Abstract
Purpose
The aim of this study was to compare the effect of the deselection of spermatozoa presenting vacuole-like structures using IMSI (intracytoplasmic morphologically selected sperm injection) with ICSI (intracytoplasmic sperm injection) by means of neonatal outcomes.
Methods
In a retrospective two-center analysis, a total of 848 successful IMSI or ICSI cycles ending with a live birth, induced abortion, or intrauterine fetal death (IUFD) were included.
Results
The IMSI and ICSI groups included 332 and 655 babies or fetuses, respectively. The parents were older in the IMSI group than in the ICSI group (mothers were 35.1 vs 32.9 years, and fathers were 39.1 vs 36.2 years). The multiple pregnancy rate was higher in the IMSI group. The mean pregnancy duration and mean birth weight were almost identical in both groups. There was no significant difference in major congenital malformations between the two groups. However, this rate was decreased in the IMSI group compared to that in the ICSI group (1.8 vs 3.2%), the difference being mainly found in singletons (1.4 vs 3.3%). Boys were more often affected than girls in both groups. The percentages of chromosomal abnormalities did not differ between the IMSI and ICSI groups (0.6 and 0.8%). The reported congenital malformations mainly affected the heart, urogenital, and musculoskeletal systems.
Conclusions
In the present study, the malformation rates observed in the IMSI and ICSI groups were not significantly different, even if slightly lower after IMSI. However, the observed difference followed the same trends observed in previous reports, indicating the possible impact of IMSI on decreasing congenital malformation occurrences. This highlights the necessity to prospectively evaluate the impact of IMSI on neonatal outcome after IVF treatment.
Keywords: Vacuole-like structures, Intracytoplasmic morphologically selected sperm injection, Major congenital malformations, Neonatal outcomes, Spermatozoa selection
Introduction
Since the first description by Antony van Leeuwenhoek in 1677, numerous authors have reported on the spermatozoon morphology, and several morphological classifications, such as those by David [1] and Kruger [2], were designed. The World Health Organization (WHO) edited its own classification, the 2010 version being currently recommended [3]. These evaluations are usually performed on fixed and stained smears of semen, which preclude the selection and use of spermatozoa for assisted reproductive treatment (ART). In 2001, Bartoov and colleagues [4] described a real-time observation method at high magnification, without any fixation or staining. This technique used Nomarski differential interference contrast (DIC) microscopic optics and was called MSOME (motile sperm organellar morphology examination). In addition to the classical morphological criteria, such as the head shape, normalcy of the intermediary piece, and flagellum insertion, Bartoov described the so-called sperm head vacuoles. These defects are characterized by crater-like depressions at the surface of the sperm head that can vary in number, size, and position. Numerous studies have attempted to understand the origins and composition of these craters or vacuole-like structures (VLS). VLS are most likely formed during spermiogenesis, as they are already observed during the spermatid elongation step [5]. VLS are a sign of impairment in spermiogenesis and are associated with abnormal chromatin organization and condensation [6–12] and higher hypermethylation [13]. Some studies have shown a link between VLS and DNA fragmentation [6, 9, 10, 14–17], as insufficiently condensed DNA is probably more vulnerable to oxidative stress. VLS were also linked to sperm aneuploidy [18].
All of these publications show that sperm VLS, together with sperm morphology, might be a marker of the genetic and epigenetic status of sperm. This hypothesis raises the issue of the safety of classical ICSI (intracytoplasmic sperm injection) that uses optics and magnifications that are incompatible with the observation of fine structures, such as VLS. The association of MSOME with ICSI, called IMSI (intracytoplasmic morphologically selected sperm injection) can allow the opportunity to select fine morphological sperm quality before oocyte injection. The advantages of deselecting sperm carrying VLS in IMSI treatments remain controversial. Some authors have reported small or no effects of VLS on fertilization and early embryo development up to day 3. However, it is now well accepted that VLS negatively affect embryo development in terms of blastocyst formation [19–23], and substantial evidence suggests an increased miscarriage risk with vacuolated sperm [24–30]. Pregnancy rates are reportedly higher after IMSI than after ICSI in select groups of patients, such as those facing previous ICSI failures [30, 31], those with advanced maternal age [32], and those with isolated teratozoospermia [33]. Two recent reports [34, 35] have shown a higher rate of congenital malformations after ICSI than after IMSI. On the other hand, several studies have shown that ART babies tend to present more congenital malformations than naturally conceived babies [36–39]. Together, these data have reinforced our interest in the deselection of sperm cells carrying VLS before oocyte injection. The aim of the present retrospective analysis was to evaluate the benefits of the IMSI technique on neonatal outcomes in IVF.
Materials and methods
Study populations
Patients from two centers were enrolled in this retrospective study on ICSI and IMSI treatments: the CPMA (Centre de Procréation Médicalement Assistée) of the University of Liège (Belgium; Center 1) and the IVF Center Prof. Zech of Salzburg (Austria, Center 2). Eligible cycles were those ending with an ongoing pregnancy (≥ 23 weeks), started between January 2009 and December 2013 in center 1 and between January 2005 and December 2012 in center 2. All induced abortions were included in the study, even those occurring before the 23rd week of gestation. All cycles using surgically retrieved and/or cryopreserved sperm and those using frozen embryo transfers were excluded.
