Abstract
Purpose
To examine current evidence of the known effects of advanced paternal age on sperm genetic and epigenetic changes and associated birth defects and diseases in offspring.
Methods
Review of published PubMed literature.
Results
Advanced paternal age (> 40 years) is associated with accumulated damage to sperm DNA and mitotic and meiotic quality control mechanisms (mismatch repair) during spermatogenesis. This in turn causes well-delineated abnormalities in sperm chromosomes, both numerical and structural, and increased sperm DNA fragmentation (3%/year of age) and single gene mutations (relative risk, RR 10). An increase in related abnormalities in offspring has also been described, including miscarriage (RR 2) and fetal loss (RR 2). There is also a significant increase in rare, single gene disorders (RR 1.3 to 12) and congenital anomalies (RR 1.2) in offspring. Current research also suggests that autism, schizophrenia, and other forms of “psychiatric morbidity” are more likely in offspring (RR 1.5 to 5.7) with advanced paternal age. Genetic defects related to faulty sperm quality control leading to single gene mutations and epigenetic alterations in several genetic pathways have been implicated as root causes.
Conclusions
Advanced paternal age is associated with increased genetic and epigenetic risk to offspring. However, the precise age at which risk develops and the magnitude of the risk are poorly understood or may have gradual effects. Currently, there are no clinical screenings or diagnostic panels that target disorders associated with advanced paternal age. Concerned couples and care providers should pursue or recommend genetic counseling and prenatal testing regarding specific disorders.
Keywords: Advanced paternal age, Genetic defects, Germ cell, De novo mutations
The epidemiology of advanced paternal age
Definition of advanced paternal age
There is no uniformly accepted definition of advanced paternal age. Currently, in the USA, the population mean paternal age is 30.9 years [1]. Guidelines for anonymous donor sperm banking outlined by clinical societies suggest that men who bank sperm be younger than 50 years of age [2, 3]. A current consensus view that is a male aged 40 years or greater at the time of conception is most frequently used to define advanced paternal age [4].
Paternal age trends
The trend toward older maternal age conceptions in Westernized countries is well described. Paralleling this is a similar aging paternity trend for fathers. According to U.S. National Vital Statistics Reports, the percent change in birth rates over a decade ending in 2013 for paternal age increased by 9% in men 35–39 years old, by 14% in men 40–44 years of age, by 16% in men 45–49 years of age, and by 8% in men 50–54 years of age [5]. Trends in the UK mirror these observations: although fewer than 15% of men fathering children in the UK were above age 35 years in 1970, this increased to 25% in 1993 and up to 40% of fathers in 2003 [4]. Clearly, advanced paternal age has paralleled trends in advanced maternal age in many Western countries over the last several decades.
Changes in testis biology and fertility with age
Several biological changes have been described in the testes as men age. Leydig cells and testosterone production show significant declines in older vs. younger men [6–8]. Daily sperm production also decreases with age as assessed histologically; however, an age-related decrease in sperm concentration on semen analyses has been more difficult to demonstrate [9, 10]. The effect of paternal age on male fertility is debated. Studies that address this issue are confounded by the variables of female factor (i.e., partner age) and the trend toward decreased coital frequency with age. However, there is reasonable evidence to suggest an increase in time to conception with paternal age [11].
Changes in sperm genetics with paternal age
Sperm chromosomal abnormalities
Sperm chromosomal abnormalities and genomic instability generally result from meiotic errors that occur during early spermatogenesis and are divided into abnormalities of chromosome number (aneuploidy) and structure (translocations, inversions, duplications) [12, 13]. Advanced paternal age increases the fraction of sperm with sex chromosomal (X,Y) aneuploidy, mainly 47,XXY Klinefelter syndrome and 47,XYY [13–15]. A pronounced relationship (r = 0.63) has also been demonstrated between paternal age and the frequency of sperm structural chromosomal or complex genomic anomalies [13, 16, 17]. However, there is little evidence that these associations contribute to an increased frequency of offspring with de novo structural chromosomal anomalies [12, 18]. The exceptions observed are the increases in inherited reciprocal translocations and trisomy 21 aneuploidy with paternal age [19]. A potential explanation for age-related chromosomal changes may be that continuing lifelong cell mitotic and meiotic divisions during spermatogenesis place germ cells at higher risk for chromosomal injury, recombination errors, and gene conversions, as cumulative exposure to cell damage and environment toxins increases with age [17, 20–22].
Sperm point mutations
A major impact of advanced paternal age on sperm involves single nucleotide changes that include pathogenic mutations in single genes (i.e., substitutions, deletions, insertions, etc.). In the sperm, these defects could stem from copy-errors formed during DNA replication (synthesis), which are then exacerbated by defects in DNA mismatch repair [23]. Frequently, such gene mutations are inherited by offspring, as discussed later [2, 17, 22, 23]. One plausible reason for the high rate of de novo mutations is that the replication machinery is prone to, and has accumulated, de novo spurious errors during 600–1000 mitotic spermatogonial stem cell divisions over 20–30 years of active reproductive life. A conservative estimate of the natural rate of de novo mutations during each replication cycle is 1.2 × 10−8 (range 1–3.8 × 10−8) mutations per nucleotide per division or nearly 7 (range 6–23) alterations per human genome per 1 mitotic division [17, 22, 23]. Therefore, considering that there are 3–4 spermatogenesis cycles per year, a 20-year-old man could acquire up to 21 (range 18–69) de novo mutations per year, subsequently accumulating 420 (range 360–1400) of de novo genomic changes over an ensuing 20-year period [24]. Even if pathogenic changes constitute a small (1-2%) fraction of this mutational load, there could be at least 4-8 novel pathogenic mutations occurring over a 20-year span. This mutational load could also increase with advancing age due to accumulation of errors in aged mismatch repair mechanisms and the synergistic effect of these errors on DNA replication.
