 |
| Bone | 1.7 | 0.8 | 1.6 |
| SYO‐1 tumor | 3.3 | 3.0 | 9.3 |
3.5. Histopathological features of α‐RIT and β‐RIT
We conducted histopathological analyses to identify any differences in the therapeutic effects of α‐RIT (211At‐OTSA101) and β‐RIT (90Y‐OTSA101) (Figure 4). The untreated SYO‐1 SS xenografts showed spindle cell proliferation with high cellularity and relatively small pleomorphism among the tumor cells, and no apparent epithelial cells. These characteristics were consistent with a spindle, monophasic type of SS. No necrosis was apparent in any of these xenografts (Figure 4A). At day 1 after α‐RIT treatment, many of the SS tumor cells became smaller with pyknotic nuclei, indicating severe damage. There were some relatively larger cells among these smaller cells, which showed milder damage. Edema was also evident (Figure 4B). At day 1 following β‐RIT, the tumor cells became round. A slight degree of edema and cell damage were also detected. Mitoses were also still evident (Figure 4C). However, the damage from β‐RIT was less severe than that induced by α‐RIT. By day 3, α‐RIT‐treated tumors were severely damaged with smaller cells and edema. Inflammatory cell infiltration was present although slight (Figure 4D). In the case of β‐RIT, the treated tumors became edematous with smaller cells showing pyknotic nuclei and severe damage, and larger cells, indicative of less damage, also found (Figure 4E). Spindle cell proliferation was found in tumors treated with both α‐RIT and β‐RIT by day 7 post‐injection, although there were still some damaged cells observed (Figure 4F,G). CD 31 and H&E staining revealed that no blood vessels were damaged at day 1 after α‐RIT treatment, suggesting that the SS cells were directly targeted by the α‐RIT (Figure 4H,I). These analyses revealed that the cellular damage induced by 211At‐OTSA101 emerges at an earlier stage and is more severe than that induced by 90Y‐OTSA101.
Figure 4.
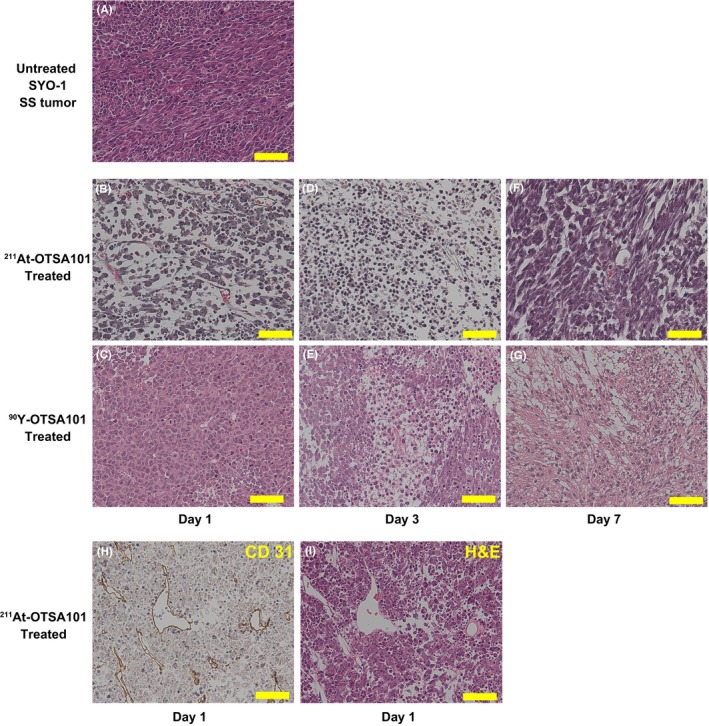
Histopathological analyses of the synovial sarcoma (SS) xenografts by immunohistochemistry and H&E staining. A, Untreated SYO‐1 SS xenograft. B,C, Day 1, D,E, day 3, F,G, day 7 after radioimmunotherapy. B,D,F, 211At‐OTSA101. C,E,G, 90Y‐OTSA101. H,I, CD31 immunostaining and its corresponding H&E staining at day 1 after 211At‐OTSA101 treatment. Scale bars, 50 μm
4. DISCUSSION
We found in our present analyses that 211At‐OTSA101, an α‐emitting anti‐FZD10 antibody, effectively suppresses the growth of SS xenografts in a mouse model without apparent toxicity. This result provides a proof‐of‐concept demonstration that α‐RIT using 211At‐OTSA101 may be a viable therapeutic option for SS. Our study findings also provide evidence that α‐RIT would be more effective for treating SS than β‐RIT. Our current data show that a 25‐μCi dose of 211At‐OTSA101 significantly suppresses SS tumor growth but that this does not occur with the same dose of 90Y‐OTSA101, a β‐emitting anti‐FZD10 antibody.