ICSI was mainly proposed to patients with male or idiopathic infertility. IMSI was recommended to patients facing mild to severe oligo-, astheno-, and/or teratozoospermia; one or more previous implantation failures after ICSI; reduced blastocyst development during previous cycles; repetitive spontaneous abortions; low oocyte counts; or advanced maternal age.
Treatment
According to the patient’s history, a long or short GnRH (gonadotropin-releasing hormone) agonist protocol or an antagonist protocol was applied for ovarian hyperstimulation. Retrieval occurred 36 h after the administration of hCG or a GnRH agonist. In center 1, a one-step medium (LifeGlobal, Ontario, Canada) was used for embryo culture. In center 2, either a one-step (LifeGlobal) or two-step medium (Irvine Scientific, Santa Ana, USA or Vitrolife, Göteborg, Sweden) was used.
The best looking embryo(s) was (were) selected for transfer. In center 1, morphological evaluation was performed at 25 h post-insemination (check for the first cleavage), on day 2 and day 3. Overall embryo quality was determined as the sum of defects, such as the presence of cytoplasmic fragments, blastomere asymmetry, cytoplasm granularity, the presence of multinucleation, and global cellular division kinetics. Embryo transfer was mainly performed on day 3. Day 2 transfer was preferred when all available embryos were transferred. In center 2, embryos were further cultured until day 5. Blastocyst quality was assessed according to the Gardner blastocyst grading scale [40], depending on blastocoel expansion and the qualities of both the inner cell mass and trophectoderm. Embryos were transferred on day 5, except in one case in which the embryo was transferred on day 4. Embryo transfer was performed using a Wallace replacement catheter (Smiths Medical International, Kent, UK) in center 2 or a Cook K-Jets catheter (Cook, Strombeek Bever, Belgium) in center 1.
All semen samples used for ICSI were prepared on a three-layer discontinuous gradient (Isolate, Irvine; All Grad Wash, LifeGlobal) except for some cryptozoospermia samples that were only washed (All Grad Wash). During ICSI, spermatozoa were selected and immobilized at × 200 magnification in a PVP droplet before injection.
The IMSI procedure was almost identical to that described for ICSI (Vanderzwalmen et al. 2008). The main difference concerned sperm selection before oocyte injection, which was performed at room temperature in a glass bottom Petri dish under × 1000 magnification (oil immersion objective) on a Leica AM6000 IMSI station. As for ICSI, spermatozoa were selected and stored in an oocyte medium drop before injection. The selection criteria were the following: 1st choice—normal form spermatozoa without any VLS on the head; 2nd choice—normal form with small VLS; 3rd choice—normal form with large VLS; 4th choice—abnormal form spermatozoa without VLS; and 5th choice—abnormal form with VLS.
Data collection
Data were collected from the databases of the two centers. When the neonatal data were incomplete, the patients received questionnaires by mail regarding the date of birth, gender, weight, height, the presence of malformations, and neonatal hospitalization. In cases that were unclear, incomplete, or missing answers, the patients and/or their physicians were contacted by phone. Malformations were classified according to the ICD-10 classification using Q-codes (Q00-Q99). Minor malformations (Q 10, Q16.2, Q17.0-17.9, Q18.0-18.2, Q18.4-18.9, Q25.0, Q27.0, Q38.1, Q51.5, Q51.6, Q52.0-Q52.7, Q53.0-Q53.9, Q66.3-Q66.6, Q69.0-Q69.9, Q70.0-Q70.9, Q81.0-Q81.9, Q82.1-Q82.9, Q83.0-Q83.9, Q84.0-Q84.9, Q85.0, Q86.0, Q95.0-Q95.2, Q95.4-Q95.5, Q95.9) were not included in the congenital malformation group [41].
Sperm sample characteristics were also retrospectively collected. The concentration and motility data were of the sample used for oocyte injection. A classical strict morphology evaluation was performed during a diagnostic sperm analysis (not on the sample used the day of oocyte injection).
Statistical analysis
The data for the parent’s age, number of oocytes, fertilized and cleaved embryos, blastocysts, transferred embryos, pregnancy duration, and neonatal weight did not follow a symmetric (Gaussian) distribution. The Mann-Whitney test was used for comparisons of means between the IMSI and ICSI groups. The comparisons of percentages between the two groups were performed with Fisher’s exact test.
Results
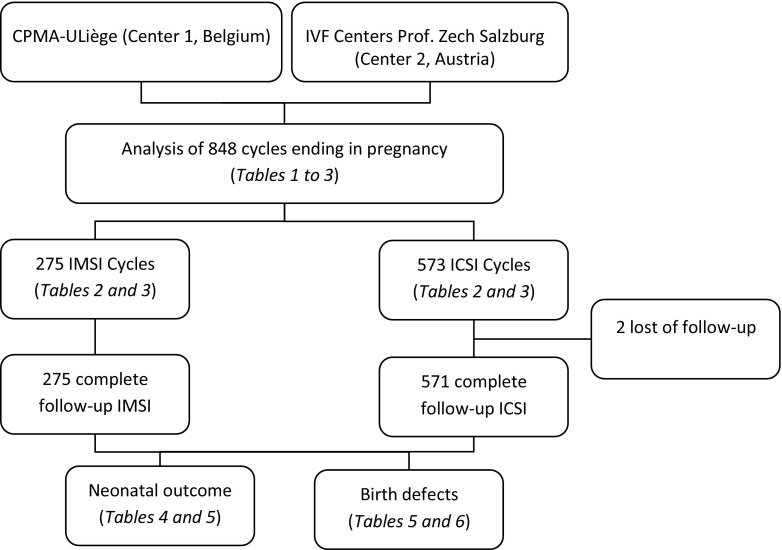
A total of 848 cycles ending in pregnancy beyond 23 weeks or in induced abortion for fetal abnormality were included in the present study: 275 in the IMSI group and 573 in the ICSI group. A flowchart of the study design is shown in Fig. 1. Most of the data were obtained with a direct phone call to patient. Only two patients were impossible to contact in this study and were classified as lost to follow-up (ICSI group). The mean ages and min/max of the mothers and fathers are detailed in Table 1. Both ages were significantly higher in the IMSI group than in the ICSI group (P < 0.0001).
