Abstract
Purpose
To determine whether a history of conception by assisted reproductive technology (ART) is associated with occurrence of one or more imprinting disorders of either maternal or paternal origin.
Methods
We implemented a systematic review of scholarly literature followed by comprehensive meta-analysis to quantitatively synthesize data from reports relating to use of ART to occurrence of any imprinting disorder of humans, including Beckwith-Wiedemann (BWS), Angelman (AS), Prader-Willi (PWS), and Silver-Russell (SRS) syndromes, as well as transient neonatal diabetes mellitus (TNDB) and sporadic retinoblasoma (RB).
Results
The systematic review identified 13 reports presenting unique data from 23 studies that related conception following ART to occurrence of imprinting disorders. Multiple studies of four disorder were identified, for which meta-analysis yielded the following summary estimates of associations with a history of ART: AS, summary odds ratio (sOR) = 4.7 (95% confidence interval (CI) 2.6–8.5, 4 studies); BWS, sOR = 5.8 (95% CI 3.1–11.1, 8 studies); PWS, sOR = 2.2 (95% CI 1.6–3.0, 6 studies); SRS, sOR = 11.3 (95% CI 4.5–28.5, 3 studies). Only one study reported on each of TNDB and RB.
Conclusion
Published data reveal positive associations between history of ART conception and each of four imprinting disorders. Reasons for these associations warrant further investigation.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1173-x) contains supplementary material, which is available to authorized users.
Keywords: Assisted reproduction, Imprinting disorder, Beckwith-Wiedemann syndrome, Angelman syndrome, Prader-Willi syndrome, Silver-Russell syndrome
Introduction
In 2002 and 2003 [1, 2], reports of Angelman syndrome (AS) in three children conceived by intracytoplasmic sperm injection (ICSI) intensified concern that assisted reproductive technology (ART) could cause epigenetic disorders. Suggested on a theoretical basis in 1998 [3, 4], this possibility gained credence in 2001 when molecular studies implicated epigenetic mechanisms in large offspring syndrome, an overgrowth phenotype of livestock produced by embryo culture [5]. At the time of these events, functional non-equivalence of genetic material in ova versus sperm had been recognized for only two decades [6], and the underlying mechanism—genetic imprinting—was even more novel. But roles in pre- and post-natal growth were already established for several human genes then known to be imprinted [7], and elevated prevalence of low birthweight following ART had been reported [8, 9]. Maher and colleagues [10] speculated that “although… imprinting disorders may be shown to be only rare complications of ART, epigenetic errors might account for a much wider spectrum of ART-related complications…”. It seemed apparent that understanding whether ART predisposes to imprinting disorders might provide insight into epigenetic processes that might influence intrauterine growth, birth weight, and proposed sequelae including insulin insensitivity and cardiovascular disease.
Epigenetics is a branch of biology with entomologic origins in “epigenesis,” development of complex organisms from single cells. Epigenetic processes govern numerous cellular functions, including changes in gene expression that occur as cell lineages emerge during development. Most autosomal genes are expressed from both alleles, but imprinted genes are preferentially expressed from alleles inherited from mothers or fathers. Parent of origin-specific expression is directed by epigenetic marks, “imprints” established specifically in DNA of sperm or eggs, of which methylation of CpG-rich differentially methylated domains [11, 12] are best described. Imprinted genes are normally inherited as one allele with the maternal imprint and one with the paternal imprint (1M:1P). Imprinting disorders can occur in children who inherit improper imprint contributions.
The eight defined human imprinting disorders are extremely rare, with collective prevalence less than one per 12,000 births [13]. AS and Prader-Willi syndrome (PWS) arise from improper contributions of the same critical region of chromosome 15q11.2–13, AS by deficient maternal contributions and PWS by deficient paternal contributions. AS can be caused by deletion of the critical region from the copy of chromosome 15 inherited from the mother (0M:1P), maternally inherited point mutations in the UBE3A gene, or paternal uniparental disomy (UPD) of chromosome 15 (0M:2P). PWS can be caused by paternal deletion of the same region (1M:0P) or maternal UPD (2M:0P). These genetic errors account for most cases, but epigenetic errors termed “imprinting defects” occur in about 1–3% of AS and 1% of PWS [14]. In AS cases with imprinting defects, the maternal imprint is partially or completely absent from the copy of chromosome 15 inherited from the mother. Because AS involving epigenetic etiology is extremely rare (approximately one per 300,000 live births [10]), it seemed remarkable that imprinting defects were noted in all three children initially reported to have AS following ICSI [1, 2], implicating imprinting defects rather than genetic errors in ART-associated imprinting disorders.
Most cases of Beckwith-Wiedemann syndrome (BWS) and some Silver-Russell (SRS) syndrome cases are determined by parental contributions of the same region of chromosome 11p15.4–.5, BWS caused by relative deficit of maternal contributions. SRS can arise from deficit of paternal contributions of chromosome 11 or chromosome 7 [15]. Imprinting defects account for approximately 50% of BWS and 50% of SRS, although etiology of both conditions is complex.
