Abstract
Purpose
To compare the effect of two different sperm preparation techniques, including swim-up and gradient methods on sperm deoxyribonucleic acid (DNA) fragmentation status of semen samples from unexplained and mild male factor subfertile patients undergoing intrauterine insemination (IUI).
Design
A prospective randomized study was conducted in 65 subfertile patients, including 34 unexplained and 31 male factor infertility to compare basal and post-procedure DNA fragmentation rates in swim-up and gradient techniques. Sperm DNA fragmentation rates were evaluated by a sperm chromatin dispersion (SCD) test in two portions of each sample of semen that was prepared with either swim-up or gradient techniques. Sperm motility and morphology were also assessed based on WHO 2010 criteria.
Results
Swim-up but not gradient method yielded a statistically significant reduction in the DNA fragmented sperm rate after preparation as compared to basal rates, in the semen samples of both unexplained (41.85 ± 22.04 vs. 28.58 ± 21.93, p < 0.001 for swim-up; and 41.85 ± 22.04 vs. 38.79 ± 22.30, p = 0.160 for gradient) and mild male factor (46.61 ± 19.38 vs. 30.32 ± 18.20, p < 0.001 for swim-up and 46.61 ± 19.38 vs. 44.03 ± 20.87, p = 0.470 for gradient) subgroups.
Conclusions
Swim-up method significantly reduces sperm DNA fragmentation rates and may have some prognostic value on intrauterine insemination in patients with decreased sperm DNA integrity.
Keywords: Sperm DNA fragmentation, DNA integrity, Swim-up, Gradient, Intrauterine insemination, Sperm chromatin dispersion
Introduction
Intrauterine insemination (IUI) plus controlled ovarian hyperstimulation (COH) with gonadotropins is used for the treatment of mild male factor and unexplained infertility [1]. The success of COH+IUI cycles is mainly dependent on female and male factors, including age, ovarian reserve, infertility period, total motile sperm count, and sperm morphology [2]. However, most of COH+IUI cycles do not result in pregnancy despite the absence of abnormality in these apparent factors. It is possible that defects of sperm DNA in some degree may cause the inability of fertilization or implantation of the fertilized ovum and have an impact on IUI cycle outcomes [3]. Sperm DNA fragmentation was also frequently found in normozoospermic men in IUI treatment population [4] and thought to be a significant predictor of the outcome of IUI cycles [5, 6]. However, limited evidence led to discuss incorporating sperm DNA fragmentation testing to infertility workup in subfertile patients undergoing COH-IUI [7, 8].
In cycles stimulated with clomiphene, basal sperm DNA fragmentation index (DFI) was related to progressive sperm motility, and use of DFI with high DNA stainability (HDS) was also effective for predicting clinical pregnancy [9]. In gonadotropin-stimulated cycles, age of partners, number of follicle, and post-wash sperm DNA fragmentation were found to be the most significant variables in predicting outcome of IUI and no pregnancy was seen when the proportion of sperm fragmentation exceeds 12% [10].
The possible underlying mechanisms of sperm DNA fragmentation are heat, radiation, abnormal lipid metabolism, and oxidative stress [11, 12]. The technique of sperm preparation for IUI may also pose a risk of sperm damage and may influence the rate of DNA fragmentation and IUI outcome [13]. This issue was evaluated in a limited number of studies with different preparation techniques and small subgroups. In a study by Younglai et al. (2001), centrifuging was not found to have a significant effect on sperm DNA fragmentation in comparison with normal (with centrifugation) and direct (without centrifugation) swim-up techniques [14]. However, Volpes A. et al. concluded that pellet swim-up technique was associated with the lowest DNA fragmentation rates in comparison of different four techniques, including direct swim-up, pellet swim-up, density gradient, and density gradient followed by swim-up in IVF/ICSI cycles [15]. In cases of teratozoospermia, although both two methods improved the results, density gradient centrifugation method was superior to swim-up regarding achievement of the specimen with a higher proportion of normal morphology and intact DNA [13].
