Highlights
-
•
Radical trachelectomy is a fertility sparing option for select stage I cervical patients.
-
•
Indications for adjuvant treatment following trachelectomy are based on standard recommendations following hysterectomy.
-
•
Case report outlining specifics of imaged-guided brachytherapy as part of the adjuvant treatment after trachelectomy.
Keywords: Trachelectomy, Cervical cancer, Cervical brachytherapy, Pelvic radiation, Fertility preservation
1. Introduction
Select patients with stage I cervical cancer may be eligible for fertility sparing surgery. Radical trachelectomy with lymphadenectomy is a reasonable option for carefully selected patients with stage IA2 to IB1 cervical cancer who desire fertility preservation and have lesions <2 cm with no radiographic evidence of nodal disease (Couchard-Fortier et al., 2014; Kato et al., 2015).
The 2018 NCCN (National Comprehensive Cancer Network) guidelines recommend adjuvant radiation therapy after radical trachelectomy for patients with intermediate or high-risk features; prior to 2018, there were no recommendations and practices varied across institutions (Sedlis et al., 1999; Peters et al., 2000; Monk et al., 2005). In a review of several large series on radical vaginal trachelectomy, 10% of patients received further treatment, in the form of either radical hysterectomy or radiation with or without chemotherapy due to positive nodes, positive margins, or unusual histologic subtypes (Beiner and Covens, 2007). In a series of 101 patients who underwent abdominal radical trachelectomy, 20% required conversion to hysterectomy and 20% received adjuvant chemotherapy and/or radiation (Wethington et al., 2012). The rates of conversion to hysterectomy or pathology requiring adjuvant treatment appear to be higher for patients with stage IB1 disease and larger tumors measuring 2-4 cm, with treatment limited to a fertility-sparing procedure in only approximately 30% of these patients (Wethington et al., 2013).
In one series, among 29 patients with stage IB1 cervical cancer eligible for fertility sparing surgery with radical trachelectomy, 40% required adjuvant chemoradiation due to risk factors found only on final pathology (Wethington et al., 2013). Intraoperative procedures recommended include frozen section of suspicious pelvic lymph nodes, a shaved endocervical margin, and endometrial curettage.
The most common indications for adjuvant therapy after trachelectomy are high-risk features, including positive nodes, parametria, or margins, as defined by Peters et al. (2000). Adjuvant therapy is also given for intermediate risk features, including lymphovascular invasion (LVI), deep stromal invasion, and large tumor diameter (Sedlis et al., 1999; Rotman et al., 2006). Cases describing the radiation treatment requirements including the use of brachytherapy after trachelectomy have not been reported.
In this case report, we will outline specifics of image-guided brachytherapy as part of the adjuvant treatment of two patients with high-risk features after radical abdominal trachelectomy.
2. Case 1
The first patient is a 35-year old G0 woman who initially presented with high-grade squamous intraepithelial lesion on screening Pap smear. She underwent colposcopy with biopsy and endocervical curettage (ECC) revealing cervical intraepithelial neoplasia (CIN) II and normal endocervical tissue. A follow up loop electrosurgical excision procedure (LEEP) revealed poorly differentiated invasive squamous cell carcinoma at least 4 mm deep and 7 mm wide with positive margins and LVI. Magnetic resonance imaging (MRI) of the pelvis showed no lymphadenopathy or parametrial extension. Computed tomography (CT) abdomen and pelvis confirmed no adenopathy.
A robotic laparoscopically-assisted radical trachelectomy and pelvic lymphadenectomy was performed. The radical trachelectomy removed the cervix, upper 2 cm of the vagina, 1/3 of the lower parametrial web and 1/3 of the uterosacral ligaments. Intraoperatively, frozen section pathology of endocervical, radial, and ectocervical margins were negative. Final pathology revealed a moderately to poorly differentiated squamous cell carcinoma with basaloid features and LVI, 1.9 cm in greatest dimension with 6 mm out of 10 mm stromal invasion (depth of invasion 60%). The cervical soft tissue margin was positive. One of 13 positive left pelvic lymph nodes and 0 of 14 positive right pelvic lymph nodes were positive. Due to the high risk features, adjuvant chemoradiation was recommended.
The patient underwent ovarian stimulation and oocyte retrieval for in vitro fertilization. She underwent left salpingo-oophoropexy for preservation of ovarian function in anticipation of adjuvant radiation therapy. A restaging positron emission tomography–computed tomography (PET/CT) prior to initiating treatment was negative.
