Abstract
Purpose
Undesirable side effects of cancer treatments are common and include damage to the ovary, and depletion of the follicle reserve, which if severe enough, can lead to infertility and early menopause. Antimetabolite drugs, such as 5-fluorouracil (5-FU), are not considered to be detrimental to the ovary, but the ovotoxicity of 5-FU has not been evaluated in any detail. The purpose of this study was to evaluate the effects of 5-FU on follicle number.
Methods
In this study, adult female C57Bl6 mice (n = 4–6 animals/group) received a single dose of saline or 5-FU (150 mg/kg) and markers of ovarian damage and follicle depletion were assessed 12 h and 7 days later.
Results
Exposure to 5-FU did not alter primordial and primary follicle numbers. Atresia of secondary and antral follicles was increased significantly 12 h after 5-FU treatment, but atresia rates returned to levels similar to that of saline treated controls at 7 days. The number of corpora lutea were reduced 7 days after exposure to 5-FU, possibly as a consequence of earlier follicular atresia.
Conclusions
These findings suggest that a single dose of 5-FU is mildly ovotoxic, but any effects on ovarian function are likely transient because the primordial follicle population is not depleted. Collectively, these data support the notion that 5-FU is unlikely to impact on the long-term fertility of women.
Keywords: 5-FU, Apoptosis, Fertility, Ovary, Follicles, Chemotherapy
Background
The ideal chemotherapy is one that specifically prevents the growth of the cancer, whilst maintaining the integrity of non-target tissues. However, undesirable side effects of cancer treatments are common and include damage to the ovary and depletion of the follicle reserve, which if severe enough, can lead to infertility and early menopause [1]. In recent years, much attention has focussed on defining the ovotoxicity and reproductive outcomes associated with exposure to chemotherapy agents belonging to the alkylating, platinum and anthracycline antibiotic classes. It has been demonstrated that these drugs, including cyclophosphamide, cisplatin and doxorubicin, damage the ovary to varying degrees and are associated with poor reproductive outcomes [2]. By contrast, the antimetabolite class of chemotherapy drugs are thought to have little impact on female fertility [3–5], but detailed studies of ovotoxicity of individual antimetabolite drugs have not been conducted and empirical evidence to support this assertion is limited.
5-Fluorouracil (5-FU) is an antimetabolite drug used to treat of variety of cancers, including breast cancer, and is most notable for its effective treatment of colorectal cancers. Colorectal cancer is amongst the most common malignancies in the western world, and epidemiological data indicate that its incidence is increasing in women of childbearing and premenopausal age [6–8]. Given that the survival rates of patients diagnosed at early stages of the disease are high [8], the effects of the anticancer treatments used to treat colorectal cancers on fertility should be considered.
5-FU is a cell-cycle specific fluorinated uracil analogue that predominantly interferes with DNA and RNA synthesis during the S phase of the cell cycle through a cascade of metabolic processes [9]. The 5-FU metabolite, fluorodeoxyuridine monophosphate (FdUMP), inhibits thymidylate synthase activity which endogenously catalyses local deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), providing a source of thymidine necessary for DNA replication. When FdUMP binds to thymidylate synthase, it blocks dUMP from accessing the enzyme, disrupting DNA replication as a consequence [9]. This interaction can destabilise the structure of the DNA, inhibit the efficiency of base excision repair mechanisms and eventually lead to double-strand DNA breaks in affected cells, which may subsequently undergo apoptosis [9].