There is also reason to believe that the “copy-error” hypothesis, which invokes an increase in random, de novo, point mutations with advanced paternal age, is insufficient to explain the increased mutation load observed in sperm with paternal age. Intriguingly, evidence now suggests that dozens of de novo heritable mutations in male germ cells may occur non-randomly [25]. Similar to natural selection, certain germ line mutations provide a selective advantage over non-mutated clones, which results in favorable mitotic geometric expansion of mutated clones during spermatogenesis [25, 26]. The selective advantage of non-random mutations has led to the idea that “selfish genes” (i.e., FGFR3 and genes in tyrosine kinase-RAS-MAPK pathways) are preferentially propagated in early germ cells. This in turn is believed to be the basis for the significantly increased incidence of sentinel diseases and neurodevelopmental disorders in offspring of advanced paternal age fathers [20, 25–29] (Tables 1 and 2).
Table 1.
Single gene dominant disorders in offspring that are associated with advanced paternal age.
| Clinical condition | Gene | Population risk | Relative risk | Adjusted risk | References |
|---|---|---|---|---|---|
| Achondroplasia | FGFR3 | 1/15,000 | 12 | 1/1,250 | [30, 31] |
| Apert syndrome | FGFR2 | 1/50,000 | 9.5 | 1/5,263 | [31, 32] |
| Crouzon syndrome | FGFR2 | 1/50,000 | 8 | 1/6,250 | [33] |
| Pfeiffer syndrome | FGFR2 | 1/100,000 | 6 | 1/16,666 | [33] |
| Aniridia | PAX6 | 1/40,000 | ? | [34] | |
| Wilms tumor | WT1 | 1/10,000 | 2.1 | 1/4,761 | [35] |
| Bilateral retinoblastomaa | RB1 | 1/15,000 | 5 | 1/3,000 | [36, 37] |
| Hemophilia A | F8 | 1/10,000 | ? | [38] | |
| Fibrodysplasia ossificans | ACVR1 | 1/2000,000 | ? | [39] | |
| Lesch-Nyhan syndrome | HPRT1 | 1/380,000 | ? | [40] | |
| Marfan syndrome | FBN1 | 1/3,000 | ? | [41] | |
| Multiple endocrine neoplasia 2A, 2Bb | RET | 1/35,000 | ? | 1/17,500 | [42, 43] |
| Neurofibromatosis 1 | NF1 | 1/3,000 | 2.9 | 1/1,034 | [31, 44] |
| Oculodentodigital syndrome | GJA1 | Rare | ? | [45] | |
| Osteogenesis imperfecta | COL1A1/2 | 1/10,000 | 2.5 | 1/4,000 | [46, 47] |
| Polycystic kidney disease | PKD1/2 | 1/1,000 | 1.2 | 1/833 | [48] |
| Gardner syndromec (adenomatous polyposis) | APC | 1/2,000 | ? | [49] | |
| Progeria syndrome (Hutchison-Gilford) | LMNA | Rare | ? | [50, 51] | |
| Thanatophoric dysplasia | FGFR3 | 1/20,000 | 3.18 | 1/6,290 | [46] |
| Treacher Collins syndrome | TCOF1 | 1/10,000 | ? | [52] | |
| Tuberous sclerosisd | TSC1,2 | 1/5,800 | ? | [53, 54] | |
| Waardenburg syndrome 1&3b | PAX3 | 1/20,000 | ? | [55, 56] |
Population risk reflects incidence of clinical conditions in general population. Relative risk represents an increased risk of the disorders in offspring of fathers with advanced age (> 40 years) compared to fathers of younger age (20–25 years). Adjusted risk represents a risk of conditions in offspring of men with advanced age. Data provided is for illustration purposes only
aRetinoblastoma appears as a dominant disorder; however, on a molecular level, it is recessive (2 alleles of RB1 carry mutations). The relative risk varies from 3 to 5 for men over 40 years
bMultiple endocrine neoplasia 2A and 2B and Waardenburg syndrome are RET and PAX3 allelic disorders that are caused by different mutations in the same gene
cConflicting results have been reported regarding paternal age effect on Gardner syndrome
dIncidence is based on the clinical diagnosis of Tuberous Sclerosis and relative risk estimates are based upon combined data from two genes, TSC1 and 2
Table 2.