She was prescribed external beam radiation therapy (EBRT) along with weekly cisplatin. An intensity-modulated radiation therapy (IMRT) plan (Fig. 1) was used to treat the pelvis and pelvic lymph nodes up to L5/S1 to 45 Gy in 25 fractions. She was switched from cisplatin to carboplatin for the last 2 weeks due to tinnitus without hearing loss. She did not experience any skin toxicity during treatment. Two weeks after completing EBRT, she received MRI-guided-high-dose-rate (HDR) brachytherapy boost with tandem and ring, 600 cGy for 3 fractions over 3 weeks. The tandem and ring (T/R) brachytherapy applicator permitted adequate dosing to the cervico-uterine junction and the adjacent parametrial tissue.
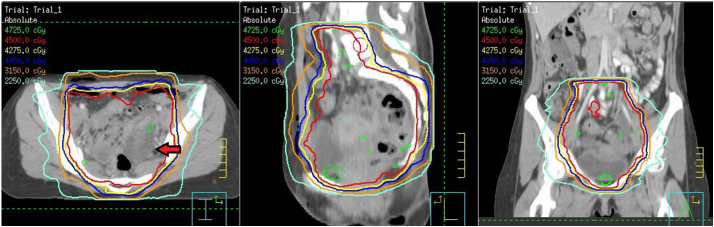
Fig. 1.
Case 1 pelvic intensity-modulated radiation therapy plan, 45 Gy in 2 fx. Arrow points to enlarged ovary due to ovarian stimulation for oocyte retrieval.
An MR scan was obtained following insertion to confirm T/R applicator placement and to plan treatment. The bladder, rectum, sigmoid and clinical target volume (CTV) structures were contoured. In order to contour the CTV, the lower uterine segment was contoured for approximately 2–3 cm along the length of the tandem to the edge of the uterus, and adjacent parametria were included (Fig. 2).
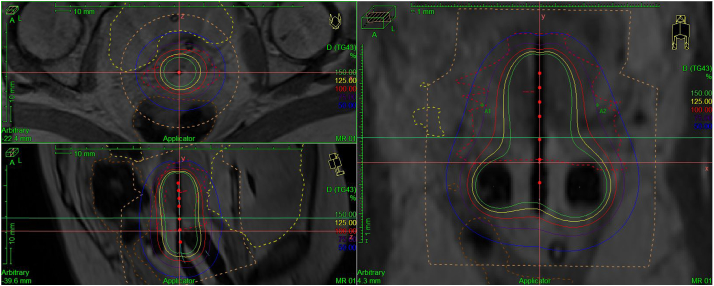
Fig. 2.
Case 1 tandem and ring high-dose-rate brachytherapy MRI treatment plan, 6 Gy. Isodose lines (solid) represent percentage of prescribed dose per fraction. Clinical target volume (red) and normal structures represented by dotted lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The patient was treated with HDR brachytherapy with iridium-192, using an MR-based plan to cover all required areas, rather than point A. The total equivalent dose in 2 Gy per fraction (EQD2) was 68.3 Gy, chosen to eradicate micrometastatic disease in the uterus and parametria, and at the positive margin. Total dose and constraints were calculated using the equivalent 2Gy doses of EBRT and brachytherapy. The cumulative dose to 2 cc or more (D2cc) of rectum was 59 Gy, bladder was 59 Gy, and sigmoid was 49 Gy. The remaining 2 fractions were delivered in 1 week intervals in a similar manner.
She was seen for 6 month and 12 month follow up without any issues related to the radiation therapy and no evidence of progression or recurrence. She was counseled on regular use of vaginal dilators to prevent vaginal stenosis and remains sexually active.
3. Case 2
The second case is a 28-year-old G0 woman who initially presented with atypical glandular cells on a Pap smear. Colposcopy confirmed adenocarcinoma in situ (AIS). She underwent cervical conization that revealed invasive adenocarcinoma in a background of AIS and CIN3. Endocervical and deep margins were negative but ectocervical margins were positive for invasive adenocarcinoma with extensive LVI and CIN3. The tumor had at least 4 mm horizontal spread and cervical stromal invasion was at least 3 out of 35 mm. An MRI of the pelvis was negative for radiographic evidence of residual primary disease and a PET/CT was negative for metastasis.
