Abstract
This is the first field study of its kind to combine radio telemetry, passive samplers, and pesticide accumulation in tissues to characterize the amphibian exposome as it relates to pesticides. Understanding how habitat drives exposure in individuals (i.e., their exposome), and how that relates to individual health is critical to managing species in an agricultural landscape where pesticide exposure is likely. We followed 72 northern leopard frogs (Lithobates pipiens) in two agricultural wetlands for insight into where and when individuals are at high risk of pesticide exposure. Novel passive sampling devices (PSDs) were deployed at sites where telemetered frogs were located, then moved to subsequent locations as frogs were radio-tracked. Pesticide concentration in PSDs varied by habitat and was greatest in agricultural fields where frogs were rarely found. Pesticide concentrations in frogs were greatest in spring when frogs were occupying wetlands compared to late summer when frogs occupied terrestrial habitats. Our results indicate that habitat and time of year influence exposure and accumulation of pesticides in amphibians. Our study illustrates the feasibility of quantifying the amphibian exposome to interpret the role of habitat use in pesticide accumulation in frogs to better manage amphibians in agricultural landscapes.
Introduction
Pesticides are one of multiple stressors that contribute to amphibian declines, but their population-level effects on health are still poorly understood1,2. Our knowledge of the impact of pesticides on amphibians is limited in part because route, timing, and rate of exposure in the field is unknown3. Pesticides occur frequently in amphibian habitats and accumulate in their tissues4–7; however, pesticide exposure relative to habitat use at the population or individual level has not been investigated. Sensitivity to pesticides varies by amphibian species and by the type of chemical or chemical mixtures to which an animal is exposed8. Timing of exposure is also important, and metabolism and excretion play a role in pesticide accumulation and residence time within tissues8,9. Compounding this complexity is the aquatic/terrestrial duality of many amphibian species. Due to their unique physiology and life history, amphibians are more vulnerable to pesticides than other wildlife species10,11. In addition, amphibians use both aquatic and terrestrial habitats, which are prone to change not only between years but also within a single season, contributing to fluctuations in pesticide presence and concentration3,12. Furthermore, amphibians have highly permeable skin which allows for dermal uptake of pesticides13,14. Contact with pesticides can cause death or a range of sub-lethal effects2. Health effects vary from immune suppression to reproductive and behavioral changes that may lead to population declines15–18. This is especially true in the agricultural landscape where pesticides are frequently applied but vary in type and amount19,20.
A better understanding of exposure potential will provide insight into threats that pesticides pose to amphibian health. Researchers have proposed that the terrestrial environment has the potential to significantly contribute to an amphibian’s pesticide accumulation and is a topic that warrants further investigation13,17,21. For example, some species (e.g., toads and wood frogs [Lithobates sylvaticus]) spend much of their adult life in terrestrial environments, and may be exposed to greater amounts of pesticides outside of wetland habitats21,22; however, this has not been tested in the field. Several field studies have noted that pesticides detected in frog tissues could not be explained by the aquatic habitats in which they were captured5–7, leaving questions about the role of habitat use in pesticide exposure and accumulation. To our knowledge, pesticide exposure based on habitat use has never been studied explicitly in any amphibian species under field conditions.
Understanding the complex relationships among amphibians, pesticides, and their environments is vital for conservation, and investigating pesticide exposure in the field is a central piece of this understanding. Specifically, to design mitigation strategies, we need to know where and when individuals are at the highest risk of pesticide exposure. Our goal was to understand the role of habitat use in determining pesticide exposure in frogs in an agricultural landscape. This information will facilitate an assessment of the potential health risks of agrochemicals under field conditions. We hypothesized that habitat use, measured as time spent in a particular habitat, would influence an individual frog’s exposure to types and amounts of pesticides, which would correlate with the types of pesticides accumulating in their tissue.
To our knowledge, this is the first field study to combine radio telemetry, passive samplers, and pesticide accumulation in tissues as a means to characterize the amphibian exposome as it relates to pesticides. We used a novel application of a silicone Passive Sampling Device (PSD)23 to assess pesticide presence near individual animals occupying different habitat types, and to facilitate comparisons of pesticides in habitats to pesticides in tissues of northern leopard frogs (Lithobates pipiens; Fig. 1). We also used radio telemetry to assess survival and determine the amount of time individual frogs spent in particular habitats relative to pesticide exposure. We compared pesticide type and concentration among different habitats and in frog tissue to determine if a relationship exists between a frog’s habitat use and their pesticide accumulation.
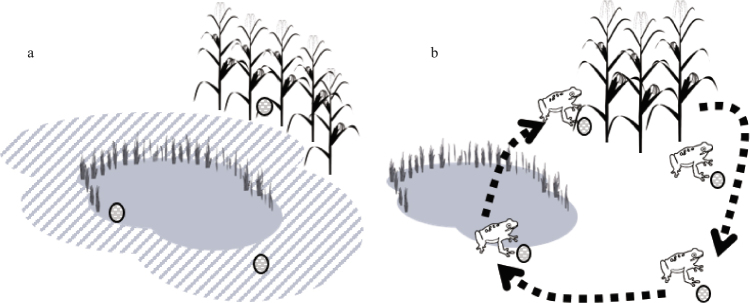
Figure 1.
Study design. (a) PSDs deployed in three habitat types (wetland, grassland, and agriculture) at both sites from May–August, 2015–2016. PSDs were placed in a single location for 2 wks, collected for analysis and replaced. Ovals = PSD, grey shaded area = wetland habitat, grey striped area = grassland buffer habitat, and corn stalks = agricultural habitat. (b) Each deployed PSD was also associated with an individual telemetered frog (n = 9). Each PSD was moved to mirror the frog’s locations as it moved among different habitat types (dashed arrow). PSDs were moved with the frog for 2 wks, collected for analysis and replaced with another PSD.
Results
Radio telemetry
We radio tracked 72 northern leopard frogs during the summers of 2015 and 2016 (M = 31, F = 41; see Supplementary Tables S1 and S2). We tracked an equal number of frogs at two constructed agricultural wetlands in Cerro Gordo and Worth Counties, IA, USA. Both sites were surrounded by a grassed buffer but were in close proximity (20–750 m) to corn and soybean fields on all sides (see Supplementary Fig. S1). Individuals were tracked for an average of 29 days (minimum = 3, maximum = 84). During the study, 20 frogs died (28%), 24 frogs could not be relocated (33%), and 28 frogs were captured and euthanized for tissue analysis (39%).
