Abstract
The use of assisted reproductive techniques for endangered species is a major goal for conservation. One of these techniques, testis tissue xenografting, allows for the development of spermatozoa from animals that die before reaching sexual maturity. To assess the potential use of this technique with endangered species, testis tissue from six Iberian lynxes (one fetus, two perinatal cubs, two 6-month-old and one 2-year-old lynx), two Cuvier’s gazelle fetuses and one 8-month-old Mohor gazelle were transplanted ectopically into nude mice. Tissue from the lynx fetus, perinatal cubs and 2-year-old donors degenerated, whereas spermatogonia were present in 15% of seminiferous tubules more than 70 weeks after grafting in transplanted testis tissue from 6-month-old donors. Seminal vesicle weights (indicative of testosterone production) increased over time in mice transplanted with tissue from 6-month-old lynxes. Progression of spermatogenesis was observed in xenografts from gazelles and was donor age dependent. Tissue from Cuvier’s gazelle fetuses contained spermatocytes 40 weeks after grafting. Finally, round spermatids were found 28 weeks after transplantation in grafts from the 8-month-old Mohor gazelle. This is the first time that xenotransplantation of testicular tissue has been performed with an endangered felid and the first successful xenotransplantation in an endangered species. Our results open important options for the preservation of biological diversity.
Additional keywords: conservation, testicular tissue, threatened species, xenografting
Introduction
The development of assisted reproductive techniques plays an important role in the conservation and management of threatened species because they could benefit free and captive populations of highly endangered taxa. Assisted reproductive techniques aid in the rescue of reproductive cells and, thus, allow for the conservation of genetic resources. The most commonly used assisted reproductive technique in males is the collection and cryopreservation of spermatozoa. Spermatozoa can be recovered from live or recently deceased adult males (Garde et al. 1998, 2003; Martinez-Pastor et al. 2005; Gañán et al. 2009a, 2010), and offspring of threatened felids and ungulates have been born after intrauterine insemination of females with frozen–thawed spermatozoa (Densmore et al. 1987; Holt et al. 1988; Garland 1989; Junior et al. 1990; Swanson et al. 1996; Johnston et al. 2002; Garde et al. 2006).
In contrast, spermatozoa cannot be collected from immature males and the death of these males represents the loss of their genetic resource forever. Relevant progress has been achieved in in vitro spermatogenesis, with the entire spermatogenic cycle from spermatogonia to spermatozoa obtained in a three-dimensional culture system (Stukenborg et al. 2009) and offspring obtained after culturing of immature mouse testis (Sato et al. 2011). Alternatively, somatic cells could be used for somatic cell nuclear transfer to clone a dead individual when host oocytes from related species are available (Lanza et al. 2000; Gómez et al. 2004). However, abnormal gene expression and epigenetic deregulation arise during cloning (Loi et al. 2007; Gómez et al. 2009), further conspiring against the success of the procedure.
Testicular tissue xenografting could provide an opportunity to rescue the genetic information of a juvenile male from an endangered species (Paris and Schlatt 2007). Testis tissue xenografting involves the transplantation of small pieces of immature testicular tissue subcutaneously to immunocompromised mice, as an in vivo culture system, to subsequently (after weeks or months) isolate spermatozoa from these tissue fragments, fertilise oocytes by intracytoplasmic sperm injection and transfer embryos into a female recipient (Honaramooz et al. 2002; Nakai et al. 2010). Xenografting of young testicular tissue has been used successfully to sustain complete spermatogenesis in several domestic animals, namely goat (Honaramooz et al. 2002), pig (Honaramooz et al. 2002), rabbit (Shinohara et al. 2002), bull (Oatley et al. 2004; Rathi et al. 2005), cat (Snedaker et al. 2004; Kim et al. 2007; Mota et al. 2012), horse (Rathi et al. 2006), sheep (Zeng et al. 2006; Arregui et al. 2008a), dog (Abrishami et al. 2010a) and bison (Abbasi and Honaramooz 2011), as well as in other non-domestic species, such as hamster (Schlatt et al. 2002), rhesus monkey (Honaramooz et al. 2004), ferret (Gourdon and Travis 2011), white-tailed deer (Abbasi and Honaramooz 2012) and humans (Wyns et al. 2008). Several of these species, namely cats, dogs, sheep, deer, bison and ferrets, have been proposed as model animals for endangered felids, canids, ungulates and small carnivores (Snedaker et al. 2004; Arregui et al. 2008a; Abrishami et al. 2010a; Abbasi and Honaramooz 2011, 2012; Gourdon and Travis 2011). However, thus far there is only one short report on xenografting of testis tissue from an endangered species: testis from the Javan banteng (Bos javanicus) were xenotransplanted, but complete spermatogenesis was not achieved (Honaramooz et al. 2005).
The world populations of Iberian lynx (Lynx pardinus), Cuvier’s gazelle (Gazella cuvieri) and Mohor gazelle (Gazella dama mhorr) have been drastically reduced in recent decades and are still decreasing. The Iberian lynx is the most endangered felid in the world, categorised as ‘critically endangered’ by the International Union for Conservation of Nature (IUCN) since 2002 (IUCN 2012). It is an endemic species of the Iberian peninsula and the current total population has been estimated to be approximately 200 individuals scattered in several isolated subpopulations in the south of Spain (Guzmán et al. 2004; Alda et al. 2008; Sarmento et al. 2009). Only two populations reproduce regularly (Guzmán et al. 2004; Von Arx and Breitenmoser-Wursten 2008). Cuvier’s gazelle has been regarded as ‘endangered’ since 1986 (IUCN 2012). It is an endemic species of the Atlas Mountains and has 1700–3000 individuals in fragmented populations in Morocco, Algeria and Tunisia, but none of them has more than 250 mature individuals (Mallon and Cuzin 2008). The current population trend is unknown. Dama gazelle (Gazella (=Nanger) dama) has been considered to be ‘critically endangered’ since 2006 (IUCN 2012), with very small and fragmented subpopulations and less than 500 individuals in the current wild population (Newby et al. 2008). The Mohor gazelle (G. dama mhorr) is considered to be extinct in the wild (Beudels et al. 2005).
Captive breeding programs have been established in Spain for these three species starting in 2004 for the Iberian lynx and between 1971 and 1975 for the gazelles. Thanks to the existence of these captive breeding programs, studies have been performed for the characterisation of sperm traits (Cassinello et al. 1998; Gañán et al. 2010), electrostimulation for sperm recovery (Cassinello et al. 1998; Garde et al. 2003; Gañán et al. 2009b) and sperm cryopreservation (Garde et al. 2003, 2008; Gañán et al. 2009b).
The premature death of young individuals is a significant problem in the conservation of these species because survival of lynx cubs and gazelle calves during the first months after birth is low. Average litter size in wild Iberian lynx is three cubs; after 3 months, 75% of cubs survive and less than 60% are alive 2 years after birth (Palomares et al. 2005). Cuvier’s and Mohor gazelle calf mortality in captive populations is close to 50% in the former and 30% in the latter during the first months of life (Abaigar and Cano 2005; Barbosa and Espeso 2005). The development of a technique to recover the germ cells of these individuals will be an important tool to maintain their alleles in the population genetic pool.
