Abstract
‘Epichromatin’, the surface of chromatin beneath the interphase nuclear envelope (NE) or at the surface of mitotic chromosomes, was discovered by immunostaining with a specific bivalent mouse monoclonal anti-nucleosome antibody (mAb PL2-6). ‘Chromomeres’, punctate chromatin particles approximately 200–300 nm in diameter, identified throughout the interphase chromatin and along mitotic chromosomes, were observed by immunostaining with the monovalent papain-derived Fab fragments of bivalent PL2-6. The specific target for PL2-6 appears to include the nucleosome acidic patch. Thus, within the epichromatin and chromomeric regions, this epitope is ‘exposed’. Considering that histones possess unstructured ‘tails’ (i.e. intrinsically disordered peptide regions, IDPR), our perception of these chromatin regions becomes more ‘fuzzy’ (less defined). We suggest that epichromatin cationic tails facilitate interactions with anionic components of NE membranes. We also suggest that the unstructured histone tails (especially, histone H1 tails), with their presumed promiscuous binding, establish multivalent binding that stabilizes each chromomere as a unit of chromatin higher order structure. We propose an ‘unstructured stability’ hypothesis, which postulates that the stability of epichromatin and chromomeres (as well as other nuclear chromatin structures) is a consequence of the collective contributions of numerous weak histone IDPR binding interactions arising from the multivalent nucleosome, analogous to antibody avidity.
Keywords: histones, nucleosomes, intrinsically disordered regions, avidity
1. Background and recent data
Although the nucleosome was discovered more than 40 years ago [1], the next level of organization, the higher order chromatin structure, remains controversial. We believe this is due to the search for a rigid organization in a highly dynamic and fluid nuclear architecture. This review will focus upon chromatin architecture at the nuclear envelope (NE), at the surface of mitotic chromosomes and, to some extent, chromatin within nuclei and mitotic chromosomes. Interphase NE chromatin, its association with inner nuclear membrane proteins, mitotic chromosome structure and post-mitotic NE reformation are discussed within numerous excellent recent articles [2–11]. This personalized brief review will primarily discuss recent discoveries from our laboratory and their possible implications to the larger issue of chromatin higher order structure.
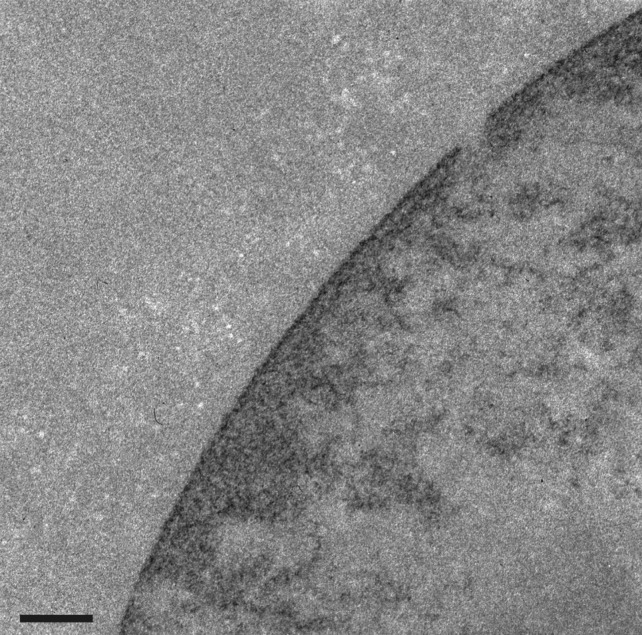
Antibodies against nuclear components and structures are a common feature of many autoimmune diseases, yielding important reagents in the study of interphase nuclei and mitotic chromosomes [12,13]. Indeed, the principal antibody tool (mAb PL2-6) of our laboratory for the past few years was discovered in the laboratory of Marc Monestier, during an analysis of mouse autoimmunity [14]. PL2-6 was developed into a hybridoma along with other anti-histone antibodies. When several of these antibodies were examined in our laboratory, we observed that PL2-6 produced a remarkably similar immunostaining pattern on cells from a variety of diverse species (human, mouse, Drosophila, Caenorhabditis elegans and tobacco) [15,16]: interphase nuclei exhibited strong staining beneath the NE, denoted ‘epichromatin’; mitotic chromosomes frequently exhibited even stronger staining at the outer edges of the chromosome surfaces (figures 1 and 2). The intense peripheral staining of the interphase nuclei was observed using thin section electron microscopy of interphase HL-60/S4 cells treated with the DNA-specific osmium ammine B (OAB) stain [17–19]; an example is shown in figure 3. This OAB stained image suggests that the DNA may be more densely packed just beneath the NE. More recently, we demonstrated that exposing interphase mammalian tissue culture cells to hyper-osmotic conditions (i.e. 320 mM sucrose in culture medium) resulted in contraction of interphase chromatin away from the NE, clearly revealing that the epichromatin epitope is separate from the NE lamina (figure 4).
