Abstract
Capnocytophaga canimorsus is a dog oral commensal bacterium that causes rare but life-threatening generalized infections in humans who have been in contact with its animal hosts. Two other dog commensals, Capnocytophaga canis and Capnocytophaga cynodegmi, cause rare, mild local infections. To date, nine capsular serovars have been described in C. canimorsus. Here, we serotyped 112 strains of Capnocytophaga spp. isolated from human infections. The C. canimorsus strains (86 of 96, 89.6%) belonged to serovars A, B, or C with relative frequencies of approximately 30% for each serovar. The high prevalence of the A, B, and C serovars in strains isolated from humans, compared to the previously described low prevalence of these serovars among dog isolates (7.6%), confirms that these three serovars are more virulent to humans than other serovars and suggests that the low incidence of disease may be linked to the low prevalence of the A, B, and C serovars in dogs. We serotyped six strains of C. canis and ten strains of C. cynodegmi and, surprisingly, found one C. canis and three C. cynodegmi strains to be of capsular serovar B. This observation prompted us to test 34 dog-isolated C. canis and 16 dog-isolated C. cynodegmi strains. We found four C. canis strains belonging to serovar A and one belonging to serovar F. In contrast, no dog-isolated C. cynodegmi strain could be typed with the available antisera. This work demonstrates that virulence-associated capsular polysaccharides (A, B, and C) are not specific to the C. canimorsus species.
Introduction
Capnocytophaga is a Gram-negative bacterial genus in the phylum Bacteroidetes that represents part of the normal oral flora of domestic animals and humans1. Four Capnocytophaga species are present in dog and cat oral cavities: C. canimorsus; C. cynodegmi; C. canis; and C. stomatis2–4. C. cynodegmi, C. canis, and C. stomatis are associated with human mild wound infections, although, recently, one case of C. canis-mediated sepsis was described5. In contrast, C. canimorsus has been known since 1961 to cause severe generalized infections in patients who have been bitten, scratched, or simply in contact with dogs and cats6–8. Despite the administration of adequate antibiotherapy, C. canimorsus-induced septicemia may evolve to septic shock with mortality as high as 30% and debilitating morbidity in survivors6.
Several predisposing factors for infection have been identified in C. canimorsus-infected patients, such as splenectomy, alcohol abuse, smoking and advanced age, although as many as 40% of patients did not present any obvious risk factor9, implying that C. canimorsus cannot solely be considered an opportunistic pathogen. To date, more than 500 cases of human infections have been reported. Two studies have reported an incidence of 0.5–0.7 cases per million inhabitants per year10,11, although, in 2016, a retrospective study performed in the Helsinki area in Finland reported an incidence of 4.1 cases per million inhabitants per year12. The discrepancy between these estimates might result from the difficulty in diagnosing C. canimorsus infections, presumably because of the slow and fastidious growth of these bacteria in culture2.
Recently, we reported the discovery of a capsular polysaccharide at the surface of C. canimorsus cells, composed of the same polysaccharide units as the lipooligosaccharide O-antigen13. While the repertoire of capsular serovars is extensive among strains isolated from dog mouths, three serovars, named A, B, and C, are predominant, worldwide, among human infections14. Here, to further investigate the correlation between capsular serovars and virulence, we serotyped the Capnocytophaga strains obtained from the Culture Collection University of Gothenburg (CCUG), the largest collection of strains of Capnocytophaga spp. isolated from human infections. Our study confirms the high prevalence of capsular serovars A, B, and C among the strains of C. canimorsus isolated from human infections (86/96, 89.6%), and we describe two new serovars (L and M). The CCUG collection contains six human-isolated C. canis and nine C. cynodegmi strains. One C. canis strain turned out to belong to serovar B, as did three C. cynodegmi strains. This unexpected finding prompted us to test our collection of 34 C. canis strains isolated from dog mouths, and we found that four strains belonged to serovar A and one to serovar F.
Thus, our study confirms the higher virulence of capsular serovars A, B, and C of C. canimorsus, but also shows that serovars A, B, and F, and presumably others, described in C. canimorsus can be found in C. canis and C. cynodegmi.