Karamahmutoglu et al. (2014) experienced in a prospective randomized study that the gradient method was superior to the swim-up method for IUI success in unexplained subfertile patients without a significant difference in the post-process standard semen parameters [16]. It is reasonable to ask whether these sperm preparation methods have different effects on sperm DNA fragmentation to influence the outcomes of IUI. However, to date, no study has compared these two most common sperm preparation technique concerning DNA fragmentation and its clinical importance on the outcomes of COH-IUI. In this prospective study, we primarily aimed to evaluate the effect of sperm preparation with swim-up or gradient technique on sperm DNA fragmentation rates in subfertile patients undergoing COH-IUI. We also evaluated whether swim-up or gradient methods have an impact on sperm DNA integrity in subgroups of IUI treatment, indicating mild male factor and unexplained infertility.
Material and methods
This prospective randomized study was conducted to compare post-wash sperm DNA fragmentation rates between two frequently used methods of sperm preparation for IUI, swim-up, and density gradient centrifugation. For this purpose, we evaluated semen samples obtained from 65 male partners of infertile couples who underwent first COH-IUI cycles between September 2011 and September 2012, because of a diagnosis of primary infertility, secondary to mild male factor and unexplained infertility, and who were not previously treated with IUI or IVF. The Local Ethical Committee, Board of Clinical Researches of Gazi University, approved the study with an approval number of 200. This study was also registered to ClinicalTrials.gov Protocol Registration and Results System with an ID of NCT01859520 and title “Swim up and Gradient Methods Used in Assisted Reproduction Techniques on DNA Fragmentation of Spermatozoa”.
Main inclusion criteria of cases were having at least two abnormal sperm analysis as low number of sperm count and/or restricted motility according to World Health Organization (WHO) 2010 criteria [17] in mild male factor group, and the patients having normal sperm parameters in addition to normal ovulatory status and patent fallopian tubes either by hysterosalpingography or laparoscopy of females in the unexplained group. The diagnosis was primary infertility, and no IUI or IVF attempts were performed previously. Exclusion criteria were a history of ovarian surgery in female and severe oligozoospermia (sperm count < 5 million per mL), and systemic diseases or therapies influencing DNA integrity in a male partner.
All IUI cycles were stimulated by recombinant gonadotropins (Gonal-F®, Merck-Serono, Istanbul, Turkey) that were started on the third day of a treatment cycle. Starting and maintaining the dose of gonadotropins were adjusted based on basal ovarian reserve parameters, including age, BMI, basal FSH and antral follicle count, and ovarian response, respectively. Ovarian response and follicular growth were monitored by serial transvaginal ultrasonography (TVU) with measurement of serum level of estradiol (E2) beginning from the eighth day of cycle to the day of ovulation trigger. Ovulation was triggered with hCG (Ovitrelle®, Merck-Serono, Istanbul, Turkey) when one or two dominant follicles reach 17–18 mm in diameter on TVU. IUI was performed 36 h after hCG administration. Vaginal progesterone (Crinone gel®, Merck-Serono, Istanbul, Turkey) was used for luteal phase support in all cycles. Pregnancy was tested 2 weeks after the IUI, and a visible gestational sac on TVU is accepted for a clinical pregnancy in patients with a positive pregnancy test.
