Abstract
Introduction:
Human papillomavirus (HPV) infection is strongly associated with risk of cervical cancer and genital warts.
Aim:
The study aimed to report the prevalence of genus alpha human papillomavirus (HPV) types in women with cervical infection and/or inflammatory in Western Iran.
Materials and Methods:
In a cross-sectional study, 435 women were clinically diagnosed with cervical infections. The method of HPVs detection was polymerase chain reaction (PCR). The majority of patients (304 patients) did Pap smear based on liquid-based specimens.
Results:
Out of 435 patients, 150 patients (34.5%) had HPV positivity that the prevalence of high-risk HPVs was 52.7%. Out of 76 patients with Pap smear positivity, 68.4% had atypical squamous cells of undetermined significance cytology class and 31.6% had low-grade squamous intraepithelial lesion cytology class. The most prevalence rate of HPV was in age ≤30 years. HPV 16 and HPV 56 were the most prevalence rate among high-risk HPVs. Overall HPV alpha-10 (59%) and HPV alpha-9 (32.2%) had the most prevalence rate.
Conclusions:
Among high-risk HPVs, HPV 16 was the most common HPV detected in our population with a very low prevalence of HPV 18. In addition, HPV 6 and then HPV 11 had the most prevalence rate in the women with uterine cervix (cervical) infection among low-risk HPVs.
Keywords: Human papillomavirus, Uterine cervical, Pap smear, Polymerase Chain Reaction
1. INTRODUCTION
Cervical cancer is the second leading cause of malignancy deaths among women worldwide (1). Persistent human papillomavirus (HPV) infection is strongly associated with risk of cervical cancer and genital warts (2) with approximately 80% of women having acquired an infection by the age of 50 (3). HPV is classified as low- or high-risk based on its association with premalignant and malignant lesions, respectively. Low-risk HPVs 6 and 11 cause 90% of the external anogenital wart cases and low-grade changes in cervical cells (4). High-risk HPVs 16 and 18 cause approximately 70% of all invasive cervical cancer cases (5) and even has been reported to be as high as 99.7% around the world (6). It is a well-established fact that high-risk HPV is an important contributor to the development of invasive cervical cancer (6). Different studies in Iran reported various prevalences, and combining their results could be important for health policy makers (7). The recently approved quadrivalent (types 6, 11, 16, and 18) HPV vaccine targets the HPV strains responsible for approximately 70% of cervical cancers and 90% of genital warts (8). Therefore, HPV typing has important prognostic and therapeutic value and the recent development of HPV vaccines makes it increasingly more important (9). The aim of the present study was to report the prevalence of genus alpha HPVs in women with cervical infection and/or inflammatory in Western Iran and the correlation between the Pap smear results and HPV status.
2. MATERIALS AND METHODS
This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran. Between April 2015 to June 2017 in a cross-sectional study among women visited to Gynecology Clinics in Kermanshah city, 435 women were clinically diagnosed with cervical infections. The physician received one liquid Pap smear sample for every patient and then the sample was sent to Razi Pathobiology Center for confirmation of disease pathologically. The information of the patients was just based the pathology report. All patients were requested for checking HPVs based on polymerase chain reaction (PCR) and/or Pap smear method. In addition, the majority of patients (304 patients) did Pap smear based on liquid-based specimens.
Pap smear method
Samples of liquid Pap smear were delivered to the lab in standard laboratory containers. Each sample was placed inside the shaker for 5 minutes and then transferred to the E-prep machine. After leaving the machine, the specimen was fixed with ethyl alcohol 96% and staining was done on it.
PCR method
This method had sensitivity & specificity of 95%. First, 400 Landa of the Pap smear sample was washed with a few times phosphate-buffered saline (PBS) buffer, so that the precipitate was obtained as much as possible, and then, depending on the amount of sediment between 25 to 50 Landa was added the buffer to the resulting precipitate and 4 Landa from the resulting solution was added to 36 Landa of master mix and then PCR method was done.