Complex disorders and birth defects in offspring associated with advanced paternal age
| Clinical condition | Population risk | Relative risk | Adjusted risk | References |
|---|---|---|---|---|
| Anatomical | ||||
| Tracheoesophageal fistula | 1/3,600 | 2.55 | 1/1,412 | [57] |
| Atrial septal defect | 1/400 | 1.9 | 1/205 | [58] |
| Ventricular septal defect | 1/200 | 1.7 | 1/118 | [59] |
| Diaphragmatic hernia | 1/4,200 | 1.08 | 1/3,888 | [58] |
| Cleft palate and cleft lip | 1/700 | 1.41 | 1/496 | [58] |
| Pulmonary stenosis | 1/20,000 | 1.08 | 1/19,607 | [58] |
| Spina bifida | 1/1,000 | 1.03 | 1/970 | [60] |
| Non-anatomical | ||||
| Multiple sclerosisa | 1/666 | 2 | 1/333 | [61] |
| Childhood CNS tumor | 1/36,000 | 1.7 | 1/21,302 | [37] |
| Childhood leukemia | 1/25,000 | 1.5 | 1/16,667 | [37, 62] |
| Prostate cancer | 1/5.9 | 1.7 | 1/3.5 | [63] |
| Breast cancer | 1/8.5 | 1.6 | 1/5.3 | [64] |
| Spontaneous miscarriage | 1/7 | 1.6 | 1/4 | [65] |
| Low birth weight | 1.40 | 1.7 | 1/23 | [66] |
| Preeclampsia | 1/62 | 1.8 | 1/34 | [67] |
| Diabetes type 1 | 1/415 | 1.5 | 1/273 | [68] |
| Epilepsy | 1/100 | 1.3 | 1/77 | [69] |
| Cognitive or developmental | ||||
| Autism | 1/1,000 | 5.75 | 1/174 | [70, 71] |
| Schizophrenia | 1/100 | 4.6 | 1/22 | [71, 72] |
| Autism spectrum disorder | 1/200 | 1.52 | 1/131 | [71, 73] |
| Dyslexia | 1/20 | ? | [71, 74, 75] | |
| Bipolar disorder | 1/38 | ? | [71, 76] | |
| Alzheimer’s disease | 1/100 | ? | [77] | |
aVariable incidence observed for different ethnic groups and countries. For illustration purposes, the highest incidence is shown
Sperm epigenetics, methylation, and DNA fragmentation
Several epigenetic alterations in sperm, particularly DNA methylation defects, have recently been correlated with advanced paternal age [78, 79]. Sperm appear to accumulate hundreds of DNA methylation defects with paternal age that are localized to specific genomic sites, such as CpG regions [3, 78–80]. Importantly, many of these defects are found in regulatory or promoter regions that govern neurological, psychiatric, and behavioral disorders, including schizophrenia, bipolar disease, autism, and mood disorders, conditions known to be increased in offspring of older fathers [3, 78, 80]. A recent genome-wide DNA methylation screen comparing epididymal sperm retrieved from young (3-month-old) and old (12-month-old) mice revealed a significant loss of methylation in regions of transcriptional regulation with age. The offspring of older fathers had reduced exploratory and startle behaviors and revealed similar brain DNA methylation abnormalities as observed in human paternal sperm [79]. Offspring from old fathers also showed dysregulation of developmental genes implicated in autism and schizophrenia [79]. At least in mice, this suggests that aberrant DNA methylation patterns occurring in the sperm of older fathers could explain some of the risks that advanced paternal age brings to bear on offspring.
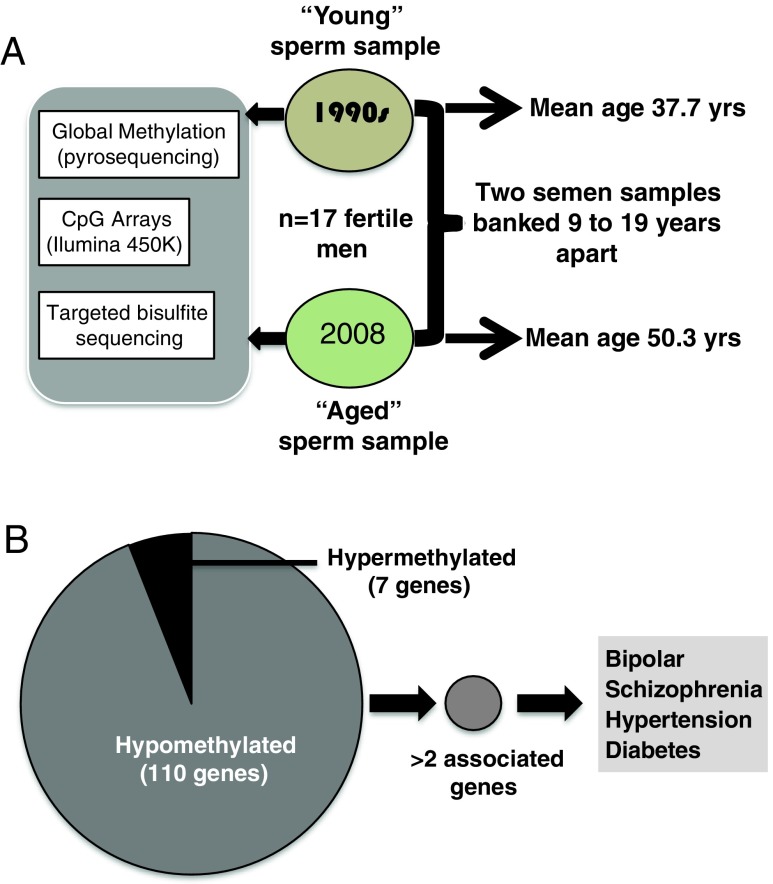
A recent human study also analyzed age-associated sperm DNA methylation patterns in sperm [78]. In addition to the type and magnitude of DNA methylation changes that occurred, the analysis examined which, if any, specific genomic regions were consistently affected with age. The design of the study is outlined in Fig. 1. Semen samples from men with known fertility were examined at two points in their lives: when they were “younger” (mean age 37.7 years) and “older” (mean age 50.3 years). Global methylation patterns were determined by pyrosequencing, and high-level CpG array analysis and targeted bisulfite sequencing were also performed. Overall, there was a significant global hypermethylation in sperm with paternal age along with localized regions of hypomethylation, which contrasts sharply with patterns of DNA methylation found in somatic tissues with age (i.e., global hypomethylation and localized hypermethylation) [81]. The authors calculated that the average fractional methylation change in sperm was 0.3% per year in hypermethylated regions and 0.28% in hypomethylated regions, both of which appear much higher than the 0.15% annual change in DNA methylation estimated to occur in somatic cells with age [78].