Results
Capsular typing of human-isolated C. canimorsus strains
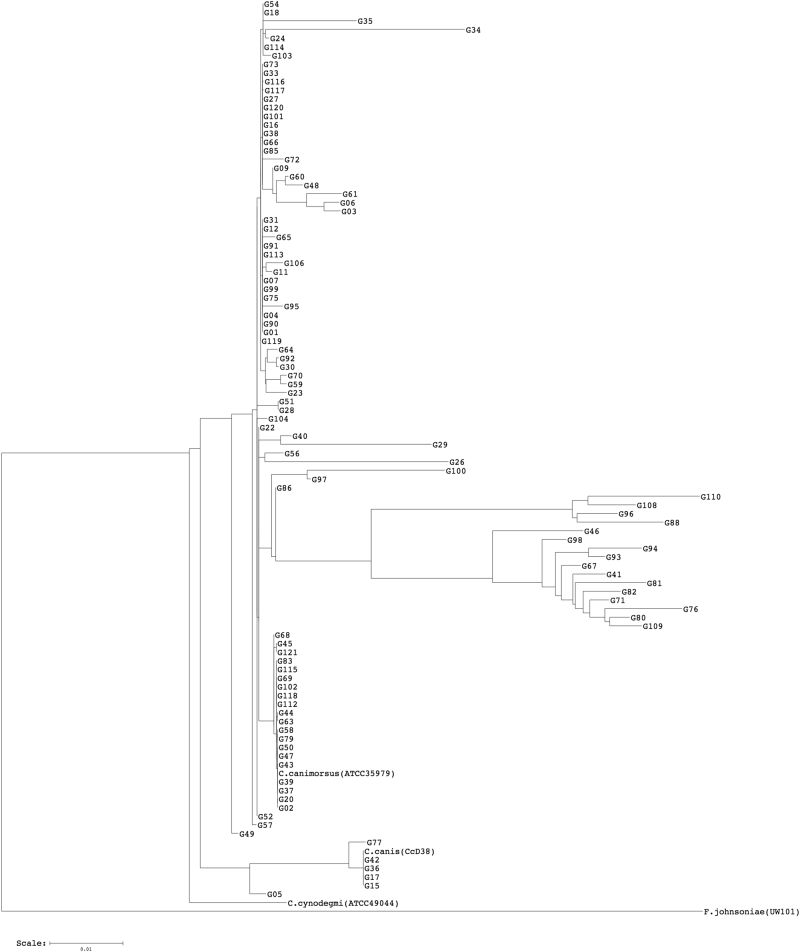
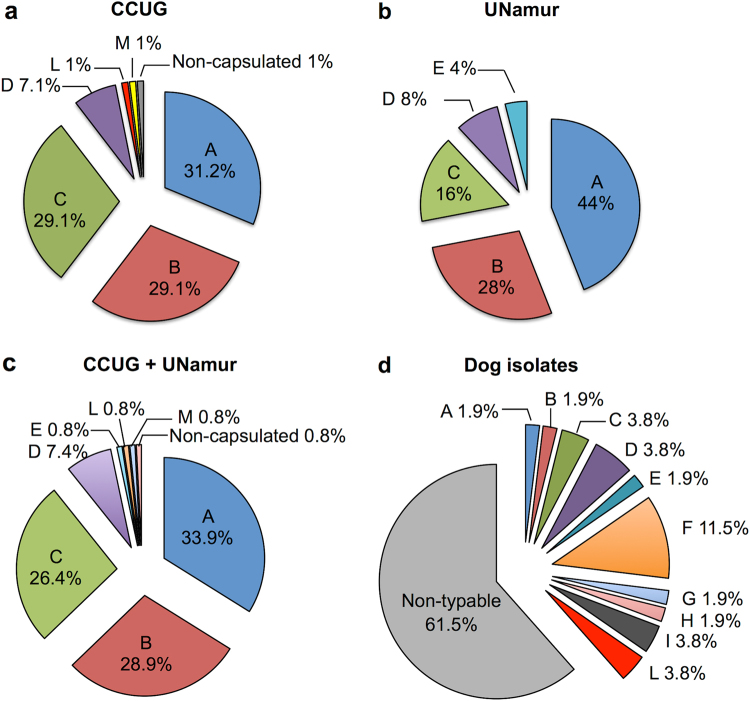
Since the C. canis species has been only recently described3–5, we tested whether any of the 102 strains from the CCUG identified as C. canimorsus might be reclassified as C. canis. To this end, we sequenced the PCR-amplified 16S rRNA genes (16S rDNAs) of these 102 strains and generated a dendrogram of their estimated phylogenetic relationships (Fig. 1). The dendrogram clearly showed that 96 strains (94.1%) clustered with the C. canimorsus type strain (ATCC 35979), while six strains (5.9%) clustered with the C. canis type strain (CcD38 = LMG 29146) (Fig. 1). According to the 16S rDNA phylogenetic analysis, these six strains were, thus, reclassified as C. canis (Supplementary Table S1). We then subjected the 96 C. canimorsus sensu stricto strains to the PCR test designed to detect the three A, B, and C capsular serovars14. These analyses showed that 87 of the 96 strains were positive and could be further typed as either A, B, or C by serovar-specific PCRs (Table 1 and Supplementary Fig. S1a–d). To confirm these results, we performed Western blot analyses on polysaccharide extracts of the 87 strains, using antisera specifically recognizing the A, B, or C capsular serovars (Table 1 and Supplementary Fig. S2a–c). This experiment confirmed the PCR typing results and identifications for all strains tested except for strain G58, which was found to belong to the B serovar by PCR but did not react with the B antiserum. In summary, 86 of the 96 C. canimorsus strains tested (89.58%) belonged to either serovars A (30/96, 31.3%), B (28/96, 29.2%), or C (28/96, 29.2%). Next, we tested all strains by PCRs detecting the D and E capsular types14. According to these analyses, seven strains belonged to serovar D and none to serovar E (Table 1 and Supplementary Fig. S1e, f), which was confirmed by Western blot (Supplementary Fig. S2d). We then tested the three strains that we could not type by the A-E PCRs (G06, G45, and G58) using Western blot for all the capsular types (A–I) we identified in previous studies14. None of the strains reacted with these antisera (Supplementary Fig. S2a–i). Three rabbit antisera were thus raised against the G06, G45, and G58 whole bacteria and adsorbed (see Methods). The anti-G06 antiserum strongly recognized the capsule of the G06 strain (Supplementary Fig. S2j). The anti-G58 serum recognized only the capsule of G58 (Supplementary Fig. S2k). Surprisingly, a Western blot analysis of a polysaccharide sample of strain G45 with G45 antiserum did not show any capsule for this strain (Supplementary Fig. S2l). Thus, we identified two new serovars that we named L (G06) and M (G58), and we found a strain without a capsule. The capsular typing results are summarized in Fig. 2. In short, we found that the 96 C. canimorsus strains from the CCUG belonged to only six serovars, with A, B, and C being heavily dominant (89.6%), followed by the D serovar (7.3%) (Fig. 2a). These results are in line with those previously observed for a collection of 25 strains isolated worldwide from human infections (Fig. 2a, b)14, with the only difference being that none of the CCUG strains belonged to the E serovar and one strain was non-capsulated.