Our biodistribution data indicate that both 111In‐OTSA101 and 211At‐OTSA101 have similar pharmacokinetics in SS xenograft mice other than in the stomach. The tumor uptake of 211At‐OTSA101 was found to be approximately 3‐fold lower than that of the 111In‐anti‐FZD10 antibody at 1‐day post‐injection. We suspect that 211At is de‐astatinated from the antibody once internalized in the cells and is then quickly excreted in the urine. We also speculate that part of the carrier‐free 211At may be accumulated preferentially in the stomach. Improving the radiochemical stability of 211At‐OTSA101 and reducing the 211At uptake in the stomach should probably be the subject of further studies, although we found no apparent abnormalities resulting from gastric toxicity in our present experiments.
Tumor growth was rapidly suppressed and the SS xenografts regressed immediately after α‐emitting 211At‐OTSA101 injection, but this only occurred several days after β‐emitting 90Y‐OTSA101 administration. Consistent with these observations, our histopathological analyses indicated more severe tumoricidal effects at an earlier stage of α‐RIT with 211At‐OTSA101 compared with β‐RIT using 90Y‐OTSA101. These dramatic tumoricidal effects were assumed to be complete even at 1 day after 211At‐OTSA101 injection, while only relatively mild pathological changes were observed in the SS xenografts at 1 day after the administration of 90Y‐OTSA101. Interestingly, our calculations showed that the absorbed dose by the SS tumor cells up to 1‐day post‐injection was almost equivalent between 211At‐OTSA101 (3.3 Gy) and 90Y‐OTSA101 (3.0 Gy). These findings strongly suggest that the radiation quality represented as linear energy transfer, rather than the absorbed dose, should be considered when determining the therapeutic effects of α‐RIT. Notably, the tumor vessels in our model mice were little affected by 211At‐OTSA101 at day 1, suggesting that this radiolabeled antibody specifically targets SS cells that are positive for FZD10.
No apparent systemic toxicities were evident in our model mice treated with 211At‐OTSA101, although the calculated absorbed doses were considerably higher in all tissues examined at 1 day after the injection of 211At‐OTSA101 compared with 90Y‐OTSA101. The body weights of the mice tended to be lower in the groups treated with both 211At‐OTSA101 and 90Y‐OTSA101 compared to the control mice, but no severe body weight loss was observed in the RIT groups and all varied within a normal range. Although hematological toxicities were not examined in our current experiments, our previous study showed transient but recoverable leukocytopenia following the systemic administration of an α‐emitting antibody.14
The potential of FZD10 as an RIT target against SS tumors has been previously suggested using an 90Y‐anti‐FZD10 antibody.10, 11 Accordingly, a Phase I trial of OTSA101 has been conducted for the treatment of advanced SS. Previous studies have also reported that α‐RIT is superior to β‐RIT in treating solid tumors because α‐particles with a higher LET may have huge advantages in terms of cytotoxicity compared to β‐particles with a lower LET.15, 16 Given that solid tumors including SS lesions are generally chemo‐resistant and radio‐resistant, α‐RIT using 211At‐OTSA101 could become a more viable therapeutic option than β‐RIT with 90Y‐OTSA101 for the treatment of SS, especially in cases of unresectable, conventional chemotherapy or radiotherapy‐resistant lesions.
