Abstract
Intramuscular injection of botulinum toxin A (BTXA) is commonly used for the treatment of forehead wrinkles. In practice, physicians often use an intradermal injection for this purpose, as they feel that there is a lower risk of adverse effects compared with intramuscular injection. However, there are no direct comparative studies between those two injection modalities. We conducted a 24-week long, double-blinded, split-face, pilot study of three participants to compare the efficacy and safety of intradermal or intramuscular injection of BTXA for the treatment of forehead wrinkles. Maximum improvement of wrinkles and the time to achieve maximum effect were similar for both methods. The brow level was lower on the intramuscular injection side throughout the follow-up period for all participants. Subjective satisfaction with wrinkles was similar on both sides, but patients felt more heaviness of the eyebrow on the intramuscular side. No serious side effects were noted. In conclusion, the anti-wrinkle effect of BTXA was not significantly different between intramuscular and intradermal injections. However, side effects such as eyebrow ptosis, and heaviness were more prominent after intramuscular injection.
Keywords: Botulinum toxins, Forehead, Intradermal injections
INTRODUCTION
Botulinum toxin A (BTXA) is a powerful neurotoxin that inhibits muscle contraction by blocking several neurotransmitters, especially acetylcholine. Nowadays, it is used in various cosmetic procedures, particularly for the treatment of facial wrinkles. Based on its mechanism of action, BTXA works well when injected into the muscular layer. However, when it comes to forehead wrinkles, many physicians claim that there are no significant differences in anti-wrinkle efficacy between intramuscular (IM) and intradermal (ID) injections1. However, no confirmatory studies have been done to compare their efficacy. A previous split-face study concluded that there are no beneficial effects of ID BTXA compared to normal saline ID injection2. However, this study was only focused on skin texture, pore size, skin tightness, and sebum production on both the cheeks and the lower face. Another study showed a significant wrinkle-soothing effect of ID BTXA in the middle and lower face3, but this was not compared to IM BTXA injection and no information was available for the forehead region.
To evaluate the efficacy and safety of ID BTXA compared with IM BTXA on forehead wrinkles, we conducted a comparative split-face study.
CASE REPORT
This study was a double-blinded, split-face, pilot study including three participants after voluntary agreement. We received the patient's consent form about publishing all photographic materials. The study was approved by the Samsung Medical Center's Institutional Review Board (IRB no. 2014-03-107). None of the patients had any contraindications for BTXA injection, such as preexisting neuromuscular diseases or medication which could be affected by BTXA injection (aminoglycosides, penicillamine, quinine, calcium channel blockers, anticoagulant drugs, etc.). Botulax® (Clostridium botulinum toxin type A, purified neurotoxin complex; Hugel, Seoul, Korea) was injected at 1 cm intervals, 2 cm above the eyebrow. The injection volume was 0.05 ml and 2 U per spot were injected. 5 spots were injected on each side. Injection was conducted by an independent dermatologist who did not participate in treatment outcome evaluation. Each side of the face from each patient was randomly assigned for injection. The end point of the injection was subepidermal wheal-like swelling of the ID side. To ensure a muscular injection on the IM side, solution was injected after the tip of the needle touched the bone. The participants were instructed to visit on days 0 and 3 and weeks 1, 2, 4, 8, 12, 16, and 24. At each visit, a medical photograph was taken with forced upward gaze to create forehead wrinkles. After photographic review, wrinkle score was graded on a 5-point scale introduced by Tsukahara et al.4, ranging from grade 1 for no wrinkles to grade 5 for severe wrinkles. The brow level was checked by measuring the eyebrow to hairline length at the level of the mid-pupil and inner epicanthus at each visit to see how much the brow level dropped (Fig. 1). Subjective satisfaction with wrinkles, eyebrow heaviness, and discomfort with facial expression was also evaluated at each visit.
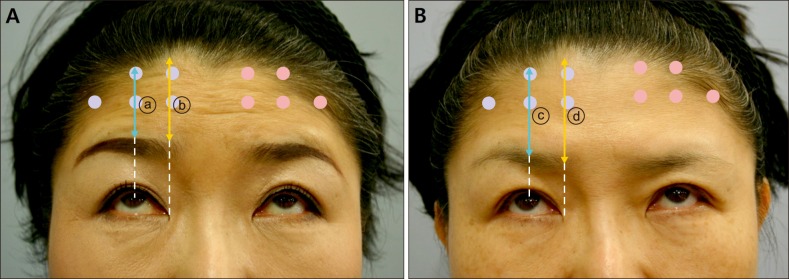
Fig. 1. (A) Photograph of Patient 1 with forced upward gaze before injection. The brow to hairline length was measured at the mid-pupillary level (a blue line denoted as ⓐ) and the inner epicanthus level (a yellow line denoted as ⓑ) on either sides. Blue dots represent intradermal injection sites and red dots represent intramuscular injection sites. (B) Four weeks after the injection of Patient 1. The brow to hairline length at week 4 was measured at the mid-pupillary level (a blue line denoted as ⓒ) and the inner epicanthus level (a yellow line denoted as ⓓ). The difference in the brow to hairline length before injection and after injection (in this case, ⓐ – ⓒfor mid-pupillary level, and ⓑ – ⓓfor inner epicanthus level) was defined as brow ptosis.