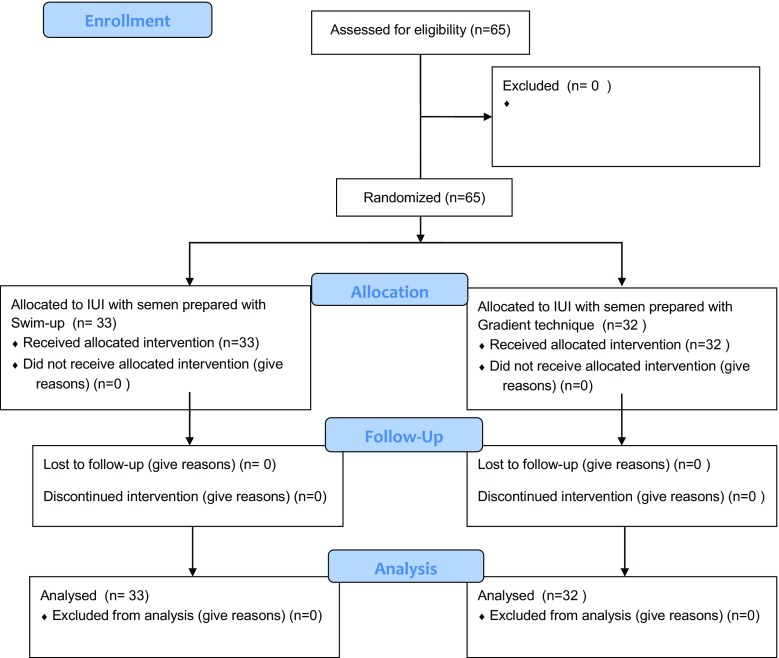
Semen samples were obtained by masturbation after 3–5 days of sexual abstinence in the IVF laboratory, a unit of Gazi University on insemination day. After basal semen volume was measured, each sample of semen was divided into two portions for washing with either swim-up or gradient density methods in all subjects. Basal and post-wash rates of sperm DNA fragmentation were assessed by using Halosperm® kit (Halotech DNA SL, Madrid, Spain) as described below. Embryologists randomly selected samples for IUI based on a randomization table created with the software The Statistical Program for Social Sciences (SPSS, version 11.5, IBM, Chicago, IL, USA). Doctors and patients were blinded to the method of sperm preparation for IUI (Fig. 1).
Fig. 1.
CONSORT flow diagram
Swim-up technique
The swim-up technique was carried out after basal seminal analyses were performed with 5 μL of seminal samples liquefied in 20 min in an incubator. The remaining semen sample was put in a falcon conical tube, and a washing medium (Sperm Rinse Solution, Vitrolife, Göteborg, Sweden) was added in 1:1 proportion. Then, the sample was centrifuged at 157g in 10 min. After the supernatant was removed, a 0.5-mL medium (Sperm Rinse Solution, Vitro Life, Sweden) was added over the pellet in a tube and kept at a 45° angle. The sample was incubated at 37°C for 1 h. A part of the sample was re-analyzed for sperm count and motility. Then, 0.3–0.5 mL of sperm solution was collected with a pipette while keeping the 45° angle of the tube constant and transferred into a 6-mL round bottom tube to be used for IUI.
Gradient technique
In this technique, sperms were separated by using a mixture of bicarbonate and HEPES-buffered silane-coated colloid silica particles (SpermGrad™, Vitrolife, Göteborg, Sweden) which were prepared both 40 and 90% in concentration by using G-IVF™ PLUS medium (Vitrolife, Göteborg, Sweden) at first. Gradient medium in 90 and 40% concentration was added sequentially to an empty falcon conical tube with preventing mixing of them. After this preparation was incubated at 37°C for 15 min, the semen sample was slowly put on to this medium with a pipette and centrifuged at 381g in 10 min. Then, the supernatant was removed, and the pellet was transferred to another 6-mL round bottom tube. A 3-mL of G-IVF™ PLUS medium (Vitrolife, Göteborg, Sweden) was put on to a pellet and centrifuged at 381g for 10 min. The pellet was resuspended to 0.3–0.5 mL of sperm wash solution and transferred into a 6-mL round bottom tube for IUI.
Evaluation of sperm DNA fragmentation
Sperm DNA integrity was tested using Halosperm® kit (Halotech DNA SL, Madrid, Spain) based on a principle that sperms with fragmented DNA fails to show big and medium halos of dispersed DNA loops that are observed in sperm with non-fragmented DNA under microscope, following acid denaturation and removal of nuclear proteins by using the following steps:
Agarose in Eppendorf tubes was kept at 37 °C for 5 min after it was melted in a beaker that was filled with water at 95–100 °C.
After sperm sample was diluted to a maximum 20 million sperm per milliliter, 25 μL of semen sample was added to the tube and 15 μL of the mixture was transferred to the precoated agarose slide of the kit.