Figure 1.
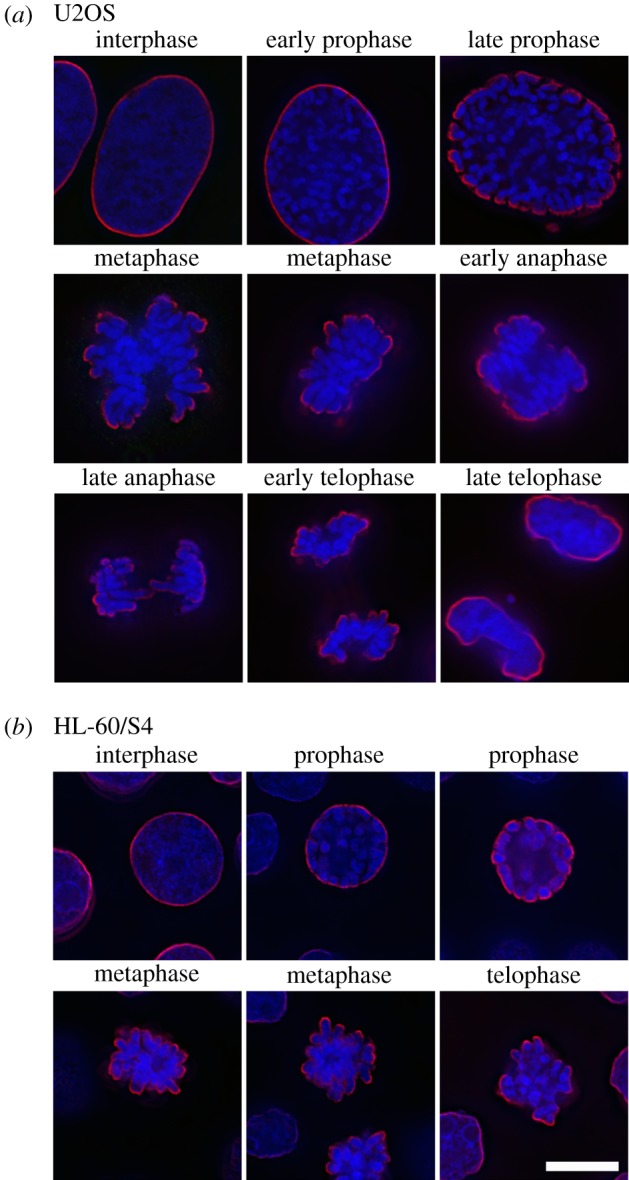
Immunostaining of the epichromatin epitope throughout the cell cycle in U2OS (a) and HL-60/S4 (b) cells using mAb PL2-6 (red) and a DNA stain (DAPI, blue). Note that epichromatin staining persists on the outer edges of the mitotic chromosomes, even following NE breakdown. The magnification bar for (a) and (b) equals 10 µm. This image has been previously published [15].
Figure 2.
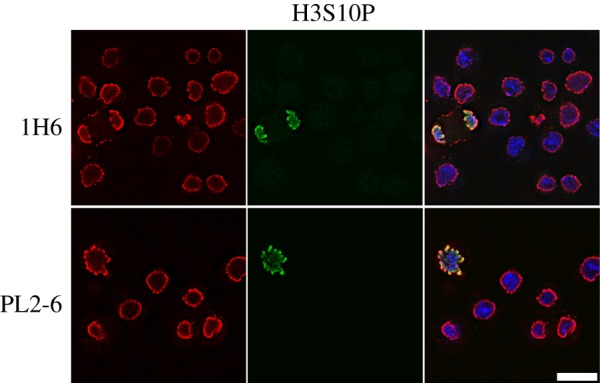
Immunostaining of the epichromatin epitope in interphase and mitotic Drosophila Kc cells using two different mAbs that stain epichromatin (PL2-6 and 1H6, red), rabbit polyclonal anti-H3S10p (green) and DNA (DAPI, blue). Note the similar staining of epichromatin as shown for human U2OS and HL-60/S4 cells (figure 1). The magnification bar equals 10 µm. This image has been previously published [16].