Therefore, the aim of the present study was to test whether testis tissue xenografting could be an option to develop spermatozoa from juvenile Iberian lynx, Cuvier’s and Mohor gazelles. The effect of donor age and freezing on testicular survival after grafting was also assessed.
Materials and methods
Lynx testes
Iberian lynx testes were obtained from necropsies at the Centro de Análisis y Diagnóstico de la Fauna Silvestre of the Junta de Andalucía (Seville, Spain) and sent to the laboratory at 5–10°C (Table 1). Donor tissue for xenografting was used from animals of different ages: one 6-week-old fetus, two perinatal cubs (1.5 and 3 days old), two 6-month-old cubs, and one 2-year-old sub-adult male. Testicular tissue from all specimens was grafted after cryopreservation, except for tissue from the 2-year-old animal, which was transplanted fresh.
Table 1. Age, phenotype, cause of death and origin of grafted tissues.
Testicular weight (TW) is for one testicle in lynxes and the average of both testes in gazelles. Testicular dimensions (TD; length × width) are for one testis in lynxes and the average of both testes in gazelles
| Species | Age | TW (g) | TD (mm) | Cause of death | Captive/freeA | Date of death | Time to laboratory (h) |
|---|---|---|---|---|---|---|---|
| Lynx pardinus | 6 week fetus | 0.011 | 3.51 × 2.04 | Maternal stress | Captive (EA) | 16 March 2007 | 36 |
| L. pardinus | 1.5 days | 0.013 | 3.99 × 1.97 | Hypothermia | Captive (EA) | 31 March 2007 | 36 |
| L. pardinus | 3 days | 0.013 | 3.86 × 1.58 | Unknown | Captive (EA) | 2 April 2007 | 12 |
| L. pardinus (1) | 6 months | – | – | Road kill | Free (SM) | 8 October 2005 | 24 |
| L. pardinus (2) | 6 months | – | – | Road kill | Free (DO) | 21 September 2006 | 24 |
| L. pardinus | 2 years | 1.32 | 15.21 × 12.33 | Feline leukaemia | Captive (LV) | 12 July 2008 | 48 |
| Gazella cuvieri | Mid-term abortion | – | 4.74 × 2.91 | Unknown | Captive (EZ) | 19 February 2008 | 24 |
| G. cuvieri | Full-term abortion | 0.07 | 5.89 × 4.58 | Unknown | Captive (EZ) | 16 October 2007 | 24 |
| G. dama | 8 months | 0.20 | 9.08 × 6.02 | Anaemia | Captive (MZ) | 16 September 2008 | 12 |
Lynxes kept in captivity were housed at El Acebuche (EA) and Los Villares (LV), whereas samples from free-ranging animals were from two populations: Sierra Morena (SM) or Doñana (DO). Gazelles were kept in captivity at the Estación Experimental de Zonas Áridas (EZ) or Madrid Zoo (MZ).
Gazelle testes
Testicular tissue was obtained from necropsies at Estación Experimental de Zonas Áridas (Consejo Superior de Investigaciones Cientificas (CSIC), Almeria, Spain) or at ZooAquarium Madrid (Madrid, Spain) and sent to the laboratory at 5–10°C (Table 1). Testes from two species of gazelles were used as donor tissue for the present study: two fetuses of Cuvier’s gazelle (a mid-term and a full-term abortion) and one 8-month-old Mohor gazelle. Testicular tissue from all specimens was grafted after cryopreservation, but tissue from one Cuvier’s gazelle was also transplanted fresh.
Testis tissue processing, cryopreservation, xenografting and recovery
After removal of the tunica albuginea, testes were cut into small fragments (~1 mm3). As a reference for testis development, a piece of testicular tissue from each donor was fixed in Bouin’s solution overnight followed by three changes of 70% ethanol before being processed for histology. Tissue was cryopreserved as described previously (Honaramooz et al. 2002). Freezing medium was prepared with fetal bovine serum (FBS; Gibco BRL, Madrid, Spain), Dulbecco’s modified Eagle’s medium (DMEM; Gibco) and dimethylsulfoxide (DMSO; Sigma, Madrid, Spain) at a ratio of 1 : 3 : 1 (v/v/v). One to 10 pieces of testicular tissue fragments were added to 0.5 mL freezing medium in 2-mL cryovials at room temperature. The vials were placed in a container with isopropyl alcohol at room temperature; the container (‘Mr Frosty’; Nalgene, ThermoFisher, Madrid, Spain) is designed to provide a controlled cooling rate of −1°C min−1 when placed in a −80°C freezer. The tissue fragments were left at −80°C overnight and were subsequently transferred to liquid nitrogen.
Cryopreserved testes were stored for at least 1 month before use in xenografting. For thawing, vials were held at room temperature for 1 min to evaporate any remaining liquid nitrogen and placed in a water bath at 25°C for 1 min. Afterwards, 1.5 mL DMEM at 25°C was added to each vial and the contents were transferred to a centrifuge tube. Then, the tissue fragments were washed twice with DMEM to remove cryoprotectant by centrifugation (300g, 2 min), resuspended in DMEM and kept in this medium until grafting.
Male immunodeficient mice (NCR-nude; 7–12 weeks old) were anaesthetised by inhalation of isofluorane, castrated and, during the same surgery, two to eight fragments of donor testis tissue were implanted under their back skin. Recipient mice were killed by CO2 inhalation and recovered grafts were fixed in Bouin’s solution and analysed by histology. Four testicular tissue fragments from a lynx fetus were transplanted to two immunodeficient mice each, two pieces from perinatal cubs testes were transplanted to 10 mice, six to eight fragments from 6-month-old males were transplanted to 17 mice and eight tissue pieces from a 2-year-old male were transplanted to six mice. Testis tissue from Cuvier’s gazelles was grafted in 15 mice, whereas testis tissue from the Mohor gazelle was transplanted to seven mice. Six to eight tissue pieces from gazelles were subcutaneously transplanted per host mouse. The number of grafted mice per donor and recovered grafts are given in Table 2. Seminal vesicle weights of recipient mice were recorded as an indicator of the presence of bioactive testosterone originating from the grafts (Schlatt et al. 2002, 2003).
Table 2.