The precise impact of 5-FU on the ovary in the clinical setting is unclear because patients frequently receive 5-FU as part of multidrug regimens, such as 5-FU/leucovorin/oxaliplatin (FOLFOX) and cyclophosphamide/methotrexate/5-FU (CMF) [10–12]. The effects of 5-FU on reproductive outcomes are believed to be negligible when used in combination with alkylators or platinum derivatives [1, 3, 11, 13–16]. However, because alkylators can result in an incidence of ovarian failure as high as 70%, it is possible that any adverse effects of 5-FU are obscured. Cercek et al. (2013) performed a retrospective study to evaluate the incidence of FOLFOX-induced amenorrhea in women aged 50 and younger and reported that 41% of patients experienced amenorrhea during treatment and 16% of these women had persistent amenorrhea 1 year after treatment [13]. However, this effect may have been due to the inclusion of oxaliplatin, a platinum derivative, in the treatment regimen, rather than 5-FU [5]. In terms of single-drug studies, a very small study conducted by Koyama and colleagues reported that menses was unaffected in all 10 patients treated with 5-FU alone [15], though lack of amenorrhea does not preclude the possibility of ovarian damage. Experimental evidence that 5-FU treatment might pose a risk to fertility independently of other treatments is limited. Exposure of rats to a single 200-mg/kg dose of 5-FU during estrus (but not other cycle stages) reduced pregnancy rates compared to saline treated controls [17], and mice receiving two 150-mg/kg doses of 5-FU (with a 5-day interval) had reduced pregnancy rates and increased resorption rate compared to controls [18], but ovarian damage was not investigated in these studies.
Because it is not possible to directly assess the impact of treatments on the ovarian follicular number in women in situ, surrogates such as amenorrhea and AMH levels are used to indirectly indicate the presence of absence of ovarian damage [19]. Importantly, these measures are unlikely to detect partial depletion of the primordial follicle pool, which has the potential to reduce the fertile life span. Therefore, this study aimed to characterise the impact of a single dose 5-FU on the ovaries of mice over time. Specifically, changes to the number of healthy and atretic follicles were examined 12 h and 7 days after treatment, and the level and presence of apoptosis in various follicular cell types were evaluated. Based on our understanding of the mode of action of antimetabolite drugs and follicle development, we hypothesised that 5-FU would damage granulosa cells of the growing follicle population and induce atresia, whilst oocytes and non-growing follicles would be unaffected.
Methods
Mice
Healthy female C57BL/6 mice, 6–8 weeks of age, were obtained from Monash University Animal Research Platform. The mice were kept in a high barrier facility that was temperature controlled and on a 12-h light versus 12-h dark cycle. All animals were allowed free access to water and mouse chow. Mice were monitored daily for appearance including coast condition, body condition, body posture and movement. The procedures and experiments undertaken were in line with the NHMRC Australian Code of Practice for the Care and Use of Animals and approved by the Monash Animal Ethics Committee.
Mice were weighed and injected intraperitoneally with a single dose of 150 mg/kg of 5-FU (Sigma-Aldrich F6627) (equivalent to 450 mg/m2) [20] dissolved in saline in a volume of 0.1 mL per 10 g mouse weight. This dose of 5-FU is within the range of that given to humans [21]. This dose is also known to suppress tumour growth in mouse models and has been used as a conditioning strategy for bone marrow engraftment studies [22, 23]. Higher doses may result in lethality [17]. Control mice received saline solution in a volume of 0.1 mL per 10-g mouse weight administered intraperitoneally. Control and 5-FU-treated mice were then randomised for treatment and euthanasia at 12 h and 7 days post-injection via cervical dislocation (n = 4–6 mice for each group).
One ovary from each mouse was fixed in Bouin’s solutions and processed into glycol-methacrylate resin. These ovaries were then serially sectioned at 20 μm, keeping every third section, and stained with period acid Schiff (PAS) and counterstained with haemotoxylin for stereological assessment. The contralateral ovary was fixed in formalin, embedded in paraffin wax and serially sectioned at 5 μm for immunofluorescence staining and TUNEL.
