Highlights
-
•
A young lady with uterine sarcoma had a successful delivery 3 years after diagnosis.
-
•
Local recurrence occurred after 8 years.
-
•
Ultrasound and endometrial biopsy can be used in the follow-up of these patients.
-
•
Patients should be counselled on risk of late recurrence.
Keywords: Fertility sparing, Adenosarcoma, Uterus
1. Introduction
Uterine adenosarcomas (AS) are rare malignancies and make up 5% of uterine sarcomas (Friedlander et al., 2014; Hensley, 2012). They are usually low-grade tumors and are characterized by a benign epithelial component with a malignant mesenchymal component, which is typically a low-grade endometrial stromal sarcoma but can also be a high-grade sarcoma (Hensley, 2012). Patients diagnosed with uterine adenosarcomas generally have a good prognosis with the exception of deeply invasive tumors or those with high-grade sarcomatous overgrowth.
The standard management is total hysterectomy and bilateral salpingo-oopherectomy. However, the difficulty arises in young patients with uterine adenosarcomas who wish to preserve their fertility. In view of the rarity of uterine adenosarcomas, there are limited data on the safety and follow-up in women opting for fertility sparing surgery. There has only one previous study (Lee et al., 2017) reporting a successful pregnancy.
We present a case report of a young lady with uterine adenosarcoma who underwent fertility sparing surgery with a successful normal delivery after. She subsequently had a local recurrence after 8 years and underwent staging surgery.
2. Case report
A 21-year-old lady presented in February 2006 with symptomatic anaemia secondary to menorrhagia. She was a virgo intacta and nulliparous. She had a significant medical history of thalassemia minor. Her haemoglobin level at presentation was 6.3 g/dL and required transfusion.
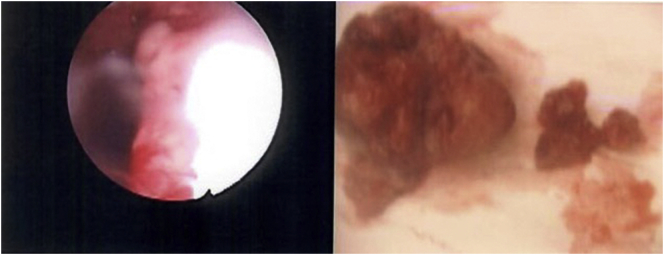
An ultrasound of the pelvis done during the admission noted focal thickening of the endometrial in the lower uterus suspicious for an endometrial polyp or submucosal fibroid. She underwent hysteroscopy, dilation and curettage and polypectomy. A 3 cm polyp protruding from cervix was noted intraoperatively (Fig. 1). Histology of the endometrial polyp showed low-grade mullerian adenosarcoma. She was diagnosed with clinical stage 1 low-grade uterine adenosarcoma.
Fig. 1.
Hysteroscopy showing polyp extruding for cervix and specimen removed.
The patient declined hysterectomy as she was keen to conserve fertility. She was monitored in the hospital's gynaecologic cancer centre with interval ultrasounds and offered hysterectomy after completion of family. Regular ultrasounds between 2006 and 2009 showed no significant abnormalities.
She had a spontaneous conception in 2009. The patient was antenatally well and underwent a term normal vaginal delivery in March 2010. Post delivery, she resumed follow-up at the cancer centre.
An ultrasound pelvis in February 2014 showed a thickened endometrium with vascularity. The patient was asymptomatic. She reported regular menses and normal flow. An endometrial sampling was performed which showed low-grade adenosarcoma. Her disease free interval was 8 years.
A magnetic resonance imaging (MRI) of the pelvis (Fig. 2) was done preoperatively which showed a polypoidal nodule is seen in the lower uterine cavity with possible involvement of the junctional zone. There was no evidence of distant metastasis on MRI and chest X-ray.
Fig. 2.
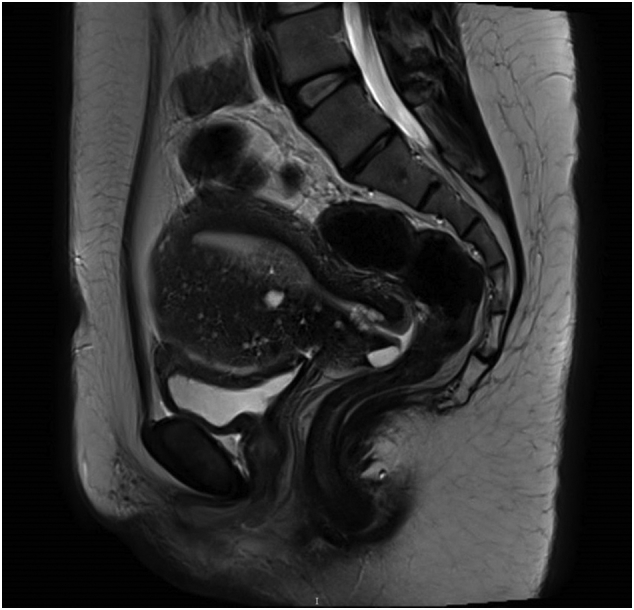
MRI pelvis showing a polypoidal tumor at the lower uterine segment.