New therapeutic approaches are now emerging for STS tumors and the development of novel treatments for SS is under active investigation.21, 22 A multi‐tyrosine kinase inhibitor pazopanib has demonstrated significant benefits in terms of extending progression‐free survival in STS patients.23 Adaptive immunotherapy targeting NY‐ESO‐1 has also demonstrated promising results against SS.24 Vaccination against the SS18‐SSX oncoprotein has been attempted and produced transient shrinkage of a metastatic SS tumor in the lung of 1 patient, although this was a small clinical trial and most of the study patients showed stable or progressive disease.25 More recently, high throughput siRNA screening has suggested that inhibition of ATR activity may be a therapeutic option for SS treatment.26 It would be of interest to investigate whether α‐RIT monotherapy using 211At‐OTSA101 or a combination therapy of 211At‐OTSA101 with other therapeutic modalities is superior to other therapeutic approaches in the treatment of localized or metastatic SS in a clinical setting.
Although our present analyses indicate that α‐RIT using 211At‐OTSA101 has therapeutic potential for the treatment of SS, there were several noteworthy limitations of our current study. First, we did not evaluate the therapeutic efficacy of a 211At‐labeled isotype control antibody because a previous study has reported that a 90Y‐labeled antibody that was non‐specific for FZD10 partially suppressed tumor growth of SS in vivo.10 However, a direct comparison using a 211At‐labeled isotype control antibody will be needed in a future study to more accurately evaluate the tumor‐suppressive effects of 211At. Second, more detailed analyses in addition to body weight measurements are warranted to assess the toxicity of 211At‐OTSA101. Third, SS often occurs in soft tissues that would differ from subcutaneous xenografts in terms of drug delivery and targeting by 211At‐OTSA101. In addition, possible immunological modification of tumor suppression should also be considered and orthotopic or immunocompetent animal models will be needed in this regard to further evaluate 211At‐OTSA101.
In conclusion, α‐RIT using 211At‐OTSA101 effectively suppresses the growth of SS xenografts in a preclinical mouse model with no apparent toxicities and with more efficiency than β‐RIT using 211Y‐OTSA101. 211At‐OTSA101 therapy may prove to be a relatively safe and effective option for the future treatment of SS.
CONFLICT OF INTEREST
Yusuke Nakamura is a stock holder and scientific advisor at OncoTherapy Science, Inc. Toyomasa Katagiri is a stock holder and external board member at OncoTherapy Science, Inc. Yosuke Harada is a stock holder and employee at OncoTherapy Science, Inc. The other authors have no financial or other competing interests to declare in relation to this study.
ACKNOWLEDGMENTS
We thank Drs Masumi Abe and Keiichiro Yoshinaga for helpful discussions and encouragement during this study. We also thank Mr Hisashi Suzuki for radioisotope production, and the support group for pathological analysis at Platform of Advanced Animal Model Support.
Li HK, Sugyo A, Tsuji AB, et al. α‐particle therapy for synovial sarcoma in the mouse using an astatine‐211‐labeled antibody against frizzled homolog 10. Cancer Sci. 2018;109:2302–2309. https://doi.org/10.1111/cas.13636
Funding information
This work was supported by a JSPS KAKENHI grant (number JP16H06276) and research grants from the National Institute of Radiological Sciences at the National Institutes for Quantum and Radiological Science and Technology.
Contributor Information
Tatsuya Higashi, Email: higashi.tatsuya@qst.go.jp.
Sumitaka Hasegawa, Email: hasegawa.sumitaka@qst.go.jp.