Quantitation of primordial and primary follicles
Stereology was used to estimate the total number of primordial and primary follicles as described [24–26]. Stereology is the gold-standard method for the unbiased and accurate quantification of cells in tissue sections [25]. Follicles were identified according to Myers et al. [27]. Briefly, primordial follicles were classified as an oocyte incompletely surrounded by squamous granulosa cells. Primary follicle oocytes were larger and completely surrounded by a single layer of cuboidal granulosa cells and a single layer of theca cells. Follicles were counted using an Olympus microscope (Olympus BX50) with a motorised stage, employing a ×100 oil immersion magnification (Immersion Oil Type-F, Olympus, IMMOIL-F30CC), equipped with the Stereo Investigator stereological system and Stereo Investigator 11 software. In short, a sampling grid, consisting of counting frames containing inclusion and exclusion boundaries, was superimposed over each section. Oocytes with visible nuclei lying on the inclusion boundary or located within the counting frame were counted. The total number of follicles within an ovary was then estimated using the following equation: N = Q∙(1/f1)∙(1/f2)∙(1/f3). N is the total primordial and primary follicle number in an ovary and Q is the sum of the raw follicle count for each section of a single ovary. F1 is the section number, in this case every third section used from a random start; thus, f1 = 3/1. F2 considers the area of the counting frame and the area it covers when shifted from each square (X axis × Y axis) or the “stepper” distance. In this experiment, the counting frame area was 2256 μm2 and stepper distance was 10,000; thus, f2 = 10,000/2256. F3 is the thickness of the section. To avoid cutting artefacts, 3 μm from the top of the section is omitted and used as a guard area, whilst the next 10 μm of the section is optically sectioned for stereology; thus, f3 = 10/20. Any oocyte nuclei that came into focus between 3 and 13 μm and were along the inclusion boundary or within the counting frame were counted.
Assessment of growing follicles, atretic follicles and corpora lutea
Every ninth 20-μm thick section was used to estimate the total number of growing follicles (secondary and antral stage) in each ovary using a light microscope employing a ×10 magnification as described [24, 26]. Follicles were counted if an oocyte nucleus was clearly visible. Atretic follicles were classified as secondary or antral follicles with 10% or more apoptotic granulosa cells (evident due to their pyknotic nuclei). Estimation of total growing follicle number and total atretic follicle number within each ovary was calculated using the following equation to correct for sections not counted: N = Q∙9, where N is the total follicle number in an ovary, Q is the sum of the raw follicle count for each section of a single ovary and 9 represents every ninth section sampled. Due to the size of corpora lutea, quantification was undertaken by obtaining images of every third section using a light microscope. Images were carefully analysed by eye to avoid double counting, and raw counts were used for analysis.
Determination of ovarian volume
To estimate the total ovarian volume, the Cavalieri estimator was employed using Stereo Investigator 11 software on an Olympus microscope with a ×10 magnification [28, 29]. Every sixth section was analysed. A series of evenly spaced points was placed over each section; any points that lie on the section were counted. An estimation of the final ovarian volume was then undertaken using the following equation: , where the area associated with each sampling point (grid spacing × block) is a(p), d is the distance between each consecutive section and (tΣPi) is the sum of each point lying on each consecutive section (Pi = P1, P2, P3 …) and multiplied by section thickness t.
Immunofluorescence
Briefly, formalin-fixed, paraffin-embedded ovarian sections were dewaxed and antigens were retrieved using a heat treatment in sodium citrate buffer (pH 6). To detect apoptotic cells, ovarian sections were stained using terminal deoxynucleotidyl transferase TdT-mediated TUNEL using the Click-iT® TUNEL Alexa Fluor® 488 Imaging Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. A positive control section treated with DNAse1 was included in every run. Sections were subsequently blocked using 10% donkey serum in PBS with 3% BSA and incubated at room temperature for 30 min. Cleaved caspase-3 antibody (CST #9661) was diluted in 1:100 in 1% BSA in PBS and incubated overnight at 4 °C. The sections were then incubated with Alexa Fluor® 488 Donkey anti-Rabbit IgG (H + L) (Thermo Fisher Scientific, #A21206) for 1 h at room temperature. The sections were coverslipped (Menzel-Glaser, 20 × 50 mm) using ProLong® Gold antifade reagent with DAPI (Thermo Fisher Scientific, #P36971).