Fig. 1. 16S rDNA majority consensus tree of CCUG clinical strains.
Flavobacterium johnsoniae (accession number M59051) was selected as an outgroup (software: RDP Tree Builder)
Table 1.
Capsular typing of 96 human-isolated C. canimorsus strains from the CCUG collection
| Strain | PCR typing | WB typing | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | A | B | C | D | E | Serovar | A | B | C | D | E | F | G | H | I | Serovar | |
| G01 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G02 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G03 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G04 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G06 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | nt |
| G07 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G09 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G11 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G12 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G16 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G18 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G20 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G22 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G23 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G24 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G26 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G27 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G28 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G29 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G30 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G31 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G33 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G34 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G35 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G37 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G38 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G39 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G40 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G41 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G43 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G44 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G45 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | nt |
| G46 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G47 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G48 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G49 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G50 | + | − | − | + | − | − | C | nd | nd | nd | nd | nd | nd | nd | nd | nd | C |
| G51 | + | − | + | − | − | − | B | nd | + | + | nd | nd | nd | nd | nd | nd | B |
| G52 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G54 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G56 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G57 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G58 | + | − | + | − | − | − | B | − | − | − | − | − | − | − | − | − | nt |
| G59 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G60 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G61 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G63 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G64 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G65 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G66 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G67 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G68 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G69 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G70 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G71 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G72 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G73 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G75 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G76 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G79 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G80 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G81 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G82 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G83 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G85 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G86 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G88 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G90 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G91 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G92 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G93 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G94 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G95 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G96 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G97 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G98 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G99 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G100 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G101 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G102 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G103 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G104 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G106 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
| G108 | + | + | − | + | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G109 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G110 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G112 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G113 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G114 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G115 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G116 | + | + | + | − | − | − | A | + | nd | nd | nd | nd | nd | nd | nd | nd | A |
| G117 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G118 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G119 | + | − | − | + | − | − | C | nd | nd | + | nd | nd | nd | nd | nd | nd | C |
| G120 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | B |
| G121 | − | − | − | − | + | − | D | nd | nd | nd | + | nd | nd | nd | nd | nd | D |
Capsular typing was determined by PCR using the oligonucleotides given in Supplementary Table S2. The results were interpreted as previously described14: strains positive for PCR A, PCR A and PCR B or PCR A and PCR C were typed as A, strains positive for only PCR B were typed as B, and strains positive for only PCR C as C. Western blot analysis on polysaccharidic structures was performed using specific polyclonal rabbit antisera. nd not determined, nt non-typable
Fig. 2. Prevalence of capsular serovars in C. canimorsus strains.
Capsular serovar prevalence in human-isolated C. canimorsus strains from the CCUG collection (a), UNamur collection (b), and CCUG and UNamur collections (c). Capsular serovar prevalence in dog-isolated C. canimorsus strains (d). The data presented in b and d were partially previously published14
Screening of dog-isolated C. canimorsus strains for the presence of the L and M capsular serovars
We tested the prevalence of the new L and M capsular serovars in a collection of 52 C. canimorsus strains isolated from the mouths of dogs from Switzerland and Belgium3,14 by an ELISA. Two strains, one isolated in Switzerland and one isolated in Belgium, were found to react with the anti-L sera (CcD20 and CcD106), while none reacted with the anti-M sera (Supplementary Table S3). These results were confirmed by Western blot analysis of polysaccharidic structures (Supplementary Fig. S3). This experiment showed that capsular serovar L is not limited to Scandinavia.
Capsular typing of C. canis strains isolated from human infections reveals the presence of a serovar B strain
As mentioned above, the strains of Capnocytophaga spp. in this study that were isolated from human clinical samples included six C. canis strains (Fig. 1 and Supplementary Table S1). This finding may seem odd because C. canis was described initially as a non-pathogenic species3, although, since then, a few cases of human infections from C. canis have been reported. Zangenah et al. reported the isolation of a C. canis from a wound caused by a cat bite, and Taki et al. recently described a human septicemia after a cat bite4,5. It was, thus, not completely unexpected to find some C. canis among the collection of clinical strains, although the limited number of C. canis strains (6) compared to the high number of C. canimorsus (96) strains in the strain collection indicates that C. canis is far less involved in human infections than C. canimorsus.
The majority of C. canis strains isolated from dogs lack several factors that might contribute to C. canimorsus pathogenicity, such as the ability to acquire iron from transferrin and proliferate in human serum, as well as the capacity to deglycosylate host glycoproteins and cytochrome-oxidase activity3. However, Zangenah et al. and Taki et al. reported that the C. canis isolates that caused human infections are endowed with cytochrome-oxidase activity, thus suggesting that these strains differ from the majority of C. canis strains found in dog mouths. We tested for oxidase activity in the six C. canis strains isolated from human clinical samples and observed two of them (G05 and G17) to be positive (data not shown).
Given the close relationship between C. canimorsus and C. canis, we assessed whether other factors, such as capsular serovars, might be shared between these two species and, in particular, among the clinical C. canis strains; we, thus, tested the six strains of C. canis isolated from human clinical samples for the presence of C. canimorsus capsular serovars by PCR, using the primers for serovars ABC, D, and E. Three strains out of six gave a positive result for the ABC PCR (G05, G36, and G77), but two of them (G36 and G77) could not be typed further by PCR using the A-specific, B-specific, and C-specific primers or by Western blot using the specific antisera. G05 was identified as serovar B (Table 2 and Supplementary Fig. S4a–f), and this result was confirmed by a Western blot analysis (Table 2 and Supplementary Fig. S5a). The remaining three C. canis strains were tested by Western blot for the presence of capsular serovars, but none of them reacted with any of the sera (Supplementary Fig. S5b–l). The finding that one of the C. canis human clinical strains (G05) has a C. canimorsus serovar B capsule is of great interest, since it shows for the first time that capsular serovar B is shared between these two closely related species. Interestingly, strain G05 is also oxidase-positive, thus suggesting that it might be more virulent than most C. canis strains, since it possesses these two factors. It is also of interest that one strain (G36) was isolated from a patient who had been bitten by a cat. This is the only cat-transmitted strain we have in our collections of human isolates.