Fig. 1.
Study of human sperm DNA methylation with age. a Schematic of study design and epigenetic investigations on sperm. The average difference in subject age between sperm samples was 12.6 years. b Schematic of study findings. Among the diseased associations with paternal age-related DNA methylation changes, only bipolar disorder reached statistical significance. Derived from [78]
Equally or more intriguing were the study findings that consistently linked altered regions of sperm DNA methylation to genes associated with specific diseases (Fig. 1). Among the 117 genes exhibiting age-associated hyper- or hypomethylation, 3 or more genes were associated with the following diseases: schizophrenia, bipolar disorder, diabetes mellitus, and hypertension. However, only bipolar disorder was more frequently associated with our identified genes (compared to background controls) and schizophrenia trended toward increased frequency in the methylated-associated gene set. This suggests that sperm DNA methylation changes observed with paternal age are not randomly distributed within the genome, but could occur more frequently in neurodevelopmental gene sets.
In summary, there is clear evidence of increases in both single gene mutations and DNA methylation changes in sperm with advanced paternal age. However, the exact relationship between paternal age-associated DNA methylation and de novo point mutation changes is unknown. It is theorized that age-associated alterations in DNA methylation might be (1) independent of single nucleotide variations, (2) a consequence of mutational events, or (3) able to influence mutation rates in certain regions [79].
Sperm from older men are known to have higher rates of DNA fragmentation, a condition known to be associated with impaired reproductive outcomes [82]. In fact, the effect of age on sperm DNA fragmentation is well modeled and appears to have no age threshold, maintaining a gradual upward trend beginning in the early reproductive years. Sperm DNA fragmentation rates double between 20 and 60 years of age and increase 5-fold in men between 20 and 80 years of age [83]. A relationship between sperm DNA fragmentation and induced point mutations is certainly possible given that postmeiotic spermatogenic cells have impaired DNA repair capabilities with age that could lead to an increased risk of mutation conversion [84].
Effect of paternal age on offspring
Effects on prenatal outcomes
Advanced paternal age has been correlated with several prenatal outcomes. Two prospective epidemiological studies of women in California (n = 5121) and in the Danish Birth cohort (n = 23,000 women) suggest that the risk of miscarriage, defined as pregnancy loss between 6 and 20 weeks gestation, increases by 27% in fathers > 35 years of age and doubles when fathers over 50 years of age are compared to younger fathers [65, 85]. Both studies controlled for lifestyle issues and maternal confounders. Preterm births, occurring before 32 weeks of gestation, have also been correlated with paternal age in several countries. In Italian and Danish studies of women aged 20–29 years followed over 10-year periods in the 1990s, the odds ratio of preterm birth ranged from 1.7 to 2.1 when fathers older than 45 years of age were compared with younger fathers [85–88]. However, a contemporary US study of women 20–35 years of age failed to show a similar correlation [85, 87]. Finally, a Danish study on fetal deaths undertaken in 23,831 births compared fetal death rates in couples in whom fathers were > 50 years old (n = 124 births) to those younger and calculated a hazard ratio of 1.88 (CI 0.93, 3.82) for fetal deaths among older fathers [85]. Thus, the preponderance of epidemiological evidence suggests that paternal age influences prenatal outcomes.
Effects on birth defects
Two large, US population-based, retrospective cohort studies have examined the occurrence of 22 birth defects in offspring fathered by men of different ages [3, 57]. A list of included birth defects is provided in Table 2. Both studies used the US birth registry to assess birth defects. The overall rate of birth defects was estimated at between 1.5–2% in the studies. In addition, 0.5% of all birth defects were attributable to advanced paternal age. More specifically, there was an additional 4% risk of birth defects associated with fathers aged 30–35 years compared to those in their twenties. This risk increased to 15% when fathers aged > 50 years were compared to 20-year-old fathers. Other research have estimated that there is a 20% increased risk of congenital anomalies (n = 86) with older paternal age, with the incidence increasing from 2% at baseline to 2.4% of births [57, 58]. In addition, the shape of the risk curve with paternal age has been postulated to be similar to that observed for aneuploid conceptions with female age [20]. Thus, US population-based data suggests that birth defect rates in offspring correlate with advanced paternal age.