Quantification of immunofluorescent follicles
TUNEL and cleaved caspase-3 immunofluorescent labelling was analysed in five sections per ovary for both control and treatment groups at 12 h (n = 3 animals/group). Sections were taken from the largest cross section and the sections on either side, separated by more than 70 μm to avoid analysing the same follicle twice. For each ovary analysed, total follicle number in each class was determined; then, the number of follicles positive for TUNEL or cleaved caspase-3 were counted and the ratio determined. Primordial and primary follicles were counted if they had a clearly visible oocyte nucleus. Secondary and antral follicles were counted if the oocyte was visible. Follicles were defined as TUNEL and cleaved caspase-3 positive if 10% of granulosa cells were positive for both or either antibody.
Statistical analysis
Statistical analysis of both stereological and immunofluorescence data between control and treated groups at each time point were assessed using a Student’s t test analysis. To test statistical significance across time points, a one-way analysis of variance (ANOVA) was used followed by Tukey’s post hoc test. Significance was determined if p < 0.05. All statistical analysis was undertaken using GraphPad Prism version 6, GraphPad Software, La Jolla, CA, USA. Data are expressed as mean ± standard error of the mean (SEM).
Results
5-FU exposure does not deplete the ovarian reserve of primordial follicles
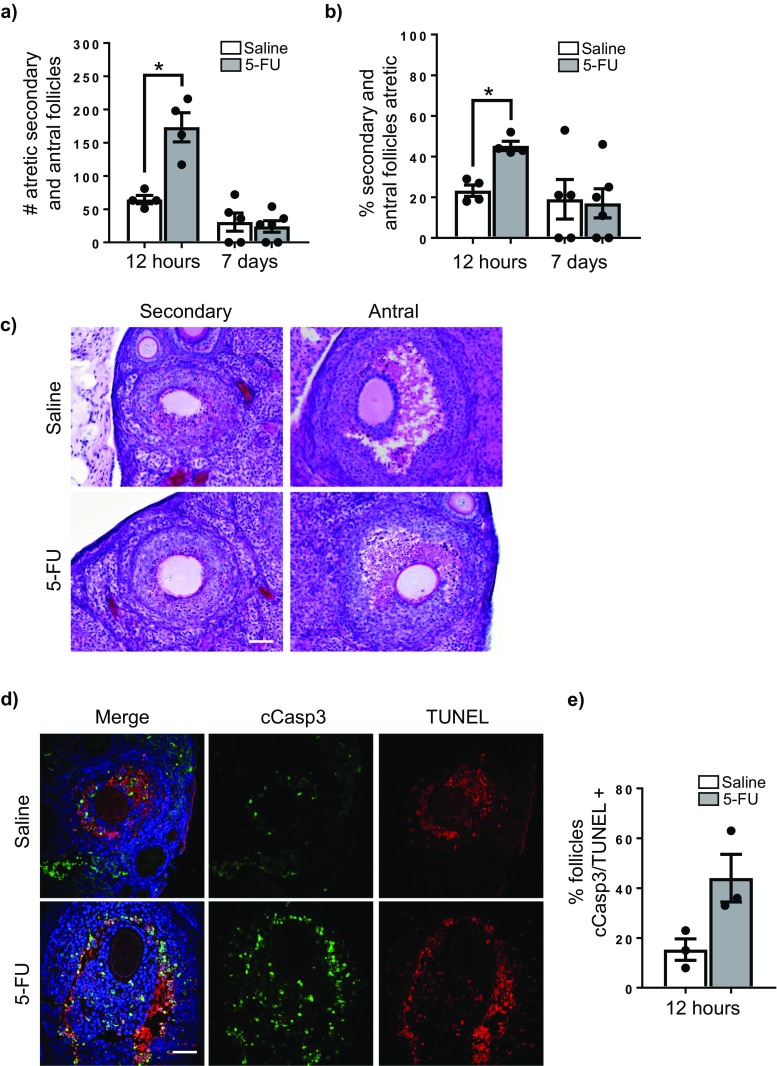
Ovaries were collected from adult female mice 12 h and 7 days after a single dose of saline or 5-FU (150 mg/kg) and follicles were enumerated (Fig. 1a–d). The number of healthy primordial and primary follicles were similar in ovaries from saline and 5-FU treated mice at both times points (Fig. 1a, b). The number of healthy secondary and antral follicles were also similar between saline and 5-FU treatment groups at each time point (Fig. 1c). Additionally, when total healthy follicle numbers were calculated (all follicle classes combined), there was no significant differences between saline and 5-FU-treated animals (Fig. 1d).