Table 2.
Capsular typing of six human-isolated C. canis strains from the CCUG collection
| Strain | PCR typing | WB typing | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | A | B | C | D | E | Serovar | A | B | C | D | E | F | G | H | I | L | M | Serovar | |
| G05 | + | − | + | − | − | − | B | nd | + | nd | nd | nd | nd | nd | nd | nd | nd | nd | B |
| G15 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| G17 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| G36 | + | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| G42 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| G77 | + | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
Capsular typing was determined by PCR using the oligonucleotides given in Supplementary Table S2. The results were interpreted as indicated in ref. 14: strains positive for PCR A, PCR A and PCR B or PCR A and PCR C were typed as A, strains positive for only PCR B were typed as B, and strains positive for only PCR C as C. Western blot analysis on polysaccharidic structures was performed using specific polyclonal rabbit antisera. nd not determined, nt non-typable
C. canis isolated from dogs includes serovar A strains
We also assessed whether other C. canimorsus capsular serovars might be shared by C. canis; we screened by PCR and ELISA our C. canis collection of 34 strains isolated from healthy dogs3. Four isolates (CcD7, CcD11, CcD46, and CcD111) were identified as capsular serovar A, and one (CcD123) was serovar F (Supplementary Fig. S6 and Table 3). None of the other strains of C. canis reacted with any of the tested sera (Table 3). We confirmed the presence of the capsular serovars A and F by Western blot analyses (Supplementary Fig. S7). Our data, thus, showed that, in addition to the serovar B capsule, C. canis can also be endowed with capsules of serovar A (four strains of 34) and of serovar F (one strain of 34). The four serovar A strains are oxidase-negative, while the serovar F strain is oxidase-positive3.
Table 3.
Capsular typing of 34 dog-isolated C. canis strains
| Strain CcD |
PCR | ELISA | WB | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | A | B | C | D | E | Serovar | A | B | C | D | E | F | G | H | I | L | M | Serovar | A | F | Serovar | |
| 1 | − | − | − | − | − | − | nt | 7.3 | 6.5 | 12.4 | 14.7 | 11.8 | 14.6 | 15.4 | 9.5 | 12.9 | 9.6 | 8.3 | nt | nd | nd | nt |
| 4 | − | − | − | − | − | − | nt | 6.1 | 6.0 | 12.8 | 16.1 | 9.26 | 14.7 | 15.4 | 9.9 | 11.8 | 10.3 | 7.9 | nt | nd | nd | nt |
| 7 | + | + | − | − | − | − | A | 102.6 | 8.6 | 10.8 | 13.8 | 9.76 | 13.2 | 15.1 | 10.6 | 10.4 | 9.1 | 7.3 | A | + | nd | A |
| 11 | + | + | − | − | − | − | A | 99.0 | 6.9 | 9.98 | 14.4 | 9.35 | 12.8 | 14.9 | 10.3 | 11.1 | 8.8 | 7.3 | A | + | nd | A |
| 15 | − | − | − | − | − | − | nt | 9.6 | 6.9 | 10.2 | 12.9 | 8.85 | 12.7 | 14.5 | 9.8 | 10.2 | 9.9 | 7.8 | nt | nd | nd | nt |
| 36 | − | − | − | − | − | − | nt | 12.3 | 6.0 | 11.8 | 13.5 | 9.35 | 13.5 | 14.3 | 9.3 | 10.6 | 9.5 | 8.0 | nt | nd | nd | nt |
| 38 | − | − | − | − | − | − | nt | 8.7 | 6.2 | 11.5 | 14.3 | 9.93 | 14.6 | 14.5 | 10.1 | 11.1 | 11.1 | 9.2 | nt | nd | nd | nt |
| 46 | + | + | − | − | − | − | A | 96.7 | 6.4 | 11.3 | 13.5 | 9.10 | 12.2 | 14.0 | 8.7 | 9.8 | 11.6 | 8.5 | A | + | nd | A |
| 50 | − | − | − | − | − | − | nt | 8.7 | 6.7 | 10.9 | 12.1 | 8.77 | 11.9 | 13.4 | 8.7 | 8.8 | 9.0 | 7.3 | nt | nd | nd | nt |
| 54 | − | − | − | − | − | − | nt | 9.3 | 5.8 | 8.09 | 15.3 | 9.14 | 14.5 | 16.3 | 12.8 | 10.8 | 7.9 | 5.7 | nt | nd | nd | nt |
| 64 | − | − | − | − | − | − | nt | 9.0 | 6.6 | 11.0 | 14.1 | 10.1 | 14.3 | 15.0 | 10.1 | 10.7 | 8.7 | 6.9 | nt | nd | nd | nt |
| 66 | − | − | − | − | − | − | nt | 11.7 | 6.7 | 11.2 | 13.9 | 10.3 | 13.7 | 14.8 | 9.5 | 11.0 | 8.8 | 7.6 | nt | nd | nd | nt |
| 75 | − | − | − | − | − | − | nt | 9.2 | 6.9 | 10.7 | 12.9 | 9.76 | 12.5 | 15.7 | 9.3 | 9.6 | 9.4 | 7.9 | nt | nd | nd | nt |
| 79 | − | − | − | − | − | − | nt | 8.9 | 6.0 | 10.3 | 13.8 | 9.39 | 15.2 | 15.6 | 9.6 | 10.1 | 9.5 | 7.8 | nt | nd | nd | nt |
| 82 | − | − | − | − | − | − | nt | 9.1 | 6.8 | 10.0 | 15.2 | 11.3 | 13.1 | 15.1 | 9.8 | 11.0 | 9.1 | 7.2 | nt | nd | nd | nt |