Tissue recovered after xenografting and weight of seminal vesicle of grafted mice
Where appropriate, data are given as the mean ± s.e.m. NA, not available
| Species | Age | Testis | Mice ~40 weeksA | Mice >40 weeksB | % RecoveryC | Seminal vesicle weightD (mg) | ||
|---|---|---|---|---|---|---|---|---|
| ~40 weeks | >40 weeks | ~40 weeks | >40 weeks | |||||
| Lynx pardinus | 6 week fetus | Cryopreserved | 1/0/0 | 1/1/0 | 0% | 0% | NA | NA |
| L. pardinus | 1.5 days | Cryopreserved | 2/2/0 | 2/1/0 | 0% | 0% | NA | NA |
| L. pardinus | 3 days | Cryopreserved | 2/0/0 | 4/0/0 | 0% | 0% | NA | NA |
| L. pardinus (1) | 6 months | Cryopreserved | 7/7/6 | 3/3/2 | 75.7 ± 8.5 | 56.3 ± 6.3 | 20.3 ± 10.3 | 158 ± 72 |
| L. pardinus (2) | 6 months | Cryopreserved | 1/1/1 | 6/4/2 | 33.3 | 33.3 ± 10.5 | NAE | 57.5 ± 42.5 |
| L. pardinus | 2 years | Fresh | 2/2/0 | 4/3/0 | 0% | 0% | NA | NA |
| Gazella cuvieri | Mid-term abortion | Cryopreserved | 2/1/0 | 4/1/0 | 0% | 0% | NA | NA |
| G. cuvieri | Full-term abortion | Fresh | 1/1/1 | 3/3/3 | 37.5 | 54.2 ± 20.8 | 10 | 64 ± 42.6 |
| G. cuvieri | Full-term abortion | Cryopreserved | NA | 5/3/0 | NA | 0% | NA | NA |
| G. dama | 8 months | Cryopreserved | 7/7/7 | NA | 50 ± 4.7 | NA | 194.9 ± 46.7 | NA |
Data show numbers of total grafted mice/grafted mice with recovered grafts/grafted mice with recovered grafts showing seminiferous tubules within 40 weeks of transplantation.
Data show total grafted mice/grafted mice with recovered grafts/grafted mice with recovered grafts showing seminiferous tubules after 40 weeks post-transplantation.
Percent recovery was calculated as (the number of recovered grafts showing seminiferous tubules/total transplanted grafts) ×100, before (~40) or after (>40) 40 weeks post-transplantation.
Seminal vesicle weight was calculated from mice with recovered successful grafts before (~40) or after (>40) 40 weeks post-transplantation. The seminal vesicle weight of castrated mice (control) was 9.3 ± 0.7 mg (n = 3).
Mouse was found dead and seminal vesicle weight could not be measured.
Animal husbandry and procedures followed European Union Regulation 2003/65 and Spanish Animal Protection Regulation RD1201/2005.
Analysis of testicular tissue
Donor and graft tissue were examined using tissue sections stained with haematoxylin and eosin. A graft was considered to be successful when seminiferous tubules could be identified. All seminiferous tubules present in one section per sample were examined under ×200 magnification and the most advanced germ cell present was recorded. When gonocytes or differentiated germ cells were not observed, the presence of spermatogonia was verified after immunostaining for protein gene product (PGP) 9.5, which is specifically expressed in germ cells of several mammalian species (Wrobel et al. 1996; Luo et al. 2006). An antibody against PGP 9.5 was used as described by Arregui et al. (2008a). Briefly, citrate antigen retrieval was used after deparaffinisation by boiling in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA, USA) for 10 min. Then, slides were treated with 3% H2O2 (Sigma) in distilled water for 10 min and blocked with 5% normal goat serum (Jackson ImmunoResearch Laboratories, Newmarket, Suffolk, UK) in phosphate-buffered saline (PBS) for 40 min at room temperature, avidin block for 10 min and biotin block for 10 min (Zymed, Invitrogen, Madrid, Spain). Subsequently, sections were incubated overnight at 4°C in a humidified chamber with the primary antibody (rabbit anti-PGP 9.5; AbD Serotec, Kidlington, Oxford, UK) diluted 1 : 500 in PBS. The following day, samples were treated for 30 min with the secondary antibody (biotinylated goat anti-rabbit IgG; 1.5 mg mL−1; Vector) diluted to 6 μg mL−1 in PBS and exposed for 30 min to streptavidin horseradish peroxidise (1 mg mL−1; Vector) at a concentration of 3 μg mL−1 in PBS. Finally, peroxidase activity was detected with a VIP substrate kit for peroxidase (Vector) for 2 min and samples were mounted.
Graft tissues from Iberian lynx and Cuvier’s gazelle were analysed at two time points: (1) within 40 weeks of grafting (grafts recovered between 25 and 38 weeks after grafting); and (2) more than 40 weeks after transplantation (range 42–71 weeks). Data between these two groups were compared using a t-test implemented in SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Mohor gazelle tissue was recovered and analysed from one mouse at 12 weeks after grafting and in two mice each at 16, 20 and 28 weeks after grafting.
Results
Testes size, cause of death and the origin of the animals in the present study are summarised in Table 1.
Lynx testes
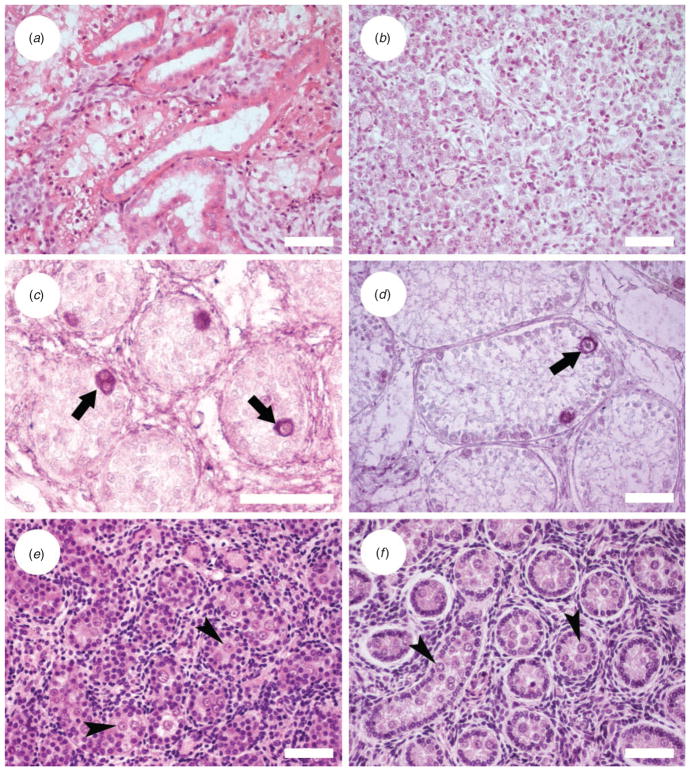
Histological analysis of testes from the 6-week-old fetus showed cubic epithelia that did not correspond to seminiferous cords (Fig. 1a). This epithelium was probably excurrent duct, such as epididymis, that in very young testes occupies a high volume. Testicular tissue from 1–3-day-old lynxes showed the formation of seminiferous cords (Fig. 1b). In 6-month-old testes, seminiferous tubules were observed in the testicular tissue; they were characterised by a lack of lumen formation and 56% of tubules had germ cells (Fig. 1c). The 2-year-old lynx presented no differentiated germ cells and some picnotic cells inside the seminiferous tubules, but premeiotic germ cells were observed after PGP 9.5 immunocytochemistry staining in 89.7% of tubules (Fig. 1d).