Fig. 1.
Flowchart of the study design
Table 1.
Maternal and paternal ages
| IMSI | ICSI | P | ||
|---|---|---|---|---|
| Number of cycles | 275 | 573 | ||
| Mothers | ||||
| Mean age (years) | 35.1 ± 3.9 | 32.9 ± 4.8 | < 0.0001 | |
| Min | 23 | 20 | ||
| Max | 44 | 45 | ||
| Fathers | ||||
| Mean age (years) | 39.1 ± 5.8 | 36.2 ± 7.0 | < 0.0001 | |
| Min | 26 | 21 | ||
| Max | 70 | 66 | ||
Mann-Whitney test for comparisons of means
Data concerning the treatments are described in Table 2. The IMSI patients were treated more readily by agonist-long protocols than the ICSI patients (P < 0.0001). The mean number of retrieved oocytes was higher in the ICSI group than in the IMSI group (P = 0.0053), but the numbers of fertilized oocytes, cleaved embryos, and blastocysts were similar between the two groups. The mean number of transferred embryos was significantly higher in the IMSI group than in the ICSI group (P < 0.0001), and more blastocyst transfers were performed in the IMSI group than in the ICSI group (P < 0.0001).
Table 2.
Treatment cycle data
| IMSI | ICSI | P | ||
|---|---|---|---|---|
| Number of cycles | 275 | 573 | ||
| Hypothalamic inhibition | ||||
| Agonist-short protocol | 26 (9.5) | 150 (26.2) | < 0.0001 | |
| Agonist-long protocol | 225 (81.8) | 308 (53.8) | < 0.0001 | |
| Antagonist | 24 (8.7) | 113 (19.7) | < 0.0001 | |
| None | 0 (0.0) | 2 (0.3) | 1 | |
| Mean number of retrieved oocytes | 10.5 ± 5.4 | 11.6 ± 6.0 | 0.0053 | |
| Mean number of fertilized (2pn) oocytes | 8.6 ± 4.7 | 8.4 ± 4.7 | 0.6928 | |
| Mean number of cleaved embryos | 7.4 ± 4.0 | 8.0 ± 4.5 | 0.0502 | |
| Mean number of blastocysts in J5/6 cycles | 4.4 ± 2.7 | 4.6 ± 2.5 | 0.2108 | |
| Number of embryo transfers | ||||
| Day 2 | 2 (0.7) | 27 (4.7) | 0.0019 | |
| Day 3 | 70 (25.5) | 318 (55.5) | < 0.0001 | |
| Day 4 | 0 | 1 (0.2) | 1 | |
| Day 5 | 203 (73.8) | 227 (39.6) | < 0.0001 | |
| Total number of transferred embryos | 511 | 923 | ||
| Mean number of transferred embryos | 1.86 ± 0.44 | 1.61 ± 0.54 | < 0.0001 | |
Values are n (%) except for means (indicated before figures). Mann-Whitney test for comparisons of means and Fisher’s exact test for percentages
The characteristics of the sperm samples used on the days of oocyte injection are described in Table 3. The mean volumes were not different between the two groups. The concentration comparisons using the Mann-Whitney test revealed a statistically significant difference between the two groups. The means were nearly identical (33.8 and 33.5 in the IMSI and ICSI groups, P = 0.0464), but the median was higher in the IMSI cycles than in the ICSI cycles (25 and 18, respectively), showing that the ICSI group was enriched with sperm samples of very low concentrations. Except for non-progressive motility, which was higher in the IMSI group (13.3 vs 11.0 in IMSI and ICSI groups; P < 0.0001), the motilities (progressive and total) were not different between the groups, while strict normal morphology was higher in the ICSI group (3.0 vs 7.4 in IMSI and ICSI groups; P < 0.0001).
Table 3.
Sperm characteristics: volume, concentration and motility of sperm samples used on the days of injection (mean ± SD), and percentage of normal morphology (on a previous diagnostic analysis)
| IMSI | ICSI | P | |
|---|---|---|---|
| Volume (ml) | 2.9 ± 1.5 | 2.9 ± 1.7 | 0.7348 |
| Concentration (millions/ml) | 33.8 ± 36.4 | 33.5 ± 41.1 | 0.0464 |
| % of progressive motility | 38.7 ± 18.7 | 38.1 ± 21.4 | 0.8791 |
| % of non-progressive motility | 13.3 ± 8.7 | 11.0 ± 10.0 | < 0.0001 |
| % total motility | 52.0 ± 18.1 | 49.1 ± 22.0 | 0.1927 |
| % of normal strict morphology | 3.0 ± 5.6 | 7.4 ± 8.1 | < 0.0001 |
Mann-Whitney test for comparisons of means
Table 4 presents the data on the pregnancies and babies. More multiple pregnancies (mainly twins) occurred in the IMSI group than in the ICSI group (34 vs 25% of multiple pregnancies in the IMSI group vs the ICSI group, P = 0.0034). The mean birth weights for either singletons or twins were not different between the IMSI and ICSI groups, nor were the pregnancy durations. The sex ratio was not different between the two groups.