Fig. 1.
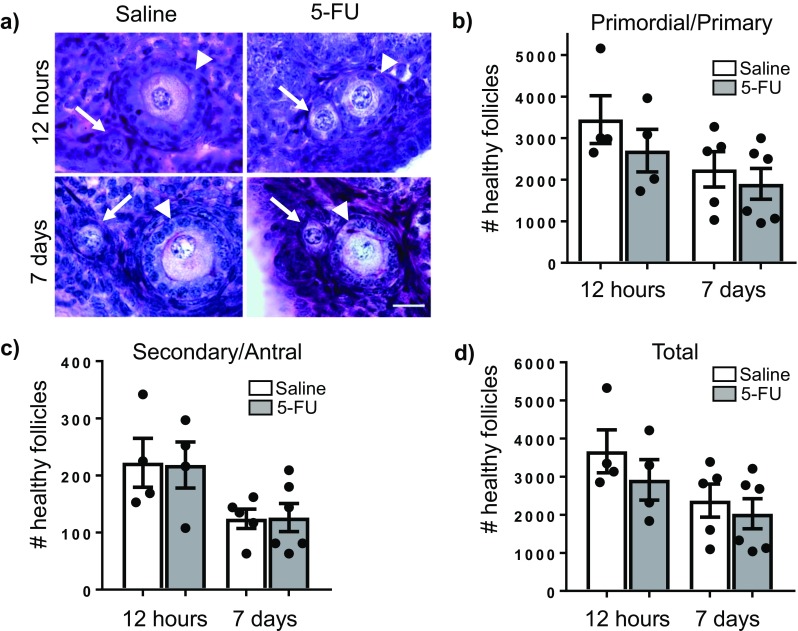
5-FU does not deplete the ovarian reserve. Representative images of primordial and primary follicles in PAS stained ovarian tissue from each treatment group (a). Arrows indicate primordial follicles, and arrowheads indicate primary follicles. Scale = 20 μm, ×100 magnification. The number of primordial and primary follicles (b), secondary and antral follicles (c) and total follicles (d) per ovary was determined 12 h and 7 days after treatment with saline or 5-FU (150 mg/kg). Data represented as mean ± SEM, n = 4 at 12 h, n = 5/6 at 7 days. Follicle numbers for individual animals are indicated by individual data points. No significant differences were found between saline and 5-FU-treated groups at each time point using Student’s t test (p > 0.05). No significant differences were found across time points within a treatment group using one-way ANOVA with Tukey’s post hoc test
5-FU exposure increases secondary and antral follicle atresia
Primordial and primary follicles are comprised of small non-growing or very slowing growing oocytes and granulosa cells with a very low mitotic index. At these early stages of development, follicles rarely show morphological signs of cellular apoptosis or follicular atresia [30]. Consistent with this characteristic, primordial and primary follicles appeared morphologically normal in all ovaries from saline and 5-FU treated at 12 h and 7 days post-treatment, with no differences observed (Fig. 1a).
In contrast, secondary and antral follicles contain rapidly growing oocytes and highly proliferative granulosa cells. Atresia of growing follicles within the ovary is a normal physiological event, and consequently, atretic secondary and antral follicles were detected in all tissues analysed (Fig. 2a–e). However, treatment with 5-FU was associated with a significant increase in the number and proportion of morphologically atretic secondary and antral follicles observed at 12 h compared to time-matched, saline-treated controls (Fig. 2a–c). Follicular atresia was confirmed by TUNEL and cleaved caspase-3 staining at 12 h post-treatment, with a greater proportion of follicles with granulosa cells staining positively for this maker of apoptosis in 5-FU than saline-treated animals (Fig. 2d, e). This effect of 5-FU on follicular atresia was transient, with no treatment-related differences in the number or proportion of atretic secondary and antral follicles observed at 7 days (Fig. 2a, b).