Disorders such as AS and BWS that arise from deficiency of maternal imprints are classified as maternal imprinting disorders, while those such as PWS and SRS are paternal imprinting disorders. Studies comparing frequency of an imprinting disorder in children conceived following ART to frequency in naturally conceived children were first published in 2003, each reporting conception following ART to be associated with BWS [16–18]. Of 14 ART-exposed cases for whom molecular etiology was reported, 13 had an imprinting defect, according with the three originally reported AS cases in two important ways: BWS and AS are maternal imprinting disorders, and imprinting defects rather than genetic error predominated in ART-exposed cases. But there was a noteworthy difference: while AS cases were conceived by ICSI, most ART-exposed BWS cases had been conceived without ICSI, implicating other procedures used in ART. Embryo culture was an attractive candidate because large offspring syndrome produced by in vitro embryo culture following in vivo conception had been shown to involve demethylation of a maternal imprint [5]. A hypothesis accounting for these observations specified that the embryo culture step of ART leads to imprinting disorders by disrupting processes that ordinarily establish maternal imprints following fertilization [10]. By this logic, embryo culture could disrupt maternal imprints, but not paternal imprints, the latter established before fertilization. A prediction of this refined hypothesis was that ART may predispose to maternal imprinting disorders, but not to paternal imprinting disorders.
In ensuing years, additional imprinting disorders were defined, approximately 200 imprinted genes [19] were recognized in humans, and epigenetic mechanisms of imprinting at numerous loci were described. Moreover, a substantial body of observational data confirmed elevated occurrence of low birthweight in infants conceived following ART [20], and additional epidemiologic studies estimating associations between ART and imprinting disorders were conducted. Summary of research on the latter topic is warranted to define associations between ART conception and imprinting disorders, and to examine the specific hypothesis that ART-related risk arises from epigenetic disruption of maternal imprints following fertilization. A review addressing these questions was conducted before numerous relevant studies were published [21], and subsequent reviews addressed the first question but did not provide quantitative synthesis [22] or overlooked contributing studies [23, 24]. We systematically reviewed scholarly literature to identify, evaluate, and quantitatively synthesize epidemiologic data estimating associations between ART and imprinting disorders, and to assess implications of summary data for causal hypotheses, accounting for contemporary understanding of imprinting.
Material and methods
To develop a focused research question, we applied the Population, Intervention, Comparison, Outcome method. We defined population as children born in the ART era, exposure as conception following any ART procedure, comparison group as children conceived spontaneously, and outcome as any defined imprinting disorder. We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study was exempt from Institutional Review Board oversight, and we did not register the protocol.
Systematic search and critical review
We searched PubMed from inception through January 2017 for articles on ART and human imprinting disorders using Medical Search Heading (MeSH) terms and keywords. Terms were developed for the exposure, which included all procedures used in ART, and for the outcome, which included established and proposed imprinting disorders (Supplementary Table 1) and retinoblastoma (RB) for which epigenetic error is a proposed etiology. The Boolean operator “AND” identified reports matching both exposure and outcome terms. The fully specified character string used in the query appears in supplementary material. Identified papers meeting inclusion criteria described below were subjected to review of cited references and citation searches implemented using Web of Science. Qualifying reports were subjected to citation searches, resulting in citation chaining in which each paper identified was subjected to citation search. We sought additional reports by querying presentations to scientific conferences and catalogued theses and dissertations. Identified reports were captured in an Endnote X7.7.1 library, with those identified by multiple searches reduced to unique entries.
Title and abstract of each unique report were reviewed independently by two investigators to identify studies that could potentially satisfy inclusion criteria. Minimum requirements were specification of (1) children with an imprinting disorder and an unaffected reference or source population and (2) history of conception following ART versus natural conception. We excluded studies not reported in English, studies without human subjects, and case reports.
Data and meta-analysis
Two investigators critically reviewed each conforming report and independently extracted study data into a structured database managed using REDCap (version 6) [25], hosted at the University of Southern California. Discrepant data detected by the REDCap double data entry procedure were resolved by consensus. Data items were study design and location, exposure according to ART procedures (ovarian stimulation/in vitro fertilization/ICSI/intrauterine insemination), outcome condition, tabular data on joint distribution of exposure and outcome, any covariates, and reference population. For two studies with exposure frequency reported for only cases [26, 27], we extracted exposure data for source populations from corresponding national registries of ART procedures, as described in supplementary methods.
Meta-analyses were implemented to quantitatively assess associations between conception following ART (versus natural conception) and each condition for which two or more studies were identified. Because occurrence of congenital conditions is measured as prevalence, the parameter estimated was the summary prevalence odds ratio, hereafter called summary odds ratio (sOR). We analyzed data on BWS using a random effects (DerSimonian-Laird) model. We analyzed data on AS, PWS, and SRS using a fixed effect model, to implement by inverse opposite sample size correction [28] continuity corrections required for one study of each of these conditions; we reanalyzed these data using random effects models to confirm compatibility of results from both models. Forest plots were created displaying each study’s contribution to summary estimates. Heterogeneity was characterized using appropriate p values and I2 statistics [29]. A funnel plot was generated for each condition to gauge publication bias. Influence analysis and cumulative meta-analysis ordered on study weight were implemented to examine impact of individual studies on summary estimates. Recognizing that ART procedures have evolved over time, we explored influence of calendar year on effect size by meta-regression, regressing estimated ln(OR) on year published. Analyses were implemented using Stata 14.2 (College Station, TX).