Table 4.
Neonatal data: multiples, sex, pregnancy duration, and mean birth weight
| IMSI | ICSI | P | ||
|---|---|---|---|---|
| Number of pregnancies | 275 | 573 | ||
| Lost to follow-up | 0 | 2 | ||
| Number of fetus and babies | Total | 332 | 655 | |
| Live born | 326 (98.2) | 640 (97.7) | 0.816 | |
| IUFD | 4 (1.2) | 8 (1.2) | 1 | |
| Induced abortion | 2 (0.6) | 7 (1.1) | 0.7257 | |
| Muliplicity | Singletons | 219 (66.0) | 491 (75.0) | 0.0034 |
| Twins | 110 (33.1) | 152 (23.2) | 0.001 | |
| Triplets | 3 (0.9) | 12 (1.8) | 0.409 | |
| Sex | Female | 146 (44.0) | 319 (48.7) | 0.1771 |
| Male | 182 (54.8) | 328 (50.1) | 0.1776 | |
| Unknown | 4 (1.2) | 8 (1.2) | 1 | |
| Mean pregnancy duration | Singletons (weeks) | 38.93 ± 2.04 | 38.61 ± 2.37 | 0.1725 |
| Twins (weeks) | 35.46 ± 3.32 | 36.03 ± 2.80 | 0.3056 | |
| Mean weight | Singletons (grams) | 3175 ± 546 | 3119 ± 613 | 0.4131 |
| Twins (grams) | 2336 ± 543 | 2335 ± 639 | 0.8501 | |
Values are n (%) except for means (indicated before figures). Mann-Whitney test for comparisons of means and Fisher’s exact test for percentages
IUFD intrauterine fetal death
Table 5 shows a higher malformation rate in the ICSI group than in the IMSI group (3.2 vs 1.8, respectively, P = 0.2235), especially in singleton babies (3.3 vs 1.4, respectively, P = 0.2086), but these differences were not significant. Congenital malformations mainly affected boys, and we did not find any difference in the sex ratio between the two groups. The live birth proportions among the malformed babies, as well as the proportions of fetuses or babies presenting chromosomal abnormality, were nearly identical in the two groups.
Table 5.
Neonatal data: chromosomal and major malformations
| IMSI | ICSI | P | ||
|---|---|---|---|---|
| Total number of babies (included IA and IUFD) | 332 | 655 | ||
| Normal | 324 (97.6) | 629 (96.0) | 0.2677 | |
| With a major malformation | 6 (1.8) | 21 (3.2) | 0.2235 | |
| With a chromosomal anomaly | 2 (0.6) | 5 (0.8) | 1 | |
| Major malformations repartition regarding multiplicity | ||||
| Singleton n/number of babies | 3/219 (1.4) | 16/491 (3.3) | 0.2086 | |
| Multiple n/number of babies | 3/113 (2.7) | 5/164 (3.0) | 1 | |
| Major malformations repartition regarding sex | ||||
| Male | 3 (50.0) | 12 (57.1) | 1 | |
| Female | 2 (33.3) | 6 (28.6) | 1 | |
| Unknown | 1 (16.7) | 3 (14.3) | 1 | |
| Outcome of malformed babies | ||||
| Alive delivery | 5 (83.3) | 17 (81.0) | 1 | |
| Induced abortion | 1 (16.7) | 4 (19.0) | 1 | |
Values are n (%) except for means (indicated before figures). Fisher’s exact test for comparisons of percentages
IA induced abortion, IUFD intrauterine fetal death
Details regarding the congenital malformations observed are compiled in Table 6. Twenty-seven malformations (22 major congenital malformations and 5 chromosomal abnormalities) were described in 26 of the 655 babies in the ICSI group (4.0%) vs 8 (6 congenital and 2 chromosomal) for 332 babies in the IMSI group (2.4%).
Table 6.