After completion of the PCR, the specimens were immediately immersed in an ice-freezer for 2 minutes, and then the hybridization according to the protocol of Kit was done. The answers are read based on the cleared points. In this method, more than thirty-five HPVs types were checked on every sample. The high-risk HPVs were included HPVs 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82. The low-risk HPVs were included HPVs 6, 11, 40, 42, 43, 44, 54, 55, 61, 67, 69, 70, 71, 72, 89, 62/81, and CP6108. In addition to, the types of unclassified (UC) HPVs were checked. HPVs 16, 31, 33, 35, 52, 58, 67, and 73 were as α9; HPVs 18, 39, 45, 59, 68, 70, and 62/81 as α7; HPVs 26, 51, 82, and 69 as α5; HPVs 53, 56, and 66 as α6; HPVs 6, 11, 44, and 55 as α10; HPVs 40, and 43 as α8; HPVs 61, 72, and 89 as α3; HPV 42 as α1; HPV 54 as α13; and HPV 71 as α15.
Statistical analysis
The data was analyzed by IBM SPSS version 22 (IBM Corp., Armonk, NY, USA) that t-test was used for comparison of means and Chi-square test for other variables. The charts were plotted by Microsoft Excel 2010. A P<0.05 was considered to be statistically significant.
3. RESULTS
The mean age at the first visit of patients for cervical infections was 36 years (range, 16-72 years) (Table 1). We divided the patients to five age groups that the most prevalence rate of patients was in range 31-40 years (39.5%), followed by ≤30 years (32.4%), 41-50 years (19.1%), 51-60 years (5.8%), and >60 years (3.2%). Out of 435 patients, PCR showed that 150 patients (34.5%) had HPV positivity. In laboratory method was reported three types of HPV (low-risk, high-risk, and unclassified (UC) HPVs) that a number of patients had two or three types of HPV together (Table 1). Among 150 patients with HPV positivity, the low-risk HPV had the most prevalence rate in patients (85/150 or 56.7%) and after that, high-risk HPV (79/150 or 52.7%), multiple-type infection (48/150 or 32%), and UC (4/150 or 2.7%). Out of 304 patients with Pap smear result, 76 patients (25%) had positive result that among them, 52 patients (68.4%) had the atypical squamous cells of undetermined significance (ASCUS) cytology class and 24 (31.6%) had the low-grade squamous intraepithelial lesion (LSIL) cytology class.
Table 1. The characteristics of the patients (n=435). Abbreviations: HPV, Human papillomavirus ; SD, Standard deviation; ASCUS, Atypical squamous cells of undetermined significance; LSIL, Low-grade squamous intraepithelial lesion; UC, Unclassified HPVs.
| Variable | Value |
|---|---|
| Age: mean, Year ± SD (range) | 36 ± 10 (16-72) |
|
Age group: N (%) ≤30 31-40 41-50 51-60 >60 |
141 (32.4) 172 (39.5) 83 (19.1) 25 (5.8) 14 (3.2) |
|
HPV DNA: N (%) Positive Negative |
150(34.5) 285 (65.5) |
|
Type of HPV: N(%), (n=150) Low risk High-risk Low-risk & High-risk UC Low-risk & UC High-risk & UC Low-risk & High-risk & UC |
50(33.3) 38(25.3) 15(16.7) 4(2.7) 7(4.7) 13(8.7) 13(8.7) |
|
Pap smear: N(%), (n=304) Positive Negative |
76 (25) 228(75) |
|
Pap smear cytology class: N(%), (n=76) ASCUS LSIL |
52(68.4) 24 (31.6) |
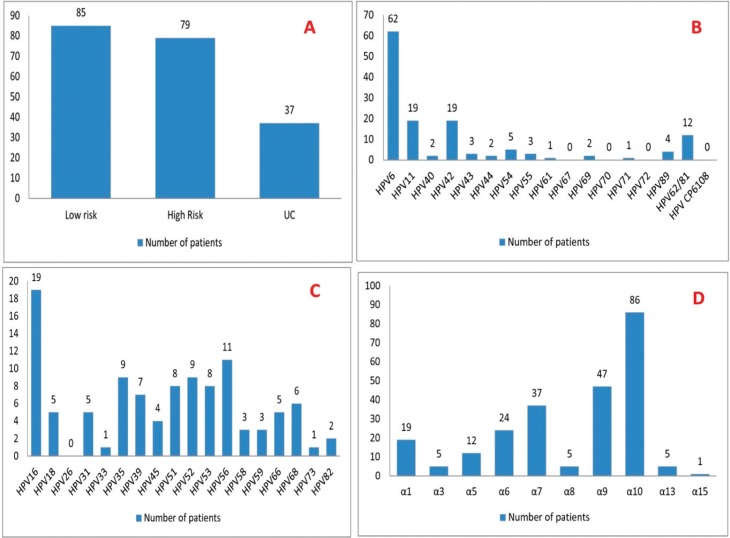
The prevalence of genus alpha HPVs types in women with cervical infections and HPV positivity based of monotype is shown in Figure 1A. Low-risk genus alpha HPV was divided to several subtypes that is shown in Figure 1B. Out of 85 patients with low-risk HPV, HPV 6 (72.9%), HPV 11 (22.3%), HPV 12 (22.3%), and HPV 62/81 (14.1%) had the prevalence more than 10%. High-risk genus alpha HPV was divided to several subtypes that is shown in Figure 1C. Out of 79 patients with high-risk HPV, HPV 16 (24.1%), HPV 56 (13.9%), HPV 52 (11.2%), HPV 35 (11.2%), HPV 51 (10.1%), and HPV 53 (10.1%) had the prevalence more than 10%. Figure 1D shows genus alpha HPVs were divided to other subtypes. Out of 146 patients with low-risk or high-risk HPV, HPVα10 (59%), HPVα9 (32.2%), HPVα7 (25.3%), HPVα6 (16.4%), and HPVα1 (13%) had the prevalence of more than 10%.