Results
Systematic search
The search identified 665 published reports (Fig. 1), and five duplicates were removed. Review of titles and abstracts of the remaining 660 identified 27 that could potentially meet inclusion criteria. Critical review of these reports revealed that one did not provide original data, two did not address relevant disorders, and nine did not include a reference group. Comparison of the remaining 15 reports with respect to outcomes, diagnosis dates, and study site identified pairs that may have included some of the same participants; for each, we excluded the report describing fewer participants (Supplementary Table 2). The remaining 13 reports [16–18, 26, 27, 30–37] provided data on 23 studies estimating associations between conception following ART and individual conditions (Table 1), four studies of AS, eight of BWS, six of PWS, three of SRS, one of RB, and one of TNDB. Authors of the RB study [34] reported that affected children were over twice as likely as unaffected children to have been conceived following ART, OR = 2.54 (95% confidence interval (CI) 1.02, 5.23). Data from the TNDM study [36] were too sparse to provide a statistically meaningful estimate of the ART-TNDM association.
Fig. 1.
Flow of information through the systematic review and meta-analysis
Table 1.
Details of studies found to meet both inclusion criteria and metrics of study quality
| Imprinting Disordera | Affected | Unaffected | Study Design | Interventionb | Reference Population | ||
|---|---|---|---|---|---|---|---|
| Exposed | Un-exposed | Exposed | Un-exposed | ||||
| DeBaun, 2003 [16] USA | |||||||
| BWS | 3 | 62 | 30285 | 3929132 | Case-control | V I U | cPrevalence of ART among live births of United States population in 1999 |
| Gicquel, 2003 [17] France | |||||||
| BWS | 6 | 143 | 9930 | 760070 | Case-control | V I F | cPrevalence of ART among live births of French population |
| Maher, 2003 [18] UK | |||||||
| BWS | 6 | 143 | 43074 | 4277408 | Case-control | V I | dPrevalence of imprinting disorder among British population 1995-2000 |
| Halliday, 2004 [31] Australia | |||||||
| BWS | 4 | 33 | 1 | 147 | Case-control | V | Matched controls (uniparity, age, maternal age) from Victoria, Australia |
| Kallen, 2005 [32] Sweden | |||||||
| PWS | 1 | 0 | 16280 | 2023663 | Cohort | V | cPrevalence of ART among live births of Swedish Medical Birth Registry 1982-2001 |
| SRS | 1 | 0 | 16280 | 2023663 | |||
| Lindegaard, 2005 [33] Denmark | |||||||
| PWS | 0 | 3 | 6052 | 436297 | Cohort | V I | dPrevalence of imprinting disorder among Danish population between 1995-2001 |
| SRS | 0 | 2 | 6052 | 436297 | |||
| Sutcliffe, 2006 [36] UK | |||||||
| AS | 2 | 73 | 68566 | 8327061 | Case-control | V I | cPrevalence of ART among live births of United Kingdom population 1991-2002 |
| BWS | 11 | 68 | 68566 | 8327061 | |||
| PWS | 9 | 154 | 68566 | 8327061 | |||
| TNDM | 0 | 23 | 68566 | 8327061 | |||
| Sanchez-Albisua, 2007 [35] Germany | |||||||
| AS | 1 | 0 | 33 | 39 | Case-control | V I | Control group not described |
| Doornboos, 2007 [30] Netherlands | |||||||
| AS | 4 | 59 | 83818 | 3954461 | Case-control | V O I U | dPrevalence of imprinting disorder among Dutch Population between 1983-2003 |
| BWS | 6 | 65 | 83818 | 3954461 | |||
| PWS | 4 | 82 | 83818 | 3954461 | |||
| Marees, 2009 [34] Netherlands | |||||||
| RB | 7 | N/A | N/A | N/A | Case series | V | Retinoblastoma cohort from Netherland national registry from 1945 |
| Wilkins-Haug, 2009 [37] USA | |||||||
| BWS | 3 | 3 | 2 | 20 | Case-control | V | Unaffected infants delivered at the same hospital |
| Hiura, 2012 [27] Japan | |||||||
| AS | 6 | 70 | 10524 | 1123610 | Case-control | V | cPrevalence of ART among live births of Japanese population in 2003 |
| BWS | 2 | 123 | 10524 | 1123610 | |||
| PWS | 4 | 261 | 10524 | 1123610 | |||
| SRS | 4 | 42 | 10524 | 1123610 | |||
| Gold, 2014 [26] USA | |||||||
| PWS | 20 | 1864 | 25015 | 3960909 | Case-control | V O I U | cPrevalence of ART among live births of United States population in 2014 |
aBWS Beckwith-Wiedemann Syndrome, AS Angelman Syndrome, PWS Prader-Willi Syndrome, SRS Silver-Russell Syndrome, RB Retinoblastoma, TNDM transient neonatal diabetes mellitus; bfor which exposure status was defined as any one or more of the specified procedures: V In Vitro Fertilization, O ovarian stimulation alone, I Intracytoplasmic sperm injection, U Intrauterine insemination; N/A data not available; reference population definition: crepresenting prevalence of ART for the base population from which cases arose, drepresenting frequency of imprinting disorder among unexposed
Meta-analysis
Both maternal imprinting disorders studied were found to be positively associated with conception following ART (Fig. 2). Four studies of AS yielded a sOR estimate of 4.7 (95% CI 2.6–8.5). Eight BWS studies yielded a sOR estimate of 5.8 (95% CI 3.1–11.1). Both paternal imprinting disorders studied were also found to be positively associated with this history (Fig. 3). Six PWS studies yielded a sOR estimate of 2.2 (95% CI 1.6–3.0). Three SRS studies yielded a sOR estimate of 11.3 (95% CI 4.5–28.5). Between-study heterogeneity was statistically significant for BWS (I2 = 66.4%, p = 0.004), expected if there are important determinants of dispersion besides random error. Whether heterogeneity of AS, PWS, and SRS did not achieve statistical significance because of fewer contributing samples, minimal underlying heterogeneity or both of these influences is unclear. For all conditions, contributing studies were too few and publication years too highly clustered for meta-regression to provide meaningful results.