We analyzed the home ranges of individuals with ≥20 recorded locations (F = 23, M = 18). Females had a larger home range than males, but total movement and average daily movement for male frogs were greater than for female frogs (Table 1). Fifty-eight percent of frog locations were in grassland, 34% in wetland, and 6% in agricultural fields (but only 7 of 72 individuals spent three or more consecutive days in agricultural fields). Frogs selected for grassland and wetland habitats (3.89 ± 0.29 Global Selection Ratio [GSR] and 3.57 ± 0.43 GSR, respectively), and avoided agriculture, forest, and developed habitats (0.09 ± 0.02; 0.17 ± 0.08; 0.03 ± 0.02 GSRs, respectively). Use of habitat by frogs changed over time (Fig. 2). Northern leopard frogs primarily used a mixture of grasslands and wetlands from mid-May until late June, then wetland habitat use declined in July and continued to decline through the summer. There was an increase in the use of grassland and agricultural habitat beginning in July.
Table 1.
Median home ranges (m2) and distances moved (m) with minimum and maximum values for female and male radio telemetered northern leopard frogs in Iowa 2015–2016.
| Core Home Range | Total Home Range | Total Movement | Daily Movement | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | Median | Range | |
| Females | 1,347 | 240 to 64,157 | 6,423 | 981 to 246,661 | 389 | 2 to 1,680 | 16 | 1 to 41 |
| Males | 3,154 | 239 to 11,800 | 4,740 | 2,640 to 70,017 | 553 | 7 to 1,405 | 18 | 2 to 59 |
Core home range = 50% quantile and total home range = 95% quantile. Total movement represents the total distance a frog moved while it was tracked and daily movement is the average distance a frog moved per day while it was tracked (total movement/number of days tracked).
Figure 2.
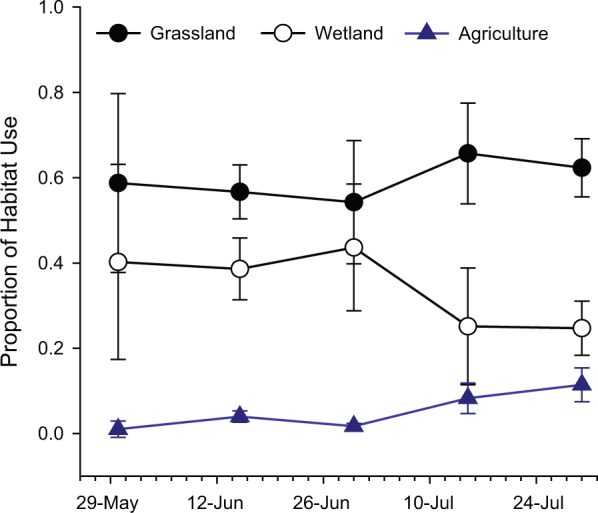
Change in the proportion of habitat used by radio tracked northern leopard frogs (n = 72) in north-central Iowa, May – August in 2015 and 2016 with associated Standard Error (SE).
Five of our 12 models (ΔAICc < 2; Table 2) were at least partially supported by the data. The model with the most support indicated that survival varied by sex and habitat type used, specifically that survival probability is lower in females, lowest in grassland habitats and highest in agricultural habitats. Survival estimates (both sexes) for the length of our study (3 mo) were extrapolated from estimated daily values by multiplying by 3 months. Three-month survival probabilities were highest in agriculture (0.62 ± 0.10), intermediate in wetlands (0.46 ± 0.92), and lowest in grasslands (0.28 ± 0.07).
Table 2.
Results of known fate models for northern leopard frogs in Iowa, 2015–2016 with support (ΔAICc < 2).
| Model Name | ΔAICc | w | k | Deviance |
|---|---|---|---|---|
| S(Sex + Habitat) | 0.00 | 0.16 | 3 | 211.45 |
| S(+Habitat) | 0.43 | 0.13 | 2 | 213.89 |
| S(Year + Habitat) | 0.55 | 0.12 | 3 | 201.12 |
| S(Group + Habitat) | 1.76 | 0.06 | 9 | 213.41 |
| S(Site + Habitat) | 1.96 | 0.06 | 3 | 221.01 |
AICc of the top model S(Sex + Habitat) was 217.50, w = AICc Weight, and k = number of parameters.
Passive Sampling Devices (PSDs)
We analyzed PSDs that were associated with telemetered frogs (n = 9) as they moved across the landscape for over 100 pesticides and pesticide degradates (Fig. 1). Twelve different pesticides were detected, and the number of pesticides to which individual frogs were potentially exposed during the time they were tracked ranged from 9–12 (median = 12; 6 fungicides, 2 herbicides and 4 insecticides; see Supplementary Table S3). The three pesticides detected at the highest concentrations in PSDs were chlorpyrifos (organophosphate insecticide), pyraclostrobin (fungicide), and azoxystrobin (fungicide).
Seventeen pesticides (8 fungicides, 6 insecticides, and 3 herbicides; see Supplementary Table S4) were observed in PSDs placed in habitats in 2015 and 2016 (single location, not moved with telemetered frogs Fig. 1a). The three pesticides detected at the highest concentrations in the PSDs were chlorpyrifos, metolachlor (herbicide), and azoxystrobin (fungicide). The highest concentration of pesticides was observed in agricultural habitats, followed by wetland and then grassland habitats (Table 3). The concentrations of pesticides detected in PSDs in grassland habitats were lower than agricultural habitats (P < 0.01) and wetlands were not different than either grassland or agricultural habitats (P = 0.06 and 0.05, respectively; Fig. 3). There were no significant differences between the total numbers of pesticides observed among habitat types.
Table 3.
Median number and concentration of pesticides (ng/PSD) found in silicone PSDs placed in grassland, wetland, and agricultural habitats in Iowa, USA 2015 and 2016 (see Supplementary Table S4 for list of pesticides detected).
| Habitat | Number of Pesticides: Median | Number of Pesticides: Range | Pesticide Concentration: Median | Pesticide Concentration: Range |
|---|---|---|---|---|
| Grassland | 4 | 2 to 11 | 60 | 7 to 250 |
| Wetland | 6 | 2 to 11 | 122 | 10 to 896 |
| Agriculture | 6 | 2 to 11 | 197 | 46 to 1,844 |
Figure 3.