Fig. 1.
Histological appearance of donor testicular tissue. (a) Iberian lynx, 6-week-old fetus; (b) Iberian lynx, 1.5-day-old cub; (c) germ cells labelled by protein gene product (PGP) 9.5 immunostaining in a 6-month-old Iberian lynx testis tissue; (d) germ cells labelled by PGP 9.5 immunostaining in a 2-year-old Iberian lynx; (e) Cuvier’s gazelle aborted fetus; and ( f ) Mohor gazelle, 8-month-old male. Arrows indicate spermatogonia; arrowheads indicate gonocytes. Scale bars = 50 μm.
Lynx xenografts
Only one and three grafts were recovered from the fetus and tissue from perinatal cubs, respectively, but none of them contained testicular tissue and recipient mouse seminal vesicles weighed ~10 mg, indicating that the grafts did not contain functional Leydig cells (Table 2).
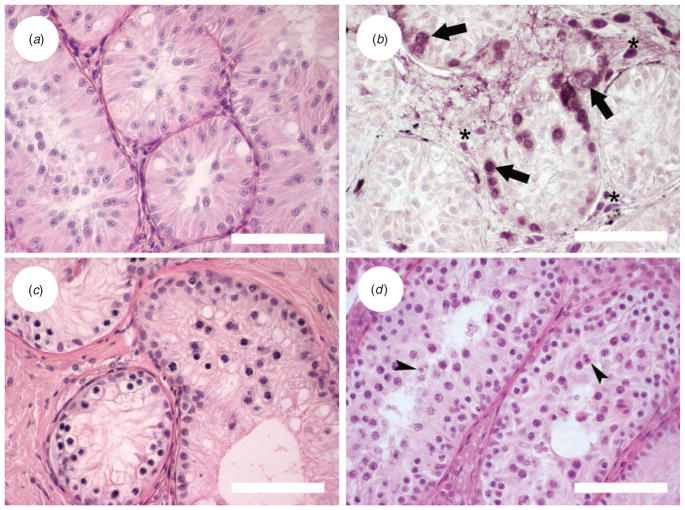
Survival of testicular grafts from 6-month-old lynxes was different from that observed in grafts of fetus and perinatal cubs. Grafts were recovered from all mice with tissue from Donor 1 (Tables 1, 2). The percentage of recovered grafts presenting seminiferous tubules was lower after 40 weeks than before 40 weeks post-grafting (P = 0.037), but seminal vesicle weight increased with time (P = 0.049; Table 2). Seminiferous tubules with a small lumen could be observed in grafts, but no differentiated germ cells were found at any time point (Fig. 2a). Six mice hosting testicular tissue from 6-month-old Donor 2 (Tables 1, 2) were kept for more than 40 weeks and grafts with seminiferous tubules were found in two of them. The histological appearance was similar to that of the other 6-month-old donor. PGP 9.5 staining of grafts recovered 28 weeks after grafting showed spermatogonia in 10% of tubules, whereas 66 weeks after transplantation, 15% of tubules contained spermatogonia (Fig. 2b).
Fig. 2.
Histological appearance of grafted testicular tissue. (a) Iberian lynx, 28 weeks after grafting; (b) germ cells labelled by protein gene product (PGP) 9.5 immunostaining in Iberian lynx testis graft, 66 weeks after transplantation; (c) Cuvier’s gazelle testis graft after 58 weeks; and (d) Mohor gazelle testis graft 28 weeks after grafting. Arrows indicate spermatogonia; arrowheads indicate round spermatids; asterisks indicate Leydig cells. Scale bars = 100 μm.
Grafts from the older Iberian lynx (2 years old) were found in five of six grafted mice. No seminiferous tubules were observed and seminal vesicle weight suggested that no testosterone was being produced (Table 2).
Gazelle testes
Testis from the mid-term Cuvier’s gazelle fetus presented 36% of seminiferous tubules with no germ cells, whereas 21%, 27% and 15% had one, two or more germ cells per round tubule section, respectively. Tissue from the full-term fetus had a similar histological appearance and presented 50% of tubules without germ cells, 27% with one, 14% with two and 9% with three or four gonocytes per round tubule section (Fig. 1e). The testis from the 8-month-old Mohor gazelle had clearly defined seminiferous tubules and 3% of round sections contained no gonocytes, 15% had one or two, 22% had three and 59% had four or more gonocytes (Fig. 1f ).
Gazelle xenografts
Grafts were recovered before and after 40 weeks from five of 11 mice grafted with cryopreserved Cuvier’s gazelle testicular tissue, but seminiferous tubules were not found in any of them. Seminal vesicle weights of these mice were not different from seminal vesicles from castrated mice that received no grafts (P >0.05; mean (±s.e.m.) 9.2 ± 0.3 vs 9.3 ± 0.7 mg, respectively; n = 11 and 3, respectively). Grafts from Cuvier’s gazelle fresh tissue showed no differentiated germ cells when recovered less than 40 weeks after grafting. When grafts were recovered after 40 weeks post-grafting (between 57 and 67 weeks), spermatocytes were the most advanced germ cells found and they were present in 82% of tubules examined (Fig. 2c). At this time, the size of seminal vesicles from grafted mice had increased (Table 2).
Transplanted tissue recovered from Mohor gazelle after 12 weeks post-grafting presented no differentiated germ cells, but seminal vesicle weight was twice that recorded for seminal vesicles from castrated mice (Fig. 3). After 16 weeks post-grafting, 62% of grafts were recovered (Table 2) and they showed 7% of tubules with spermatocytes and 1% with round spermatids. Seminal vesicle weight increased 10-fold at this time point (Fig. 3). At 20 weeks after transplantation, round spermatids were not observed but 10% of tubules had spermatocytes. Finally, at 28 weeks after grafting, 8% of seminiferous tubules in graft tissue contained spermatocytes and 1% contained round spermatids (Fig. 2d). Seminal vesicles weighed ≥300 mg (Fig. 3).
Fig. 3.