There has been speculation about potential genetic mechanisms that could underlie a relationship between paternal age and birth defects in offspring. As reviewed earlier, the relationship between advanced paternal age and sperm chromosomal abnormalities is well defined. Sperm sex chromosomal diploidy (XX, XY, or YY) increases with paternal age and may be linked to Klinefelter syndrome in offspring [15, 16, 89]. Similarly, an increase in chromosome 21 aneuploidy may lead to Down syndrome in offspring [14, 89]. However, with the exception of these two disorders, no consistent patterns of paternal, chromosomally-based birth defects in offspring have been reported. In addition, although sperm chromosomal structural abnormalities increase with male age, this again appears not to be associated with birth defects in offspring [8, 11, 14]. Thus, no consistent correlation has been described between paternal age-related sperm chromosomal genetics and birth defects in offspring.
Importantly, when single gene mutations are examined, there is a strong correlation between paternal age and birth defects in offspring [8, 18, 19]. Classic “sentinel phenotypes” in offspring are well described and consist of rare but highly penetrant diseases transmitted by point mutations (Table 1). Many of these 40 known disorders have associated birth defects or have significant debilitation and the need for lifelong care. These diseases occur approximately 10-fold more frequently in fathers > 50 years old compared to those 20 to 30 years old. Consequently, at least some of the increased risk of birth defects in offspring associated with paternal age can be attributed to single gene mutations.
Effects on adult disease in offspring
There is a large, emerging literature that implicates advanced paternal age as a cause of adult disease in offspring. A genetic mechanism involving the paternal transmission of de novo point mutations has been implicated in disease transmission in many cases [20]. A landmark study by Malaspina et al. (2001) surmised that increased DNA mutation rates in sperm associated with paternal age may lead to an increase in neurodevelopmental disease in offspring [90]. In a study of the Israeli national psychiatric disease registry of 87,907 births, they observed a 2.96-fold relative risk of schizophrenia among offspring of fathers > 50 years of age compared to those 20–24 years of age. In addition, the relative risk increased almost linearly above a paternal age of 30 years. These findings have subsequently been confirmed in studies of national disease registries in Sweden and the USA [80, 91].
In what may be a rare collusion of evidence, molecular genetics has validated epidemiological findings on the correlation between paternal age and schizophrenia in offspring. In a rigorous study of 78 Icelandic families that were genetically extremely well characterized, Kong and colleagues compared the whole-genome sequences of trios of a mother, father, and child [23]. Importantly, the children in the trios were diagnosed with either schizophrenia or autism spectrum disorder, but neither disorder was observed in parents. The authors examined de novo mutations in children that were not present in either parent’s DNA. They observed that fathers passed on nearly 4-fold as many new mutations to offspring as mothers: on average, 55 vs. 14, a finding consistent with the literature and within the limits of known variability among examined foci [92]. Father’s age also accounted for nearly all of the new mutations (97%) in the child’s genome, and the number of new mutations passed on rose exponentially with paternal age [23]. The research estimated that a 36-year-old father passes on twice as many mutations to his child as a man 20 years of age, and a 70-year-old man produces eight times as many. Given that most de novo mutations in offspring are neurodevelopmental in nature [90], it is probable that these mutations account for a large proportion of schizophrenia and autism among children in the study.
In addition to schizophrenia, the risk of other neurodevelopmental diseases in offspring such as autism and psychosis has been studied with respect to paternal age. A recent study by D’Onofrio et al. [80] from the Swedish National Birth Registry examined the entire population of Swedish births (n = 2,615,081) between 1973 and 2010 to assess the link between offspring with autism and father’s age at conception. They observed that compared with offspring born to fathers 20–24 years old, offspring of fathers > 45 years old were 3.45-fold (CI 1.6, 4.7) more likely to develop autism, 13.1-fold (CI 6.8, 25.2) more likely to develop attention deficit hyperactivity disorder, and 2.07-fold (CI 1.5, 3.2) more likely to develop psychosis. In summary, evidences from both molecular-genetic and epidemiologic studies support a strong association between advanced paternal age and the occurrence of what is termed “psychiatric morbidity” in offspring [80].
Most of the research describing new mutation rates in offspring with paternal age compares fathers > 45 years of age to those 20–30 years of age and show linear increases that become exponential as paternal age further increases. Based on such modeling, one might anticipate that younger fathers would exhibit the lowest mutation rates and that the mutation rates among young fathers might be similar to that of young mothers. However, recent research suggests that even very young fathers contribute a significant load of point mutations to offspring [92]. In a study of 24,097 parental and validated proband DNA samples from Europe, the Middle East, and Africa, Forster et al. evaluated microsatellite repeats to study new mutation rates between generations. The youngest father in the cohort was 12.1 years old and the oldest was 70.1 years at conception. Even among the youngest cohort of teenage fathers, the relative mutation rates in offspring were observed to be 5-fold higher than those of teenage mothers. Evolutionarily, this suggests that the mutational burden inherited by human offspring is derived mainly from paternal germ cells across the spectrum of parental ages.
Summary
Advanced paternal age is associated with significantly increased genetic risk to offspring. However, the precise age at which risk develops and the magnitude of the risk are poorly understood or may have complex and/or gradual effects.
Observed increased rates of sperm DNA fragmentation and altered epigenetic profiles of sperm are associated with advanced paternal age.
Increased risk to offspring of fathers with advance paternal age may occur in the form of miscarriage, fetal loss, rare single gene disorders, and congenital anomalies.
Risk rates of autism, schizophrenia, and other forms of “psychiatric morbidity” are substantially increased in offspring with advanced paternal age.