Figure 3.
DNA-specific staining (OAB) of an interphase HL-60/S4 cell imaged by thin section transmission electron microscopy. OAB shows a region of intense staining at the periphery of the interphase nucleus, suggesting a higher concentration of ordered DNA in the epichromatin region. The magnification bar equals 0.2 µm.
Figure 4.
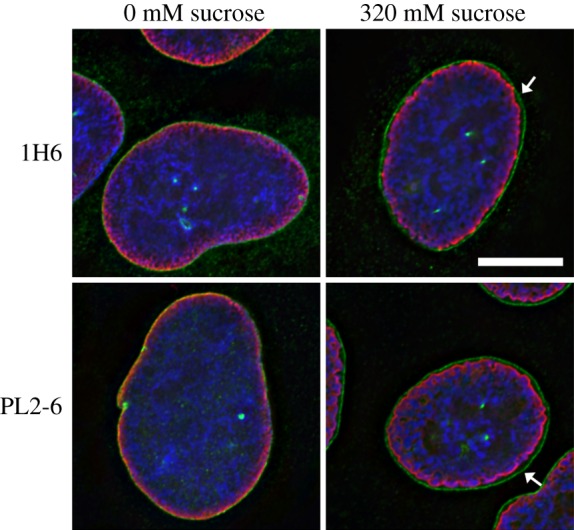
Immunostaining of the epichromatin epitope in interphase U2OS cells exposed to hyper-osmotic conditions (320 mM sucrose), compared to iso-osmotic conditions (0 mM sucrose), employing two different mAbs that stain epichromatin (PL2-6 and 1H6, red), two rabbit antibodies that stain NE proteins (emerin and lamin A, green) and a DNA stain (DAPI, blue). The arrows point to a gap between the NE (lamina) and epichromatin, observed in 320 mM sucrose. The magnification bar equals 10 µm. This image has been previously published [16].
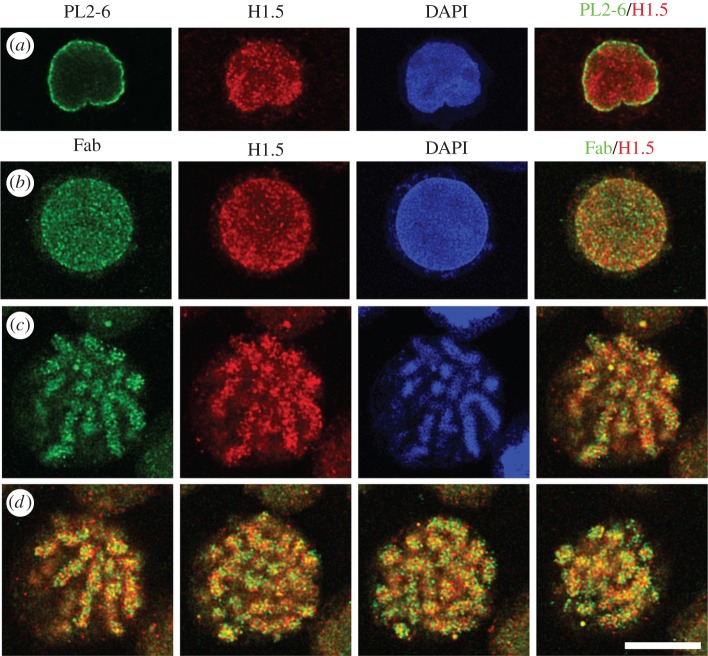
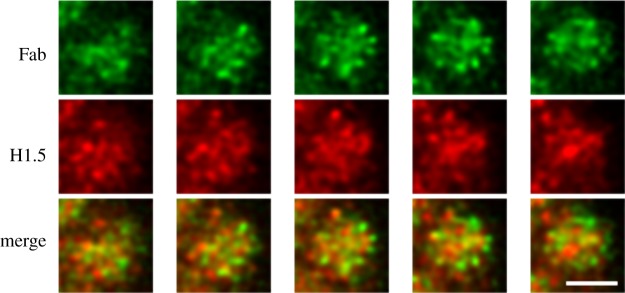
PL2-6 is a bivalent mouse IgG2b antibody. In order to examine the significance of antibody bivalency to the remarkable peripheral chromatin nuclear staining, we generated a monovalent form of PL2-6, employing papain digestion [20]. To our surprise, the monovalent Fab fragments yielded a punctate immunostaining pattern throughout interphase nuclei and along mitotic chromosome arms (figure 5). These (formaldehyde-fixed) punctate structures are approximately 200–300 nm in diameter and have been named ‘chromomeres’ [20], in deference to the pioneering observations of mitotic chromosome granules observed at the end of the nineteenth century [21–23] (figure 6). In favourable microscopic views, chromomeres appear to radiate out of a central region in the chromosome arms (figure 7). During the initial discovery of mAb PL2-6 [14], the authors demonstrated that histones H2A and H2B include the epitope site. By comparison of the peptide sequence of the PL2-6 (heavy chain variable region 3, hv3) to those of known nucleosome ‘acidic patch’-binding proteins (LANA and CENP-C), we predicted that the acidic patch (an ‘exposed’ juxtaposition of acidic amino acids in histones H2A and H2B) would include the epitope (figure 8) [20].