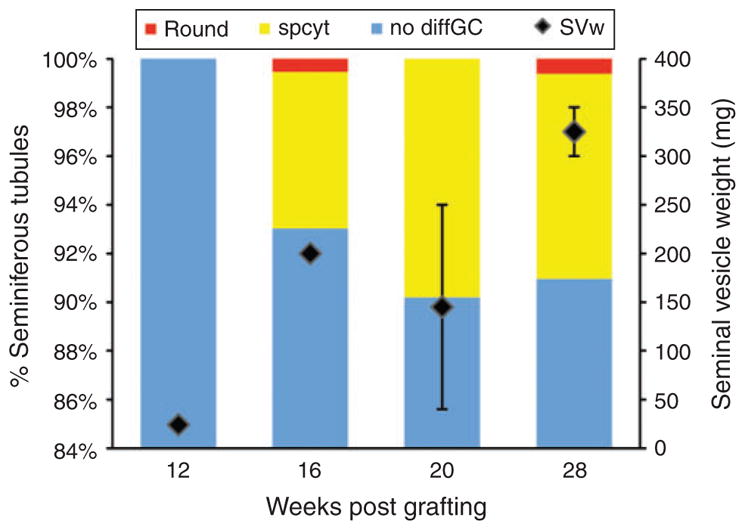
Percentage of seminiferous tubules with the most advanced germ cell type in grafts and seminal vesicle weight (SVw) of mice hosting Mohor gazelle testis tissue. Round, round spermatids; spcyt, spermatocytes; nodiffGC, with no differentiated germ cells.
Discussion
Testis tissue xenografting has been used in several species but, to our knowledge, this is the first successful testicular tissue xenotransplantation, where haploid germ cells have been found, in endangered species and the first attempt at xenotransplantation in an endangered felid. In the present study, testis tissue and spermatogonia from 6-month-old Iberian lynx survived more than 70 weeks after grafting. Tissue from a Cuvier’s gazelle fetus exhibited spermatocytes after 40 weeks post-grafting, whereas round spermatids could be found 16 weeks after transplantation of 8-month-old Mohor gazelle testis tissue.
Xenografting of testis tissue from prepubertal mammals of different species has resulted in complete spermatogenesis (Honaramooz et al. 2002, 2004; Schlatt et al. 2002; Shinohara et al. 2002; Oatley et al. 2004; Snedaker et al. 2004; Rathi et al. 2006; Zeng et al. 2006; Abrishami et al. 2010a; Abbasi and Honaramooz 2011; Gourdon and Travis 2011). However, until now, there has been only a preliminary, unsuccessful attempt of xenografting in a threatened ungulate. Javan banteng testis tissue presented spermatocytes at 9 months after grafting and did not proceed further through meiosis; at 15 months after transplantation, spermatocytes were still the most advanced germ cell observed (Honaramooz et al. 2005). In the present study, xenografts from prepubertal Iberian lynx tissue showed spermatogonia, with the percentage of tubules containing spermatogonia increasing from 28 to 66 weeks after grafting. In addition, seminal vesicle weights in mice carrying Iberian lynx grafts increased after 40 weeks post-transplantation. These findings indicate that spermatogonial proliferation takes place 1 year after grafting and that testosterone secretion increases in that period of time. Based on this finding, it could be proposed that progression of spermatogenesis and sperm production could, potentially, be observed at a later sampling point. In xenotransplanted gazelle testis, we observed that spermatogenesis occurred and round spermatids were recorded in Mohor gazelle grafts after 16 weeks post-grafting.
In gazelle testicular grafts, the onset of spermatogenesis and androgen production occurred earlier in tissue from prepubertal donors than in that from the fetus. When testis tissue is grafted, an initial loss of germ cells takes place, probably due to a transient lack of blood supply (Rathi et al. 2005). This may affect fetal and prepubertal tissues differently. Early in puberty spermatogonia experience a proliferative phase; hence, the number of spermatogonia per tubule, or per Sertoli cell, is higher in prepubertal than in fetal tissues (Vergouwen et al. 1991), as was observed in the present study. Therefore, the effect of the initial loss of spermatogonia will be more pronounced in fetal than prepubertal testis and the onset of spermatogenesis would be delayed in grafts from fetal testicular tissue.
Previous studies on xenografting fetal testicular tissue have focused mainly on humans (Povlsen et al. 1974; Skakkebæk et al. 1974; Yu et al. 2006; Mitchell et al. 2010), whereas only one study has reported work on bovine fetal testis tissue (Rodriguez-Sosa et al. 2011). Therefore, the present study is the first to use fetal testicular tissue from endangered species as donor material. Human and bovine fresh fetal tissue survived after being xenografted into nude mice, and human tissue showed normal structure and function (Yu et al. 2006; Mitchell et al. 2010; Rodriguez-Sosa et al. 2011). However, in humans, differentiated germ cells were not found, although perhaps the recovery time (maximum 19 weeks) was not sufficient to reach the onset of spermatogenesis. In contrast, bovine fetal testis xenografts started spermatogenesis and spermatocytes at the pachytene stage were observed at 10 months after grafting (Rodriguez-Sosa et al. 2011). Similarly, in the present study we observed that Cuvier’s gazelle grafts from fetal testes survived and spermatogenesis progressed, but only with freshly grafted tissue, whereas cryopreserved tissue did not contain seminiferous tubules. Iberian lynx cryopreserved fetus tissue was transplanted in two mice, but seminiferous tubules were not observed in grafts, although young lynx tissue cryopreserved by the same protocol showed survival of spermatogonia. In addition, protocols for the cryopreservation of adult or fetal human testes were applied to prepubertal human tissue and different results were obtained, with more tissue damage observed when the protocol for fetal tissue was used (Keros et al. 2007). Therefore, specific protocols for fetal testicular tissue cryopreservation will need to be developed for endangered species.
Cryopreserved neonatal or prepubertal tissue used for xeno-transplantation initiated spermatogenesis in pig, rabbit and rhesus monkey (Honaramooz et al. 2002; Shinohara et al. 2002; Orwig and Schlatt 2005; Jahnukainen et al. 2007; Abrishami et al. 2010b) and allowed survival of spermatogonia in humans (Wyns et al. 2007, 2008). Conversely, no germ cells survived after cryopreservation and xenografting of prepubertal and pubertal cats (Mota et al. 2012). Hence, a species effect may underlie differences in survival.
In contrast with the ability of young testis tissue to reinitiate spermatogenesis when grafted, transplantation of adult mammal testicular tissue does not result in germ cell differentiation and, usually, the tissue degenerates (Schlatt et al. 2002, 2006; Geens et al. 2006; Rathi et al. 2006; Kim et al. 2007; Arregui et al. 2008b; Abrishami et al. 2010a). However, suppression of spermatogenesis before grafting enhances survival of spermato-gonia in human adult testis tissue xenografts (Schlatt et al. 2006) and allows sperm recovery in adult mouse testis tissue allografts (Arregui et al. 2012). Sub-adult Iberian lynx testes without differentiated germ cells were grafted, but testicular tissue degenerated completely.
