In the United States each year 150,000 patients die and 1.5 million develop a medical complication after surgery1. Postoperative complications cause a two-fold increase in 30-day mortality and incur an annual cost of $25 billion, thus even a small reduction in their occurrence could save lives and reduce healthcare costs2. Postoperative acute kidney injury (AKI) affects up to 30% of patients and is associated with increased risk for other major complications and increased costs of care. Postoperative AKI that does not resolve is associated with the development of chronic critical illness, chronic kidney disease and permanent disability3–6.
The risk for postoperative complications arises from the complex interaction between a patient’s preoperative health and the organ-specific physiologic capacity to withstand surgery-related stress. The type and quality of surgery and anesthesia that the patient undergoes modulate that risk. Patients in good health, with high organ-specific physiologic reserve, survive even high risk procedures associated with acute stress (such as hemodynamic instability due to blood loss, prolonged hypoperfusion of major organs, inflammation and drug toxicity) and may not have any significant postoperative organ dysfunction. For patients in poor health, with low organ-specific physiologic reserve, even minor surgery may result in significant organ dysfunction. Optimal perioperative care requires personalized management to minimize the effects of the acute stress of surgery and anesthesia, informed by precise and timely identification of patients at the greatest risk of individual complications.
Intraoperative hypotension is a well-recognized risk factor for multiple postoperative complications, including AKI, with biologic plausibility that is well accepted by anesthesiologists and surgeons. There is a shared understanding that the autoregulatory capacity of organs such as the kidneys can be impaired in patients with baseline hypertension, and may render them susceptible to injury at higher blood pressures than would otherwise be accepted. Yet no consensus exists regarding optimal blood pressure targets to support perfusion of critical organs during surgery, and in practice both systolic and mean arterial pressure thresholds vary widely. The type and dose of drugs (fluids and vasopressors) used to achieve these variable thresholds is also highly variability.
The Intraoperative Norepinephrine to control arterial PRESSure (INPRESS) Study is a multicenter, prospective, randomized, stratified, parallel-group clinical trial conducted in nine French hospitals to evaluate whether an individualized blood pressure management strategy could reduce postoperative organ dysfunction among a selected group of high-risk patients undergoing major surgery7. The cohort included 298 high-risk patients aged 50 years or older undergoing surgery under general anesthesia with an expected duration of 2 hours or longer. Patients were randomly assigned to two different blood pressure management strategies during and for 4 hours following surgery. The individualized management strategy aimed at achieving a systolic blood pressure (SBP) within 10% of the reference value (the patient’s resting SBP) using a titration protocol for continuous infusion of norepinephrine. The standard management strategy used intravenous ephedrine administered in 6 mg boluses (maximum dose 60 mg), for any decrease in SBP below 80 mm Hg or lower than 40% from the patient’s reference value. The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and at least one organ dysfunction within seven days after surgery: renal as measured by risk-injury-failure-loss-end-stage AKI (RIFLE AKI), respiratory (need for invasive or non-invasive mechanical ventilation), cardiovascular (cardiac ischemia or failure), neurologic (stroke or Glascow Coma Scale<=14) and coagulation (platelets < 100,000).
The authors used the AKI risk index, a score developed in a retrospective analysis of the NSQIP database, to measure the susceptibility for postoperative complications and thus to enrich the study population. This is an interesting approach since the primary outcome was not AKI but a composite outcome of SIRS and one major organ dysfunction. The AKI risk index is an insensitive logistic regression model predicting only the 1% of surgical patients who will develop very severe AKI (the overall incidence of RIFLE AKI in the INPRESS cohort was 40%). Since there was no difference in the incidence of RIFLE-F AKI between the study and control groups, one has to question the use of the AKI risk index as an enrichment tool.
The intervention resulted in a 14% decrease in absolute risk for primary outcome. By the 7th postoperative day 38.1% of patents with individualized intraoperative SBP management had a positive composite outcome compared to 51.7% with standard management. Only renal (32.7% vs. 49%) and neurologic (5.4% vs. 15.9%) complications varied significantly between the two groups. The decrease was only in RIFLE-R AKI while there was no difference in more severe AKI or in the need for RRT. There was no difference in the incidence of SIRS or the other complications. By the 30th postoperative day patients in the individualized treatment group had less overall organ dysfunction (adjusted hazard ratio 0.66; P = .001)) and less sepsis (adjusted relative risk 0.54; P = .009) but mortality was not different.