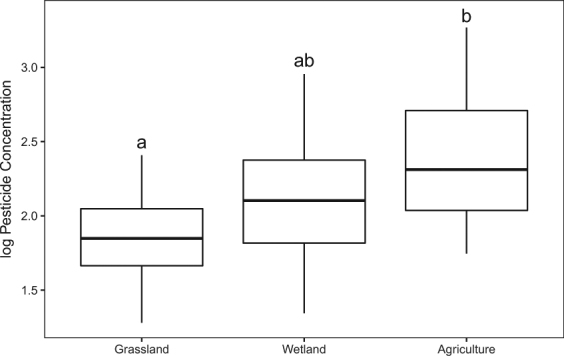
Log normalized concentrations of pesticides detected in environmental PSDs (ng/PSD) placed in different habitat types in Iowa, 2015–2016. Letters above boxplots indicate differences (P < 0.05) among habitat types (see Supplementary Table S4 for list of pesticides detected).
Concentrations of pesticides did not vary over time within a particular habitat, based on data from the PSDs placed independent of frogs and collected at 2 wk intervals for 3 mos during the summer (see Supplementary Table S4). The number of pesticides in agricultural fields also did not differ from May to August, but the number of pesticides in wetlands and in grassland habitats varied over time. In both cases the number of pesticides detected peaked in July and was higher (P < 0.01) than the number detected earlier in the spring or later in the summer.
In the winter samples (PSDs placed in aquatic locations; see Supplementary Fig. S1), the herbicides atrazine and metolachlor, and the insecticide chlorpyrifos, were detected at both the Cerro Gordo (20.2, 16.6, and 2.6 ng/PSD, respectively) and Worth wetlands (16.0, 17.5, and 0.42 ng/PSD, respectively). The pyrethroid insecticide bifenthrin was detected at the Worth wetland only (0.50 ng/PSD).
Frog tissues
The number of pesticides in frog livers and gonads collected in 2016 ranged from 0–3 (median = 3) and included 2 fungicides (fenbuconazole and tebuconazole), 1 insecticide (bifenthrin), and 1 breakdown product of DDT (p, p’-DDE; Table 4, see Supplementary Table S5). Frogs were collected in mid-May and early August in 2016 to characterize potential differences in pesticide accumulation as frogs moved from the wetland to the terrestrial environment. Both the number of pesticides detected in the livers and gonads and their respective concentrations were higher in frogs collected in May compared to those collected in August (P < 0.01 and < 0.01, respectively; Fig. 4). p, p’- DDE was not included in further analyses of the tissue data, because it is persistent in the environment and would not degrade within the time frame of our study24.
Table 4.
Total pesticide concentrations (sum of all pesticide detected) and the number of pesticides in the liver and gonad tissue of telemetered northern leopard frogs vs their associated PSDs that “followed” them over time and the proportion of time the frogs and their PSDs spent in each habitat type (Fig. 1b).
| Site | Frog ID | Concentration of Pesticides | Number of Pesticides | Proportion of habitat (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Frog Tissue (ng/g) | PSDs (ng/PSD) | Frog Tissue (ng/g) | PSDs (ng/PSD) | Grass | Wetland | Agriculture | Other | ||
| CG | 60.3 | 20.1 | 86.3 | 1 | 9 | 0.82 | 0.18 | 0.00 | 0.00 |
| CG | 89.2 | 9.6 | 73.6 | 3 | 9 | 0.68 | 0.00 | 0.21 | 0.11 |
| W | 26.3 | 6.65 | 159 | 2 | 12 | 0.84 | 0.16 | 0.00 | 0.00 |
| W | 18 | ND | 148 | ND | 12 | 0.85 | 0.06 | 0.00 | 0.09 |
| W | 4.4 | ND | 160 | ND | 12 | 0.84 | 0.16 | 0.00 | 0.00 |
| W | 13.2 | ND | 123 | ND | 10 | 0.11 | 0.17 | 0.72 | 0.00 |
| W | 42 | 21.1 | 147 | 1 | 12 | 0.04 | 0.78 | 0.00 | 0.00 |
| W | 45 | ND | 147 | ND | 12 | 0.84 | 0.14 | 0.02 | 0.00 |
| W | 48 | ND | 69.3 | ND | 10 | 0.65 | 0.00 | 0.35 | 0.00 |
Frog ID = Individual frog identification number. Pesticide concentrations are in ng/g for frog tissue and ng/PSD for PSDs, Site: CG = Cerro Gordo and W = Worth, “ND” = Not detected.
Figure 4.
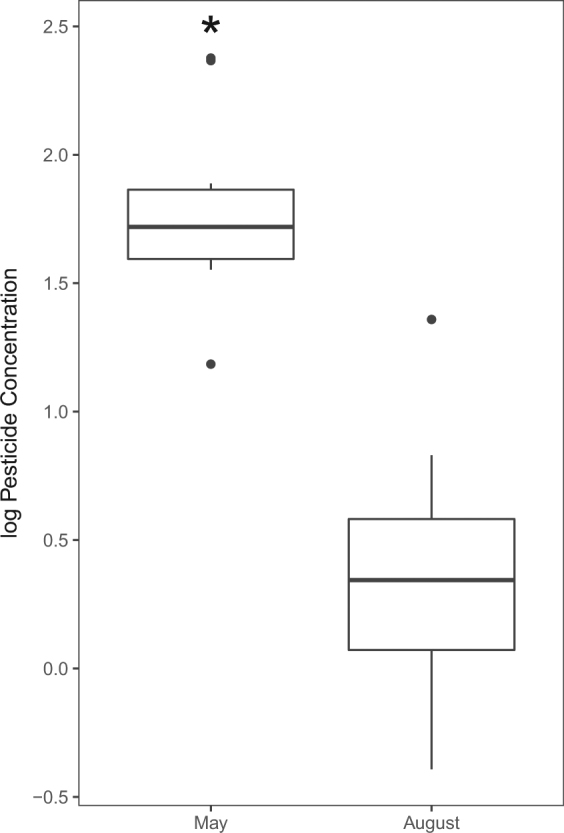
Log normalized concentrations of pesticides detected in frog gonad and liver tissues (ng/g) May vs August, 2016 in Iowa, USA. Asterisk (*) indicates difference (P < 0.05; see Supplementary Table S5 for list of pesticides detected).