The slide was put in a fridge at 4 °C for 5 min to allow solidification of agarose.
Meanwhile, denaturing solution was mixed with 10 mL of distilled water.
Then the slide was taken out from the fridge at 4 °C, and its cover was removed.
The slide was sequentially incubated in a denaturing solution for 7 min, lysis solution for 25 min, distilled water for 5 min, 70% ethanol for 2 min, 90% ethanol for 2 min, and 100% ethanol for 2 min.
The slide was left for drying at room temperature and then, stained with Diffquick.
Sperms were evaluated under a bright field microscope with × 40 objective whether they have a big, medium and small, or no halo, or they show degradation. Because denaturing steps permits to prevent denaturation of sperm tail, sperms were easily discriminated from other cells. Percentage of sperms showing small halo, degradation, and no halo reveals DNA fragmentation rate as described on the manufacturer’s website (http://www.halotechdna.com/wp-content/uploads/2015/09/Halosperm-web.pdf). A minimum number of 500 spermatozoa were assessed per one slide for one patient.
Main outcome measure was the comparison of basal and post-procedure DNA fragmentation rates in swim-up and gradient techniques in unexplained and mild male factor infertility. Secondary outcome measures were the comparison of basal and post-wash sperm DNA fragmentation rates, concentration, and motility of sperms between cycles resulted with and without pregnancy. Comparison of sperm DNA fragmentation rates among different age periods of patients was also considered as secondary outcome measures.
Recorded data were analyzed with the Statistical Program for Social Sciences (SPSS, version 11.5, IBM, Chicago, IL, USA). Demographic characteristics were compared with Mann-Whitney U test. Pre- and post-washing sperm values were analyzed with Wilcoxon signed rank test in paired group comparisons. Kruskal-Wallis test is used for comparisons of three or more groups. Data were presented as mean ± standard error mean (SEM) and p value was set to < 0.05 for statistical significance.
Results
A total number of 65, 34 (52.3%), and 31 (42.7%) subfertile cases were recruited to unexplained and mild male factor groups, respectively. Demographic characteristics, including the age of partners and infertility period between unexplained and mild male factor groups, were similar and presented with basal and post-washing sperm analyses and DNA fragmentation rates in Table 1. Mean basal, post-swim-up, and post-gradient sperm DNA fragmentation rate was found to be similar between the male factor and unexplained infertility groups. Comparison of basal and post-washing sperm analyses and DNA fragmentation rates between 20 and 29, 30–39, and ≥ 40 years of male age groups in all cases of the study also revealed no any significant difference between these different 10 years of age intervals (Table 2).
Table 1.
Comparison of demographic data and basal and post-washing sperm analyses between groups of unexplained and male factor infertility
| Variable | Unexplained (n = 34) | Male factor (n = 31) | p* value |
|---|---|---|---|
| Age, male (year) | 30 ± 0.5 | 31.7 ± 1.1 | 0.670 |
| Age, female (year) | 28 ± 0.8 | 29.6 ± 1.1 | 0.300 |
| Infertility period (month) | 35.1 ± 4.1 | 51.1 ± 9.1 | 0.710 |
| Basal semen volume (mL) | 2.4 ± 0.1 | 3.0 ± 0.1 | 0.027 |
| Basal number of sperm count (n × 106) | 38.8 ± 3.4 | 8.8 ± 0.7 | 0.0001 |
| Basal progressive sperm motility (%) | 48.4 ± 2.6 | 39.4 ± 2.8 | 0.005 |
| Basal sperm DNA fragmentation (%) | 41.8 ± 3.7 | 46.6 ± 3.4 | 0.313 |
| Basal normal sperm morphology (%) | 1.156 ± 0.1 | 0.4 ± 0.1 | 0.009 |
| Post-swim-up number of sperm count (n × 106) | 22.3 ± 3 | 5.1 ± 0.5 | 0.0001 |
| Post-swim-up progressive sperm motility (%) | 73.9 ± 3 | 59.5 ± 3.4 | 0.002 |
| Post-swim-up sperm DNA fragmentation (%) | 28.5 ± 3.7 | 30.3 ± 3.2 | 0.373 |
| Post-gradient number of sperm count (n × 106) | 26.4 ± 3.9 | 6.4 ± 0.8 | 0.0001 |
| Post-gradient progressive sperm motility (%) | 72.2 ± 2.4 | 55.1 ± 3.3 | 0.0001 |
| Post-gradient sperm DNA fragmentation (%) | 38.7 ± 3.8 | 44 ± 3.7 | 0.245 |
Data were presented as mean ± standard error mean (SEM)
*Mann-Whitney U test and italicized p values indicate statistical significance
Table 2.