Fig. 2.
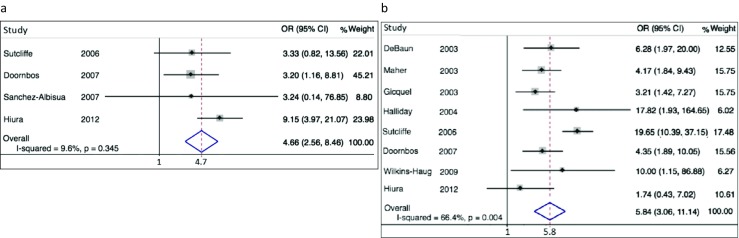
Forrest plot displaying results of meta-analysis relating history of conception by ART to imprinting disorders of maternal origin. Contributing studies ordered within stratum by year of publication and relative weight (%) of study (point and 95% confidence interval [CI] estimates of individual study odds ratio [OR] are small black diamonds and horizontal bars; relative weights of individual studies are area of gray squares; point and interval sOR estimates are vertical and horizontal apices of open diamonds). a Four studies of Angelman syndrome, sOR = 4.7 (95% CI 2.6–8.5). b Eight studies of Beckwith-Wiedemann syndrome, sOR = 5.8 (95% CI 3.1–11.1)
Fig. 3.
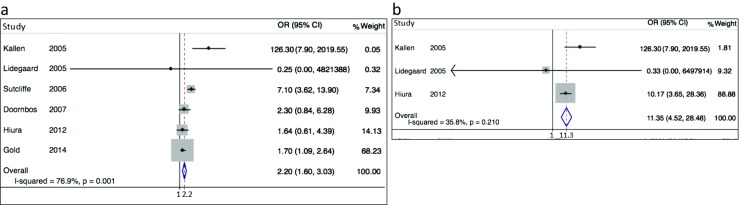
Forrest plot displaying results of meta-analysis relating history of conception by ART to imprinting disorders of paternal origin. Contributing studies ordered within stratum by year of publication and relative weight (%) of study (point and 95% confidence interval [CI] estimates of individual study odds ratio [OR] are small black diamonds and horizontal bars; relative weights of individual studies are area of gray squares; point and interval sOR estimates are vertical and horizontal apices of open diamonds). a Six studies of Prader-Willi syndrome, sOR = 2.2 (95% CI 1.6–3.0). b Three studies of Silver-Russell syndrome, sOR = 11.3 (95% CI 4.5–28.5)
Several results contradict publication bias as an explanation for these associations. Forest plots (Figs. 2 and 3) displaying contributing studies in order of publication reveal no pattern of extreme results in early, small studies that could account for positive associations. Funnel plots (Supplementary Figure 1) show no conspicuous over-representation of studies in the lower right portion of the figure, compared to lower left.
Further analyses demonstrate that sOR estimates for all four conditions are statistically robust. Influence analyses show that none of the associations relies on any one study, estimates of the sOR remaining little changed in magnitude and statistically significant after removing contributing studies one at a time. (Supplementary Figure 2). Meta-analyses cumulated on reverse weight of contributing studies show that for each condition, the single largest study provides an OR estimate compatible with the reported sOR; adding studies sequentially does not at any stage alter the impression that ART is strongly associated with outcome (Supplementary Figure 2.).
Discussion and conclusions
We identified strong positive associations between a history of conception following ART and two imprinting disorders of maternal origin, AS and BWS, and two of paternal origin, PWS and SRS. These results contradict the hypothesis articulated in the early 2000s that etiology entails specific disruption of maternal imprints following fertilization, but at least three explanations for the observed associations seem plausible. They could be causal if one or more ART procedures influence both maternal and paternal imprints, thereby predisposing offspring to imprinting disorders. They could reflect confounding by indication, if aberrant imprints on gamete DNA compromise both male and female fertility. And they could reflect conventional confounding by risk factors for both maternal and paternal imprinting disorders. Each explanation has separate implications for rational intervention; fortunately, each has a distinct set of predictions that may allow future research to distinguish between them.
Both maternal and paternal imprinting disorders could be caused by ART if procedures impact either both sperm and ova, or cells of the conceptus following fertilization. Molecular studies of affected children provide insight into these possibilities. Studying children with BWS and SRS, Hiura and colleagues [27] reported that compared to naturally conceived patients, those conceived following ART more frequently exhibited both aberrant DNA methylation at multiple loci and cellular mosaicism in methylation errors. Reasoning that events impacting only some cells of multi-celled embryos could explain mosaicism, these authors inferred that imprinting defects occurred after fertilization. Consistent with this timing, some patients with BWS and SRS who were conceived following ART exhibit alterations in both maternally and paternally methylated imprints [27, 38]. Although specific mechanisms that preserve imprints in somatic cells during embryonic development are poorly understood [38, 39], ART entails procedures such as in vitro culture and cryopreservation that may disrupt maintenance DNA methylation through perturbations in folate and methionine cycling [40]. If ART causes these disorders by disrupting properly established imprints, children born with imprinting disorders following ART conception could harbor epigenetic errors set on genetic material inherited from any parent, even those with proven fertility including gamete donors, and disruptions might in some instances be traceable to both parents. Moreover, any cellular mosaicism in such errors would point to disruptive events likely to have occurred in somatic cells of multicellular embryos. Future research reporting such findings would implicate stressors imparted by ART as causal and could inform efforts to identify and refine any ART-related procedures responsible for disrupting maintenance of imprints.