Currently, there are no screening or diagnostic panels that target disorders and conditions associated with advanced paternal age.
Concerned couples or care providers should pursue or recommend genetic counseling and prenatal testing regarding specific genetic disorders [1].
Contributor Information
Alexander N. Yatsenko, Phone: 412-641-6290, Email: yatsenkoan@mwri.magee.edu
Paul J. Turek, Phone: 415-392-3200, Email: DrPaulTurek@gmail.com
References
- 1.Khandwala YS, Zhang CA, Lu Y, Eisenberg ML. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod. 2017;32(10):2110–2116. doi: 10.1093/humrep/dex267. [DOI] [PubMed] [Google Scholar]
- 2.Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10(6):457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of American Society for Reproductive Medicine; Practice Committee of Society for Assisted Reproductive Technology. Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril. 2013;99(1):47-62. 10.1016/j.fertnstert.2012.09.037. [DOI] [PubMed]
- 4.Bray I, Gunnell D, Davey SG. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–853. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 6.Kaler LW, Neaves WB. Attrition of the human Leydig cell population with advancing age. Anat Rec. 1978;192(4):513–518. doi: 10.1002/ar.1091920405. [DOI] [PubMed] [Google Scholar]
- 7.Petersen PM, Pakkenberg B. Stereological quantitation of Leydig and Sertoli cellsin the testis from young and old men. Image Anal Stereol. 2000;9:215–218. [Google Scholar]
- 8.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 9.Johnson L, Petty CS, Neaves WB. Age-related variation in seminiferous tubules in men. A stereologic evaluation. J Androl. 1986;7(5):316–322. doi: 10.1002/j.1939-4640.1986.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 10.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, et al. The association of age and semen quality in healthy men. Hum Reprod. 2003;18(2):447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 11.Ford WCL, North K, Taylor H, Farrow A, Hull MGR, Golding J, et al. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. Hum Reprod. 2000;15(8):1703–1708. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 12.Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17(7):1826–1832. doi: 10.1093/humrep/17.7.1826. [DOI] [PubMed] [Google Scholar]
- 13.Martin RH, Rademaker AW. The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet. 1987;41(3):484–492. [PMC free article] [PubMed] [Google Scholar]
- 14.McInnes B, Rademaker A, Martin R. Donor age and the frequency of disomy for chromosomes 1, 13, 21 and structural abnormalities in human spermatozoa using multicolour fluorescence in-situ hybridization. Hum Reprod. 1998;13(9):2489–2494. doi: 10.1093/humrep/13.9.2489. [DOI] [PubMed] [Google Scholar]
- 15.Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69(5):1046–1054. doi: 10.1086/323763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubes J, Vozdova M, Oracova E, Perreault SD. Individual variation in the frequency of sperm aneuploidy in humans. Cytogenet Genome Res. 2005;111(3–4):229–236. doi: 10.1159/000086893. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Fan HC, Behr B, Quake SR. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell. 2012;150(2):402–412. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 19.Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, et al. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum ReprodHum Reprod. 2002;17(10):2600–2614. doi: 10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- 20.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1(1):40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 21.Moolgavkar SH, Knudson AG., Jr Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 1981;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- 22.Conrad DF, Keebler JEM, DePristo MA, Lindsay SJ, Zhang YJ, Casals F, et al. Variation in genome-wide mutation rates within and between human families. Nat Genet. 2011;43(7):712–U137. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahbari R, Wuster A, Lindsay SJ, Hardwick RJ, Alexandrov LB, Turki SA, et al. Timing, rates and spectra of human germline mutation. Nat Genet. 2016;48(2):126–133. doi: 10.1038/ng.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goriely A, McVean GA, Rojmyr M, Ingemarsson B, Wilkie AO. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science (New York, NY) 2003;301(5633):643–646. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- 26.Maher GJ, Goriely A, Wilkie AO. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology. 2014;2(3):304–314. doi: 10.1111/j.2047-2927.2013.00175.x. [DOI] [PubMed] [Google Scholar]
- 27.Makova KD, Li WH. Strong male-driven evolution of DNA sequences in humans and apes. Nature. 2002;416(6881):624–626. doi: 10.1038/416624a. [DOI] [PubMed] [Google Scholar]
- 28.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goriely A, McGrath JJ, Hultman CM, Wilkie AO, Malaspina D. “Selfish spermatogonial selection”: a novel mechanism for the association between advanced paternal age and neurodevelopmental disorders. Am J Psychiatry. 2013;170(6):599–608. doi: 10.1176/appi.ajp.2013.12101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiemann-Boege I, Navidi W, Grewal R, Cohn D, Eskenazi B, Wyrobek AJ, et al. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc Natl Acad Sci U S A. 2002;99(23):14952–14957. doi: 10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risch N, Reich EW, Wishnick MM, Mccarthy JG. Spontaneous mutation and parental age in humans. Am J Hum Genet. 1987;41(2):218–248. [PMC free article] [PubMed] [Google Scholar]