Figure 5.
Immunostaining patterns of bivalent PL2-6 and monovalent Fab fragments, derived from PL2-6. (a) An undifferentiated HL-60/S4 interphase nucleus stained with PL2-6 (green), anti-histone H1.5 (red), DAPI (blue) and merged red and green (R + G). (b) An undifferentiated HL-60/S4 interphase nucleus stained with Fab (green), anti-histone H1.5 (red), DAPI (blue) and merged (R + G). (c) A single confocal Z-slice from a mitotic HL-60/S4 cell stained with Fab, anti-histone H1.5, DAPI and the merged (R + G). (d) Various Z-slices from the merged mitotic R + G stack are presented. For all images, the magnification bar equals 10 µm. This image has been previously published in part [20].
Figure 6.
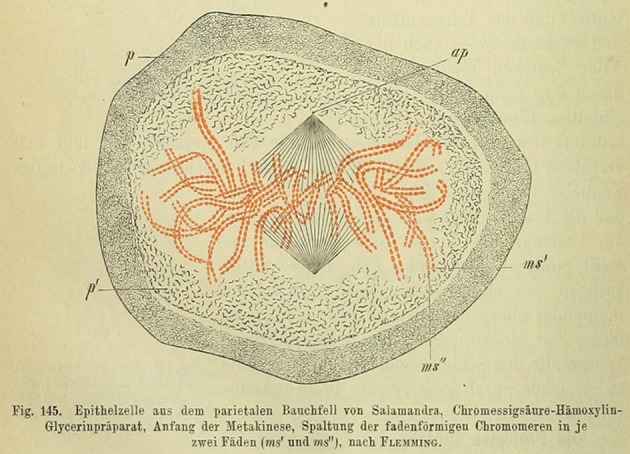
Mitotic figure from a stained salamander epithelial cell, drawn by Walther Flemming, originally published in ‘Zellsubstanz, Kern und Zelltheilung’ (1882, Tafel IIIb, Fig. 41). In the figure caption, Flemming specifically noted the chromosome substructure ‘Körnelung sehr deutlich’ (granulation very distinct). In 1896, this drawing was reprinted by H. Fol [22], who is generally credited with introducing the term ‘chromomere’. However, in his own caption to the reprinted figure, he appears to be describing the chromatid fibres not the chromatin granules. Simultaneously [23], in his classic book ‘The Cell in Development and Inheritance’ (1896), E. B. Wilson wrote in Chapter VI, p.221 the following: ‘The facts are now well established (1) that in a large number of cases the chromatin-thread consists of a series of granules (chromomeres) embedded in and held together by the linin-substance, (2) that the splitting of the chromosomes is caused by the division of these more elementary bodies … . These facts point unmistakably to the conclusion that these granules are perhaps to be regarded as independent morphological elements of a lower grade than the chromosomes.’ It is in that spirit that we have denoted our immunostained chromatin granules as ‘chromomeres’.
Figure 7.
Immunostaining patterns of monovalent Fab fragments on alternate sequential Z-slices along a mitotic chromosome arm, revealing a radial ‘chromomeric’ pattern. The top row is Fab (green); middle row, anti-histone H1.5 (red). The bottom row contains the merged ‘red + green (R + G)’ slices. Chromomeres are approximately 300 nm in diameter. The magnification bar equals 2 µm. This image has been previously published [20].
Figure 8.