| 85 | − | − | − | − | − | − | nt | 11.6 | 6.2 | 9.5 | 13.2 | 10.2 | 14.1 | 14.9 | 9.7 | 11.1 | 8.5 | 6.9 | nt | nd | nd | nt |
| 88 | − | − | − | − | − | − | nt | 10.3 | 6.2 | 10.6 | 12.8 | 10.2 | 13.1 | 14.3 | 8.8 | 10.3 | 10.2 | 7.6 | nt | nd | nd | nt |
| 93 | − | − | − | − | − | − | nt | 6.7 | 5.7 | 12.7 | 14.7 | 9.8 | 13.3 | 14.8 | 9.4 | 10.6 | 9.8 | 7.6 | nt | nd | nd | nt |
| 94 | − | − | − | − | − | − | nt | 8.2 | 6.76 | 8.63 | 12.81 | 9.31 | 12.6 | 13.7 | 9.6 | 10.2 | 6.8 | 5.8 | nt | nd | nd | nt |
| 95 | − | − | − | − | − | − | nt | 7.1 | 6.3 | 10.6 | 14.1 | 9.14 | 12.8 | 15.0 | 9.9 | 10.1 | 19.8 | 7.6 | nt | nd | nd | nt |
| 97 | − | − | − | − | − | − | nt | 6.7 | 6.6 | 10.7 | 13.5 | 9.02 | 13.8 | 15.7 | 9.8 | 9.8 | 9.9 | 8.2 | nt | nd | nd | nt |
| 102 | − | − | − | − | − | − | nt | 10.5 | 6.0 | 9.44 | 14.9 | 9.31 | 14.1 | 16.8 | 11.0 | 10.4 | 8.7 | 6.1 | nt | nd | nd | nt |
| 103 | − | − | − | − | − | − | nt | 7.7 | 9.6 | 8.57 | 15.3 | 10.8 | 14.4 | 16.5 | 10.8 | 10.9 | 9.5 | 7.0 | nt | nd | nd | nt |
| 108 | − | − | − | − | − | − | nt | 9.6 | 7.6 | 9.20 | 14.55 | 9.51 | 12.3 | 13.8 | 9.1 | 8.8 | 7.8 | 7.2 | nt | nd | nd | nt |
| 109 | − | − | − | − | − | − | nt | 7.9 | 6.5 | 10.1 | 13.0 | 10.14 | 12.5 | 15.1 | 8.7 | 9.2 | 10.0 | 7.4 | nt | nd | nd | nt |
| 110 | − | − | − | − | − | − | nt | 8.0 | 5.7 | 10.5 | 17 | 9.89 | 13.6 | 14.7 | 9.4 | 9.9 | 7.8 | 6.7 | nt | nd | nd | nt |
| 111 | + | + | − | − | − | − | A | 104.5 | 9.4 | 10.4 | 14.7 | 10.6 | 13.4 | 16.3 | 10.4 | 11.3 | 9.5 | 7.0 | A | + | nd | A |
| 112 | − | − | − | − | − | − | nt | 7.1 | 6.5 | 9.32 | 15.5 | 9.47 | 12.1 | 14.1 | 9.3 | 10.3 | 8.8 | 6.7 | nt | nd | nd | nt |
| 114 | − | − | − | − | − | − | nt | 8.9 | 6.8 | 11.4 | 15 | 9.85 | 13.4 | 16.2 | 9.8 | 9.9 | 11.3 | 7.7 | nt | nd | nd | nt |
| 121 | − | − | − | − | − | − | nt | 8.1 | 6.3 | 11.4 | 15.1 | 9.51 | 11.6 | 14.9 | 9.6 | 10.7 | 9.5 | 7.5 | nt | nd | nd | nt |
| 123 | − | − | − | − | − | − | nt | 10.3 | 9.2 | 7.3 | 21.9 | 9.16 | 81.2 | 13.7 | 12.0 | 10.7 | 21.4 | 10.3 | F | nd | + | F |
| 125 | − | − | − | − | − | − | nt | 7.4 | 7.8 | 11.1 | 13.8 | 7.29 | 12.5 | 15.4 | 13.3 | 9.0 | 8.2 | 6.5 | nt | nd | nd | nt |
| 127 | − | − | − | − | − | − | nt | 8.1 | 7.1 | 12.5 | 18.9 | 7.87 | 12.1 | 14.2 | 12.4 | 9.9 | 8.8 | 7.9 | nt | nd | nd | nt |
| 128 | − | − | − | − | − | − | nt | 7.4 | 16.8 | 12.3 | 13. | 6.9 | 11.2 | 13.4 | 10.8 | 10.6 | 9.1 | 7.9 | nt | nd | nd | nt |
Capsular typing was determined by PCR using the oligonucleotides given in Supplementary Table S2. The results were interpreted as indicated in ref. 14: strains positive for PCR A, PCR A and PCR B or PCR A and PCR C were typed as A, strains positive for only PCR B were typed as B, and strains positive for only PCR C as C. Capsular serotyping was determined by ELISA on entire heat-killed bacteria using specific polyclonal rabbit antisera. The readout of the ELISA was absorbance, but the results are expressed here as percentage of reactivity calculated with respect to the absorbance value obtained for the capsular type strain. Values are expressed as the means. Isolates with high reactivities are in bold. Western blot analysis on polysaccharidic structures was performed using specific polyclonal rabbit antisera. nd not determined, nt non-typable
Capsular serovar B is also found in C. cynodegmi human isolates
The finding that C. canis strains may have capsules of the same serovars that identify more virulent C. canimorsus strains raised the question of whether C. cynodegmi, another closely related species, might also have capsules of these C. canimorsus serovars. We tested 10 C. cynodegmi strains isolated from human infections and 16 C. cynodegmi strains isolated from healthy dogs (Supplementary Figs. S8, S9, S10) by PCR and Western blot. As summarized in Table 4, three of the 10 C. cynodegmi strains isolated from human infections were found to possess serovar B capsules (Supplementary Fig. S9). The remaining 7 human-isolated strains and the 16 dog-isolated strains could not be typed with the 11 C. canimorsus antisera (Table 4, Supplementary Figs. S9, S10). In conclusion, our data show that C. cynodegmi, as well as C. canis, may share capsule serovar B.