The patient underwent laparoscopic total hysterectomy, bilateral salpingo-oopherectomy with bilateral pelvic lymph node dissection in May 2014 (Fig. 3). Histology showed a low-grade mullerian adenosarcoma with 81% myometrial invasion. All 15 pelvic lymph nodes resected were negative for malignancy. She has recovered well postoperatively and is currently disease free for 3 years and 7 months.
Fig. 3.
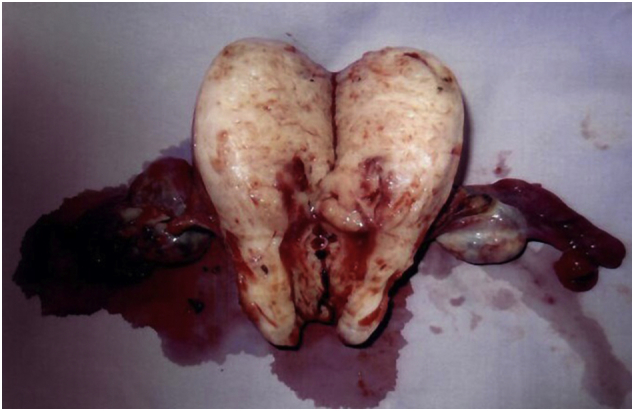
Cut specimen of the uterus and tumor during staging surgery.
3. Discussion
In a review of cases in our centre from 2005 to 2015, there were 23 uterine adenosarcomas diagnosed among 125 patients with uterine sarcomas (18.4%). 16 patients (69.6%) had low-grade disease. This is higher than the reported frequency of 5–10% in literature (Friedlander et al., 2014; Khatib et al., 2014), although the reason for this difference is uncertain. Patients of a variety of ages are affected: the largest series reported 100 cases in patients aged 14–89 years, with the median age at presentation 58 years (Carroll et al., 2014). The median age of diagnosis of patients with uterine AS in our centre was 48 years (range 21 to 87). The study population in the abovementioned studies involved non-Asian patients. More studies need to be done to determine if there is potential genetic predispositions among Asians and if the differences in frequency and age of presentation are significant.
In literature, the majority of patients with uterine AS presented with early-stage tumors and had a favorable outcome (Friedlander et al., 2014; Arend et al., 2010). A review of survival outcomes of Stage 1 uterine AS in USA reported a five-year survival of 79% (95% CI, 75–84%) for stage 1 adenosarcomas. The results from our centre correspond with those in literature. In our centre, 19 out of 23 patients (82.6%) were diagnosed with Stage 1 disease. Among the stage 1 disease, most (74.7%) were low-grade adenosarcomas. The 5-year survival of patients with Stage 1 disease in our centre was 92.6%. The 5-year survival the subset of patients with Stage 1 low-grade adenosarcoma was 100%.
Literature on fertility preservation in patients with uterine AS is limited. There is only one case series on the safety and feasibility of uterine preservation in patients with uterine AS (Lee et al., 2017). The retrospective study done in Korea reported 7 patients opting for uterine preservation among the 31 patients were diagnosed with Stage 1 uterine AS. One out of the 7 patients had a successful vaginal delivery and remained disease free for 77 months. 2 out of 7 patients had recurrences. One of the patients had disease recurrence with peritoneal seeding 10 months after adjuvant chemotherapy. She was the only patient in the group of 7 to have sarcomatous overgrowth. The disease free intervals of the two patients with recurrence were 13 months and 27 months. The median follow-up time was 32 months (range: 12–77 months). The strength of our case report is the long follow-up interval. She has been on follow-up in our centre for 11 years and had a DFI of 8 years before recurrence. This highlights the risk of late recurrence in patients with uterine AS. Thus, adequate counseling and long-term follow-up are pertinent in the care of patients opting for uterine preservation.
Ovarian preservation may have a potential role in fertility preservation in premenopausal patients with uterine AS. A recent retrospective review by Nasioudis D et al. (Nasioudis et al., 2017) on the safety of ovarian preservation in premenopausal women with Stage I uterine sarcoma found that ovarian preservation was not associated with worse oncological outcomes. However, the limitations of the study include the retrospective nature as well as the low prevalence of AS (162/1482). The authors conclude that further multi-institutional prospective collaborations are warranted to further clarify the oncologic safety of ovarian preservation for women with AS.
Previous studies (Lee et al., 2017; Carroll et al., 2014) have shown that sarcomatous overgrowth and lymphovascular invasion are associated with increased risk of recurrence. These factors should be considered in selection of appropriate patients for uterine preservation. Patients with high-risk features such as sarcomatous overgrowth should not be offered uterine preservation for fertility sparing and should be offered hysterectomy as gold standard treatment.
There is currently insufficient evidence in literature on the monitoring of patients opting for fertility preservation. Our centre performs interval ultrasounds with endometrial sampling in the follow-up of these patients. In this case report, the recurrence was diagnosed while patient was still asymptomatic, allowing for early diagnosis and treatment.