One of the issues to consider for testis tissue xenografting is the age at which full spermatogenesis is established in the intact animal compared with that observed after grafting. Grafts have been found to shorten the time required to recover haploid spermatids in monkeys (Honaramooz et al. 2004), whereas, interestingly, in bull, sheep, bison, deer and ferret (Oatley et al. 2004; Arregui et al. 2008a; Abbasi and Honaramooz 2011, 2012; Gourdon and Travis 2011) xenografts and intact tissues had shown similar timing of sperm production. For domestic cats, there have been discrepancies between studies (Snedaker et al. 2004; Kim et al. 2007), although donors of different ages have been used. Donors of 2.5 weeks of age showed elongated spermatids 35 weeks after grafting (Snedaker et al. 2004), corresponding to control cats that present complete spermatogenesis by 32 weeks of age (Sánchez et al. 1993). Spermatozoa in semen obtained by electroejaculation in Iberian lynx are first observed at 2 years of age (N. Gañan and E. R. S. Roldan, unpublished observations), in agreement with the presence of spermatozoa in Eurasian lynx of similar age (Lynx lynx; Axnér et al. 2009). After 70 weeks post-grafting (>1 year and 4 months) no differentiated germ cells were found in Iberian lynx testis tissue grafts. It is likely that at least 2 years will be needed for the establishment of full spermatogenesis, or longer if spermatogenesis is delayed in felid xenografts, as proposed by some authors (Kim et al. 2007). Hence, the lifespan of nude mice would be shorter than the period of time required to ensure complete germ cell differentiation in Iberian lynx grafted tissue. In addition, mouse health may deteriorate over time, reducing the number of grafts available (Schlatt et al. 2002; Snedaker et al. 2004; Abrishami et al. 2010a). Nevertheless, testicular maturation could be accelerated by gonadotropin supplementation, as was observed in monkey xenografts (Rathi et al. 2008) and in isolated cells cografted ectopically with testicular tissue (Arregui et al. 2008a); further studies are required to test this possibility.
The youngest males of Cuvier’s and Mohor gazelles fathering offspring have been recorded at 1–1.5 years of age (Espeso 2007). The Mohor gazelle male donor for this experiment (8 months old) exhibited round spermatids 28 weeks (~6–7 months) post-transplantation. Therefore, it could be speculated that full spermatogenesis would occur after 8–9 months post-grafting, at the same time as in the intact animal, in agreement with results in other ungulates (Oatley et al. 2004; Arregui et al. 2008a; Abbasi and Honaramooz 2011, 2012).
In conclusion, we found that spermatogonia survive in Iberian lynx grafts for more than 70 weeks post-grafting and although, theoretically, spermatozoa could be obtained after longer periods of time, the lifespan of nude mice may limit the applicability of this approach. Acceleration of testicular maturation by supplementation with gonadotropins may potentially overcome this limitation. Progression of spermatogenesis in gazelle grafts was dependent on donor age. Although spermatocytes were found 40 weeks after transplantation of fresh fetal Cuvier’s gazelle testes, round spermatids were obtained from cryopreserved testicular tissue of prepubertal Mohor gazelle after 16 weeks post-grafting. These results represent an important step in the conservation of these three critically endangered species.
Acknowledgments
The authors greatly appreciate the work of everyone involved in collecting testicular tissue at the participating institutions: Gerardo Espeso at the Estación Experimental de Zonas Áridas (CSIC), Astrid Vargas and Fernando Martinez from the ‘El Acebuche’ Iberian Lynx Captive Breeding Centre, Irene Zorrilla and staff from the Centro de Análisis y Diagnóstico de la Fauna Silvestre, Junta de Andalucía, and Eva Martínez and María Delclaux from ZooAquarium Madrid. The authors thank the Consejería de Medio Ambiente, Junta de Andalucía, for Iberian lynx samples. The authors are grateful to Angel Naranjo, Javier Martin and staff of the animal house at the Centro Nacional de Biotecnología (CSIC, Madrid, Spain) for animal care. This study was supported by the Spanish Ministry of Science and Innovation (grants CGL2006-13340 and CGL2009-11606). LA was a recipient of a predoctoral studentship from the Spanish Ministry of Education (BES-2004-4112).
References
- Abaigar T, Cano M. International Studbook. Management and Conservation of Cuvier’s Gazelle (Gazella cuvieri Ogilby, 1841) in Captivity. Estación Experimental de Zonas Áridas (CSIC); Almeria, Spain: 2005. [Google Scholar]
- Abbasi S, Honaramooz A. Xenografting of testis tissue from bison calf donors into recipient mice as a strategy for salvaging genetic material. Theriogenology. 2011;76:607–614. doi: 10.1016/J.THERIOGENOLOGY.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Abbasi S, Honaramooz A. Feasibility of salvaging genetic potential of post-mortem fawns: production of sperm in testis tissue xenografts from immature donor white-tailed deer (Odocoileus virginianus) in recipient mice. Anim Reprod Sci. 2012;135:47–52. doi: 10.1016/J.ANIREPROSCI.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Abrishami M, Abbasi S, Honaramooz A. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology. 2010a;73:512–522. doi: 10.1016/J.THERIOGENOLOGY.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Abrishami M, Anzar M, Yang Y, Honaramooz A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology. 2010b;73:86–96. doi: 10.1016/J.THERIOGENOLOGY.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Alda F, Inogés J, Alcaraz L, Oria J, Aranda A, Doadrio I. Looking for the Iberian lynx in central Spain: a needle in a haystack? Anim Conserv. 2008;11:297–305. doi: 10.1111/J.1469-1795.2008.00185.X. [DOI] [Google Scholar]
- Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan E, Dobrinski I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008a;136:85–93. doi: 10.1530/REP-07-0433. [DOI] [PubMed] [Google Scholar]
- Arregui L, Rathi R, Zeng W, Honaramooz A, Gomendio M, Roldan ERS, Dobrinski I. Xenografting of adult mammalian testis tissue. Anim Reprod Sci. 2008b;106:65–76. doi: 10.1016/J.ANIREPROSCI.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui L, Rathi R, Modelski M, Zeng W, Roldan ERS, Dobrinski I. Suppression of spermatogenesis before grafting increases survival and supports resurgence of spermatogenesis in adult mouse testis. Fertil Steril. 2012;97:1422–1429. doi: 10.1016/J.FERTNSTERT.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axnér E, Uhlhorn H, Ågren E, Mörner T. Reproductive maturation in the male Eurasian lynx (Lynx lynx): a study on 55 reproductive organs collected from carcasses during 2002–2005. Reprod Domest Anim. 2009;44:467–473. doi: 10.1111/J.1439-0531.2008.01130.X. [DOI] [PubMed] [Google Scholar]