Table 4.
Capsular typing of C. cynodegmi isolates
| Strain | PCR typing | WB typing | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | A | B | C | D | E | Serovar | A | B | C | D | E | F | G | H | I | L | M | Serovar | ||
|
Human strains |
G14 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| G25 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| G55 | + | − | + | − | − | − | B | − | + | − | − | − | − | − | − | − | − | − | B | |
| G78 | + | − | + | − | − | − | B | − | + | − | − | − | − | − | − | − | − | − | B | |
| G84 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| G89 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| G107 | + | − | + | − | − | − | B | − | + | − | − | − | − | − | − | − | − | − | B | |
| G122 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| G123 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy4 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
|
Dog strains |
Gd02 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt |
| Gd12 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Gd13 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Gd14 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Gd16 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Gd17 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy1 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy19 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy46 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy74 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy121 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy118 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy110 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy113 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccy281 | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
| Ccyn2B | − | − | − | − | − | − | nt | − | − | − | − | − | − | − | − | − | − | − | nt | |
Capsular typing was determined by PCR using the oligonucleotides given in Supplementary Table S2. The results were interpreted as described previously14: strains positive for PCR A, PCR A and PCR B or PCR A and PCR C were typed as A, strains positive for only PCR B were typed as B, and strains positive for only PCR C as C. Western blot analysis on polysaccharidic structures was performed using specific polyclonal rabbit antisera. nt non-typable
Discussion
In our previous work, we showed that C. canimorsus is endowed with capsular polysaccharides and that the majority of human-isolated strains exhibit capsule type A, B, or C, suggesting that these three capsular serovars are more virulent to humans13,14. In this study, we confirmed the prevalence of C. canimorsus capsular serovars A, B, and C in a larger collection of human-isolated strains. Among the 102 strains within the CCUG, 16S rDNA sequence analyses showed that 96 were C. canimorsus sensu stricto, while 6 belonged to the closely related species C. canis; the misclassifications were due to the recognition of C. canis as a distinct species 26 years after the valid publication of C. canimorsus2,3. Serotyping the 96 C. canimorsus strains confirmed the prevalence of capsular serovars A, B, and C in strains isolated from human infections, with 86 of 96 strains (89.6%) belonging to these three serovars, with approximately 30% of each serovar. No significant difference was found in the prevalence of the A, B, and C serovars (Fisher’s exact test, with significance levels of p < 0.05, p = 0.2441 for A, p = 1 for B, and p = 0.589 for C) between the human-isolated C. canimorsus of the CCUG collection and the previously studied collection of strains isolated worldwide14. These data also confirmed that there are significantly more strains belonging to serovars A, B, and C among human isolates than among dog isolates (7.6%), confirming that these three serovars are more virulent than any of the others (Fig. 2c, d). In addition, our study confirms that the A, B, and C serovars are not restricted to one geographical area. In addition to the serovar A, B, and C strains, the CCUG collection contained seven (7.3%) C. canimorsus strains of serovar D. These data are in agreement with the finding that the D serovar is the fourth most prevalent serovar among C. canimorsus human isolates14. Thus, although they represent only 7–8% (ref. 14 and this study) of the human clinical isolates, serovar D strains should be considered to be virulent and considered for prophylaxis. In contrast to our previous findings14, we could not detect any serovar E strain among the CCUG collection, although we discovered two new capsular serovars, L and M, each with a limited (1%) prevalence in human infections. We also found one non-capsulated strain among the CCUG collection. Unfortunately, due to the lack of clinical data regarding the patients from whom all CCUG strains were isolated, at this point, we cannot draw any conclusions regarding correlation of the severity of the disease with the capsular serovars. Nevertheless, the present study confirms that serovars other than A–D may cause human infection but with a very low frequency. These serovars may be less virulent and infectious only for immunocompromised patients, although we have no data to test this hypothesis here. We also tested the two new L and M antisera against the collection of strains isolated from Swiss and Belgian dogs3 and found two Swiss C. canimorsus strains endowed with an L-type capsule. Thus, as we have shown before14, the serovars are internationally distributed. With 11 C. canimorsus serovars (A–M) characterized to date, we can type only 38.5% of the dog-isolated strains of our collection (20 of 52), thus reinforcing a hypothesis of the existence of a large repertoire of capsular serovars in dog-hosted C. canimorsus.