Figure 1. (A) The prevalence of genus alpha HPVs types in women with cervical infections and HPV positivity based of monotype (n=150). (B) The prevalence of low-risk genus alpha HPV subtypes in women with cervical infections and low-risk HPV positivity (n=85). (C) The prevalence of high-risk genus alpha HPV subtypes in women with cervical infections high-risk HPV positivity (n=79). (D) The prevalence of alpha HPV subtypes in women with cervical infections and high-risk or low-risk HPV (n=146). Abbreviations: HPV, Human papillomavirus ; UC, Unclassified HPVs.
The comparison of age and the results of Pap smear with HPV status have been shown in Table 2. There were significant differences between the variables with HPV status. The mean age of patients with HPV positivity was lower than HPV negativity (P<0.001), the most patients with HPV positivity were younger than the patients with HPV negativity (≤40 years: 85.6% versus 64.9%; >40 years: 14.7% versus 35.1%); (P<0.001). There was a significant direct correlation between Pap smear result and HPV status that the most patients with Pap smear positivity had HPV positivity (63/76) and the most patients with Pap smear negative had HPV negativity (179/228); (P<0.001). There were two Pap smear cytology classes in the patients that HPV positivity in LSIL was more than ASCUS (P=0.037).
Table 2. Correlation of age and Pap smear status with HPV status (n=435). Abbreviations: HPV, Human papillomavirus; SD, Standard deviation; ASCUS, Atypical squamous cells of undetermined significance; SIL, Low-grade squamous intraepithelial lesion.
| Variables | HPV+ (n=150) | HPV- (n=285) | P-value |
|---|---|---|---|
| Age: mean, Year ± SD | 32.46 ± 8.01 | 37.85 ± 10.44 | <0.001 |
|
Age group: N(%), year ≤30 31-40 41-50 51-60 >60 |
63 (42) 65(43.3) 16 (10.7) 5 (3.3) 1(0.7) |
78(27.4) 107(37.5) 67(23.5) 20(7) 13(4.6) |
<0.001 |
|
Pap smear: : N(%), (n=304) Positive Negative |
63(56.3) 49(43.8) |
13(6.8) 179(93.2) |
<0.001 |
|
Pap smear cytology class: N(%), (n=76) ASCUS LSIL |
40 (63.5) 23 (36.5) |
12 (92.3) 1 (7.7) |
0.037 |
We do two other analyses. The first, there was a correlation between HPV status and age groups in the patients. The most prevalence rate of HPV was in age ≤30 (44.7%) years and the least prevalence was in age >60 years (7.1%) with a new increase was seen over the age of 51-60 years (44.4%). The second, the comparison of age and HPV status in 52 patients having ASCUS in cytology showed that the patients with HPV positivity were younger than HPV negativity (mean age ± SD: 32.27±6.61 vs. 40.16±9.04 years, P=0.002), but the prevalence of patients in HPV positivity and negativity based on age group was similar (P=0.051). We couldn’t do the comparison for patients having LSIL in cytology because there was one patient with HPV negativity in LSIL.
4. DISCUSSION
The present study showed that 34.5% women with cervical infection and/or inflammatory had HPV positivity that the prevalence of high-risk HPV was 52.7%. Among the patients with Pap smear result, 68.4% had ASCUS cytology class and 31.6% had LSIL cytology class.