Details on babies presenting a major malformation
| ICD-10 Q code class | IMSI | ICSI | |
|---|---|---|---|
| 00–07 | CM of the nervous system | ||
| Spina bifida, hydrocephalus | |||
| Anencephaly | |||
| 10–18 | CM of the eye, ear, face and neck | ||
| Pinna dysplasia and meatus acusticus atresia right | |||
| 20–28 | CM of the circulatory system | ||
| Aorta coarctation and truncus arteriosus | |||
| Left heart hypoplasia | |||
| Tetralogy of Fallot (2)a | |||
| 30–34 | CM of the respiratory system | ||
| Imperforate nostrils | |||
| 38–45 | Other CM of the digestive system | ||
| Esophageal atresia | Anal atresia | ||
| Anal stenosis | |||
| 50–56 | CM of the genital system | ||
| Hypospadias | |||
| 60–64 | CM of the urinary system | ||
| One kidneya | |||
| Ureter constriction and renal congestion | |||
| Bilateral Hydronephroses | |||
| Double renal pelvis | |||
| Vesicoureteral reflux Grade IV | |||
| 65–79 | CM/D of the musculoskeletal system | ||
| Clubfoot—both feet | Franceschetti syndrome | ||
| Prune-belly syndrome | Club feet | ||
| Omphalocele | Eye malformation, Treacher-Collins syndrome suspected | ||
| Caudal regression syndrome | |||
| 80–89 | Other CM | ||
| Prader-Willi syndrome | Silver-Russell syndrome | ||
| William-Beuren syndrome | |||
| Malformation syndrome | |||
| 90–99 | Chromosomal abnormalities | ||
| Trisomy 21 | Trisomy 21 (3) | ||
| Trisomy 13 | Trisomy 13, complex malformation syndrome | ||
| Trisomy 18 | |||
(#) no. of babies presenting this malformation
CM congenital malformation, CM/D congenital malformation and deformations
aThese two malformations concerned the same baby
Discussion
In this study, we retrospectively collected clinical outcome data after ICSI or IMSI in 2 IVF centers. In a cohort of 987 babies, the neonatal data were quite similar between the two groups. With the exception of a higher multiplicity rate in the IMSI cycles, no differences were statistically significant. We did not find a significant difference between the congenital malformation rates after ICSI compared to after IMSI. However, the congenital malformation rate seemed to be higher after ICSI than after IMSI, and this trend confirmed earlier results obtained by Cassuto [34] and Hersko-Klement [35]. Most of the reports comparing ICSI and IMSI did not include the health statuses of babies after birth. Recently, two teams [34, 35] described lower congenital malformation rates with IMSI. In the present retrospective study, we also observed a trend toward a lower malformation rate in IMSI vs ICSI, but the comparison failed to reach statistical significance. A power analysis (Fisher’s exact test) of our data revealed that the calculated sample sizes required for statistically significant differences when comparing the major congenital malformation rates in ICSI and IMSI were 2235 babies for all babies and 1154 babies for singletons alone. Pooling the data from the three studies increased the number of babies up to 4000, and the malformation rate was approximately two times lower among babies born from IMSI cycles than those born from ICSI cycles. In the study by Cassuto and colleagues, congenital malformation rates were 3.80% in ICSI and 1.33% in IMSI, and in the study by Hersko-Klement, congenital malformation rates were 3.9 and 2.3% for ICSI and IMSI, respectively. Our results show a reduced difference between ICSI and IMSI, with 3.2 and 1.8% of congenital malformations, respectively. It is worth noting that some minor malformations were not included in our study, as described in the “Materials and methods” section. Moreover, both maternal and paternal ages were higher in our IMSI group than in our ICSI group, in contrast to the previous studies mentioned above, in which the parents’ ages did not differ between the two groups. These unfavorable age differences in the IMSI group may compensate, at least partially, for the impact of IMSI in our laboratories, even if the impacts of maternal age on congenital abnormalities other than chromosomal abnormalities are discussed [42–44]. Regarding semen data, we observed an enrichment in the ICSI group with samples of very low concentrations. This can be explained by the fact that IMSI sperm selection is very difficult and can even be impossible in patients presenting cryptozoospermia. In these cases, ICSI is sometimes the only option. In contrast to the concentration, the percentage of sperm with normal morphology is higher in the ICSI group than in the IMSI group. This observation seems logical as teratozoospermia is a proposed IMSI indication. Concerning the MSOME quality of the sperm injected in the IMSI group, the selection of 1st and 2nd choice sperm (no large vacuoles) was possible in a large majority of cycles (data not shown). In only 14 cycles, 3rd choice sperm had been used as not enough 1st and 2nd choice were present. All of these 14 pregnancies ended in the births of normal babies.
There was no difference in the sex ratio in terms of the malformed babies between the 2 groups. The pregnancy multiplicity seemed to impact our malformation rate, in accordance with the observations of Hersko-Klement [35]: 1.4 and 3.3% in the IMSI and ICSI singletons, respectively, versus 2.7 and 3.0% in the IMSI and ICSI multiple pregnancies, respectively (not significant). The higher incidence of malformations in male babies confirms the findings of previous studies [45]. The types of malformation varied widely, but chromosomal abnormalities were nearly identical between the two groups. We observed six congenital malformations of the urogenital system and four of the circulatory system in the ICSI group; however, none of these types of malformation was reported in the IMSI group. These high incidences of urogenital and cardiac malformations are in accordance with the findings of previous studies [34, 35, 46, 47], as are their lower incidences after IMSI [34, 35].
Moreover, ART is suspected to increase the risk of congenital malformation by overcoming natural gamete selection. ICSI alone does not seem to increase the malformation risk [48], but a question regarding the possible benefit of better sperm selection to lower the male infertility impact arises. The main role of the spermatozoon is to transmit intact paternal DNA that will guarantee a suitable gene expression pattern, to ensure that normal fertilization ends with a healthy baby. As a consequence, one essential idea to consider is whether the presence of VLS has an impact on the quality of the DNA and the subsequent rate of malformations in babies. With aniline blue staining, Boitrelle demonstrated that isolated sperm with large nuclear VLS presented lower levels of chromatin condensation [7], and these data were confirmed by three other studies for large and small nuclear VLS [8, 10, 11] using the same technique. These precise studies, performed at the one-cell level, reinforce the conclusions of previous publications that highlight a correlation between vacuolization and insufficient chromatin condensation [6, 9, 12].