Comparisons of basal and post-washing sperm analyses and DNA fragmentation between different age groups in all cases
| Variable | 20–29 years (n = 30) | 30–39 years (n = 31) | ≥ 40 years (n = 4) | p* value |
|---|---|---|---|---|
| Basal number of sperm count (n × 106) | 25.1 ± 3.9 | 26 ± 3.8 | 7.7 ± 1.4 | 0.101 |
| Basal progressive sperm motility (%) | 43.8 ± 2.7 | 45.7 ± 3.1 | 36.2 ± 7.7 | 0.572 |
| Basal sperm DNA fragmentation (%) | 40.9 ± 3.9 | 47.2 ± 3.5 | 43.7 ± 12.4 | 0.419 |
| Post-swim-up number of sperm count (n × 106) | 15.9 ± 3.1 | 13.7 ± 2.5 | 3.5 ± 0.9 | 0.065 |
| Post-swim-up progressive sperm motility (%) | 69 ± 3.9 | 66.4 ± 3.2 | 56.2 ± 5.5 | 0.223 |
| Post-swim-up sperm DNA fragmentation (%) | 27.4 ± 3.9 | 31.6 ± 3.4 | 27.5 ± 7.5 | 0.482 |
| Post-gradient number of sperm count (n × 106) | 20 ± 4.1 | 15.4 ± 3.0 | 4.2 ± 1.1 | 0.084 |
| Post-gradient progressive sperm motility (%) | 64.7 ± 3.6 | 65.7 ± 3 | 46.2 ± 8.5 | 0.110 |
| Post-gradient sperm DNA fragmentation (%) | 39.3 ± 4.4 | 42.4 ± 3.1 | 47.5 ± 16.1 | 0.623 |
Data were presented as mean ± standard error mean (SEM)
y years
*Kruskal-Wallis test
Paired samples statistics showed that the mean basal sperm DNA fragmentation rate was significantly improved with swim-up (44.1 ± 2.5 vs. 29.4 ± 2.4, p < 0.0001, respectively) but was not improved with gradient method of sperm preparation technique (44.1 ± 2.5 vs. 41.2 ± 2.6, p = 0.111, respectively) among all cases. Table 3 shows paired comparison of basal, post-swim-up and post-gradient number of sperm count, motility, and DNA fragmentation rates within unexplained and male factor groups. Analyses revealed that post-procedure sperm DNA fragmentation rate was improved better by swim-up than by gradient method in both unexplained (28.5 ± 3.7 vs. 38.7 ± 3.8, p = 0.0001, respectively) and male factor groups (30.3 ± 3.2 vs. 44 ± 3.7, p = 0.0001, respectively). Sperm DNA fragmentation was not significantly different from basal values by gradient method in both unexplained (41.8 ± 3.7 vs. 38.7 ± 3.8, p = 0.160) and male factor groups (46.6 ± 3.4 vs. 44 ± 3.7, p = 0.470).
Table 3.