- Barbosa A, Espeso G. International Studbook for Gazella dama mhorr. Estación Experimental de Zonas Áridas (CSIC); Almeria, Spain: 2005. [Google Scholar]
- Beudels RC, Devillers P, Lafontaine R-M, Devillers-Terschuren J, Beudels M-O. Sahelo-Saharan Antelopes. Status and Perspectives. Report on the Conservation of the Six Sahelo-Saharan Antelopes. UNEP/CMS Secretariat; Bonn, Germany: 2005. [Google Scholar]
- Cassinello J, Abaigar T, Gomendio M, Roldan ERS. Characteristics of the semen of three endangered species of gazelles (Gazella dama mhorr, G. dorcas neglecta and G. cuvieri) J Reprod Fertil. 1998;113:35–45. doi: 10.1530/JRF.0.1130035. [DOI] [PubMed] [Google Scholar]
- Densmore MA, Bowen MJ, Magyar SJ, Amoss MSJ, Robinson RM, Harms PG, Kraemer DC. Artificial insemination with frozen, thawed semen and pregnancy diagnosis in Addax (Addax nasomaculatus) Zoo Biol. 1987;6:21–29. doi: 10.1002/ZOO.1430060104. [DOI] [Google Scholar]
- Espeso G. Aspectos reproductivos de dos especies de gacelas norteafricanas, Gacela dama (Gazella dama mhorr) y Gacela cuvieri (Gazella cuvieri) Departamento de Ciencia y Tecnología Agroforestal y Genética, Universidad de Castilla-La Mancha; Ciudad Real, Spain: 2007. [Google Scholar]
- Gañán N, Gomendio M, Roldan ERS. Effect of storage of domestic cat (Felis catus) epididymides at 5°C on sperm quality and cryopreservation. Theriogenology. 2009a;72:1268–1277. doi: 10.1016/J.THERIOGENOLOGY.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Gañán N, Gonzalez R, Garde JJ, Martinez F, Vargas A, Gomendio M, Roldan ERS. Assessment of semen quality, sperm cryopreservation and heterologous IVF in the critically endangered Iberian lynx (Lynx pardinus) Reprod Fertil Dev. 2009b;21:848–859. doi: 10.1071/RD08226. [DOI] [PubMed] [Google Scholar]
- Gañán N, Sestelo A, Garde JJ, Martinez F, Vargas A, Sanchez I, Perez-Aspa MJ, Lopez-Bao JV, Palomares F, Gomendio M, Roldan ERS. Reproductive traits in captive and free-ranging males of the critically endangered Iberian lynx (Lynx pardinus) Reproduction. 2010;139:275–285. doi: 10.1530/REP-09-0259. [DOI] [PubMed] [Google Scholar]
- Garde JJ, Ortiz N, Garcia AJ, Gallego L, Landete-Castillejos T, Lopez A. Postmortem assessment of sperm characteristics of the red deer during the breeding season. Arch Androl. 1998;41:195–202. doi: 10.3109/01485019808994891. [DOI] [PubMed] [Google Scholar]
- Garde JJ, Soler AJ, Cassinello J, Crespo C, Malo AF, Gomendio M, Roldan ERS. Sperm cryopreservation in three species of endangered gazelles (Gazella cuvieri, G. dama mhorr and G. dorcas neglecta) Biol Reprod. 2003;69:602–611. doi: 10.1095/BIOLREPROD.102.012914. [DOI] [PubMed] [Google Scholar]
- Garde JJ, Gomendio M, Espeso G, Roldan ERS. Live birth of a Mohor gazelle (Gazella dama mhorr) calf following intrauterine insemination of mother with frozen–thawed semen. Reprod Fertil Dev. 2006;18:218. doi: 10.1071/RDV18N2AB220. Abstract. [DOI] [Google Scholar]
- Garde JJ, del Olmo A, Soler AJ, Espeso G, Gomendio M, Roldan ERS. Effect of egg yolk, cryoprotectant, and various sugars on semen cryopreservation in endangered Cuvier’s gazelle (Gazella cuvieri) Anim Reprod Sci. 2008;108:384–401. doi: 10.1016/J.ANIREPROSCI.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Garland P. Artificial insemination of scimitar-horned oryx (Oryx dammah) Bull Zoo Manage. 1989;27:29–30. [Google Scholar]
- Geens M, de Block G, Goossens E, Frederickx V, van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum Reprod. 2006;21:390–396. doi: 10.1093/HUMREP/DEI412. [DOI] [PubMed] [Google Scholar]
- Gómez MC, Pope CE, Giraldo A, Lyons LA, Harris RF, King AL, Cole A, Godke RA, Dresser BL. Birth of African wildcat cloned kittens born from domestic cats. Cloning Stem Cells. 2004;6:247–258. doi: 10.1089/clo.2004.6.247. [DOI] [PubMed] [Google Scholar]
- Gómez MC, Pope CE, Ricks DM, Lyons J, Dumas C, Dresser BL. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod Fertil Dev. 2009;21:76–82. doi: 10.1071/RD08222. [DOI] [PubMed] [Google Scholar]
- Gourdon JC, Travis AJ. Spermatogenesis in ferret testis xenografts: a new model. Comp Med. 2011;61:145–149. [PMC free article] [PubMed] [Google Scholar]
- Guzmán JN, García FJ, Garrote G, Pérez R, Iglesias C, editors. El lince ibérico (Lynx pardinus) en España y Portugal. Censodiagnóstico de sus poblaciones. Dirección General para la Biodiversidad; Madrid, Spain: 2004. [Google Scholar]
- Holt WV, Moore HDM, North RD, Hartman TD, Hodges JK. Hormonal and behaviour detection of oestrus in blackbuck, Antilope cervicapra, and successful artificial insemination with fresh and frozen semen. J Reprod Fertil. 1988;82:717–725. doi: 10.1530/JRF.0.0820717. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/NATURE00918. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MCT, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–1503. doi: 10.1095/BIOLREPROD.103.025536. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Zeng W, Rathi R, Koster J, Ryder O, Dobrinski I. Testis tissue xenografting to preserve germ cells from a cloned banteng calf. Reprod Fertil Dev. 2005;17:247. doi: 10.1071/RDV17N2AB193. Abstract. [DOI] [Google Scholar]
- IUCN. IUCN Red List of Threatened Species. Version 2012.1. 2012 Available from: http://www.iucnredlist.org [Verified 25 June 2012]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22:1060–1067. doi: 10.1093/HUMREP/DEL471. [DOI] [PubMed] [Google Scholar]
- Johnston SD, McGowan MR, Blyde D. Birth of a banteng (Bos javanicus) calf at Western Plains Zoo after fixed time artificial insemination. Aust Vet J. 2002;80:94–95. doi: 10.1111/j.1751-0813.2002.tb12061.x. [DOI] [PubMed] [Google Scholar]
- Junior SM, Armstrong DL, Hopkins SH, Simmons LG, Schiewe MC, Gross TS. Semen cryopreservation and the first successful artificial insemination of gaur (Bos gaurus) Theriogenology. 1990;33:262. doi: 10.1016/0093-691X(90)90686-N. Abstract. [DOI] [Google Scholar]
- Keros V, Hultenby K, Borgström B, Fridström M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/HUMREP/DEL508. [DOI] [PubMed] [Google Scholar]
- Kim Y, Selvaraj V, Pukazhenthi B, Travis AJ. Effect of donor age on success of spermatogenesis in feline testis xenografts. Reprod Fertil Dev. 2007;19:869–876. doi: 10.1071/RD07056. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, Diaz F, Moraes CT, Farin PW, Farin CE, Hammer CJ, West MD, Damiani P. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning. 2000;2:79–90. doi: 10.1089/152045500436104. [DOI] [PubMed] [Google Scholar]