The study has many strengths. Risk assessment went well beyond the usual ASA score and the authors used a sophisticated perioperative protocol. Hypertensive medications were discontinued 24 hours prior to surgery, anesthesia induction was standardized and then titrated using Bispectral Index, fluid management was standardized using stroke volume index, and ventilator management was standardized. Some variations from current practice (the use of hetastarch for fluid boluses, and liberal transfusion triggers) don’t detract from the validity of the findings.
This study offers a window into a new mode of practice, by demonstrating that personalized intraoperative management can improve outcomes. The evidence is mounting that standardized prevention bundles, based on published consensus guidelines applied to patients identified at high risk for AKI, can decrease the incidence of AKI in the postoperative period4, 8, 9. Although studies to date have not demonstrated a mortality benefit, the message emerging is clear. A combination of existing clinical, biological and imaging studies can be used to standardize intra- and post-operative management and thus to reduce the incidence of AKI and other complications. The INPRESS investigators have added a personalized tool to our arsenal of preventive strategies for the patient at risk. The next step is to incorporate real-time data streams and analysis into the kind of guideline approach demonstrated in the INPRESS study. We recommend a large platform clinical trial, using real-time perioperative analytics as well as current practice guidelines, to advance our ability to prevent surgical complications (Figure 1)10.
Figure 1.
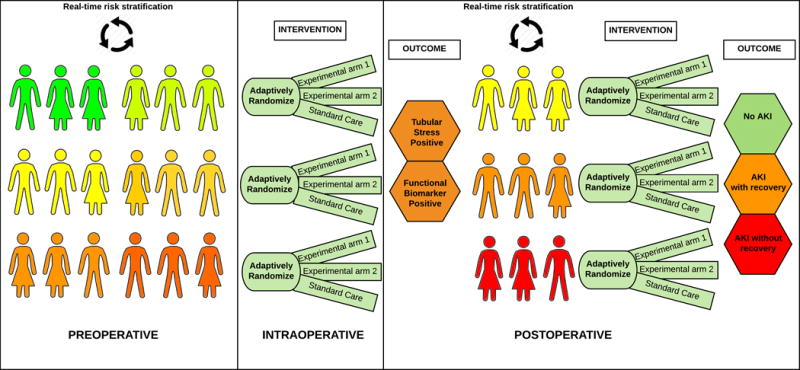
Framework for platform adaptive clinical trial for prevention of perioperative AKI. The risk stratification occurs in preoperative period using real time intelligent engine processing clinical data from electronic health records and medical devices. Patients are subsequently and sequentially adaptively randomized to multiple arms. The first sequence involves intraoperative interventions aimed to prevent immediate postoperative renal stress (determined using real time analytics and biomarkers). Based on renal stress patients are adaptively randomized in the next sequence to intervention aimed to prevent AKI and renal recovery.
Standfirst.
The INPRESS study demonstrates that personalized intraoperative management can improve outcomes. We already know that standardized prevention bundles applied to patients identified at high risk for AKI can decrease the incidence of AKI. The authors show that personalized management, guided by risk stratification, can improve perioperative practice and thus outcomes.
Acknowledgments
We thank Daniel Freeman for his help with the figure.
Funding: A.B. is supported by R01 GM110240 and P50 GM-111152 from the National Institute of General Medical Sciences from the National Institute of General Medical Sciences.
Footnotes
Reprints will not be available from the author(s).
References
- 1.Weiser TG, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. The Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Hall BL, et al. Does Surgical Quality Improve in the American College of Surgeons National Surgical Quality Improvement Program: An Evaluation of All Participating Hospitals. Annals of Surgery. 2009;250:363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 3.Hobson CE, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207–14. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobson C, Ruchi R, Bihorac A. Perioperative Acute Kidney Injury: Risk Factors and Predictive Strategies. Crit Care Clin. 2017;33:379–396. doi: 10.1016/j.ccc.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozrazgat-Baslanti T, et al. Acute and Chronic Kidney Disease and Cardiovascular Mortality After Major Surgery. Ann Surg. 2016;264:987–996. doi: 10.1097/SLA.0000000000001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile LF, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. Journal of Trauma-Injury Infection & Critical Care. 2012;72:1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Futier E, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA. 2017;318:1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meersch M, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017 doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gocze I, et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 10.Feng Z, et al. ArXiv e-prints. 2017 [Google Scholar]