Paired comparisons of variables between basal, post-swim-up, and post-gradient procedures within each group
| Variable | Unexplained (n = 34) | Male factor (n = 31) | ||||||
|---|---|---|---|---|---|---|---|---|
| Basal (n = 34) | Swim-up (n = 34) | Gradient (n = 34) | p* value | Basal (n = 31) | Swim-up (n = 31) | Gradient (n = 31) | p* value | |
| Number of sperm count (n × 106) | 38.8 ± 3.4 | 22.3 ± 3 | – | 0.0001 | 8.8 ± 0.7 | 5.1 ± 0.5 | – | 0.0001 |
| 38.8 ± 3.4 | – | 26.4 ± 3.9 | 0.001 | 8.8 ± 0.7 | – | 6.4 ± 0.8 | 0.001 | |
| – | 22.3 ± 3 | 26.4 ± 3.9 | 0.195 | – | 5.1 ± 0.5 | 6.4 ± 0.8 | 0.002 | |
| Progressive sperm motility (%) | 48.4 ± 2.6 | 73.9 ± 3 | – | 0.0001 | 39.4 ± 2.8 | 59.5 ± 3.4 | – | 0.0001 |
| 48.4 ± 2.6 | – | 72.2 ± 2.4 | 0.0001 | 39.4 ± 2.8 | – | 55.1 ± 3.3 | 0.0001 | |
| – | 73.9 ± 3 | 72.2 ± 2.4 | 0.293 | – | 59.5 ± 3.4 | 55.1 ± 3.3 | 0.027 | |
| Sperm DNA fragmentation (%) | 41.8 ± 3.7 | 28.5 ± 3.7 | – | 0.0001 | 46.6 ± 3.4 | 30.3 ± 3.2 | – | 0.0001 |
| 41.8 ± 3.7 | – | 38.7 ± 3.8 | 0.160 | 46.6 ± 3.4 | – | 44 ± 3.7 | 0.470 | |
| – | 28.5 ± 3.7 | 38.7 ± 3.8 | 0.0001 | – | 30.3 ± 3.2 | 44 ± 3.7 | 0.0001 | |
Data were presented as mean ± standard error mean (SEM)
*Wilcoxon signed rank test and italicized p values indicate statistical significance
Comparison of basal and post-preparation progressive sperm motility, morphology, and DNA fragmentation rates between cycles with and without clinical pregnancy is shown in Table 4. In this analysis, any variable did not show a significant difference between groups resulted with and without pregnancy.
Table 4.
Comparisons of basal and post-washing sperm analyses and DNA fragmentation between cycles resulted with and without clinical pregnancy
| Variable | No pregnancy (n = 55) | Clinical pregnancy (n = 10) | p* value |
|---|---|---|---|
| Basal progressive sperm motility (%) | 45.1 ± 1.9 | 39.6 ± 7.3 | 0.184 |
| Basal sperm DNA fragmentation (%) | 43.2 ± 2.8 | 49 ± 5.2 | 0.295 |
| Basal total motile sperm count (106) | 29.4 ± 4.5 | 34 ± 14.2 | 0.763 |
| Post-swim-up progressive sperm motility (%) | 67.6 ± 2.7 | 63.8 ± 5.2 | 0.387 |
| Post-swim-up sperm DNA fragmentation (%) | 28.9 ± 2.8 | 32 ± 4.8 | 0.317 |
| Post-gradient progressive sperm motility (%) | 63.7 ± 2.5 | 65.7 ± 6 | 0.964 |
| Post-gradient sperm DNA fragmentation (%) | 40.3 ± 2.9 | 46.5 ± 6.6 | 0.380 |
| Basal normal sperm morphology (%) | 0.83 ± 0.1 | 1.0 ± 0.4 | 0.813 |
Data were presented as mean ± standard error mean (SEM)
*Mann-Whitney U test
Discussion
In this study, our primary objective was to compare swim-up and gradient methods of sperm preparation regarding sperm DNA fragmentation rates in semen samples of a subfertile population undergoing their first IUI cycle. The main finding was that swim-up method significantly decreases sperm DNA fragmentation rate by sperm chromatin dispersion (SCD) test (Halosperm kit) in both unexplained and mild male factor infertility. Also, gradient method did not result in significant improved sperm DNA quality among the population undergoing IUI.