In healthy spermatozoa chromatin, approximately 85% of the histones are replaced by protamines, resulting in hard DNA condensation that facilitates sperm mobility and DNA protection against oxidative stress [49]. The remaining 15% of DNA is organized by histones or is linked to the nuclear matrix [50]. During the fertilization process, protamines are replaced by oocyte histones. The ratio of histone to protamine is important because these histone-bound regions are maintained in specific areas of the genome at the levels of the gene promotors implicated in early embryonic development [50–53]. Therefore, it appears very likely that chromatin condensation plays an epigenetic role in embryo development. Jenkins and Carrell [54] reported that the usual replacement of protamines by oocyte histones prior to pronucleus formation may be deregulated when the protamine/histone ratio in the spermatozoon does not comply to the normal 85/15. Gene expression in the early embryo genome might also be impaired. Moreover, differences in methylation levels have been observed between vacuolated and non-vacuolated spermatozoa [13], adding further support to the hypothesis of a link between vacuoles, chromatin disorganization, and epigenetic abnormalities. This link has not yet been fully evaluated, but it might be hypothesized that abnormal condensation provokes abnormal epigenetic pattern. An abnormal epigenetic background may be a feature of infertility origins and of condensation problems. For example, hypermethylation is associated with poor quality semen, probably due to abnormal epigenetic reprogramming in the presence of deficient demethylation during fetal life [55, 56]. Such epigenetic abnormalities are supposedly linked to some congenital abnormalities or childhood defects, such as autism [57, 58]. Taken together, these data highlight the need for a better understanding of chromatin packaging and control during spermiogenesis. All of these data reinforce our interest in vacuole deselection to improve ART outcomes, especially infant health.
In conclusion, the present study did not demonstrate a statistically significant effect of IMSI on congenital malformation occurrence. However, we observed a lower congenital malformation rate in IMSI singleton babies than in ICSI singleton babies, despite the higher parental age of the IMSI group. Although not significant, these data reinforce the idea presented by Cassuto and Hersko-Klement that the deselection of spermatozoa carrying VLS is a possible way to improve the neonatal outcome of babies. The present retrospective study confirms the results of these two other teams and increases the total cohort up to 4000 babies. The limits of this study are its retrospective and non-randomized characteristics. The parental ages are higher in the IMSI group, and the stimulation protocols used and the durations of embryo culture are quite different between the groups. Moreover, some differences must be noted concerning semen quality. Despite very close means, the sperm concentration comparison revealed an enrichment of very low concentrations in the ICSI group, and a lower % of normal morphology in the IMSI group. None the three studies that analyzed comparative neonatal data from IMSI and ICSI cycles were prospectively randomized, so inclusion bias related to infertility cause is suspected as IMSI is more readily proposed to couples facing male infertility, advanced maternal age or recurrent ICSI failures. Further prospective randomized studies with careful pediatric follow-up are needed to sustain the hypothesis regarding the impact of IMSI on neonatal data.
Acknowledgments
The authors thank Dr S Bulk from the Department of Genetics (Centre Hospitalier Universitaire de Liège) in Belgium for her help regarding the analysis of the babies’ neonatal data.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.David G, Bisson J, Czyglik F, Jouannet P, Gernigo N. Anomalies morphologiques du spermatozoïde humain. (1) Propositions pour un système de classification. J Gynecol Obstet Biol Reprod. 1975;4:17–36. [Google Scholar]
- 2.Kruger T, Menkveld R, Stander F, Lombard C, Van der Merwe J, van Zyl J, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–1123. doi: 10.1016/S0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 3.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 4.Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–1068. doi: 10.1056/NEJM200110043451416. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka A, Nagayoshi M, Tanaka I, Kusunoki H. Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil Steril. 2012;98:315–320. doi: 10.1016/j.fertnstert.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Garolla A, Fortini D, Menegazzo M, De Toni L, Nicoletti V, Moretti A, et al. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod BioMed Online. 2008;17:610–616. doi: 10.1016/S1472-6483(10)60307-0. [DOI] [PubMed] [Google Scholar]
- 7.Boitrelle F, Ferfouri F, Petit JM, Segretain D, Tourain C, Bergere M, Bailly M, Vialard F, Albert M, Selva J. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–1658. doi: 10.1093/humrep/der129. [DOI] [PubMed] [Google Scholar]
- 8.Boitrelle F, Albert M, Petit JM, Ferfouri F, Wainer R, Bergere M, Bailly M, Vialard F, Selva J. Small human sperm vacuoles observed under high magnification are pocket-like nuclear concavities linked to chromatin condensation failure. Reprod BioMed Online. 2013;27:201–211. doi: 10.1016/j.rbmo.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Franco JG, Baruffi RLR, Mauri AL, Petersen CG, Oliveira JBA, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod BioMed Online. 2008;17:42–45. doi: 10.1016/S1472-6483(10)60291-X. [DOI] [PubMed] [Google Scholar]