She underwent a radical abdominal trachelectomy and bilateral pelvic lymphadenectomy. The cervix, bilateral parametria and upper 2 cm of the vagina were removed. Final pathology of the trachelectomy specimen revealed a minute focus of invasive adenocarcinoma with associated LVI. Margins were negative. One of 3 right parametrial lymph nodes, 0 of 1 left parametrial lymph nodes, 1 of 4 right pelvic lymph nodes, and 0 of 4 left pelvic lymph nodes were involved.
Adjuvant chemo-radiation was recommended. After extensive counseling, she opted to forego preservation of ovarian function with transposition due to the increased risk of ovarian metastasis with adenocarcinoma of the cervix. Additionally, she was not interested in having a biologic child through assisted reproductive technology (ART) and surrogacy and so did not proceed with oocyte stimulation or retrieval. She was treated with external beam radiation along with weekly cisplatin. IMRT plan was used to treat the pelvis and pelvic lymph nodes up to L4 to 4500 cGy in 25 fractions. One week after completing EBRT, she started MRI guided HDR T/R brachytherapy, 500 cGy for 3 fractions over 3 weeks (Fig. 3) for a total EQD2 of 63 Gy. Rectal D2cc was 53 Gy, bladder 53 Gy, sigmoid 53 Gy, and bowel 56 Gy. The final dose was selected to eradicate micrometastatic disease in the setting of negative margins, and to spare the bowel, while covering the parametria and uterus appropriately.
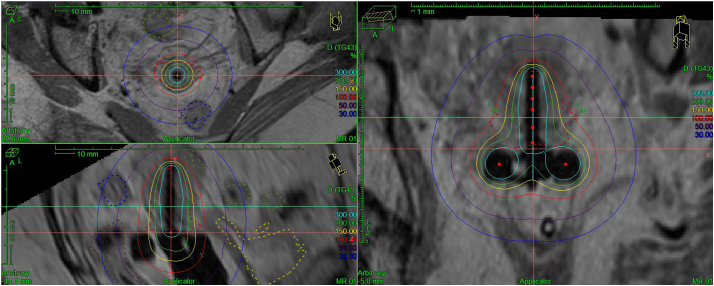
Fig. 3.
Case 2 tandem and ring high-dose-rate brachytherapy MRI treatment plan, 5 Gy. Isodose lines represent percentage of prescribed dose per fraction. Clinical target volume (red) and normal structures represented by dotted lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
She was seen for 3 and 6 month follow up without any evidence of progression or recurrence. She did have persistent vaginal discharge, likely related to cerlage erosion. This was treated by transvaginal removal of extruded suture material. She remains amenorrheic since completing radiation and was prescribed conjugated estrogens/medroxyprogesterone acetate for hormonal replacement and remains sexually active.
4. Discussion
While there are limited data to guide recommendations for adjuvant treatment following radical trachelectomy, there are data supporting radical radiation after radical hysterectomy or for inoperable cervical cancer. The role of adjuvant radiation therapy was first established in early stage 1B cervical cancer patients treated with radical hysterectomy with lymph node dissection with at least two of the following risk factors: >1/3 stromal invasion, LVI, and large clinical tumor diameter (Sedlis et al., 1999). The study demonstrated a local control benefit with the addition of adjuvant pelvic radiation therapy compared to observation; similar results, including improved survival, were seen with chemoradiation for patients with positive lymph nodes, positive margins or positive residual parametria (Peters et al., 2000; Rotman et al., 2006). Patients with stage IA2-IIA who received concurrent chemotherapy with whole pelvis radiation therapy had significantly higher 4-year overall survival compared to those receiving radiation therapy alone (81% vs. 71%) (Peters et al., 2000).
In support of standardizing these generalizable concepts, radiation is indicated for post-trachelectomy patients based on high or intermediate risk features in the 2018 NCCN guidelines (National Comprehensive Cancer Network, 2018). Completion hysterectomy after trachelectomy is not recommended in patients that require adjuvant radiation as the presence of an intact uterus pushes small bowel from the pelvic radiation field and results in fewer acute bowel side effects and long-term risk of fistula.
In one retrospective review comparing outcomes of radical trachelectomy versus radical hysterectomy for stage IB1 patients, there was no difference in 5-year recurrence-free survival (Diaz et al., 2008). Patients in both groups who met intermediate-risk and high-risk characteristics were counseled to undergo adjuvant radiation therapy. Patients undergoing radical trachelectomy should always be counseled on the possibility of finding metastatic or high-intermediate risk stage I disease, which would require radical hysterectomy and/or adjuvant chemoradiation.