Previously, only a few study compared swim-up and gradient methods with regard to sperm DNA fragmentation. In a survey among 35 semen samples, DNA fragmentation rates have been found to be lower by gradient than swim-up method, although characteristics of cases and basal values of DNA fragmentation were not mentioned [18]. In another study, although gradient method was found to be better, both swim-up and gradient methods improved DNA fragmentation in teratozoospermic semen samples [13]. The inconsistency of the results between the present and previous studies may result from evaluation of different population, different assessment methods of DNA fragmentation, and/or small sample sizes of the studies. Concordantly, it was concluded by Younglai et al. and Chenlo PH et al. that sperm DNA fragmentation rates are not increased but also decreased while processing semen samples with a swim-up technique in infertile and fertile cases [14, 19].
Higher sperm DNA fragmentation rates were found to be correlated with abnormal sperm parameters, including oligozoospermia, teratozoospermia, and asthenozoospermia when compared to normozoospermia, and also the severity of these abnormalities [4, 20]. Furthermore, lower sperm DNA integrity was shown in normozoospermic infertile men undergoing IUI than fertile controls [4]. Since unexplained and mild male infertility is the primary diagnoses of men enrolled to IUI, we also aimed to evaluate whether a difference exists between basal and post-washing sperm DNA fragmentation rates between unexplained and mild male factor infertility. Our data revealed similar basal and post-washing sperm DNA fragmentation rates between unexplained and mild male factor infertility.
Karamahmutoglu H. et al. previously found that gradient method was superior to swim-up regarding biochemical, clinical, and ongoing pregnancies as the outcomes of IUI without any significant difference in basal and post-preparation sperm analyses [16]. This finding suggested the probability of presence of any underlying molecular mechanisms that cause different outcomes and may be differently influenced by two sperm preparation methods. Swim-up and gradient techniques have proven effects in increasing sperm motility and morphology [21] as well as may influence the outcome of IUI cycles by improving DNA fragmentation rates. However, as a secondary result of our study, the improvement in DNA fragmentation was not reflected on the outcomes of IUI cycles, since basal and post-process sperm DNA fragmentation rates were similar between cycles resulted with and without clinical pregnancy among the study population. In addition, contrary to expectation from the results of the study by Karamahmutoglu H. et al., swim-up technique yielded better DNA integrity than gradient method in this study. The literature lacks data on this matter. Only a previous study by Duran EH et al. concluded that post-washing sperm DNA integrity was better in IUI cycles resulted in pregnancy than failure [10]. In that study by Duran EH et al., just gradient method was used and non-statement of basal values makes impossible to comment whether sperm preparation technique has some role in cycle outcomes via DNA fragmentation rates.
This study is also unique regarding comparing basal and post-process sperm DNA fragmentation rates on IUI cycle outcomes. In contrast to previous reports evaluating only basal [9] and post-washing [10] sperm DNA fragmentation, we found no significant difference in mean basal (49 ± 5.2 vs. 43.2 ± 2.8%, p = 0.295), post-swim-up (32 ± 4.8 vs. 28.9 ± 2.8%, p = 0.317), and post-gradient (46.5 ± 6.6 vs. 40.3 ± 2.9%, p = 0.380) DNA fragmentation rates between pregnancy and failure groups, respectively. In previous studies, the significance of DNA fragmentation was presented by comparison of pregnancy rates at any selected cutoff values instead of the comparison of mean values between pregnancy and failure groups [9, 10]. The variable sensitivity of the assessment methods of DNA fragmentation may also be responsible from controversial results as SCD test was found to be more sensitive than TUNEL method [22] that were used in the present and previous study, respectively [10]. The importance of basal and post-wash DNA fragmentation might also be low in subfertile population. Because of incorporation of the low number of pregnant participants into the analyses, it is also difficult to conclude the role of DNA fragmentation rates on IUI cycle outcomes.
In conclusion, because swim-up method significantly yielded better sperms with DNA integrity in subfertile population, the use of swim-up sperm processing method seems to be valuable for patients expected to have higher rates of DNA fragmentation.
Acknowledgements
We thank Gazi University Unit of Scientific Research Projects for supporting our study.