In theory, imprints could be aberrantly set in parents’ gametes by disruption of processes that normally erase then reestablished DNA methylation in gender-specific states during development of parents’ germ cell lineage [38]. DNA of gametes produced at sexual maturity would be hypermethylated at sites that escaped erasure, causing males to produce sperm that retain maternal imprints and females to produce ova that retain paternal imprints. Whether such gametes are produced cannot practically be assessed in ova, but maternal imprints have been reported in DNA of sperm from men with abnormal semen parameters, suggestive of subfertility [41], but whether this reflects improper erasure or somatic cell contamination remains controversial. Conversely, incomplete reestablishment of methylation could result in hypomethylation of sites that should be methylated to establish proper imprints. Both types of aberrant imprints—hypermethylated and hypomethylated—are reported in children conceived by ART [42]. If parents have subfertility due to improper imprints, they may elect ART, transmitting aberrant imprints to offspring who consequently develop imprinting disorders. In this scenario, ART would be associated with imprinting disorders through confounding by indication. Excess imprinting errors in children with imprinting disorders following ART would be traceable to subfertile parents, but not to genetic parents of proven fertility—including gamete donors. Such findings would implicate disordered epigenetic programming of germline cells in subfertility, and could help identify stages of development vulnerable to such disruption. In this instance, predictive preconception testing of sperm could be contemplated.
Most studies included in the meta-analysis showed no effort to control confounding by risk factors for imprinting disorders [16–18, 26, 27, 32–35, 37]. Parents’ ages are potential confounders, because couples who elect ART tend to be older than those who conceive naturally, and advanced parental age strongly predisposes to types of genetic errors that cause imprinting disorders, UPD and de novo mutation. Rescue of aneuploidy is the predominant mechanism producing UPD [43], and maternal age is highly associated with aneuploidy. At 10-week gestation, trisomy 21 is reportedly 15 and 65 times more frequent in women 35 and 45 years of age, respectively, than in those of 20 years [44]. Age-related mechanisms may also cause trisomy 7 and trisomy 15, which could resolve to disomy by chromosome loss. One third of random losses would produce maternal UPD 7 or 15, causes of SRS and PWS. This rationale whereby advanced maternal age could predispose to PWS accords with clinical observations. In fetuses with trisomy 15 detected at amniocentesis for advanced maternal age, trisomy is traced frequently to maternal meiosis I [45]; in approximately one third of samples demonstrating mosaic trisomy 15, substantial proportions of cells have resolved as maternal UPD [46, 47], and mothers of PWs patients with maternal UPD tend to be older than mothers of PWS patients with other etiologies [48, 49]. Through associations with both use of ART and frequency of UPD, advanced maternal age may impart strong positive confounding between ART and each of PWS and SRS. Positive confounding by advanced paternal age also seems possible based on predisposition to de novo mutation: chromosome gains and losses are more common in sperm produced by older men [50], and advanced paternal age is the primary determinant of de novo mutations responsible for several autosomal dominant disorders [51]. Moreover, conditions associated with de novo deletions are independently associated with advanced age of both fathers and mothers [52]. If confounding by parental age is responsible for ART-imprinting disorder associations, careful control of such confounding in future research is expected to yield credible null estimates of the associations (e.g., narrow confidence intervals containing the null value, 1.0), and correlative analyses of case-only data could reveal explanatory patterns between parental age and molecular etiology, for example, proportion maternal UPD-attributable PWS monotonically associated with maternal age. Such findings would allay concern that ART-related procedures may cause imprinting disorders and facilitate refinement of counseling regarding parental age-related risks.