C. canis was originally isolated from healthy dogs and presumed to be a non-pathogenic species because it was found exclusively in dogs and not among the strains of Capnocytophaga spp. isolated from severe human infections. In addition, C. canis strains are missing several factors that are suspected to contribute to C. canimorsus pathogenicity, such as the ability to acquire iron from transferrin and proliferate in human serum, as well as the capacity to deglycosylate host glycoproteins and a lack of oxidase activity3. Since the description of the C. canis species in 20153, only a few cases of human infection from C. canis have been reported in the literature. However, a C. canis strain was isolated from a cat wound, and another C. canis was recently reported to cause a human septicemia4,5. It was, thus, not totally unexpected to find some C. canis among the CCUG collection of clinical strains, although their limited number (6) compared to that of C. canimorsus (96) indicates that C. canis is much less involved in human infections than is C. canimorsus.
The C. canis strains previously isolated from human infections were both oxidase-positive4,5, differing from the majority of C. canis strains found in dog mouths3.
Among the six C. canis strains of the CCUG collection, we found two that were oxidase-positive, supporting the hypothesis that oxidase-positive C. canis strains are more virulent to humans than strains lacking this activity. We found one C. canis strain to be endowed with a serovar B capsule. This observation is of great interest, because it shows for the first time that this capsular serovar is not restricted to the C. canimorsus species. In addition, we identified four dog-isolated C. canis strains belonging to capsular serovar A and one isolate belonging to the F serovar, showing that several C. canimorsus serovars are present in the closely related C. canis species. We found 11.7% of dog-isolated C. canis belonging to the A serovar, indicating that this serovar is even more frequent in C. canis than in dog-isolated C. canimorsus (1.9%). In contrast, among the six human-isolated C. canis strains, we could not find any serovar A strain. Considering the limited number of clinical C. canis strains analyzed, we cannot draw any definitive conclusion, but we can hypothesize that, in C. canis, capsular serovar A strains are not more virulent than other serovars, as is the case for C. canimorsus. The same conclusion cannot be drawn for serovar B C. canis, which we found in one clinical isolate but not in dog-isolated C. canis. Most C. canis strains could not be typed with the 11 sera we generated, suggesting that, as in C. canimorsus, there is a large reservoir of capsular serovars in C. canis. In line with this hypothesis, we note C. canis strains G36 and G77, which are positive for PCR ABC but do not belong to the A, B, and C serovars. Their genomes likely encode the wfdR glycosyl transferase detected by the ABC PCR but not one or more genes that determine the A, B, or C capsular serovars.
Whether some C. canis serovars might be more virulent to humans remains unknown and will require further investigation.
The finding of C. canis strains having similar capsules as C. canimorsus raised the question as to whether the other closely related dog-hosted species, C. cynodegmi, might also have capsules with similar epitopes. Interestingly, the typing of 10 human-isolated C. cynodegmi strains revealed the presence of three serovar B strains, indicating that this capsular serovar is not restricted to the C. canimorsus and C. canis species. Among the dog-isolated C. cynodegmi strains, none could be typed with our 11 sera. The finding of 3 of 10 (30%) C. cynodegmi strains of serovar B among clinical isolates and none among dog isolates suggests that C. cynodegmi endowed with a B capsule is more virulent to humans (Fisher’s exact test with a significance level of p < 0.05, p = 0.0462). As stated before, these conclusions should be taken with caution, considering the limited number (26) of C. cynodegmi strains analyzed in this study.
In conclusion, our study confirms that C. canimorsus strains belonging to serovars A, B, and C are more virulent to humans than other strains. Since these serovars represent a low percentage of dog-isolated strains, we can infer that few dogs carry these dangerous strains. This conclusion is of great importance, because it contributes to an explanation for the paradox of the low incidence of the disease in spite of the abundance of dogs carrying C. canimorsus. This conclusion is important for the prevention of the disease, because the PCR test we have developed could be adapted to identify potentially more dangerous dogs, keeping in mind, however, that some C. canis strains do harbor an A capsule and some C. cynodegmi strains harbor a B capsule. A real-time PCR test could be developed, for example, to detect virulent Capnocytophaga strains directly in dog saliva samples. A step forward would then be to eradicate the strains from these animals, leading to a significant reduction in pathogen shedding and, finally, in human infections. Furthermore, we show, for the first time, that several capsular serovars (A, B, and F) are shared by the three dog-hosted Capnocytophaga species. The commonality of some capsular serovars in these three species might result from genetic transfers of the capsular genes. This hypothesis is supported by the genetic organization of the C. canimorsus capsular genes, which are organized in loci including genes coding for transposases13. Whether Capnocytophaga species exchange their capsular genes is an open question that requires further investigation.
Materials and methods
Bacterial strains and growth conditions
The C. canimorsus, C. canis, and C. cynodegmi bacterial strains used in this study are listed in Supplementary Table S1. For serotyping, bacteria were grown on heart infusion agar (BD Difco, Franklin Lakes, NJ, USA) plates supplemented with 5% sheep blood (Oxoid, Basingstoke, UK) and 20 µg/ml gentamicin (Sigma-Aldrich, Darmstadt, Germany) and incubated for 48 h at 37 °C with 5% CO2.