4. Conclusion
Uterine preservation in low-grade uterine adenosarcoma is controversial in view of the risk of recurrence. In selected young female patients with low-grade early stage disease wishing to maintain fertility and opting for fertility preservation, adequate counseling and close follow-up is required for many years. While there is chance of future successful pregnancy, one should still bear in mind the risk of late recurrence.
The following are the supplementary data related to this article.
Supplementary Fig. S1.
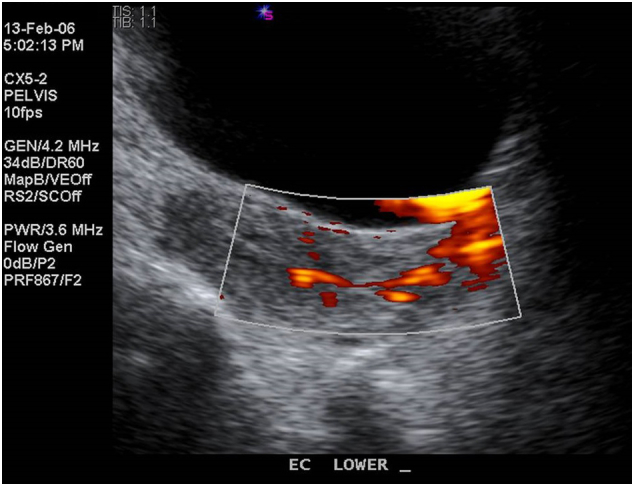
Transabdominal ultrasound done in 2006 showing focal thickening at lower uterine segment with vascularity, suggestive of a polyp.
Supplementary Fig. S2.
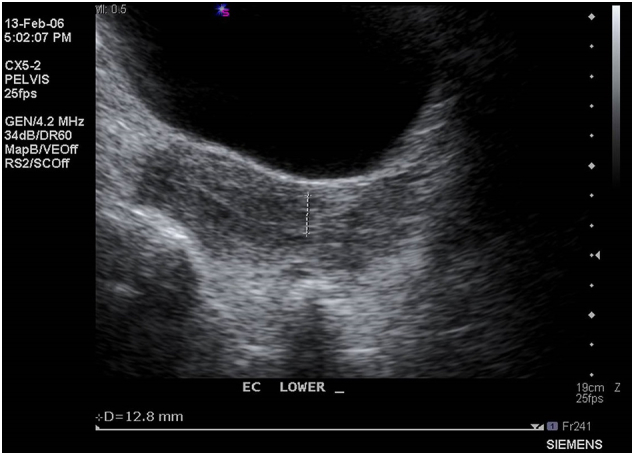
Transvaginal ultrasound in 2014 showing a thickened endometrium with increased vascularity suspicious for recurrence.
Funding
This project has not received any funding.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Arend R., Bagaria M., Lewin S.N., Sun X., Deutsch I., Burke W.M., Herzog T.J., Wright J.D. Long-term outcome and natural history of uterine adenosarcomas. Gynecol. Oncol. 2010;119(2):305–308. doi: 10.1016/j.ygyno.2010.07.001. Nov. [DOI] [PubMed] [Google Scholar]
- Carroll A., Ramirez P.T., Westin S.N., Soliman P.T., Munsell M.F., Nick A.M., Schmeler K.M., Klopp A.H., Fleming N.D. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol. Oncol. 2014;135(3):455–461. doi: 10.1016/j.ygyno.2014.10.022. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M.L., Covens A., Glasspool R.M., Hilpert F., Kristensen G., Kwon S., Selle F., Small W., Witteveen E., Russell P. Gynecologic Cancer InterGroup (GCIG) consensus review for mullerian adenosarcoma of the female genital tract. Int. J. Gynecol. Cancer. 2014;24(9 Suppl. 3):S78–S82. doi: 10.1097/IGC.0000000000000239. Nov. [DOI] [PubMed] [Google Scholar]
- Hensley M.L. Uterine sarcomas: histology and its implications on therapy. Am. Soc. Clin. Oncol. Educ. Book. 2012:356–361. doi: 10.14694/EdBook_AM.2012.32.7. [DOI] [PubMed] [Google Scholar]
- Khatib G., Guzel A.B., Gulec U.K., Gumurdulu D., Vardar M.A., Altintas A. Clinicopathological features and prognostic factors of the uterine sarcomas: 20 years of experience at Cukurova University. Eur. J. Gynaecol. Oncol. 2014;35(6):646–654. [PubMed] [Google Scholar]
- Lee Y.J., Kim D.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T., Nam J.H. Feasibility of uterine preservation in the management of early-stage uterine adenosarcomas: a single institute experience. World J. Surg. Oncol. 2017;15(1):87. doi: 10.1186/s12957-017-1137-0. Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasioudis D., Chapman-Davis E., Frey M., Holcomb K. Safety of ovarian preservation in premenopausal women with stage I uterine sarcoma. J. Gynecol. Oncol. 2017;28(4):e46. doi: 10.3802/jgo.2017.28.e46. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]