REFERENCES
- 1. Spillane AJ, A'Hern R, Judson IR, Fisher C, Thomas JM. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18:3794‐3803. [DOI] [PubMed] [Google Scholar]
- 2. Sultan I, Rodriguez‐Galindo C, Saab R, Yasir S, Casanova M, Ferrari A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537‐3547. [DOI] [PubMed] [Google Scholar]
- 3. Lewis JJ, Antonescu CR, Leung DH, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087‐2094. [DOI] [PubMed] [Google Scholar]
- 4. Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16:437‐444. [DOI] [PubMed] [Google Scholar]
- 5. Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502‐508. [DOI] [PubMed] [Google Scholar]
- 6. Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT‐SSX fusion type on the clinical behavior of synovial sarcoma: a multi‐institutional retrospective study of 243 patients. Cancer Res. 2002;62:135‐140. [PubMed] [Google Scholar]
- 7. Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M. Molecular cloning of Frizzled‐10, a novel member of the Frizzled gene family. Biochem Biophys Res Commun. 1999;262:39‐43. [DOI] [PubMed] [Google Scholar]
- 8. Nagayama S, Katagiri T, Tsunoda T, et al. Genome‐wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002;62:5859‐5866. [PubMed] [Google Scholar]
- 9. Nagayama S, Fukukawa C, Katagiri T, et al. Therapeutic potential of antibodies against FZD 10, a cell‐surface protein, for synovial sarcomas. Oncogene. 2005;24:6201‐6212. [DOI] [PubMed] [Google Scholar]
- 10. Fukukawa C, Hanaoka H, Nagayama S, et al. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci. 2008;99:432‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanaoka H, Katagiri T, Fukukawa C, et al. Radioimmunotherapy of solid tumors targeting a cell‐surface protein, FZD10: therapeutic efficacy largely depends on radiosensitivity. Ann Nucl Med. 2009;23:479‐485. [DOI] [PubMed] [Google Scholar]
- 12. Zalutsky MR, Pruszynski M. Astatine‐211: production and availability. Curr Radiopharm. 2011;4:177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kodaira S, Li HK, Konishi T, Kitamura H, Kurano M, Hasegawa S. Validating alpha‐particle emission from 211At‐labeled antibodies in single cells for cancer radioimmunotherapy using CR‐39 plastic nuclear track detectors. PLoS One. 2017;12:e0178472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li HK, Morokoshi Y, Nagatsu K, Kamada T, Hasegawa S. Locoregional therapy with alpha‐emitting trastuzumab against peritoneal metastasis of human epidermal growth factor receptor 2‐positive gastric cancer in mice. Cancer Sci. 2017;108:1648‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behr TM, Behe M, Stabin MG, et al. High‐linear energy transfer (LET) alpha versus low‐LET beta emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose‐limiting toxicity of 213Bi‐ versus 90Y‐labeled CO17‐1A Fab' fragments in a human colonic cancer model. Cancer Res. 1999;59:2635‐2643. [PubMed] [Google Scholar]
- 16. Song H, Hobbs RF, Vajravelu R, et al. Radioimmunotherapy of breast cancer metastases with alpha‐particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69:8941‐8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawai A, Naito N, Yoshida A, et al. Establishment and characterization of a biphasic synovial sarcoma cell line, SYO‐1. Cancer Lett. 2004;204:105‐113. [DOI] [PubMed] [Google Scholar]
- 18. Nagatsu K, Minegishi K, Fukada M, Suzuki H, Hasegawa S, Zhang MR. Production of (211)At by a vertical beam irradiation method. Appl Radiat Isot. 2014;94:363‐371. [DOI] [PubMed] [Google Scholar]
- 19. Sugyo A, Tsuji AB, Sudo H, et al. Evaluation of efficacy of radioimmunotherapy with 90Y‐labeled fully human anti‐transferrin receptor monoclonal antibody in pancreatic cancer mouse models. PLoS One. 2015;10:e0123761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckerman KF, Endo A. MIRD: radionuclide data and decay schemes. Reston, VA: Society of Nuclear Medicine; 2008. [Google Scholar]
- 21. Nielsen TO, Poulin NM, Ladanyi M. Synovial sarcoma: recent discoveries as a roadmap to new avenues for therapy. Cancer Discov. 2015;5:124‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stacchiotti S, Van Tine BA. Synovial sarcoma: current concepts and future perspectives. J Clin Oncol. 2018;36:180‐187. [DOI] [PubMed] [Google Scholar]
- 23. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2012;379:1879‐1886. [DOI] [PubMed] [Google Scholar]
- 24. Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY‐ESO‐1. J Clin Oncol. 2011;29:917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawaguchi S, Tsukahara T, Ida K, et al. SYT‐SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci. 2012;103:1625‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones SE, Fleuren EDG, Frankum J, et al. ATR is a therapeutic target in synovial sarcoma. Cancer Res. 2017;77:7014‐7026. [DOI] [PMC free article] [PubMed] [Google Scholar]