- 10.Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, Mousset-Simeon N, Mace B, Rives N. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58. doi: 10.1093/humrep/deq297. [DOI] [PubMed] [Google Scholar]
- 11.Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, Jellad S, Plouchart JM, Selva J, Yazbeck C. Correlation between DNA defect and sperm-head morphology. Reprod BioMed Online. 2012;24:211–218. doi: 10.1016/j.rbmo.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Franco Jr JG, Mauri AL, Petersen CG, Massaro FC, Silva LFI, Felipe V, et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl. 2011;35:46–51. doi: 10.1111/j.1365-2605.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 13.Cassuto NG, Montjean D, Siffroi J, Bouret D, Marzouk F, Copin H, et al. Different levels of DNA methylation detected in human sperms after morphological selection using high magnification microscopy. Biomed Res Int Hindawi Publishing Corporation. 2016;2016:1–7. doi: 10.1155/2016/6372171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilding M, Coppola G, di Matteo L, Palagiano A, Fusco E, Dale B. Intracytoplasmic injection of morphologically selected spermatozoa (IMSI) improves outcome after assisted reproduction by deselecting physiologically poor quality spermatozoa. J Assist Reprod Genet. 2011;28:253–262. doi: 10.1007/s10815-010-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammoud I, Boitrelle F, Ferfouri F, Vialard F, Bergere M, Wainer B, Bailly M, Albert M, Selva J. Selection of normal spermatozoa with a vacuole-free head (x6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation rates. Andrologia. 2013;45:163–170. doi: 10.1111/j.1439-0272.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 16.Utsuno H, Oka K, Yamamoto A, Shiozawa T. Evaluation of sperm head shape at high magnification revealed correlation of sperm DNA fragmentation with aberrant head ellipticity and angularity. Fertil Steril. 2013;99:1573–1580.e1. doi: 10.1016/j.fertnstert.2013.01.100. [DOI] [PubMed] [Google Scholar]
- 17.Komiya A, Kato T, Kawauchi Y, Watanabe A, Fuse H. Clinical factors associated with sperm DNA fragmentation in male patients with infertility. ScientificWorldJournal. 2014;2014:868303. doi: 10.1155/2014/868303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garolla A, Sartini B, Cosci I, Pizzol D, Ghezzi M, Bertoldo A, Menegazzo M, Speltra E, Ferlin A, Foresta C. Molecular karyotyping of single sperm with nuclear vacuoles identifies more chromosomal abnormalities in patients with testiculopathy than fertile controls: implications for ICSI. Hum Reprod. 2015;30:2493–2500. doi: 10.1093/humrep/dev202. [DOI] [PubMed] [Google Scholar]
- 19.Vanderzwalmen P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, Uher P, Zintz M, Lejeune B, Vanderzwalmen S, Cassuto G, Zech NH. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod BioMed Online. 2008;17:617–627. doi: 10.1016/S1472-6483(10)60308-2. [DOI] [PubMed] [Google Scholar]
- 20.Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, Larue L, Barak Y. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–1625. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 21.Knez K, Zorn B, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reprod Biol Endocrinol. 2011;9:123. doi: 10.1186/1477-7827-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knez K, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. Developmental dynamics of IMSI-derived embryos: a time-lapse prospective study. Reprod BioMed Online. 2013;27:161–171. doi: 10.1016/j.rbmo.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Neyer A, Zintz M, Stecher A, Bach M, Wirleitner B, Zech NH, Vanderzwalmen P. The impact of paternal factors on cleavage stage and blastocyst development analyzed by time-lapse imaging—a retrospective observational study. J Assist Reprod Genet. 2015;32:1607–1614. doi: 10.1007/s10815-015-0558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, Artzi S, Gross M, Barak Y. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–1419. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–1790. doi: 10.1093/humrep/del049. [DOI] [PubMed] [Google Scholar]
- 26.Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, Bartoov B. How to improve IVF-ICSI outcome by sperm selection. Reprod BioMed Online. 2006;12:634–638. doi: 10.1016/S1472-6483(10)61191-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Yoon HJ, Jang JM, Oh HS, Lee YJ, Lee WD, Yoon SH, Lim JH. Comparison between intracytoplasmic sperm injection and intracytoplasmic morphologically selected sperm injection in oligo-asthenoteratozoospermia patients. Clin Exp Reprod Med. 2014;41:9–14. doi: 10.5653/cerm.2014.41.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shalom-Paz E, Anabusi S, Michaeli M, Karchovsky-Shoshan E, Rothfarb N, Shavit T, Ellenbogen A. Can intra cytoplasmatic morphologically selected sperm injection (IMSI) technique improve outcome in patients with repeated IVF-ICSI failure? A comparative study. Gynecol Endocrinol. 2015;31:247–251. doi: 10.3109/09513590.2014.982085. [DOI] [PubMed] [Google Scholar]
- 29.Hazout A, Dumont-Hassan M, Junca A-M, Bacrie PC, Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online. Reproductive Healthcare Ltd, Duck End Farm, Dry Drayton, Cambridge CB23 8DB, UK; 2006;12:19–25. [DOI] [PubMed]
- 30.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, D’Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod BioMed Online. 2008;16:835–841. doi: 10.1016/S1472-6483(10)60150-2. [DOI] [PubMed] [Google Scholar]