Our participants were all females, aged 52, 71, and 56 years. None of the enrolled subjects experienced serious adverse effects, such as an allergic reaction, facial palsy, or severe paralysis of muscles adjacent to the point of injection during or after this study.
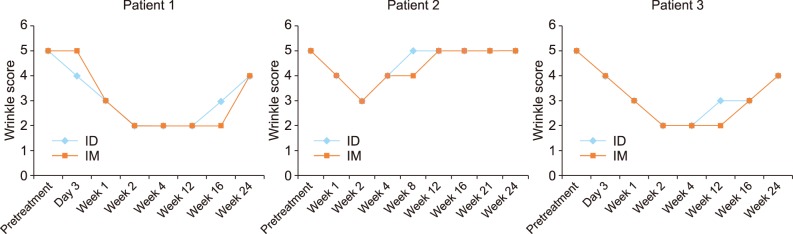
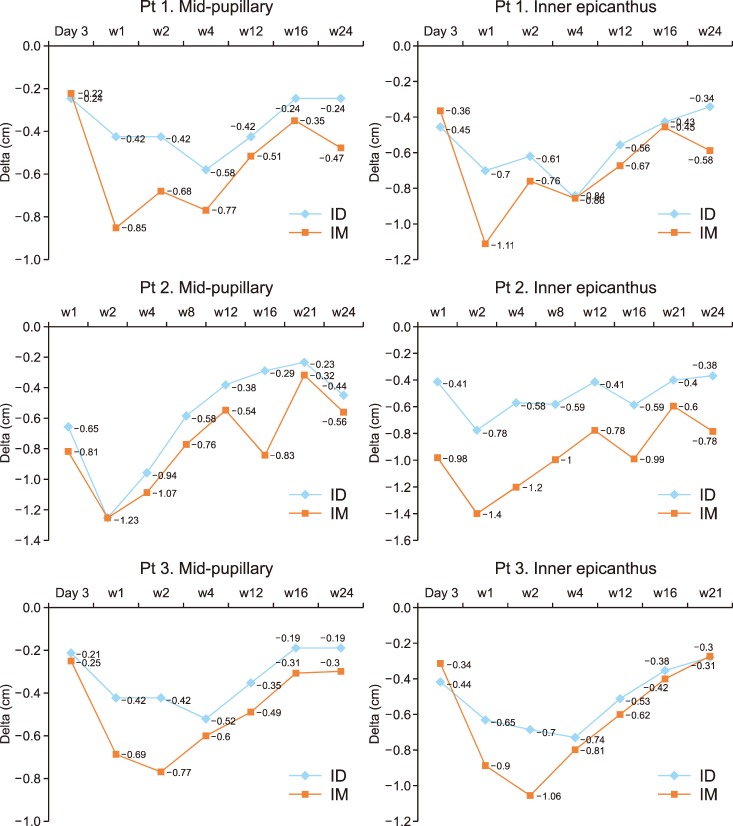
For all participants, the anti-wrinkle effect was most effective at week 2 either on the IM or ID side (Fig. 2). The IM injection side showed a slightly longer duration of effect, but the maximum improvement and time-to-achieve maximum effect were similar. The brow level was lowest weeks 1 to 2 on the IM side and weeks 2 to 4 on the ID side (Fig. 3). The IM side showed more brow ptosis throughout the follow-up period for all participants. All three participants were satisfied with the anti-wrinkle effect and they did not feel a significant difference between sides. However, they felt more eyebrow heaviness and difficulty making facial expressions on the IM side at weeks 1 to 12 (Fig. 4).
Fig. 2. Wrinkle scores of Patients 1, 2, and 3. The blue line is the wrinkle score of the intradermally injected side (ID). The orange line is the wrinkle score of the intramuscularly injected side (IM). Patient 1: 52 years (female), Patient 2: 71 years (female), Patient 3: 56 years (female).
Fig. 3. Brow ptosis of Patients (Pt) 1, 2, and 3 at the mid-pupillary level and the inner epicanthus level. The blue line shows brow ptosis on the intradermally injected side (ID). The orange line shows brow ptosis on the intramuscularly injected side (IM). The difference in the brow to hairline lengths were measured in centimeters.