Overall, HPV testing is superior to traditional screening for the detection of high-grade cervical lesions, and efforts are focused on improving its sensitivity, increasing its cut-off for positivity or selecting those subgroups where HPV testing is expected to have higher positive predictive value for the cervical disease, or by seeking to optimize triage tests after a positive HPV result (10). Although women with age≥40 years are not specifically considered high-risk for HPV infection, many women are testing positive in this age group and are facing the impact of an HPV diagnosis that implicates a sexually transmitted disease and is known to be a precursor to cervical cancer (1).
Oncogenic or high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, and 58) are associated with cervical, vulvar, vaginal, and anal cancers, and non-oncogenic or low-risk types (6, 11, 40, 42, 43, 44, and 54) that are associated with genital warts (11). HPV 16 is the most oncogenic, accounting for almost half of all cervical cancers, and HPVs 16 and 18 together account for approximately 70% of cervical cancers (12). HPVs 6 and 11 are the most common strains associated with genital warts and are responsible for approximately 90% of these lesions (8). The prevalence of high-risk HPV in the USA women (57-85 years) was 6.0%, corresponding with 1.8 million women, and is stable across older age subgroups (13). A meta-analysis in 2016 showed that the total prevalence of HPV and its high-risk genotypes (16 and 18) among healthy noncancerous Iranian women was very high (7).
One study in Tehran, Iran (14), on 851 women aged 18-65 years, showed that in 265 (31.1%) women 19 different HPV types were detected. Overall HPV infection as well as infection with high-risk HPV types was highest in women aged 18–25 years and decreased with age. Type-specific prevalence of HPVs 16 and 18 was 7.3% and 2.8%, respectively. Another study (15) obtained cervical specimens from 825 married women aged 18-59 years from the general population of Tehran, Iran. HPV prevalence in the general population was 7.8% (5.1% of high-risk types), with no significant variation by age. In India (16), a total of 2501 women between 25-65 years old and without cervical cancer were screened by Pap smear cytology. Prevalence of HPV 18 (1.4%) was greater than that of HPV 16 (0.6%) in the overall screened population. In Brazil (17), 2300 women (15-65 years old) self-referred to cervical cancer screening between February 2002 and March 2003. High-risk genital HPV infection prevalence in this population was 17.8% that the highest prevalence of HPV infection was seen in women under 25 years old and then a new increase was seen over the age of 55-65 years. Out of 16748 women with cervical abnormalities or infections for HPV, 24.2% were infected with any HPV type at study entry, mainly HPV 16 and HPV 18. HPV infection was most common in participants aged 16 or 17 years (18). The present study reported that among age groups, the most prevalence rate of HPV was in age ≤30 years and the least prevalence was in age >60 years (P<0.001).
In a study in China with a sample size of 4987 (19), the total prevalence of HPV and prevalence of high-risk HPV genotype were 13.3% and 10.2%, respectively. HPV52 (3.1%), HPV16 (2.5%), HPV58 (2.1%), HPV68 (1%), and HPV81 (0.9%) were the most common genotypes. One study among 2362 Spanish women identified 34 different genotypes of HPV (20). The most common genotypes were HPV16 (19.18%), HPV53 (11.26%), HPV58 (7.66%), and HPV18 (4.02%). HPVs 16 and 18 were detected among 24.3% and 5.1% of the high-risk infections, respectively. Both HPV16 and HPV18 were responsible for 30% of the high-risk infections in Spanish clinical centers. Another study in Brazil, Canada, and the USA showed on 3204 healthy 15–25-year-old women (21), reported that the total HPV infection was 26.6%. The most common types were HPV16 (5.2%), HPV51 (3.3%), HPV52 (3.3%), HPV31 (2.9%), HPV66 (2.3%), and HPV39 (2%). In Hawaii (22) among women with cervical infection alone, oncogenic HPV 16 was the most common HPV detected followed by oncogenic HPV types 53 (7%), 58 (6%), and 52 (6%) and nononcogenic type 62 (6%). In Italian women with cervical cytological abnormalities (23), HPV prevalence was 52.6%; high-risk genotypes were found in 68.9% of women and multiple-type infection in 36.1% of HPV-positive women. The commonest types were HPV 52 (23.4%), HPV 53 (15.7%), HPV 16 (15.4%) and HPV 6 (12.4%). Among high-risk HPVs, HPV 16 (24.1%) had the most prevalence rate followed by HPV 56 (13.9%), HPV 52 (11.2%), HPV 35 (11.2%), HPV 51 (10.1%), and HPV 53 (10.1%). Among low-risk HPVs, HPV 6 (72.9%) had the most prevalence rate followed by HPV 11 (22.3%), HPV 12 (22.3%), and HPV 62/81 (14.1%).