Discussion
Information from this study using a combination of radio telemetry and PSDs to document exposure and accumulation in tissues expands our understanding of the amphibian exposome as it relates to pesticides. Our study is likely the first to assess questions of pesticide exposure and habitat selection on adult animals based on field data, and supports the importance of the terrestrial environment and the notion that amphibians are at risk of exposure in terrestrial habitats13,21. Data showing higher tissue pesticide concentration detected in May relative to August suggest that the greatest vulnerability of adult frogs to pesticide accumulation, in the agricultural landscape, may be during winter hibernation and spring breeding when their use of wetlands is most intense (Fig. 2). Although, transient exposures in agricultural fields are likely to be of long-term importance and should not be discounted despite the low percentage of time that frogs spend there. Pesticide research with amphibians typically focuses on larval stages, but a reduction in adult (and juvenile) survival rates is more likely to presage population decline25, suggesting that our information on adults is timely.
Results supported our expectation of an influence of habitat use on survival rates. However, we suggest that this was because of increased predation in grassland habitats rather than differences in pesticide exposure. Pesticide exposure in the field is generally sublethal for amphibians26,27 including leopard frogs26, and it is unlikely that we would have captured sublethal effects on survival in our 3 mo survival probabilities. Pesticides observed in amphibian habitats with documented sublethal effects include herbicides, atrazine and metalochlor, the organophosphate insecticide, chlopyrifos, and several fungicides. Atrazine is responsible for a variety of estrogenic effects, reduced immune response, and behavior modification28. Metolachlor slows development and growth and has negative effects on the thymus gland27,29, and synergistically impacts the effects of atrazine27,28. Chlorpyrifos causes increased time to metamorphosis30 while exposure to fungicide formulations containing pyraclostrobin decreases growth and development in larval amphibians31.
Frogs in our study spent most of their time (58%) in grasslands, the habitat where pesticide exposure was the lowest, but where predation risk to amphibians is typically higher than in wetlands32,33. Additionally, the highest percentage of radio-tracked frogs that died of predation were found in grassland habitats (47%). Thus, for this short-term study, it is likely that differences in survival relative to habitat are indicative of predation risk rather than exposure to pesticides. However, in terms of management, frogs did spend a small but measurable amount of time in agricultural fields (on average frogs spent 6% of their time in a corn or soybean field) and because the effects of pesticides are accumulative2,27, even short periods of time spent in habitats where pesticide exposure is high can have negative consequences over their lifetime, especially when use of agricultural fields coincides with pesticide application2,34.
Northern leopard frogs, like many ranid species, hibernate at the bottom of lakes and ponds, resting on the substrate and covered with a thin layer of silt that allows for cutaneous uptake of O2. Understanding potential exposure during hibernation is another piece of the exposome that is understudied. Amphibian skin is highly permeable, a characteristic that is magnified during hibernation, when frogs undergo physiological changes at low temperatures (<5 °C). These changes lower metabolism which reduces the metabolism of pesticides35–37. Liver condition and function is also lowest at the end of hibernation (early spring), which makes it more difficult for an individual to efficiently metabolize contaminants38. In our study, four pesticides (two herbicides, atrazine and metolachlor, and two insecticides, bifenthrin and chlorpyrifos) were identified in overwintered (aquatic) PSDs, with atrazine and metolachlor at the highest concentrations. These results indicate that exposure during winter hibernation is significant for northern leopard frogs.
In the spring, northern leopard frogs selected for aquatic habitats coincident with the application of herbicides (May, June) and then moved into terrestrial habitats (July, August) when insecticides are commonly applied (i.e., chlorpyrifos peaked in August in all habitat types). Frogs used agricultural fields infrequently indicating that the pesticide application rates (exposure concentrations) tested in laboratory studies21 may not represent the actual environmental concentrations to which frogs are exposed in their preferred grassland habitats.
Pesticide accumulation in tissues was assessed in telemetered frogs in May that were spending a high proportion of their time in aquatic habitats and again in August in frogs spending a high proportion of their time in terrestrial environments (grasslands and agricultural fields; Fig. 2). Higher pesticide concentrations were observed in frog tissue collected in May compared to August. Other studies have reported the accumulation of similar types and concentrations of pesticides in frog tissues (liver and whole bodies) collected from wetland habitats early in the summer5–7, however, no information on pesticide exposure and resulting accumulation in frogs occupying more terrestrial habitats is available for comparison. Anurans have been shown to exhibit reduced dermal uptake of pesticides from soil while dehydrated39, a potential contributing factor in our study where pesticide concentration in leopard frog tissue was lowest while they were occupying terrestrial habitats later in the summer.
A number of pesticides were identified in PSDs (representing potential exposure) that were not detected in frog tissues. Other studies have reported differences in pesticides identified in the environment (water and sediment) compared to those accumulating in tissue5–7. These differences can be attributed to several factors, (1) the physical-chemical properties of the pesticides (may not be accumulated), (2) metabolism/elimination by the frog (accumulated but quickly broken down), or (3) limited time spent in each habitat (slow assimilation into tissues). In contrast, two pesticides (fenbuconazole and p, p’-DDE) were only identified in frog tissues. Discrepancies between pesticides identified in tissues versus the environment could be related to low environmental concentrations, limitations in the ability of PSDs to sequester more hydrophobic compounds – although this is unlikely23,40, or effect of maternal transfer rather than dermal uptake.