Comprehensive synthesis of human data on ART-imprinting disorder associations is a great strength of this research. Interpreting prevalence odds ratios as exposure effects requires the assumption that ART does not influence likelihood of surviving imprinting disorders long enough to be diagnosed, which seems reasonable. Frequency of spontaneous abortion is elevated following ART conception, but differential loss could spuriously create the observed positive associations only if survival of conceptuses otherwise destined to develop each of the four imprinting disorders were substantially more advantageous following ART than following natural conception, which seems unlikely. Cumulative meta-analyses and funnel plots indicate that publication bias is unlikely to explain observed associations, but results of any meta-analysis must be interpreted in light of limitations of contributing studies. Several compared frequency of ART exposure of cases in disease registries to exposure frequency in corresponding source populations based on national reports of livebirths. If definition employed by a registry included more procedures than definition used in the corresponding national report (e.g., intrauterine insemination included in the former but not the latter), the resulting differential misclassification of ART would be expected to bias sOR estimates upward. But this potential source of bias cannot fully explain positive associations, which were reported even for studies wherein investigators specified that these definitions were identical [17, 18, 30–33, 37]. Because diagnostic features can be subtle [42], some affected children are likely to have remained undiagnosed, particularly those with BWS of SRS. Were this equally common for children conceived by ART or naturally, estimates of the ART associations are expected to bias toward the null value. But if those conceived by ART were more likely to be diagnosed, due to unrecognized influences such as access to medical care, estimates could be biased upward, although it seems unlikely that resulting bias could create the strong associations observed with all four conditions. Similarly, if ART-conceived cases were more likely than naturally conceived cases to be enrolled in disease registries, resulting bias would be upward but would not fully explain positive associations, which were observed in studies that enrolled cases and non-cases from identical sources [27, 30–33, 37]. Finally, confounding by unrecognized risk factors cannot be ruled out completely, since demonstrably complex etiology of both subfertility and imprinting disorders have yet to be fully defined. The theoretical possibility that factors predisposing to both parental subfertility and imprinting disorders of offspring could be confounders is illustrated by NLRP5 mutations associated with both maternal subfertility and imprinting disorders [53], although described mutations at this locus are collectively too rare to explain associations reported here. Thus, apart from possible confounding by parental age, important spurious influences on the reported associations seem unlikely. We did not explore associations with individual ART procedures, because most reports did not provide these details of exposure history.
Research to resolve candidate hypotheses for the associations, described above, may be feasible in the near future because contemporary guidelines for registering imprinting disorders include molecular etiology, parental ages, and detailed exposure history [42]. Should results favor the causal hypothesis, efforts to identify and ameliorate processes whereby ART procedures may disrupt epigenetic marks would be warranted. Conversely, should they favor confounding by indication, new research addressing epigenetic state of gamete DNA in the etiology of both male- and female-factor subfertility would be further justified. Finally, should confounding by parental age appear to largely explain the associations, counseling of couples who consider ART could be revised to include specific risks of imprinting disorders if one or both partners are of advanced age. Until such studies are completed, associations estimated in the work reported here can be the basis of more general counseling. We estimated risk following ART as the product of the published upper estimate of population frequency of each condition [42] and point estimate of sOR. Resulting expectations are that among 10,000 live births following ART, there will be 3.9 children diagnosed with AS, 3.9 with BWS, 2.2 with PWS, and 1.5 with SRS—or 11.5 cases of any of these disorders. Based on the same sources, frequency of any of these disorders is estimated to be 2.0 per 10,000 children in the general population, but is not available for children born to subfertile couples after natural conception. Accordingly, couples can currently be counseled that while frequency may be several fold higher following ART, absolute risk is low.
Electronic supplementary material
(PDF 633 kb)
Acknowledgments
The authors gratefully acknowledge expert instruction in systematic search provided by Lynn 1. Kysh, MLIS, and Robert E. Johnson, MLIS. This work was supported in part by grants R56ES017091 and P30 ES07048 from the National Institute of Environmental Health Sciences, and LG-99-17-0069 from the Institute of Museum and Library Sciences.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1173-x) contains supplementary material, which is available to authorized users.
References
- 1.Cox GF, Burger J, Lip V, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orstavik KH, Eiklid K, van der Hagen CB, et al. Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet. 2003;72:218–219. doi: 10.1086/346030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesarik J, Sousa M, Greco E, Mendoza C. Spermatids as gametes: indications and limitations. Hum Reprod. 1998;13(Suppl 3):89–107. doi: 10.1093/humrep/13.suppl_3.89. [DOI] [PubMed] [Google Scholar]
- 4.Manning M, Lissens W, Bonduelle M, Camus M, de Rijcke M, Liebaers I, et al. Study of DNA-methylation patterns at chromosome 15q11-q13 in children born after ICSI reveals no imprinting defects. Mol Hum Reprod. 2000;6:1049–53. [DOI] [PubMed]
- 5.Young LE, Fernandes K, McEvoy TG, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153–4. [DOI] [PubMed]
- 6.Surani MA, Barton SC, Norris ML. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- 7.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 8.Buitendijk SE. Children after in vitro fertilization. An overview of the literature. Int J Technol Assess Health Care. 1999;15:52–65. doi: 10.1017/S0266462399015160. [DOI] [PubMed] [Google Scholar]
- 9.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 10.Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18:2508–2511. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- 11.Kanber D, Buiting K, Zeschnigk M, Ludwig M, Horsthemke B. Low frequency of imprinting defects in ICSI children born small for gestational age. Eur J Hum Genet. 2009;17:22–29. doi: 10.1038/ejhg.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eroglu A, Layman LC. Role of ART in imprinting disorders. Semin Reprod Med. 2012;30:92–104. [DOI] [PMC free article] [PubMed]
- 13.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horsthemke B, Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am J Med Genet A. 2008;146A:2041–2052. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Amero S, Monk D, Frost J, Preece M, Stanier P, Moore GE. The genetic aetiology of Silver-Russell syndrome. J Med Genet. 2008;45:193–199. doi: 10.1136/jmg.2007.053017. [DOI] [PubMed] [Google Scholar]
- 16.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc Y. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet. 2003;40:62–4. [DOI] [PMC free article] [PubMed]
- 19.Jirtle RL. Imprinted gene databases. http://geneimprint.com/site/genes-by-species.Homo+sapiens.imprinted-All. Accessed on May 10, 2017. 2017.