Fig. 2.
5-FU induces atresia of growing follicles. The number of atretic secondary and antral follicles per ovary was determined 12 h and 7 days after treatment with saline or 5-FU (150 mg/kg) (a). Data represented as mean ± SEM, *p < 0.0004, Student’s t test, n = 4 at 12 h, n = 5/6 at 7 days. Follicle numbers for individual animals are indicated by individual data points. The proportion of all secondary and antral follicles that were atretic was also calculated (b). Data represented as mean ± SEM, *p < 0.0009, Student’s t test. Representative images of PAS stained ovarian tissue from each treatment group demonstrating presence of atretic follicles in both saline and 5-FU-treated groups (c). Representative images of TUNEL (red) and immunofluorescent staining for cleaved capase-3 (green) marking apoptotic cells in 5-FU-treated and saline ovarian tissue (d). The mean percentage of secondary and antral follicles with positive staining was calculated at 12 h (e). Data summarise results from five sections/ovary, three ovaries/treatment. Percentages for individual animals are indicated by individual data points. Scale = 100 μm, ×20 magnification
5-FU exposure reduces corpora lutea number
The number of corpora lutea were counted in order to determine if exposure to 5-FU was associated with reduced ovulation. There were no differences in the number of corpora lutea between saline and 5-FU-treated ovaries at 12 h (Fig. 3a, b). However, at 7 days after 5-FU treatment, significantly fewer corpora lutea were observed when compared with time-matched, saline-treated controls, indicating a disruption to ovulation (Fig. 3a, b). Despite a reduction in corpora lutea number, which typically make up a large fraction of actively cycling ovaries, there were no differences in volume between saline-treated control ovaries and 5-FU-treated ovaries at 7 days (Fig. 3c). These data also indicate an absence of shrinkage that is often associated with chemotherapy-induced ovarian damage (Fig. 3c).
Fig. 3.
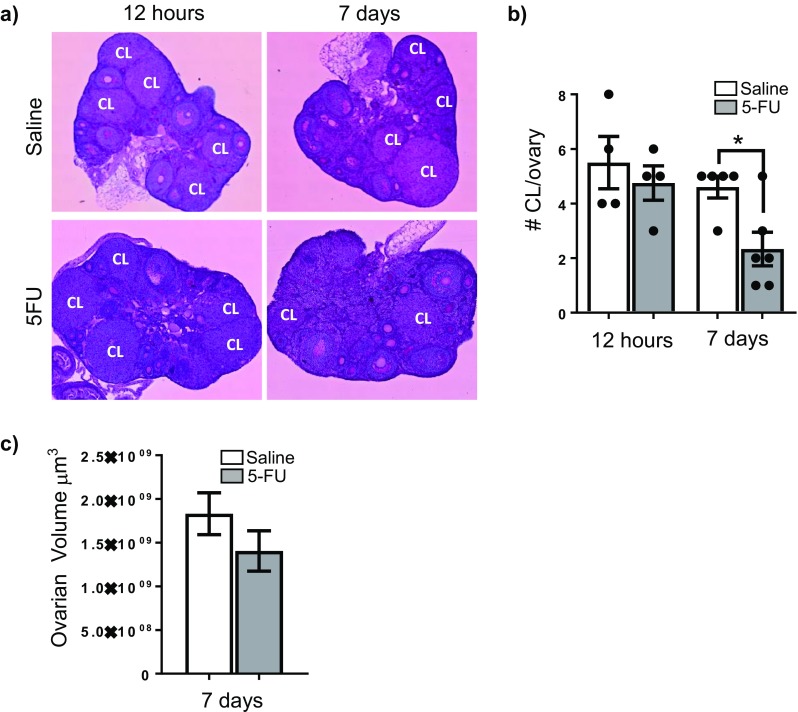
5-FU reduces the number of corpora lutea. Representative images of PAS stained ovarian tissue from each group (a). CL indicates corpora lutea. The number of corpora lutea was calculated 12 h and 7 days after treatment with saline or 5-FU (b). Data represented as mean ± SEM and were analysed at each time point using Student’s t test (*p = 0.0163), n = 4 at 12 h, n = 5/6 at 7 days. Numbers for individual animals are indicated by individual data points. Ovarian volume was also calculated at 7 days, and is expressed as mean ± SEM (c). No significant difference in ovarian volume was observed between saline and 5-FU treatments
Discussion
A full understanding of the off-target effects of chemotherapy on the female reproductive system is important for girls and women of reproductive age being treated for cancer. The aim of this study was to examine the effects of 5-FU on the ovarian follicular reserve in a mouse model. We found that a single 150-mg/kg dose of 5-FU does not induce apoptosis in the oocytes or granulosa cells of primordial and primary follicles and does not result in depletion of the primordial and primary follicle reserve within in 7 days of exposure. These results are consistent with a study in mice showing that the primordial follicle population was not depleted in mice 72 h after treatment with 200 mg/kg of the antimetabolite drug gemcitabine [5]. Because 5-FU has a biological half-life of 10–20 min in mouse plasma, it is likely that if any primordial and primary follicles directly damaged by 5-FU would have died before the final 7-day time point used in our study. However, in order to confirm this assumption and rule out the possibility of delayed damage and follicle loss by 5-FU, an additional later time point (e.g. 14 days) could be evaluated in the future.