Our results corroborate the importance of multiple habitat types to anurans, highlighting the need to protect wetlands and surrounding habitats (e.g., grass buffers). We assessed pesticide exposure in the environment and differences in the use of habitat by frogs, relative to pesticides found in their tissues, to understand how these factors interact to affect the persistence of frogs on the landscape. These data illustrate that understanding the extent and magnitude of pesticide exposure is foundational in assessing risk and developing mitigation strategies for the conservation of frogs; and our study was unique in its innovative use of silicone PSDs to facilitate these assessments. More broadly, the distances frogs moved, patterns of habitat use, and home range sizes are similar to previous telemetry studies on northern leopard frogs in the Midwestern United States41,42, such that our findings may be applicable to other altered (i.e., agricultural) landscapes. This study provides initial steps towards quantifying the amphibian exposome and interpreting the role of habitat use in pesticide accumulation as it contributes to population declines.
Methods
Study Sites and Species
This study was conducted in northern Iowa, USA at two constructed wetlands (see Supplementary Fig. S2). Wetlands were embedded in a highly agricultural landscape that consisted mainly of annual row crops (corn and soybeans). Each wetland had an associated grass buffer (buffer:wetland ratio = 4:1), but were surrounded on all sides by agricultural fields (ranging from 20–750 m away; see Supplementary Table S6). Wetlands were built to catch and hold subsurface tile drainage from surrounding agricultural fields to remove nitrate from local surface water bodies43.
We chose northern leopard frogs as our study species because they are (1) appropriately sized for radio telemetry, (2) relatively abundant in our study area, and (3) similar to other anuran species in their use of both aquatic and terrestrial habitat. Northern leopard frogs are distributed widely across the United States and parts of Canada44. Although considered a fairly common species, declines or extinctions have been observed in parts of their range45,46. Northern leopard frogs overwinter by resting on the substrate in waterbodies that do not freeze completely47. Breeding and egg deposition occur in aquatic environments during the spring48. Post-breeding, adults move to grassy, terrestrial habitats for feeding before returning to waterbodies for hibernation in the fall44,49.
Radio telemetry
We used radio telemetry to assess survival probability, home range, distance moved, and habitat types used by frogs in 2015 and 2016. In May we captured 10 adult frogs at each study site (see Supplementary Table S2). Frogs appeared healthy (no observable disease) and were taken to a field laboratory and implanted with an internal radio transmitter (17 × 8.5 × 5.5 mm, 1.8 g; BD-2H in 2015 and 27 × 8.5 × 5.5 mm, 1.8g; BD-2HX in 2016, Holohil Systems Ltd., Canada)50. Frogs were located daily, from May release, until August. We recorded the habitat type where frogs were observed and marked their location with GPS (Garmin eTrex® 30). Frogs were recorded in 1 of 5 habitats: grassland (grass buffers, pastures, or prairies); wetland (wetlands, streams and other small water bodies); agriculture (corn and soybean fields); forest (large groups of deciduous trees); or developed (man-made structures such as roads). If individuals died or could not be located for >2 wks they were replaced with newly captured and telemetered individuals (see Supplementary Table S2). This study was approved by the Iowa State University Institutional Animal Care and Use Committee (protocol #3-15-7989-D) and performed in accordance with protocol guidelines.
Passive Sampling Device (PSD) deployment and analysis
We used a novel application of a silicone PSD technique23 to quickly and easily screen for potential pesticide exposure. Commercially available silicone wristbands were purchased (width: 2.5 cm; inner diameter 6.7 cm; 24hourwristbands.com, Houston, TX) and cleaned23. Three PSDs were set aside as “cleaning blanks”, frozen, and extracted with the environmental samples. Additionally, during each PSD field deployment a “field blank” was also deployed at the site to assess potential contamination. Clean latex gloves were worn when handling PSDs. In 2016, we assigned each telemetered frog (initial n = 10/site) a PSD (Fig. 1b). That PSD was secured to the substrate at the frog’s initial location, then relocated each time the frog moved (see Supplementary Fig. S3). Because individual frogs were relocated daily, PSDs were deployed at a particular location no more than 24 hrs longer than a frog. Thus, the PSD “experienced” the same habitat encountered by that frog and provided daily information on an individual’s exposure to pesticides as it moved across the landscape. PSDs were moved with each frog for 2 wks then collected and replaced throughout the tracking period 2–4 times depending on how long the frog was tracked.
We also used PSDs to assess the potential pesticide exposure by placing PSDs (independent of frogs) in habitats where we most frequently observed our telemetered animals (Fig. 1a). We deployed PSDs in four locations at each site in a combination of grassland, wetland, and agricultural habitats (e.g., two in grasslands, one in wetland, and one in agriculture), in July–August in 2015, and June–August in 2016 (see Supplementary Fig. S1). PSDs were anchored to the substrate using sterilized landscape staples. They were left in the environment for 2 wks and then collected and replaced with new PSDs in the same location.
Finally, we assessed the potential pesticide exposure in overwintering habitat by placing PSDs at four aquatic locations in each wetland from November 2015 to April 2016. PSDs were zip-tied to a metal O-ring that was attached to a metal T-post driven into the substrate in 1 m of water (see Supplementary Fig. S4). The PSDs were submerged and in contact with the substrate as a northern leopard frog would be during hibernation51,52. PSDs were not collected and replaced during the winter because sites were inaccessible due to ice cover.
All PSDs were sent to the USGS California Water Science Center for analysis. PSDs were extracted and analyzed for over 100 pesticides and pesticide degradates. In the laboratory, PSDs were rinsed gently with deionized water to remove particulates and allowed to dry in the fume hood. Prior to extraction PSDs were weighed and placed in a pre-cleaned flask. Samples were spiked with d14-trifluralin-, ring 13C4-fipronil, ring-13C12-p, p’-DDE and phenoxy-13C6-cis-permethrin as recovery surrogates, extracted twice with 50 mL of ethyl acetate using sonication for 2 hrs. Each extract was reduced to 1 mL, combined, reduced to 200 µL and analyzed for pesticides and pesticide degradates on an Agilent 7890 GC coupled to an Agilent 7000 MS/MS operating in electron ionization (EI) mode or an Agilent 1260 bio-inert LC coupled to an Agilent 6430 MS/MS. Data was collected in multiple reaction monitoring (MRM) mode with each compound having 1 quantifier MRM and at least 1 qualifier MRM. Limits of detection (LODs) for all compounds ranged from 2–5 ng/PSD.