Unique in this report, we describe the use of MR-guided brachytherapy after trachelectomy and chemoradaition. MR allows more precise delineation of residual disease than CT (Viswanathan et al., 2014a) and MR-brachytherapy results in excellent clinical outcomes (Kamran et al., 2017). In one resource-constrained country, the societal cost-benefit in womens' lives saved due to MR-based brachytherapy exceeds the cost of buying an MR scanner (Chakraborty et al., 2017).
The ABS recommends radiographic localization with high geometric precision (Viswanathan and Thomadsen, 2012). CT and MR-based localization allows for correlation of anatomic data with source positioning and results in more accurate contouring. CT can be used for planning, but does not provide adequate delineation of the tumor due to poor tissue contrast and leads to overestimating the lateral extent of the cervix compared to MRI (Viswanathan et al., 2014b).
Dose limits in the setting of microscopic disease were based on the general principle to treat as low as feasible to normal tissues while covering areas of potential disease spread. Furthermore, doses to the CTV including the uterus and parametria were extrapolated from the intermediate-risk clinical target volume (IR-CTV) recommendation of at least an EQD2 of 60 Gy (Viswanathan and Thomadsen, 2012). Though parametrial boosts are no longer routinely required in all cases that receive image-guided brachytherapy, ensuring adequate dose coverage to the uterus and parametria is crucial to preventing a potential life-threatening central recurrence.
Conflicts of interest
The authors have no conflicts of interest.
References
- Beiner M.E., Covens A. Surgery insight: radical vaginal trachelectomy as a method of fertility preservation for cervical cancer. Nat. Clin. Pract. Oncol. 2007;4:353–361. doi: 10.1038/ncponc0822. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Mahantshetty U., Chopra S. Income generated by women treated with magnetic resonance imaging-based brachytherapy: a simulation study evaluating the macroeconomic benefits of implementing a high-end technology in a public sector healthcare setting. Brachytherapy. 2017;16(5):981–987. doi: 10.1016/j.brachy.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Couchard-Fortier G., Reade C.J., Covers A. Non-radical surgery for small early-stage cervical cancer. Is it time? Gynelcol. Oncol. 2014;132:624–627. doi: 10.1016/j.ygyno.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Diaz J.P., Sonoda Y., Leitao M.M. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol. Oncol. 2008;111(2):255–260. doi: 10.1016/j.ygyno.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Kamran S.C., Manuel M.M., Cho L.P. Comparison of outcomes for MR-guided versus CT-guided high-dose-rate interstitial brachytherapy in women with locally advanced carcinoma of the cervix. Gynecol. Oncol. 2017;145(2):284–290. doi: 10.1016/j.ygyno.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Takashima A., Kasamatsu T. Clinical tumor diameter and prognosis of patients with FIGO stage IB1 cervical cancer (JCOG0806-A) Gynecol. Oncol. 2015;137:34–39. doi: 10.1016/j.ygyno.2015.01.548. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Wang J., Im S. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol. Oncol. 2005;96(3):721–728. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network Cervical Cancer (Version 1.2018) 2018. https://www.nccn.org/professionals/physician_gls/pdf/cervical_blocks.pdf
- Peters W.A., Liu P.Y., Barrett R.J. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- Rotman M., Sedlis A., Piedmonte M.R. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(1):169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sedlis A., Bundy B.N., Rotman M.Z. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. Gynecol. Oncol. 1999 May;73(2):177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- Viswanathan A.N., Thomadsen B. American brachytherapy society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012;11(1):33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Viswanathan A.N., Erickson B., Gaffney D.K. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;90(2):320–328. doi: 10.1016/j.ijrobp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A.N., Erickson B., Gaffney D.K. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;90(2):320–328. doi: 10.1016/j.ijrobp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethington S.L., Cibula D., Duska L.R. An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int. J. Gynecol. Cancer. 2012;22(7):1251. doi: 10.1097/IGC.0b013e318263eee2. [DOI] [PubMed] [Google Scholar]
- Wethington S.L., Sonoda Y., Park K.J. Expanding the indications for radical trachelectomy: a report on 29 patients with stage IB1 tumors measuring 2 to 4 centimeters. Int. J. Gynecol. Cancer. 2013;23(6):1092–1098. doi: 10.1097/IGC.0b013e318296034e. [DOI] [PMC free article] [PubMed] [Google Scholar]