- 32.Glaser RL, Broman KW, Schulman RL, Eskenazi B, Wyrobek AJ, Jabs EW. The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm. Am J Hum Genet. 2003;73(4):939–947. doi: 10.1086/378419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaser RL, Jiang W, Boyadjiev SA, Tran AK, Zachary AA, Van Maldergem L, et al. Paternal origin of FGFR2 mutations in sporadic cases of Crouzon syndrome and Pfeiffer syndrome. Am J Hum Genet. 2000;66(3):768–777. doi: 10.1086/302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hingorani M, et al. Aniridia. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle: University of Washington; 2003. [Google Scholar]
- 35.Olson JM, Breslow NE, Beckwith JB. Wilms’ tumour and parental age: a report from the National Wilms’ Tumour Study. Br J Cancer. 1993;67(4):813–818. doi: 10.1038/bjc.1993.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dockerty JD, Draper G, Vincent T, Rowan SD, Bunch KJ. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30(6):1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 37.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35(6):1495–1503. doi: 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 38.Stonebraker JS, Bolton-Maggs PH, Soucie JM, Walker I, Brooker M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16(1):20–32. doi: 10.1111/j.1365-2516.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 39.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 40.Nyhan WL, O'Neill JP, Jinnah HA, Harris JC, et al. Lesch-Nyhan syndrome. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle: University of Washington; 2000. [Google Scholar]
- 41.Keane MG, Pyeritz RE. Medical management of Marfan syndrome. Circulation. 2008;117(21):2802–2813. doi: 10.1161/CIRCULATIONAHA.107.693523. [DOI] [PubMed] [Google Scholar]
- 42.Carlson KM, Bracamontes J, Jackson CE, Clark R, Lacroix A, Wells SA, Jr, et al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am J Hum Genet. 1994;55(6):1076–1082. [PMC free article] [PubMed] [Google Scholar]
- 43.Schuffenecker I, Ginet N, Goldgar D, Eng C, Chambe B, Boneu A, et al. Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma Le Groupe d’Etude des Tumeurs a Calcitonine. Am J Hum Genet. 1997;60(1):233–237. [PMC free article] [PubMed] [Google Scholar]
- 44.Bunin GR, Needle M, Riccardi VM. Paternal age and sporadic neurofibromatosis 1: a case-control study and consideration of the methodologic issues. Genet Epidemiol. 1997;14(5):507–516. doi: 10.1002/(SICI)1098-2272(1997)14:5<507::AID-GEPI5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol. 2002;249(5):584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- 46.Orioli IM, Castilla EE, Scarano G, Mastroiacovo P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. Am J Med Genet. 1995;59(2):209–217. doi: 10.1002/ajmg.1320590218. [DOI] [PubMed] [Google Scholar]
- 47.Carothers AD, McAllion SJ, Paterson CR. Risk of dominant mutation in older fathers: evidence from osteogenesis imperfecta. J Med Genet. 1986;23(3):227–230. doi: 10.1136/jmg.23.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera B, Gonzalez S, Sanchez-Tome E, Blanco I, Mercadillo F, Leton R, et al. Clinical and genetic characterization of classical forms of familial adenomatous polyposis: a Spanish population study. Ann Oncol. 2011;22(4):903–909. doi: 10.1093/annonc/mdq465. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon LB, Brown WT, Collins FS. Hutchinson-Gilford Progeria Syndrome. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews. [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. 2003 Dec 12 [updated 2015 Jan 8]. [PubMed]
- 52.Splendore A, Jabs EW, Felix TM, Passos-Bueno MR. Parental origin of mutations in sporadic cases of Treacher Collins syndrome. Eur J Hum Genet. 2003;11(9):718–722. doi: 10.1038/sj.ejhg.5201029. [DOI] [PubMed] [Google Scholar]
- 53.Northrup H, Koenig MK, Au KS. Tuberous sclerosis complex. Seattle: University of Washington; 2003. [PubMed] [Google Scholar]
- 54.Sampson JR, Scahill SJ, Stephenson JB, Mann L, Connor JM. Genetic aspects of tuberous sclerosis in the west of Scotland. J Med Genet. 1989;26(1):28–31. doi: 10.1136/jmg.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamayo ML, Gelvez N, Rodriguez M, Florez S, Varon C, Medina D, et al. Screening program for Waardenburg syndrome in Colombia: clinical definition and phenotypic variability. Am J Med Genet A. 2008;146A(8):1026–1031. doi: 10.1002/ajmg.a.32189. [DOI] [PubMed] [Google Scholar]
- 56.Milunski JM, et al. Waardenburg syndrome type I. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews. Seattle: University of Washington; 2001. [Google Scholar]
- 57.Yang Q, Wen SW, Leader A, Chen XK, Lipson J, Walker M. Paternal age and birth defects: how strong is the association? Hum Reprod. 2007;22(3):696–701. doi: 10.1093/humrep/del453. [DOI] [PubMed] [Google Scholar]
- 58.Lian ZH, Zack MM, Erickson JD. Paternal age and the occurrence of birth defects. Am J Hum Genet. 1986;39(5):648–660. [PMC free article] [PubMed] [Google Scholar]
- 59.Olshan AF, Schnitzer PG, Baird PA. Paternal age and the risk of congenital heart defects. Teratology. 1994;50(1):80–84. doi: 10.1002/tera.1420500111. [DOI] [PubMed] [Google Scholar]
- 60.Green RF, Devine O, Crider KS, Olney RS, Archer N, Olshan AF, et al. Association of paternal age and risk for major congenital anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann Epidemiol. 2010;20(3):241–249. doi: 10.1016/j.annepidem.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montgomery SM, Lambe M, Olsson T, Ekbom A. Parental age, family size, and risk of multiple sclerosis. Epidemiology. 2004;15(6):717–723. doi: 10.1097/01.ede.0000142138.46167.69. [DOI] [PubMed] [Google Scholar]