Frog tissue collection and analysis
In 2016 we captured and euthanized individuals in mid-May prior to tracking while they were breeding and residing at the wetlands (n = 10). In August we captured and euthanized all remaining radio telemetered frogs (n = 10). Frogs were euthanized with an overdose of Tricaine Methanesulfonate (0.5 g/1 L water)53. Individual liver and gonads were dissected in the field, frozen, and sent to the USGS California Water Science Center for analysis6.
All tissues were extracted using pressurized fluid extraction and analyzed for 98 pesticides and pesticide degradates using an Agilent 7890 GC coupled to an Agilent 7000 MS/MS operating in EI mode. Method detection limits (MDLs) for all compounds ranged from 0.5 to 4.2 μg/kg wet weight5 (see Supplementary Methods).
Statistical analysis
We performed a known fate analysis in Program MARK (V6.1)54 to estimate survival probabilities of the radio tracked frogs (n = 72). We included daily habitat use (i.e., the habitat type of each location for individual frogs) as an individual covariate to evaluate its influence on frog survival. Each frog was assigned to 1 of 8 groups that reflected site, year, and sex (see Supplementary Table S7). Twelve models were fit to the data to explore survival among groups and habitat types (see Supplementary Table S8). We considered models where survival was constant or differed between site, sex, year, or among groups. We also fit models to include a daily habitat covariate using our classification of the five habitat types. Survival was not estimated in forest or developed habitats because of low use (Forest = 0.008% and Developed = 0.003% of recorded locations). Model selection was based on Akaike’s Information Criterion for small sample sizes (AICc)55.
We estimated core home range (50% quantile) and total home range (95% quantile) of 41 frogs (18 M, 23 F; see Supplementary Table S2) that had ≥20 recorded GPS locations56. We used the “kde” and “isopleth” functions in Geospatial Modeling Environment (GME; V0.7.4.0)57 using kernel density estimates.
We estimated the daily distances (m) each frog moved using the “pointdistances” function in GME, which assumes a straight-line moved between successive locations. We summed the daily distances each frog moved and divided the total by the number of days the frog was tracked to get average daily movement.
We determined the proportional use of habitat types, relative to their availability by calculating GSRs using the Manly Resource Selection function in R (V3.3.3)58. Available habitat was calculated within a 1 km buffer41,42. GSRs reflect the proportion of time an animal uses each habitat relative to the proportion of that available habitat. For example, a habitat with a GSR > 1 indicates that the animal shows positive selection for that habitat.
We combined PSD data from 2015 and 2016 and used Analysis of Variances (ANOVAs) in R to test for a difference in number of pesticides and total pesticide concentration in the PSDs among the habitat types (whether PSD was placed in an agricultural, wetland, or grassland habitat). We also performed ANOVAs to test for a difference in number of pesticides and pesticide concentrations within habitat types over time (PSDs placed in early, mid, or late summer). Data were log transformed to meet assumptions of constant variance and normality. All non-detect values received ½ MDL for statistical analyses.
We assessed differences in pesticide presence in frog gonad and liver tissue using a Kruskal-Wallis test59 and an independent t-test to test for differences in concentration of pesticides found in individuals collected in May vs August 2016. Data were log transformed to meet assumptions of constant variance and normality. All non-detect values received ½ MDL for statistical analyses.
Data availability
Data generated in this study are available at the U.S. Geological Survey data release, 10.5066/F7D799PV.
Electronic supplementary material
Acknowledgements
This study was funded by the Fort Collins Science Center as a part of ongoing technical assistance to the Farm Service Agency, the U.S. Geological Survey Toxic Substances Hydrology Program, and the U.S. Geological Survey Amphibian Research and Monitoring Initiative. We thank C. Brocka and C. Corwin for field assistance, P. Bartelt for advice, and T. Yaw and B. Zaffarano for veterinary consultation, and M. Reeves for constructive comments on an earlier draft. We thank M. McWayne, C. Sanders, M. De Parsia, and S. Stout at the U.S. Geological Survey California Water Science Center for the pesticide extraction and analyses. We thank the Iowa Department of Agriculture and Land Stewardship (IDALS) and private landowners for wetland access. This is contribution number 651 of the U.S. Geological Survey Amphibian Research and Monitoring Initiative. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Data generated in this study are available online at the U.S. Geological Survey data release, 10.5066/F7D799PV.
Author Contributions
J.E.S. conducted the field work, J.E.S., E.M. and K.L.S. participated in planning the project, the data analysis, and in writing the manuscript. C.L.P. and M.W.V. aided field work and planning of field work. S.J.D. aided in the statistical analysis. M.L.H. conducted the pesticide analysis. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28132-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davidson C. Declining downwind: Amphibian population declines in California and historical pesticide use. Ecol. Appl. 2004;14:1892–1902. doi: 10.1890/03-5224. [DOI] [Google Scholar]
- 2.Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: Review of the risks. Environ. Pollut. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Fryday, S. & Thompson, H. Toxicity of pesticides to aquatic and terrestrial life stages of amphibians and occurrence, habitat use and exposure of amphibian species in agricultural environments. EFSA supporting Publications, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2012.EN-343/epdf (2012).