- 20.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 21.Cortessis VK. Imprinting errors and IVF. In: Van Voorhis BJ, editor. Biennial review of infertility. Dordrecht: Springer; 2009. pp. 239–246. [Google Scholar]
- 22.Vermeiden JP, Bernardus RE. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril. 2013;99:642–651. doi: 10.1016/j.fertnstert.2013.01.125. [DOI] [PubMed] [Google Scholar]
- 23.Lazaraviciute G, Kauser M, Bhattacharya S, Haggarty P, Bhattacharya S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2014;20:840–852. doi: 10.1093/humupd/dmu033. [DOI] [PubMed] [Google Scholar]
- 24.Lazaraviciute G, Kauser M, Bhattacharya S, Haggarty P, Bhattacharya S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2015;21:555–557. doi: 10.1093/humupd/dmv017. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold JA, Ruth C, Osann K, Flodman P, McManus B, Lee HS, et al. Frequency of Prader-Willi syndrome in births conceived via assisted reproductive technology. Genet Med. 2014;16:164–9. [DOI] [PMC free article] [PubMed]
- 27.Hiura H, Okae H, Miyauchi N, Sato F, Sato A, van de Pette M, et al. Characterization of DNA methylation errors in patients with imprinting disorders conceived by assisted reproduction technologies. Hum Reprod. 2012;27(8):2541–8. [DOI] [PubMed]
- 28.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22:2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 31.Halliday J, Oke K, Breheny S, Algar E, JA D. Beckwith-Wiedemann syndrome and IVF: a case-control study. Am J Hum Genet. 2004;75:526–528. doi: 10.1086/423902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: risk for congenital malformations after different IVF methods. Birth Defects Res Part A Clin Molec Teratol. 2005;73:162–169. doi: 10.1002/bdra.20107. [DOI] [PubMed] [Google Scholar]
- 33.Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod. 2005;20(4):950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 34.Marees T, Dommering CJ, Imhof SM, Kors WA, Ringens PJ, van Leeuwen FE, et al. Incidence of retinoblastoma in Dutch children conceived by IVF: an expanded study. Hum Reprod. 2009;24:3220–4. [DOI] [PubMed]
- 35.Sanchez-Albisua I, Borell-Kost S, Mau-Holzmann UA, Licht P, Krageloh-Mann I. Increased frequency of severe major anomalies in children conceived by intracytoplasmic sperm injection. Dev Med Child Neurol. 2007;49:129–134. doi: 10.1111/j.1469-8749.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 36.Sutcliffe AG, Peters CJ, Bowdin S, Temple K, Reardon W, Wilson L, et al. Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Hum Reprod. 2006;21:1009–11. [DOI] [PubMed]
- 37.Wilkins-Haug L, Porter A, Hawley P, Benson CB. Isolated fetal omphalocele, Beckwith-Wiedemann syndrome, and assisted reproductive technologies. Birth Defects Res A Clin Mol Teratol. 2009;85:58–62. doi: 10.1002/bdra.20547. [DOI] [PubMed] [Google Scholar]
- 38.Chiba H, Hiura H, Okae H et al. DNA methylation errors in imprinting disorders and assisted reproductive technology. Pediatr Int. 2013;55:542–9. [DOI] [PubMed]
- 39.Pinborg A, Loft A, Romundstad LB, Wennerholm UB, Söderström-Anttila V, Bergh C, et al. Epigenetics and assisted reproductive technologies. Acta Obstet Gynecol Scand. 2016;95:10–5. [DOI] [PubMed]
- 40.Hoeijmakers L, Kempe H, Verschure PJ. Epigenetic imprinting during assisted reproductive technologies: the effect of temporal and cumulative fluctuations in methionine cycling on the DNA methylation state. Mol Reprod Dev. 2016;83:94–107. doi: 10.1002/mrd.22605. [DOI] [PubMed] [Google Scholar]
- 41.Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, Sokol RZ. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One. 2007;2:e1289. doi: 10.1371/journal.pone.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggermann T, Netchine I, Temple IK, Tümer Z, Monk D, Mackay D, et al. Congenital imprinting disorders: EUCID.net—a network to decipher their aetiology and to improve the diagnostic and clinical care. Clin Epigenetics. 2015;7:23. [DOI] [PMC free article] [PubMed]
- 43.Conlin LK, Thiel BD, Bonnemann CG, Medne L, Ernst LM, Zackai EH, et al. Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum Mol Genet. 2010;19:1263–75. [DOI] [PMC free article] [PubMed]
- 44.Snijders RJ, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13:167–170. doi: 10.1046/j.1469-0705.1999.13030167.x. [DOI] [PubMed] [Google Scholar]
- 45.Zaslav AL, Fallet S, Brown S, Ebert R, Fleischer A, Valderama E, et al. Prenatal diagnosis of low level trisomy 15 mosaicism: review of the literature. Clin Genet. 1998;53:286–92. [DOI] [PubMed]
- 46.Christian SL, Smith AC, Macha M, et al. Prenatal diagnosis of uniparental disomy 15 following trisomy 15 mosaicism. Prenat Diagn. 1996;16:323–332. doi: 10.1002/(SICI)1097-0223(199604)16:4<323::AID-PD856>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Chen CP, Chern SR, Chen YN, Wu PS, Yang CW, Chen LF, et al. Mosaic trisomy 15 at amniocentesis: prenatal diagnosis, molecular genetic analysis and literature review. Taiwan J Obstet Gynecol. 2015;54:426–31. [DOI] [PubMed]
- 48.Robinson WP, Bottani A, Xie YG, Balakrishman J, Binkert F, Mächler M, et al. Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am J Hum Genet. 1991;49:1219–34. [PMC free article] [PubMed]
- 49.Mitchell J, Schinzel A, Langlois S, Gillessen-Kaesbach G, Schuffenhauer S, Michaelis R, et al. Comparison of phenotype in uniparental disomy and deletion Prader-Willi syndrome: sex specific differences. Am J Med Genet. 1996;65:133–6. [DOI] [PubMed]
- 50.Sartorelli EM, Mazzucatto LF, de Pina-Neto JM. Effect of paternal age on human sperm chromosomes. Fertil Steril. 2001;76:1119–1123. doi: 10.1016/S0015-0282(01)02894-1. [DOI] [PubMed] [Google Scholar]
- 51.Wiener-Megnazi Z, Auslender R, Dirnfeld M. Advanced paternal age and reproductive outcome. Asian J Androl. 2012;14:69–76. doi: 10.1038/aja.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. 2016;21:693–700. [DOI] [PMC free article] [PubMed]
- 53.Docherty LE, Rezwan FI, Poole RL, et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun. 2015;6:8086. doi: 10.1038/ncomms9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Technology SfAR . Assisted Reproductive Technology National Summary Report 2014. Atlanta: US Dept of Health and Human Services; 2016. [Google Scholar]
- 55.United Nations Statistics Division. United Nations report on vital statistics, Series A, Vol LVIII, No 1, 2006.