- 31.Klement AH, Koren-Morag N, Itsykson P, Berkovitz A. Intracytoplasmic morphologically selected sperm injection versus intracytoplasmic sperm injection: a step toward a clinical algorithm. Fertil Steril. 2013;99:1290–1293. doi: 10.1016/j.fertnstert.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Setti AS, Figueira RCS, Braga DPAF, Aoki T, Iaconelli A, Borges E. Intracytoplasmic morphologically selected sperm injection is beneficial in cases of advanced maternal age: a prospective randomized study. Eur J Obstet Gynecol Reprod Biol. 2013;171:286–290. doi: 10.1016/j.ejogrb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Knez K, Tomazevic T, Zorn B, Vrtacnik-Bokal E, Virant-Klun I. Intracytoplasmic morphologically selected sperm injection improves development and quality of preimplantation embryos in teratozoospermia patients. Reprod BioMed Online. 2012;25:168–179. doi: 10.1016/j.rbmo.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Cassuto NG, Hazout A, Bouret D, Balet R, Larue L, Benifla JL, Viot G. Low birth defects by deselecting abnormal spermatozoa before ICSI. Reprod BioMed Online. 2014;28:47–53. doi: 10.1016/j.rbmo.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Hershko-Klement A, Sukenik-Halevy R, Biron Shental T, Miller N, Berkovitz A. Intracytoplasmic morphologically selected sperm injection and congenital birth defects: a retrospective cohort study. Andrology. 2016;4:887–893. doi: 10.1111/andr.12221. [DOI] [PubMed] [Google Scholar]
- 36.Pinborg A, Henningsen A-KA, Malchau SS, Loft A. Congenital anomalies after assisted reproductive technology. Fertil Steril. 2013;99:327–332. doi: 10.1016/j.fertnstert.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Sö derström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 38.Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333:679. doi: 10.1136/bmj.38919.495718.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tararbit K, Lelong N, Thieulin a-C, Houyel L, Bonnet D, Goffinet F, et al. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Hum Reprod. 2013;28:367–374. doi: 10.1093/humrep/des400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 41.Chaabane S, Sheehy O, Monnier P, Bissonnette F, Trasler JM, Fraser W, et al. Ovarian stimulators, intrauterine insemination, and assisted reproductive technologies use and the risk of major congenital malformations—the AtRISK study. Dev Reprod Toxicol. 2016;107:136–147. doi: 10.1002/bdrb.21178. [DOI] [PubMed] [Google Scholar]
- 42.Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk factors for birth defects. Benjamin Obstet Gynecol Surv. 2017;72:123–135. doi: 10.1097/OGX.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 43.Loane M, Dolk H, Morris JK. Maternal age-specific risk of non-chromosomal anomalies. BJOG An Int J Obstet Gynaecol. 2009;116:1111–1119. doi: 10.1111/j.1471-0528.2009.02227.x. [DOI] [PubMed] [Google Scholar]
- 44.Nybo Andersen A-M, Urhoj SK. Is advanced paternal age a health risk for the offspring? Fertil Steril. 2017;107:312–318. doi: 10.1016/j.fertnstert.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Sokal R, Tata LJ, Fleming KM. Sex prevalence of major congenital anomalies in the United Kingdom: a national population-based study and international comparison meta-analysis. Birth Defects Res A Clin Mol Teratol. 2014;100:79–91. doi: 10.1002/bdra.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, Liu J, Hu Z. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97:1331–1337.e4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 47.Silver R, Rodriguez R, Chang T, Gearhart J. In vitro fertilization is associated with an increased risk of hypospadias. J Urol. 1999;161:1954–1957. doi: 10.1016/S0022-5347(05)68863-5. [DOI] [PubMed] [Google Scholar]
- 48.Fauser BCJM, Devroey P, Diedrich K, Balaban B, Bonduelle M, Delemarre-van de Waal HA, Estella C, Ezcurra D, Geraedts JP, Howles CM, Lerner-Geva L, Serna J, Wells D, Evian Annual Reproduction (EVAR) Workshop Group 2011 Health outcomes of children born after IVF/ICSI: a review of current expert opinion and literature. Reprod BioMed Online. 2014;28:162–182. doi: 10.1016/j.rbmo.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barratt CLR, Aitken RJ, Björndahl L, Carrell DT, De Boer P, Kvist U, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications-a position report. Hum Reprod. 2010;25:824–838. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 51.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287–301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 52.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–1126. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 53.Oliva R, Luís Ballescà J. Altered histone retention and epigenetic modifications in the sperm of infertile men. Asian J Androl. 2012;14:239–240. doi: 10.1038/aja.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins TG, Carrell DT. Dynamic alterations in the paternal epigenetic landscape following fertilization. Front Genet. 2012;3:1–8. doi: 10.3389/fgene.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, Sokol RZ. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One. 2007;2:e1289. doi: 10.1371/journal.pone.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schagdarsurengin U, Paradowska A, Steger K. Analysing the sperm epigenome: roles in early embryogenesis and assisted reproduction. Nat Rev Urol. 2012;9:609–619. doi: 10.1038/nrurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 57.Feinberg JI, Bakulski KM, Jaffe AE, Tryggvadottir R, Brown SC, Goldman LR, Croen LA, Hertz-Picciotto I, Newschaffer CJ, Daniele Fallin M, Feinberg AP. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. Int J Epidemiol. 2015;44:1199–1210. doi: 10.1093/ije/dyv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi H, Hiura H, John RM, Sato A, Otsu E, Kobayashi N, Suzuki R, Suzuki F, Hayashi C, Utsunomiya T, Yaegashi N, Arima T. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet. 2009;17:1582–1591. doi: 10.1038/ejhg.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]