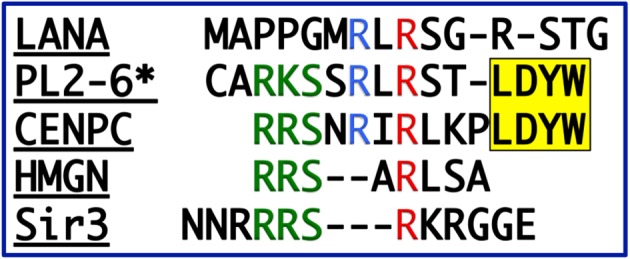
Peptide sequence comparisons. The PL2-6 heavy chain variable region 3 (‘*’, Hv3) compared to various acidic patch binding proteins. The second arginine (red R) in the LANA sequence (… RLRS …) forms salt bridges with H2A E61, D90 and E92 [24]. The yellow (… LDYW …) motif is a common hydrophobic structural feature of many Hv3 regions. This image has been previously published [20].
Several major questions arise from the unusual chromatin surface binding of interphase epichromatin by bivalent PL2-6: What are the properties of this chromatin? Do these properties change during cell differentiation? What type of DNA is present in interphase epichromatin? These questions were explored employing ChIP-Seq on the human myeloid leukaemia HL-60/S4 cell line, which can be differentiated in vitro into granulocytes and macrophage forms [25]. In summary, ChIP-Seq revealed considerable similarity in the DNA composition comparing interphase epichromatin from undifferentiated and differentiated cells. Epichromatin contains only approximately 4–5% of the total DNA sequences. In the HL-60/S4 cells, it is GC-enriched, highly methylated and exhibits a significant enrichment in retrotransposon Alu. Mapping epichromatin regions along the human chromosomes demonstrated considerable similarity in its discrete and discontinuous distribution, comparing the three cell states. Furthermore, epichromatin exhibits a high nucleosome density and a paucity of various histone post-translational modifications associated with transcription or repression of transcription [26]. Indeed, it appears that interphase epichromatin represents a unique unmodified (except for DNA methylation) chromatin ‘surface’ facing the inner membrane of the existing (or reforming) NE. It is important to stress that PL2-6 is not an anti-DNA antibody and that the DNA sequences enriched from HL-60/S4 cells are possibly unique to these cells. Certainly, Drosophila, C. elegans and tobacco cells do not have retrotransposon Alu, among other differences. A recent study [27], employing acridine orange staining, argues that epichromatin DNA possesses an A-form conformation more readily than internal chromatin.
Much less can be said about chromomeres, visualized with the monovalent Fab fragments of PL2-6. Chromomeres appear to have ‘exposed’ nucleosome acidic patches which are not highlighted by bivalent PL2-6. It is possible that some chromomeres present transcriptional ‘target regions’ on their surface, maintaining a more ‘open’ display of surface nucleosomes [28], but we have no information on the relationship of chromomeres to transcription. Chromomeres can also be visualized using bivalent anti-H1 antibodies [20], which implies that chromomere surfaces exhibit ‘exposed’ H1 epitopes. There appears to be some level of chromomere heterogeneity, with some chromomeres exhibiting particular H1 isotypes and others showing absence of particular H1 isotypes. Present immunostaining data are not sufficient to say whether chromomeres contain more than one H1 isotype. HL-60/S4 cells (undifferentiated and differentiated) have been demonstrated to contain primarily three H1 isotypes, H1.2, H1.4 and H1.5 [29]. Depending upon image ‘thresholding’ constraints, the number of HL-60/S4 Fab-stained undifferentiated interphase chromomeres (per diploid nucleus) is approximately 2791 (at 20% threshold) and less (approx. 1150) in mitotic chromosomes [20]. It is not clear whether this apparent reduction in chromomeres during metaphase is due to chromatin conformational changes or to the epichromatin epitope becoming ‘hidden’, or both. It is clear that loops in mitotic chromosomes, whether nested or tightly packaged, are highly condensed [11] and could appear as chromomeres in formaldehyde-fixed chromosomes.