Species identification by 16S rDNA sequencing
For 16S rRNA gene (16S rDNA) amplification by PCR, a single colony was resuspended in 100 µl ddH2O and heated for 10 min at 98 °C. One microliter was used as template for amplification of a 1.07 kb fragment of the 16S rDNA. The primers 27F and 1100R (Supplementary Table S2) were used at 0.4 mM concentration with 200 mM dNTP and 1 U Taq polymerase (NEB, Ipswich, MD, USA). PCR was carried out for five initial cycles (94 °C for 30 s, 60 °C for 2 min, 72 °C for 3 min), in which the annealing temperature was reduced by 1.5 °C/cycle, followed by 30 cycles (94 °C for 30 s, 52 °C for 90 s, 72 °C for 3 min) and a final elongation for 10 min at 72 °C. The 1.07 kb PCR product was extracted from a 1.2% agarose gel by NucleoSpin (Macherey Nagel, Düren, Germany). The cleaned PCR products were sequenced by Eurofins Genomics with the primers 27F, 685R, and 1100R (ref. 15 and ref. 16) (Supplementary Table S2). The consensus sequences were obtained using BioEdit software and then used to build a 16S phylogenetic tree using the Ribosomal Database Project Tree Builder tool (http://rdp.cme.msu.edu/).
Antiserum production and adsorption
Anti-A to anti-I antiserum production is described in ref. 14. Rabbit polyclonal sera anti-L (strain G06) and anti-M (strain G58) and anti-G45 strain were prepared likewise according to ref. 14. Immunizations were performed at the Centre d’Economie Rurale (CER Groupe, Aye, Belgium). The CER animal welfare committee approved the animal handling and procedures. Sera were then adsorbed with a mix of 25 strains isolated from patients (Cc1 to Cc25, Supplementary Table S1) to obtain polyclonal antibodies specifically recognizing the capsular serovars against which the sera were raised. Adsorptions were performed by incubating 250 µl of antiserum with 6 × 109 PFA-fixed bacteria on a rotating wheel at room temperature for at least 2 h. Bacteria were removed by successive centrifugations. The incubation and following centrifugations were repeated four times.
ELISA
Capsular typing of Capnocytophaga strains by PCR, Western blotting and ELISA was performed as described in ref. 14.
Electronic supplementary material
Acknowledgements
This work was supported by grant SOC 1510582 from the Walloon Region and by Proof of Concept grant 780540 “CANITEST” from the ERC.
Authors’ contributions
F.R. and E.H. conceived the study, analyzed and interpreted data, and wrote the manuscript. M.D., D.K., E.C., and M.O. performed the experiments. M.O. and E.M. provided CCUG strains, and E.M. revised the manuscript. G.R.C. conceived and designed the study, analyzed and interpreted the data, and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Francesco Renzi, Estelle Hess
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41426-018-0126-x).
References
- 1.Leadbetter ER, Holt SC, Socransky SS. Capnocytophaga: new genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch. Microbiol. 1979;122:9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hollis DG, Fanning GR, Weaver RE. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J. Clin. Microbiol. 1989;27:231–235. doi: 10.1128/jcm.27.2.231-235.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renzi, F., Dol, M., Raymackers, A., Manfredi, P. & Cornelis, G. R. Only a subset of C. canimorsus strains is dangerous for humans. Emerg. Microbes Infect.4, e48 (2015). [DOI] [PMC free article] [PubMed]
- 4.Zangenah S, Abbasi N, Andersson AF, Bergman P. Whole genome sequencing identifies a novel species of the genus Capnocytophaga isolated from dog and cat bite wounds in humans. Sci. Rep. 2016;6:22919. doi: 10.1038/srep22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taki M, et al. Sepsis caused by newly identified Capnocytophaga canis following cat bites: C. canis is the third candidate along with C. canimorsus and C. cynodegmi causing zoonotic infection. Intern. Med. 2018;57:273–277. doi: 10.2169/internalmedicine.9196-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1271–1280. doi: 10.1007/s10096-015-2360-7. [DOI] [PubMed] [Google Scholar]
- 7.Butler T, et al. Unidentified gram-negative rod infection. A new disease of man. Ann. Intern. Med. 1977;86:1–5. doi: 10.7326/0003-4819-86-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Zajkowska J, Krol M, Falkowski D, Syed N, Kamienska A. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite—review of literature. Przegl. Epidemiol. 2016;70:289–295. [PubMed] [Google Scholar]
- 9.Gaastra W, Lipman LJ. Capnocytophaga canimorsus. Vet. Microbiol. 2010;140:339–346. doi: 10.1016/j.vetmic.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin. Infect. Dis. 1996;23:71–75. doi: 10.1093/clinids/23.1.71. [DOI] [PubMed] [Google Scholar]
- 11.van Dam AP, Jansz A. Capnocytophaga canimorsus infections in The Netherlands: a nationwide survey. Clin. Infect. Dis. 2011;17:312–315. doi: 10.1111/j.1469-0691.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 12.Hastbacka J, Hynninen M, Kolho E. Capnocytophaga canimorsus bacteremia: clinical features and outcomes from a Helsinki ICU cohort. Acta Anaesthesiol. Scand. 2016;60:1437–1443. doi: 10.1111/aas.12752. [DOI] [PubMed] [Google Scholar]
- 13.Renzi F, et al. Evidence for a LOS and a capsular polysaccharide in Capnocytophaga canimorsus. Sci. Rep. 2016;6:38914. doi: 10.1038/srep38914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess E, Renzi F, Koudad D, Dol M, Cornelis GR. Identification of virulent Capnocytophaga canimorsus isolates by capsular typing. J. Clin. Microbiol. 2017;55:1902–1914. doi: 10.1128/JCM.00249-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, J. L. Similarity Analysis of rRNAs. In P. Gerhard, R.G.E. Murray, G.A. Wood, N.R. Krieg (Eds.), Methods for General and Molecular Bacteriology, American Society for Microbiology, Washington, DC., 683–700, (1994).
- 16.Lane, D. J. 16S/23S rRNA Sequencing. In E.G. Stackebrandt, M. Goodfellow (Eds.), Nucleic Acid Techniques in Bacterial Systematics, John Wiley & Sons, NewYork.,115–174, (1991).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.