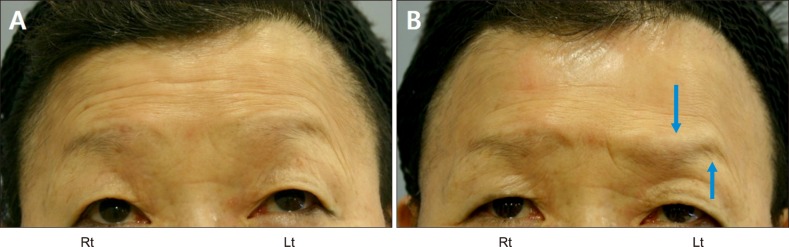
Fig. 4. (A) Pretreatment photograph of Patient 2 with forced upward gaze. (B) One week after injection in Patient 2. The right side (Rt) was the intradermal injection side and the left side (Lt) was the intramuscular injection side. Eyebrow ptosis is more prominent at the mid-to inner eyebrow on the intramuscular side, and this looks like “samurai eyebrow” when the patient attempted an upward gaze (arrows).
DISCUSSION
In this pilot study, the anti-wrinkle effect of BTXA was not significantly different between IM and ID injection for all three participants. However, side effects such as eyebrow ptosis, eyebrow heaviness, and difficulty making facial expressions were more prominent on the IM injection side. It is possible that this clinical differences are caused by the disparity in initial wrinkle severity, not by the injection techniques. Nevertheless, all three patients consistently showed more brow ptosis on the IM injection side throughout the whole follow up period, thus supporting many physicians' long experience that ID injection is better than IM injection on the forehead in order to avoid brow ptosis5. The exact mechanism by which ID injection exerts similar effects to IM injection, but less brow ptosis, is unknown. A previous report showed that ID BTXA injection improves forehead wrinkles but not effective for wrinkles of other parts of face6. Considering this former result, we can hypothesize that the forehead skin is thin enough so that ID BTXA can diffuse readily into the superficial muscle fibers to reduce the wrinkles, but sparing deeper muscles that maintain the resting tone required to lift the eyebrow. Dermis and subcutis of other parts of face is too thick so that ID BTXA cannot reach the superficial muscle fibers. Elderly people with weak frontalis muscles have a higher risk for brow ptosis. Direct injection of BTXA into the frontalis muscle may be too strong for those people, as it impedes the resting tone necessary to maintain brow level. Our participants were all over 50 and experienced significant brow ptosis on both sides. Nevertheless, the ID injection side showed less ptosis and it was much more tolerable than IM injection side.
Besides the low risk of brow ptosis, ID BTXA injection has its own merit of improving skin texture and lift while also reducing sebum production and pore size2,3,6,7.
Possible demerits of ID BTXA injection compared to IM injection is more pain and shorter duration of the toxin effect. Pain can be alleviated by pre-procedural topical anesthetics or ice pack application. The difference of the duration of the toxin effect between the two injection techniques was subtle, with similar total duration and similar time span for maximal effect.
We suggest that it is effective and safer to choose ID BTXA injection for forehead wrinkles especially in elder people. This study is the first study that directly compares the effect of IM and ID BTXA on forehead wrinkles. However, further studies with more cases are necessary to obtain a statistically significant outcome.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Carruthers A, Carruthers J. Botulinum toxin. 3rd ed. London: Saunders Elsevier; 2013. p. 183. [Google Scholar]
- 2.Kapoor R, Shome D, Jain V, Dikshit R. Facial rejuvenation after intradermal botulinum toxin: is it really the botulinum toxin or is it the pricks? Dermatol Surg. 2010;36(Suppl 4):2098–2105. doi: 10.1111/j.1524-4725.2010.01703.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang SP, Tsai HH, Chen WY, Lee WR, Chen PL, Tsai TH. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47:1287–1294. doi: 10.1111/j.1365-4632.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsukahara K, Takema Y, Kazama H, Yorimoto Y, Fujimura T, Moriwaki S, et al. A photographic scale for the assessment of human facial wrinkles. J Cosmetic Sci. 2000;51:127–139. [Google Scholar]
- 5.Redaelli A, Forte R. How to avoid brow ptosis after forehead treatment with botulinum toxin. J Cosmet Laser Ther. 2003;5:220–222. doi: 10.1080/14764170310023254. [DOI] [PubMed] [Google Scholar]
- 6.Sapra P, Demay S, Sapra S, Khanna J, Mraud K, Bonadonna J. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin a formulas to reduce signs of facial aging. J Clin Aesthet Dermatol. 2017;10:34–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SK. Multiple intradermal small bolus injection of botulinum toxin: the limit and the potentiality. J Cosmet Laser Ther. 2012;14:304–306. doi: 10.3109/14764172.2012.738914. [DOI] [PubMed] [Google Scholar]