- Loi P, Galli C, Ptak G. Cloning of endangered mammalian species: any progress? Trends Biotechnol. 2007;25:195–200. doi: 10.1016/J.TIBTECH.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–1540. doi: 10.1002/MRD.20529. [DOI] [PubMed] [Google Scholar]
- Mallon DP, Cuzin F. Gazella cuvieri. IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. 2008 Available from: http://www.iucnredlist.org [Verified 25 June 2012]
- Martinez-Pastor F, Guerra C, Kaabi M, Diaz AR, Anel E, Herraez P, de Paz P, Anel L. Decay of sperm obtained from epididymes of wild ruminants depending on postmortem time. Theriogenology. 2005;63:24–40. doi: 10.1016/J.THERIOGENOLOGY.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Saunders PTK, Childs AJ, Cassidy-Kojima C, Anderson RA, Wallace WHB, Kelnar CJH, Sharpe RM. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod. 2010;25:2405–2414. doi: 10.1093/HUMREP/DEQ183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota PC, Ehmcke J, Westernströer B, Gassei K, Ramalho-Santos J, Schlatt S. Effects of different storage protocols on cat testis tissue potential for xenografting and recovery of spermatogenesis. Theriogenology. 2012;77:299–310. doi: 10.1016/J.THERIOGENOLOGY.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction. 2010;139:331–335. doi: 10.1530/REP-09-0509. [DOI] [PubMed] [Google Scholar]
- Newby J, Wacher T, Lamarque F, Cuzin F, de Smet K. Nanger dama. IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. 2008 Available from: http://www.iucnredlist.org [Verified 25 June 2012]
- Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod. 2004;71:494–501. doi: 10.1095/BIOLREPROD.104.027953. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;2005:51–56. doi: 10.1093/JNCIMONOGRAPHS/LGI029. [DOI] [PubMed] [Google Scholar]
- Palomares F, Revilla E, Calzada J, Fernández N, Delibes M. Reproduction and pre-dispersal survival of Iberian lynx in a subpopulation of the Doñana National Park. Biol Conserv. 2005;122:53–59. doi: 10.1016/J.BIOCON.2004.06.020. [DOI] [Google Scholar]
- Paris MCJ, Schlatt S. Ovarian and testicular tissue xenografting: its potential for germline preservation of companion animals, non-domestic and endangered species. Reprod Fertil Dev. 2007;19:771–782. doi: 10.1071/RD07038. [DOI] [PubMed] [Google Scholar]
- Povlsen CO, Skakkebaek NE, Rygaard J, Jensen G. Heterotransplantation of human foetal organs to the mouse mutant nude. Nature. 1974;248:247–249. doi: 10.1038/248247A0. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I. Germ cell fate and seminiferous tubule development in bovine testis xenografts. Reproduction. 2005;130:923–929. doi: 10.1530/REP.1.00912. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I. Germ cell development in equine testis tissue xenografted into mice. Reproduction. 2006;131:1091–1098. doi: 10.1530/REP.1.01101. [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–5296. doi: 10.1210/EN.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Rathi R, Wang Z, Dobrinski I. Development of bovine fetal testis tissue after ectopic xenografting in mice. J Androl. 2011;32:271–281. doi: 10.2164/JANDROL.110.010322. [DOI] [PubMed] [Google Scholar]
- Sánchez B, Pizarro M, Garcia P, Flores JM. Postnatal development of seminiferous tubules in the cat. J Reprod Fertil Suppl. 1993;47:343–348. [PubMed] [Google Scholar]
- Sarmento P, Cruz J, Monterroso P, Tarroso P, Ferreira C, Negröes N, Eira C. Status survey of the critically endangered Iberian lynx Lynx pardinus in Portugal. Eur J Wildl Res. 2009;55:247–253. doi: 10.1007/S10344-008-0240-5. [DOI] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/NATURE09850. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/REP.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boinai M, Schöler HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331–2335. doi: 10.1095/BIOLREPROD.102.014894. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rübben H, Dhir R, Dobrinski I, Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum Reprod. 2006;21:384–389. doi: 10.1093/HUMREP/DEI352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, Honjo T, Ogura A. Birth of offspring following transplantation of cryopreserved immature pieces and in-vitro microinsemination. Hum Reprod. 2002;17:3039–3045. doi: 10.1093/HUMREP/17.12.3039. [DOI] [PubMed] [Google Scholar]
- Skakkebæk NE, Jensen G, Povlsen CO, Rygaard J. Heterotransplantation of human foetal testicular and ovarian tissue to the mouse mutant nude. Acta Obstet Gynecol Scand. 1974;53(s29):73–75. doi: 10.3109/00016347409157196. [DOI] [PubMed] [Google Scholar]
- Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15:521–529. doi: 10.1093/MOLEHR/GAP052. [DOI] [PubMed] [Google Scholar]
- Swanson WF, Howard JG, Roth TL, Brown JL, Alvarado T, Burton M, Starnes D, Wildt DE. Responsiveness of ovaries to exogenous gonadotrophins and laparoscopic artificial insemination with frozen–thawed spermatozoa in ocelots (Felis pardalis) J Reprod Fertil. 1996;106:87–94. doi: 10.1530/JRF.0.1060087. [DOI] [PubMed] [Google Scholar]
- Vergouwen RPFA, Jacobs SGPM, Huiskamp R, Davids JAG, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. doi: 10.1530/JRF.0.0930233. [DOI] [PubMed] [Google Scholar]
- Von Arx M, Breitenmoser-Wursten C. Lynx pardinus. IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. 2008 Available from: http://www.iucnredlist.org [Verified 25 June 2012]
- Wrobel KH, Bickel D, Schimmel M, Kujat R. Immunohistochemical demonstration of nerve growth factor receptor in bovine testis. Cell Tissue Res. 1996;285:189–197. doi: 10.1007/S004410050636. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human crypt-orchid testicular tissue grafting to immunodeficient mice. Hum Reprod. 2007;22:1603–1611. doi: 10.1093/HUMREP/DEM062. [DOI] [PubMed] [Google Scholar]
- Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xeno-grafted immature human testicular tissue. Hum Reprod. 2008;23:2402–2414. doi: 10.1093/HUMREP/DEN272. [DOI] [PubMed] [Google Scholar]
- Yu J, Cai ZM, Wan HJ, Zhang FT, Ye J, Fang JZ, Gui YT, Ye JX. Development of neonatal mouse and fetal human testicular tissue as ectopic grafts in immunodeficient mice. Asian J Androl. 2006;8:393–403. doi: 10.1111/J.1745-7262.2006.00189.X. [DOI] [PubMed] [Google Scholar]
- Zeng W, Avelar GF, Rathi R, Franca LR, Dobrinski I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl. 2006;27:527–533. doi: 10.2164/JANDROL.05143. [DOI] [PubMed] [Google Scholar]