In a routine pap smear of 2470 Korean women (24), HPV was detected in 44.8% of the patients and in 58.7% of the 861 atypical lesions based on the Bethesda system, including 52.6% of 627 ASCUS, 69.0% of 168 LSIL, and 89.4% of 66 HSIL cases. HSIL cases were mostly infected by sole HPV16 whereas LSIL that by various HPV types, suggesting a certain type may become dominant over others as the disease progresses. Two studies (25, 26) showed that high-risk HPV had the less prevalence in ASCUS cases compared with LSIL cases. One study (27) reported that HPV was found in 83.5% of LSIL patients. Tang et al. (28) concluded that among HPV-positive women, 22.5% had ASCUS, 0.2% had LSIL. The present study showed percent of ASCUS cases was 68.5% and LSIL was 31.6%. The prevalence any genus alpha HPV type was more in ASCUS cases compared with LSIL cases.
6. CONCLUSION
The prevalence of HPV types was variable in different areas in women with uterine cervix (cervical) infection/inflammatory. HPV 16 was the most common HPV detected in the population followed by HPV 56, HPV 52, HPV 35, HPV 51, and HPV 53 with a very low prevalence of HPV 18 among high-risk HPVs. In addition, HPV 6 and after that HPV 11 had the most prevalence rate in the women with low-risk HPV. Therefore, knowledge of the epidemiological distribution is necessary to predict the efficacy of vaccines on the incidence of infection and assess cross-protection from current vaccines against infection with other types.
Author’s contribution:
Two authors participated in each step of article and gave final improvement for submission of revised version.
Conflict of interest:
none declared.
REFERENCES
- 1.Montgomery K, Bloch JR. The human papillomavirus in women over 40: implications for practice and recommendations for screening. Journal of the American Association of Nurse Practitioners. 2010;22(2):92–100. doi: 10.1111/j.1745-7599.2009.00477.x. [DOI] [PubMed] [Google Scholar]
- 2.Stanley M. Preventing cervical cancer and genital warts–How much protection is enough for HPV vaccines? Journal of Infection. 2016;72:S23–S8. doi: 10.1016/j.jinf.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Control CfD, Prevention. Rockville, Md: CDC National Prevention Information Network; 2004. Genital HPV infection Fact Sheet. [visitado en 2004, Sept. 21] [Google Scholar]
- 4.Stanley M. Prophylactic HPV vaccines: prospects for eliminating ano-genital cancer. British journal of cancer. 2007;96(9):1320. doi: 10.1038/sj.bjc.6603695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International journal of cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Malary M, Moosazadeh M, Hamzehgardeshi Z, Afshari M, Moghaddasifar I, Afsharimoghaddam A. The prevalence of cervical human papillomavirus infection and the most at-risk genotypes among Iranian healthy women: A systematic review and meta-analysis. International journal of preventive medicine. 2016;7 doi: 10.4103/2008-7802.181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braaten KP, Laufer MR. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Reviews in obstetrics and gynecology. 2008;1(1):2. [PMC free article] [PubMed] [Google Scholar]
- 9.Martín P, Kilany L, García D, López-García AM, Martín-Azaña MJ, Abraira V, et al. Human papillomavirus genotype distribution in Madrid and correlation with cytological data. BMC infectious diseases. 2011;11(1):316. doi: 10.1186/1471-2334-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agorastos T, Sotiriadis A, Chatzigeorgiou K. Can HPV testing replace the pap smear? Annals of the New York Academy of Sciences. 2010;1205(1):51–56. doi: 10.1111/j.1749-6632.2010.05661.x. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England Journal of Medicine. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 12.Clifford G, Smith J, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. British journal of cancer. 2003;89(1):101. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindau ST, Drum ML, Gaumer E, Surawska H, Jordan JA. Prevalence of high-risk human papillomavirus among older women. Obstetrics and gynecology. 2008;112(5):979. doi: 10.1097/AOG.0b013e31818b0df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefzadeh A, Mostafavizadeh SM, Jarollahi A, Raeisi M, Garshasbi M, Siavashvahabi Z, et al. Human papillomavirus (HPV) prevalence and types among women attending regular gynecological visit in Tehran, Iran. Clin Lab. 2014;60(2):267–273. doi: 10.7754/clin.lab.2013.130221. [DOI] [PubMed] [Google Scholar]