- 62.Murray L, McCarron P, Bailie K, Middleton R, Davey Smith G, Dempsey S, et al. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. 2002;86(3):356–361. doi: 10.1038/sj.bjc.6600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Kreger BE, Dorgan JF, Cupples LA, Myers RH, Splansky GL, et al. Parental age at child’s birth and son’s risk of prostate cancer. Framingham Study Am J Epidemiol. 1999;150(11):1208–1212. doi: 10.1093/oxfordjournals.aje.a009947. [DOI] [PubMed] [Google Scholar]
- 64.Choi JY, Lee KM, Park SK, Noh DY, Ahn SH, Yoo KY, et al. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer. 2005;5:143. doi: 10.1186/1471-2407-5-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slama R, Bouyer J, Windham G, Fenster L, Werwatz A, Swan SH. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol. 2005;161(9):816–823. doi: 10.1093/aje/kwi097. [DOI] [PubMed] [Google Scholar]
- 66.Reichman NE, Teitler JO. Paternal age as a risk factor for low birthweight. Am J Public Health. 2006;96(5):862–866. doi: 10.2105/AJPH.2005.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harlap S, Paltiel O, Deutsch L, Knaanie A, Masalha S, Tiram E, et al. Paternal age and preeclampsia. Epidemiology. 2002;13(6):660–667. doi: 10.1097/00001648-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Cardwell CR, Carson DJ, Patterson CC. Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood type 1 diabetes: a UK regional retrospective cohort study. Diabet Med. 2005;22(2):200–206. doi: 10.1111/j.1464-5491.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 69.Vestergaard M, Mork A, Madsen KM, Olsen J. Paternal age and epilepsy in the offspring. Eur J Epidemiol. 2005;20(12):1003–1005. doi: 10.1007/s10654-005-4250-2. [DOI] [PubMed] [Google Scholar]
- 70.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, et al. Advancing paternal age and autism. Arch Gen Psychiat. 2006;63(9):1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 71.de Kluiver H, Buizer-Voskamp JE, Dolan CV, Boomsma DI. Paternal age and psychiatric disorders: a review. Am J Med Genet B Neuropsychiatr Genet. 2017;174(3):202–213. doi: 10.1002/ajmg.b.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rasmussen F. Paternal age, size at birth, and size in young adulthood—risk factors for schizophrenia. Eur J Endocrinol. 2006;155:S65–SS9. doi: 10.1530/eje.1.02264. [DOI] [Google Scholar]
- 73.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 74.Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6(3):e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayasekara R, Street J. Parental age and parity in dyslexic boys. J Biosoc Sci. 1978;10(3):255–261. doi: 10.1017/S002193200001172X. [DOI] [PubMed] [Google Scholar]
- 76.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1034–1040. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 77.Bertram L, Busch R, Spiegl M, Lautenschlager NT, Muller U, Kurz A. Paternal age is a risk factor for Alzheimer disease in the absence of a major gene. Neurogenetics. 1998;1(4):277–280. doi: 10.1007/s100480050041. [DOI] [PubMed] [Google Scholar]
- 78.Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet. 2014;10(7):e1004458. doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milekic MH, Xin Y, O'Donnell A, Kumar KK, Bradley-Moore M, Malaspina D, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry. 2015;20(8):995–1001. doi: 10.1038/mp.2014.84. [DOI] [PubMed] [Google Scholar]
- 80.D'Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjolander A, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71(4):432–438. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Day K, Waite LL, Thalacker-Mercer A, West A, Bamman MM, Brooks JD, et al. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biol. 2013;14(9):R102. doi: 10.1186/gb-2013-14-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–1408. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 83.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103(25):9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchetti F, Wyrobek AJ. Mechanisms and consequences of paternally-transmitted chromosomal abnormalities. Birth Defects Res C Embryo Today. 2005;75(2):112–129. doi: 10.1002/bdrc.20040. [DOI] [PubMed] [Google Scholar]
- 85.Nybo Andersen AM, Hansen KD, Andersen PK, Davey SG. Advanced paternal age and risk of fetal death: a cohort study. Am J Epidemiol. 2004;160(12):1214–1222. doi: 10.1093/aje/kwh332. [DOI] [PubMed] [Google Scholar]
- 86.Ramasamy R, Chiba K, Butler P, Lamb DJ. Male biological clock: a critical analysis of advanced paternal age. Fertil Steril. 2015;103(6):1402–1406. doi: 10.1016/j.fertnstert.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuhnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update. 2004;10(4):327–339. doi: 10.1093/humupd/dmh030. [DOI] [PubMed] [Google Scholar]
- 88.Lambert SM, Masson P, Fisch H. The male biological clock. World J Urol. 2006;24(6):611–617. doi: 10.1007/s00345-006-0130-y. [DOI] [PubMed] [Google Scholar]
- 89.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16(1):65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 90.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58(4):361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 91.Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, et al. Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002;159(9):1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forster P, Hohoff C, Dunkelmann B, Schurenkamp M, Pfeiffer H, Neuhuber F, et al. Elevated germline mutation rate in teenage fathers. Proc Biol Sci. 2015;282(1803):20142898. doi: 10.1098/rspb.2014.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]