- 4.Fellers GM, McConnell LL, Pratt D, Datta S. Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada Mountains of California, USA. Environ. Toxicol. Chem. 2014;23:2170–2177. doi: 10.1897/03-491. [DOI] [PubMed] [Google Scholar]
- 5.Smalling KL, Fellers GM, Kleeman PM, Kuivila KM. Accumulation of pesticides in pacific chorus frogs (Pseudacris regilla) from California’s Sierra Nevada Mountains, USA. Environ. Toxicol. Chem. 2013;32:2026–2034. doi: 10.1002/etc.2308. [DOI] [PubMed] [Google Scholar]
- 6.Smalling KL, et al. Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Sci. Total Environ. 2015;502:80–90. doi: 10.1016/j.scitotenv.2014.08.114. [DOI] [PubMed] [Google Scholar]
- 7.Battaglin WA, et al. Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Sci. Total Environ. 2016;566–567:320–332. doi: 10.1016/j.scitotenv.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 8.Shah PV, Monroe RJ, Guthrie FE. Comparative penetration of insecticides in target and non-target species. Drug Chem. Toxicol. 1983;6:155–179. doi: 10.3109/01480548309016022. [DOI] [PubMed] [Google Scholar]
- 9.Bridges CM, Semlitsch RD. Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conserv. Biol. 2000;14:1490–1499. doi: 10.1046/j.1523-1739.2000.99343.x. [DOI] [Google Scholar]
- 10.De Lange HJ, Lahr J, Van der Pol JJC, Wessels Y, Faber JH. Ecological vulnerability in wildlife: An experiment judgement and multicriteria analysis tool using ecological traits to assess relative impact of pollutants. Environ. Toxicol. Chem. 2009;28:2233–2240. doi: 10.1897/08-626.1. [DOI] [PubMed] [Google Scholar]
- 11.Quaranta A, Bellantuono V, Cassano G, Lippe C. Why amphibians are more sensitive than mammals to xenobiotics. Plos One. 2009;4:1–4. doi: 10.1371/journal.pone.0007699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashauer R, Boxall A, Brown C. Predicting effects on aquatic organisms from fluctuating or pulsed exposure to pesticides. Environ. Toxicol. Chem. 2006;25:1899–1912. doi: 10.1897/05-393R.1. [DOI] [PubMed] [Google Scholar]
- 13.Van Meter RJ, et al. Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environ. Pollut. 2014;193:262–268. doi: 10.1016/j.envpol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Van Meter RJ, et al. Pesticide uptake across the amphibian dermis through soil and overspray exposures. Arch. Environ. Contam. Toxicol. 2015;69:545–556. doi: 10.1007/s00244-015-0183-2. [DOI] [PubMed] [Google Scholar]
- 15.Brodkin MA, Madhoun H, Rameswaran M, Vatnick I. Atrazine is an immune disruptor in adult northern leopard frogs (Rana pipiens) Environ. Toxicol. Chem. 2007;26:80–84. doi: 10.1897/05-469.1. [DOI] [PubMed] [Google Scholar]
- 16.Hayes T, et al. Atrazine-inducted hermaphroditism at 0.1 ppb in american leopard frogs (Rana pipiens): Laboratory and field evidence. Environ. Health Perspect. 2003;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brühl CA, Schmidt T, Pieper S, Alscher A. Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci. Rep. 2013;3:1–4. doi: 10.1038/srep01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denoël M, et al. Effects of a sublethal pesticide exposure on locomotor behavior: A video-tracking analysis in larval amphibians. Chemosphere. 2013;90:945–951. doi: 10.1016/j.chemosphere.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Handy RD. Intermittent exposure to aquatic pollutants: assessment, toxicity and sublethal responses in fish and invertebrates. Comp. Biochem. Physiol. C. 1994;107:171–184. doi: 10.1016/0300-9629(94)90290-9. [DOI] [Google Scholar]
- 20.Reinert KH, Giddings JM, Judd L. Effects analysis of time-varying or repeated exposures in aquatic ecological risk assessment of agrochemicals. Environ. Toxicol. Chem. 2002;21:1977–1992. doi: 10.1002/etc.5620210928. [DOI] [PubMed] [Google Scholar]
- 21.Brühl CA, Pieper S, Weber B. Amphibians at risk? Susceptibility of terrestrial amphibian life stages to pesticides. Environ. Toxicol. Chem. 2011;30:2465–2472. doi: 10.1002/etc.650. [DOI] [PubMed] [Google Scholar]
- 22.Bartelt PE, Peterson CR, Klaver RW. Sexual differences in the post-breeding movements and habitats selected by western toads (Bufo boreas) in southeastern Idaho. Herpetologica. 2004;60:455–467. doi: 10.1655/01-50. [DOI] [Google Scholar]
- 23.O’Connell SG, Kincl LD, Anderson KA. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 2014;2014:3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas JE, Ou LT, All-Agely A. DDE remediation and degradation. Rev. Environ. Contam. Toxicol. 2008;194:55–69. doi: 10.1007/978-0-387-74816-0_3. [DOI] [PubMed] [Google Scholar]
- 25.Biek R, Funk WC, Maxell BA, Mills LS. What is missing in amphibian decline research: Insights from ecological sensitivity analysis. Conserv. Biol. 2002;16:728–734. doi: 10.1046/j.1523-1739.2002.00433.x. [DOI] [Google Scholar]
- 26.Allran J, Karasov W. Effects of atrazine on embryos, larvae, and adults of anuran amphibians. Environ. Toxicol. Chem. 2001;20:769–775. doi: 10.1002/etc.5620200411. [DOI] [PubMed] [Google Scholar]
- 27.Hayes TB, et al. Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ. Health Perspect. 2006;114:40–50. doi: 10.1289/ehp.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman, C. M. & Williams, B. K. Effects of current-use pesticides on amphibians in Ecotoxicology of Amphibians and Reptiles, Second Edition (eds Sparling, D. W., Linder, G., Bishop, C. A. & Krest, S. K.) 167–202 (Society of Environmental Toxicology and Chemistry, 2010).
- 29.Osano O, Admiraal W, Otieno D. Developmental disorders in embryos of the frog Xenopus laevis induced by chloroacetanilide herbicides and their degradation products. Environ. Toxicol. Chem. 2002;21:375–379. doi: 10.1002/etc.5620210221. [DOI] [PubMed] [Google Scholar]
- 30.Sparling DW, Fellers GW. Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environ. Toxicol. Chem. 2009;28:169–1703. doi: 10.1897/08-336.1. [DOI] [PubMed] [Google Scholar]
- 31.Hartman EAH, Belden JB, Smith LM, McMurry ST. Chronic effects of strobilurin fungicides on development, growth and mortality of larval great plains toads (Bufo cognatus) Ecotoxicology. 2014;23:396–403. doi: 10.1007/s10646-014-1203-0. [DOI] [PubMed] [Google Scholar]
- 32.Gregory PT. Predator avoidance behavior of the red-legged frog (Rana aurora) Herpetologica. 1979;35:175–184. [Google Scholar]
- 33.Russell, A. P. & Bauer, A. M. The amphibians and reptiles of Alberta (University of Calgary Press and University of Alberta Press, 1993).