- 15.Khodakarami N, Clifford GM, Yavari P, Farzaneh F, Salehpour S, Broutet N, et al. Human papillomavirus infection in women with and without cervical cancer in Tehran, Iran. International journal of cancer. 2012;131(2) doi: 10.1002/ijc.26488. [DOI] [PubMed] [Google Scholar]
- 16.Dutta S, Begum R, Mazumder D, Mandal SS, Mondal R, Biswas J, et al. Prevalence of human papillomavirus in women without cervical cancer: a population-based study in Eastern India. International Journal of Gynecological Pathology. 2012;31(2):178–183. doi: 10.1097/PGP.0b013e3182399391. [DOI] [PubMed] [Google Scholar]
- 17.Rama CH, Roteli-Martins CM, Derchain SFM, Longatto-Filho A, Gontijo RC, Sarian LOZ, et al. Prevalence of genital HPV infection among women screened for cervical cancer. Revista de saude publica. 2008;42(1):123–130. doi: 10.1590/s0034-89102008000100016. [DOI] [PubMed] [Google Scholar]
- 18.Bahmanyar ER, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, et al. Prevalence and risk factors for cervical HPV infection and abnormalities in young adult women at enrolment in the multinational PATRICIA trial. Gynecologic oncology. 2012;127(3):440–450. doi: 10.1016/j.ygyno.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Cheng X, Chen X, Ye F, Lü W, Xie X. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virology journal. 2010;7(1):66. doi: 10.1186/1743-422X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Roman JJ, Echevarria C, Salas S, Gonzalez-Moran MA, Perez-Mies B, Garcia-Higuera I, et al. A type-specific study of human papillomavirus prevalence in cervicovaginal samples in three different Spanish regions. Apmis. 2009;117(1):22–27. doi: 10.1111/j.1600-0463.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Roteli-Martins CM, de Carvalho NS, Naud P, Teixeira J, Borba P, Derchain S, et al. Prevalence of human papillomavirus infection and associated risk factors in young women in Brazil, Canada, and the United States: a multicenter cross-sectional study. International Journal of Gynecological Pathology. 2011;30(2):173–184. doi: 10.1097/PGP.0b013e3181f38dfe. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez BY, McDuffie K, Zhu X, Wilkens LR, Killeen J, Kessel B, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiology and Prevention Biomarkers. 2005;14(11):2550–2556. doi: 10.1158/1055-9965.EPI-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meloni A, Pilia R, Campagna M, Usai A, Masia G, Caredda V, et al. Prevalence and molecular epidemiology of human papillomavirus infection in italian women with cervical cytological abnormalities. Journal of public health research. 2014;3(1) doi: 10.4081/jphr.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang H-S, Park M, Lee S-Y, Kwon K-H, Pang M-G. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiology and Prevention Biomarkers. 2004;13(12):2153–2156. [PubMed] [Google Scholar]
- 25.Brismar-Wendel S, Froberg M, Hjerpe A, Andersson S, Johansson B. Age-specific prevalence of HPV genotypes in cervical cytology samples with equivocal or low-grade lesions. British journal of cancer. 2009;101(3):511. doi: 10.1038/sj.bjc.6605165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson H, Bjelkenkrantz K, Darlin L, Dilllner J, Forslund O. Presence of high-risk HPV mRNA in relation to future high-grade lesions among high-risk HPV DNA positive women with minor cytological abnormalities. PloS one. 2015;10(4):e0124460. doi: 10.1371/journal.pone.0124460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brebi P, Ili CG, Andana A, Menzel D, Lopez J, Guzman P, et al. Frequency of Human papillomavirus in women attending cervical cancer screening program in Chile. BMC cancer. 2017;17(1):518. doi: 10.1186/s12885-017-3496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Zheng L, Yang S, Li B, Su H, Zhang L-p. Epidemiology and genotype distribution of human papillomavirus (HPV) in Southwest China: a cross-sectional five years study in non-vaccinated women. Virology journal. 2017;14(1):84. doi: 10.1186/s12985-017-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]