Compliance with ethical standards
The Local Ethical Committee, Board of Clinical Researches of Gazi University, approved the study with an approval number of 200. This study was also registered to ClinicalTrials.gov Protocol Registration and Results System with an ID of NCT01859520 and title “Swim up and Gradient Methods Used in Assisted Reproduction Techniques on DNA Fragmentation of Spermatozoa”.
References
- 1.Fritz MA, Speroff L. Male infertility. In: Fritz MA, Speroff L, editors. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Lippincott Williams & Wilkins, A Wolters Kluwer Business; 2011. pp. 1249–1293. [Google Scholar]
- 2.Erdem A, Erdem M, Atmaca S, Korucuoglu U, Karabacak O. Factors affecting live birth rate in intrauterine insemination cycles with recombinant gonadotrophin stimulation. Reprod BioMed Online. 2008;17(2):199–206. doi: 10.1016/S1472-6483(10)60195-2. [DOI] [PubMed] [Google Scholar]
- 3.Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology. 2013;1(3):357–360. doi: 10.1111/j.2047-2927.2012.00041.x. [DOI] [PubMed] [Google Scholar]
- 4.Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30(11):1519–1524. doi: 10.1007/s10815-013-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samplaski MK, Dimitromanolakis A, Lo KC, Grober ED, Mullen B, Garbens A. The relationship between sperm viability and DNA fragmentation rates. Reprod Biol Endocrinol. 2015;13:42. doi: 10.1186/s12958-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod BioMed Online. 2006;12(4):466–472. doi: 10.1016/S1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 7.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57(1–2):78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 8.Drobnis EZ, Johnson MH. Are we ready to incorporate sperm DNA-fragmentation testing into our male infertility work-up? A plea for more robust studies. Reprod BioMed Online. 2015;30(2):111–112. doi: 10.1016/j.rbmo.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–1408. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 10.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17(12):3122–3128. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93(4):1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Thomson LK, Zieschang JA, Clark AM. Oxidative deoxyribonucleic acid damage in sperm has a negative impact on clinical pregnancy rate in intrauterine insemination but not intracytoplasmic sperm injection cycles. Fertil Steril. 2011;96(4):843–847. doi: 10.1016/j.fertnstert.2011.07.356. [DOI] [PubMed] [Google Scholar]
- 13.Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH, Guo BZ, Qin Z. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet. 2014;31(9):1161–1166. doi: 10.1007/s10815-014-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younglai EV, Holt D, Brown P, Jurisicova A, Casper RF. Sperm swim-up techniques and DNA fragmentation. Hum Reprod. 2001;16(9):1950–1953. doi: 10.1093/humrep/16.9.1950. [DOI] [PubMed] [Google Scholar]
- 15.Volpes A, Sammartano F, Rizzari S, Gullo S, Marino A, Allegra A. The pellet swim-up is the best technique for sperm preparation during in vitro fertilization procedures. J Assist Reprod Genet. 2016;33(6):765–770. doi: 10.1007/s10815-016-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamahmutoglu H, Erdem A, Erdem M, Mutlu MF, Bozkurt N, Oktem M, Ercan DD, Gumuslu S. The gradient technique improves success rates in intrauterine insemination cycles of unexplained subfertile couples when compared to swim up technique: a prospective randomized study. J Assist Reprod Genet. 2014;31(9):1139–1145. doi: 10.1007/s10815-014-0274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 18.Amiri I, Ghorbani M, Heshmati S. Comparison of the DNA fragmentation and the sperm parameters after processing by the density gradient and the swim up methods. J Clin Diagn Res. 2012;6(9):1451–1453. doi: 10.7860/JCDR/2012/4198.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chenlo PH, Curi SM, Pugliese MN, Ariagno JI, Sardi-Segovia M, Furlan MJ. Fragmentation of sperm DNA using the TUNEL method. Actas Urol Esp. 2014;38(9):608–612. doi: 10.1016/j.acuro.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009;74(4):789–793. doi: 10.1016/j.urology.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007;4:CD004507. doi: 10.1002/14651858.CD004507.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Feijo CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101(1):58–63. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]