- 34.Lenhardt PP, Brühl CA, Berger G. Temporal coincidence of amphibian migration and pesticide applications on arable fields in spring. Basic Appl. Ecol. 2014;16:54–63. doi: 10.1016/j.baae.2014.10.005. [DOI] [Google Scholar]
- 35.Tattersall GJ, Ultsch GR. Physiological ecology of aquatic overwintering in Ranid frogs. Biol. Rev. Camb. Philos. Soc. 2008;83:119–140. doi: 10.1111/j.1469-185X.2008.00035.x. [DOI] [PubMed] [Google Scholar]
- 36.Jackson DC, Ultsch GR. Physiology of hibernation under the ice by turtles and frogs. J. Exp. Zool. 2010;313A:311–327. doi: 10.1002/jez.603. [DOI] [PubMed] [Google Scholar]
- 37.Maniero GD, Carey C. Changes in selected aspects of immune function in the Leopard Frog, Rana pipiens, associated with exposure to cold. J. Comp. Physiol. B. 1997;167:256–263. doi: 10.1007/s003600050072. [DOI] [PubMed] [Google Scholar]
- 38.Pasanen S, Koskela P. Seasonal and age variation in the metabolism of the common frog, Rana temporaria L. in northern Finland. Comp. Biochem. Physiol. A. 1974;47:635–654. doi: 10.1016/0300-9629(74)90027-9. [DOI] [PubMed] [Google Scholar]
- 39.Glinski, D. A., Henderson, W. M., Van Meter, R. J. & Purucker, S. T. Effect of hydration status on pesticide uptake in anurans following exposure to contaminated soils. Environ. Sci. Pollut. Res., 10.1007/s11356-018-1830-8 (2018). [DOI] [PMC free article] [PubMed]
- 40.Kile ML, et al. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res. 2016;147:365–372. doi: 10.1016/j.envres.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pember, B. C., Knights, B. C., Knutson, M. G. & Weick, S. E. Effects of agricultural and urban land use on movement and habitat selection by northern Leopard Frogs (Rana pipiens). Report submitted to the Legistlative Commission on Minnesota Resources, St. Paul. US Geological Survey, Upper Midwest Environmental Sciences Center, La Crosse, WI, https://www.umesc.usgs.gov/terrestrial/amphibians/mknutson_5003869_final_report.html (2002).
- 42.Bartelt PE, Klaver RW. Response of anurans to wetland restoration on a Midwestern agricultural landscape. J. Herpetol. 2017;51:504–514. doi: 10.1670/16-113. [DOI] [Google Scholar]
- 43.Iovanna R, Hyberg S, Crumpton W. Treatment wetlands: Cost-effective practice for intercepting nitrate before it reaches and adversely impacts surface waters. J. Soil Water Conserv. 2008;63:14A–15A. doi: 10.2489/jswc.63.1.14A. [DOI] [Google Scholar]
- 44.Rorabaugh, J. C. Rana pipiens In Amphibian Declines: The Conservation Status of United States Species (ed. Lannoo, M. C.) 570–577 (University of California Press, 2005).
- 45.Gibbs EL, Nace GW, Emmons MB. The live frog is almost dead. Bioscience. 1971;21:1027–1034. doi: 10.2307/1296057. [DOI] [Google Scholar]
- 46.Corn PS, Fogleman JC. Extinction of montane populations of the Northern Leopard Frog (Rana pipiens) in Colorado. J. Herpetol. 1984;18:147–152. doi: 10.2307/1563742. [DOI] [Google Scholar]
- 47.Wagner, G. Status of the Northern Leopard Frog (Rana pipiens) in Alberta. Alberta Environmental Protection, Wildlife Management Division. No. 9. Wildlife Status Report, https://open.alberta.ca/dataset/ec34c5da-630b-40f2-a65a-229ddcf9d282/resource/e9f1b274-5a8e-4a0e-a8ea-e4d6f62cb38a/download/1997-SAR-StatusNorthernLeopardFrog-1997.pdf (1997).
- 48.Merrell DJ. Migration and gene dispersal in Rana pipiens. Amer. Zool. 1970;10:47–52. doi: 10.1093/icb/10.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Pope SE, Fahrig L, Merriam HG. Landscape complementation and metapopulation effects on leopard frog populations. Ecology. 2000;81:2498–2508. doi: 10.1890/0012-9658(2000)081[2498:LCAMEO]2.0.CO;2. [DOI] [Google Scholar]
- 50.Yaw T, et al. Placement of intracoelomic radio transmitters and silicone passive sampling devices in northern leopard frogs (Lithobates pipiens) J. Herpetol. Med. Surg. 2017;27:111–115. doi: 10.5818/16-10-090.1. [DOI] [Google Scholar]
- 51.Cunjak R. Winter habitat of northern leopard frogs, Rana pipiens, in a southern Ontario stream. Can. J. Zool. 1986;64:255–257. doi: 10.1139/z86-040. [DOI] [Google Scholar]
- 52.Stewart ER, Reese SA, Ultsch GR. The physiology of hibernation in Canadian leopard frogs (Rana pipiens) and bullfrogs (Rana catesbeiana) Physiol. Biochem. Zool. 2004;77:65–73. doi: 10.1086/378921. [DOI] [PubMed] [Google Scholar]
- 53.Fellers, G. M. & Freel, K. L. A standardized protocol for surveying aquatic amphibians. NPS/WRUC/NRTR-95-001. Technical Report. Davis, CA, USA: National Biological Service, Cooperative Park Studies Unit, University of California, http://elkhornsloughctp.org/uploads/files/1172879165Fellers_Standardized_Survey_Protocol.pdf (1995).
- 54.White GC, Burnham KP. Program MARK: Survival estimate from populations of marked animals. Bird Study. 1999;46:120–138. doi: 10.1080/00063659909477239. [DOI] [Google Scholar]
- 55.Burnham KP, Anderson DR. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manage. 2002;66:912–918. doi: 10.2307/3803155. [DOI] [Google Scholar]
- 56.Seaman DE, et al. Effects of sample size on kernel home range estimates. J. Wildl. Manage. 1999;63:739–747. doi: 10.2307/3802664. [DOI] [Google Scholar]
- 57.Beyer, H. L. Geospatial Modelling Environment (Version 0.7.4.0) (2015).
- 58.R Core Team. R: A language and environment for statistical computing (2013).
- 59.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952;47:583–621. doi: 10.1080/01621459.1952.10483441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study are available at the U.S. Geological Survey data release, 10.5066/F7D799PV.