- 56.Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91:2281–2294. doi: 10.1016/j.fertnstert.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Irahara M, Kuwahara A, Iwasa T, Ishikawa T, Ishihara O, Kugu K, et al. Assisted reproductive technology in Japan: a summary report of 1992–2014 by the Ethics Committee, Japan Society of Obstetrics and Gynecology. Reprod Med Biol. 2017;16:126–32. [DOI] [PMC free article] [PubMed]
- 58.Kultursay N, Senrencber S, Arcasoy M, Capanoglu R, Yuce G. DiGeorge syndrome after in vitro fertilization. J Assist Reprod Genet. 1993;10:380–381. doi: 10.1007/BF01213435. [DOI] [PubMed] [Google Scholar]
- 59.Sutcliffe AG, D'Souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B. Minor congenital anomalies, major congenital malformations and development in children conceived from cryopreserved embryos. Hum Reprod. 1995;10:3332–3337. doi: 10.1093/oxfordjournals.humrep.a135915. [DOI] [PubMed] [Google Scholar]
- 60.Koudstaal J, Braat DD, Bruinse HW, Naaktgeboren N, Vermeiden JP, Visser GH. Obstetric outcome of singleton pregnancies after IVF: a matched control study in four Dutch university hospitals. Hum Reprod. 2000;15:1819–1825. doi: 10.1093/humrep/15.8.1819. [DOI] [PubMed] [Google Scholar]
- 61.Olivennes F, Mannaerts B, Struijs M, Bonduelle M, Devroey P. Perinatal outcome of pregnancy after GnRH antagonist (ganirelix) treatment during ovarian stimulation for conventional IVF or ICSI: a preliminary report. Hum Reprod. 2001;16:1588–1591. doi: 10.1093/humrep/16.8.1588. [DOI] [PubMed] [Google Scholar]
- 62.Orstavik KH. Intracytoplasmic sperm injection and congenital syndromes because of imprinting defects. Tidsskr Nor Laegeforen. 2003;123:177. [PubMed] [Google Scholar]
- 63.Ludwig M, Katalinic A, Gross S, Sutcliffe A, Varon R, Horsthemke B. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet. 2005;42:289–291. doi: 10.1136/jmg.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lidegaard O, Pinborg A, Andersen AN. Imprinting disorders after assisted reproductive technologies. Curr Opin Obstet Gynecol. 2006;18:293–296. doi: 10.1097/01.gco.0000193006.42910.ee. [DOI] [PubMed] [Google Scholar]
- 65.Bowdin S, Allen C, Kirby G, Brueton L, Afnan M, Barratt C, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22:3237–40. [DOI] [PubMed]
- 66.Kagami M, Nagai T, Fukami M, Yamazawa K, Ogata T. Silver-Russell syndrome in a girl born after in vitro fertilization: partial hypermethylation at the differentially methylated region of PEG1/MEST. J Assist Reprod Genet. 2007;24:131–136. doi: 10.1007/s10815-006-9096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim D, Bowdin SC, Tee L, et al. Clinical and molecular genetic features of Beckwith-Wiedemann syndrome associated with assisted reproductive technologies. Hum Reprod. 2009;24:741–747. doi: 10.1093/humrep/den406. [DOI] [PubMed] [Google Scholar]
- 68.King JL, Yang B, Sparks AE, Mains LM, Murray JC, Van Voorhis BJ. Skewed X inactivation and IVF-conceived infants. Reprod BioMed Online. 2010;20:660–663. doi: 10.1016/j.rbmo.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuentz P, Bailly A, Faure AC, Blagosklonov O, Amiot C, Bresson JL, et al. Child with Beckwith-Wiedemann syndrome born after assisted reproductive techniques to an human immunodeficiency virus serodiscordant couple. Fertil Steril. 2011;96:e35–8. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 633 kb)