Whilst we did not detect depletion in the number of healthy secondary and antral follicles, there was a significant increase in the atretic secondary and antral follicle populations 12 h after 5-FU treatment compared to controls, with atresia levels returning to normal by day 7. This finding was supported by our results showing an increase in the proportion of follicles with TUNEL and cleaved caspase-3-positive granulosa cells at the 12-h time point in treated mice compared to time-matched controls. Furthermore, our data shows fewer corpora lutea in the ovaries of mice treated 7 days earlier with 5-FU treatment. This decline in corpora lutea may be the result of the increased atresia of secondary and antral follicles caused by 5-FU at the earlier time point. Collectively, our study suggests that the secondary and antral follicle populations are likely more susceptible to 5-FU apoptosis than the primordial and primary follicle populations, likely due to the increase in granulosa cell mitotic activity. Importantly, any effect of 5-FU on secondary and antral follicles is transient, likely due to the acute and relatively short-lived action of the drug.
It was surprising that the increased number of atretic secondary and follicles 12 h after 5-FU treatment was not accompanied by a concomitant depletion in the number of healthy secondary and antral follicles. The reason for this is not clear, but it could reflect complex changes in follicle dynamics (e.g. growth rates, survival/death rates and clearance times). For example, the atresia of secondary follicles caused by 5-FU treatment could have triggered rapid development of primary follicles into secondary follicles to replace those lost. Indeed, there was a slight (but non-significant) reduction in the mean primordial and primary follicle counts in ovaries in 5-FU-treated animals compared controls that may reflect this occurrence. Another possibility is that inter-animal variability in the number of healthy secondary and antral follicles, combined with the inability to track follicle loss in situ in individual animals over time, may have obscured any depletion of healthy secondary and antral follicle numbers in treated animals. Regardless, these questions do not detract from our main finding that 5-FU does not deplete the ovarian reserve of primordial follicles and is therefore unlikely to have a long-term effect on fertility.
Whist in humans treatment protocols including 5-FU vary considerably, a typical initial intravenous bolus dose of 5-FU is between 400 and 600 mg/m2 [21]. 5-FU is then administered repeatedly over several months. Repetitive doses of chemotherapy could cause cumulative insults to the ovaries that would not be seen in our study, and those of others, using a lower dose of 5-FU [17]. Therefore, future studies examining the effects of repeated 5-FU doses would provide additional important insight in the ability of 5-FU to damage the ovary. In addition, 5-FU is usually given in combination with other drugs, and therefore, future studies identifying the combined effects of a chemotherapy would be useful in elucidating the effects typical regimens have on the ovary.
In summary, the results obtained in this study provide novel insight into the effects of 5-FU on the ovaries in a mouse model. The primordial and primary follicle populations were not significantly affected by 5-FU. The secondary and antral follicle populations show increased atresia, likely mediated by granulosa cell apoptosis, and reduced corpora lutea number. This study suggests that 5-FU is mildly ovotoxic, but is unlikely to have significant long-term impacts on fertility because the ovarian reserve of primordial follicles remained unaffected.
Acknowledgements
We thank the Monash Micro Imaging and the Monash Histology Platform.
Authors’ contributions
KJH and HA designed the experiments; ML, SHL, KH and JMS performed the experiments; ML and KJH interpreted the data; ML and KJH wrote the manuscript; HA, SHL, KH and JMS critically revised the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the National Health and Medical Research Council (KJH #1050130). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. The funding bodies played no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Compliance with ethical standards
Conflicts of interests
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169(1):123–131. doi: 10.1016/S0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 2.Meirow D, et al. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 3.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill MT, Ni Dhonnchu T, Brannigan AE. Topic update: effects of colorectal cancer treatments on female fertility and potential methods for fertility preservation. Dis Colon Rectum. 2011;54(3):363–369. doi: 10.1007/DCR.0b013e31820240b3. [DOI] [PubMed] [Google Scholar]
- 5.Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015;30(12):2926–35. [DOI] [PubMed]
- 6.Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(18):4354–4359. doi: 10.1002/cncr.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomark Prev. 2009;18(6):1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 8.DeSantis CE, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 9.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 10.Brincker H, Mouridsen H, Andersen K. Adjuvant chemotherapy with cyclophosphamide or CMF in premenopausal women with stage II breast cancer. Breast Cancer Res Treat. 1983;3(1):91–95. doi: 10.1007/BF01806239. [DOI] [PubMed] [Google Scholar]
- 11.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 12.Hosein PJ, Rocha-Lima CM. Role of combined-modality therapy in the management of locally advanced rectal cancer. Clin Colorectal Cancer. 2008;7(6):369–375. doi: 10.3816/CCC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 13.Cercek A, Siegel CL, Capanu M, Reidy-Lagunes D, Saltz LB. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer. 2013;12(3):163–167. doi: 10.1016/j.clcc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Dynes, J., The effects of chemotherapy schedules on ovarian function. 2014.
- 15.Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y, Terasawa T, et al. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–9. [DOI] [PubMed]
- 16.Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control. 2002;9(6):466–472. doi: 10.1177/107327480200900603. [DOI] [PubMed] [Google Scholar]
- 17.Hrushesky WJ, Vyzula R, Wood PA. Fertility maintenance and 5-fluorouracil timing within the mammalian fertility cycle. Reprod Toxicol. 1999;13(5):413–420. doi: 10.1016/S0890-6238(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 18.Tal, R., et al., A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology, 2016: p. en20161418. [DOI] [PMC free article] [PubMed]
- 19.Dunlop CE, Anderson RA. Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–250. doi: 10.1016/j.maturitas.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101(22):1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 22.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187(2):217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollander GA, et al. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373(6512):350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 24.Liew SH, Vaithiyanathan K, Cook M, Bouillet P, Scott CL, Kerr JB, et al. Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol Reprod. 2014;90(4):77. [DOI] [PubMed]
- 25.Geuna S, Herrera-Rincon C. Update on stereology for light microscopy. Cell Tissue Res. 2015;360(1):5–12. doi: 10.1007/s00441-015-2143-6. [DOI] [PubMed] [Google Scholar]
- 26.Liew SH, Nguyen QN, Strasser A, Findlay JK, Hutt KJ. The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF. Cell Death Dis. 2017;8(8):e2971. doi: 10.1038/cddis.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 28.Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 29.Howard, V. and M. Reed, Unbiased stereology: three-dimensional measurement in microscopy. 2004: Garland Science.
- 30.Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol Reprod. 2009;81(1):16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]