How do we explain the different immunostaining patterns of bivalent PL2-6 and of monovalent Fab fragments? Conceivably, at least four factors might affect the bivalent versus the monovalent antibody staining pattern: (i) epitope local concentration; (ii) geometric arrangements of the multivalent epitopes; (iii) blocking by competing binders; and (iv) unique properties of epichromatin as a ‘surface’ (e.g. chromatin on one side; no chromatin on the other side). It is interesting that a zig–zag arrangement of nucleosomes along a single chromatin fibre (a view quite common in surface spread chromatin fibres; see [1]) yields adjacent nucleosomes exhibiting acidic patches related by an external dyad axis, which could match complementary bivalent PL2-6 binding sites (also related by an internal dyad axis; figure 9 and the electronic supplementary material, Movie). We suggest that the intense localized immunostaining pattern of PL2-6 is an example of high antibody ‘avidity’ (i.e. the binding of a bivalent antibody to a multivalent antigen, resulting in an increased ‘association constant’) binding to ‘exposed’ epitopes present at a high local concentration [30]. The monovalent Fab fragment does not have the property of avidity (with its high association constant) and is less dependent upon local geometry. But it is still influenced by epitope concentration and blocking by competitors. In sum, we suggest that bivalent PL2-6 is greatly responsive to the nucleosome–nucleosome geometry at the epichromatin surface, benefiting by its capability for avidity. The monovalent Fab can detect ‘exposed’ epitopes scattered throughout the nuclear and chromosome environment, revealing clustered epitopes defining the punctate chromomeres.
Figure 9.
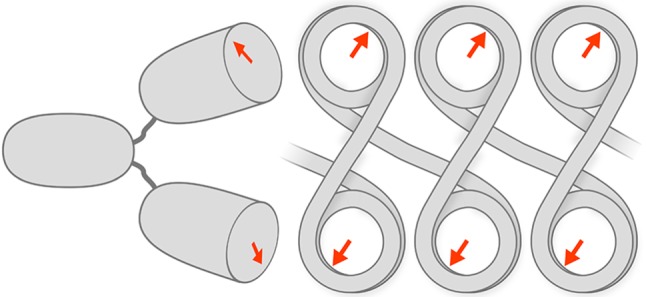
Cartoon sketch depicting a bivalent PL2-6 molecule binding to a zig-zag arrangement of nucleosomes. This sketch does not display accurate molecular dimensions. Rather, it is exaggerated to show the potential sites of interaction. A more accurate model can be visualized in the electronic supplementary material, Movie. The cartoon arrows on the nucleosome ‘faces’ represent the nucleosome acidic patches (AP). Notice that in this zig-zag arrangement, for sequential nucleosomes, the AP orientations are related by a dyad axis (perpendicular to the linker DNA and to the plane of the cartoon). Adjacent to the zig-zag chromatin is a representation of the bivalent PL2-6, also displaying the complementary binding regions as arrows. A bivalent IgG has a dyad axis running between the two Fab regions and along the midline of the Fc region. The junction between the Fab and Fc regions is very flexible, tolerating a wide range of angles between the Fab ‘arms’ and allowing the arms to rotate with considerable freedom. The geometry of the nucleosomes presents two binding sites to the bivalent IgG, exploiting the avidity principle.
2. Discussion
The 1950s and 1960s were the beginning of exciting times for structural biology: deduction of the protein α-helix and β-sheet from X-ray diffraction data of polypeptides, determination of the collagen triple helix, the DNA double helix, haemoglobin subunit allosteric interactions, predictability of RNAse peptide folding, tRNA structure, etc. In many respects, the cell became regarded as a ‘bag’ of highly regular macromolecular structures, functioning by ‘lock-and-key’ mechanisms with predictable secondary, tertiary and quaternary higher order folding, while being immersed in defined intracellular solutions. An amazing documentation of the successes with structure determination can be seen in the beautiful poster available online from the PDB (Molecular Machinery: A Tour of the Protein Data Base). This confidence in structural predictability, combined with fibre X-ray diffraction data (i.e. the method employed to decipher the DNA double helix) employed on isolated chromatin fibres led to two proposals for helical models of chromatin, both of which were wrong (aspects of this part of chromatin history, viewed through the eyes of the present authors, have been previously published) [1]. It is now clear that much of cellular structure, including chromatin, is more complex and disorganized than we had hoped. Soon after the discovery of the nucleosome in 1973/1974, helical arrangements of nucleosomes into approximately 30 nm fibres became the favoured next level of chromatin organization. Numerous studies, including electron microscopy and X-ray scattering, have eroded our confidence in the general existence of such structures [31–36]. Instead, current views emphasize less well-defined punctate super-nucleosomal clusters (with various names; e.g. fractal globules, topologically associating domains, contact domains, compact domains and 1 Mbp chromatin domains) [37–44], structures that we suggest are correlates with our fixed ‘chromomeres’ [20]. These structures undoubtedly reflect an underlying genetic organization and are reminiscent of the earlier studies of lampbrush and polytene chromosomes [45–49].
Describing the internal nucleosomal organization of chromomeres presents a distinct challenge. Histone H1 is a particularly attractive candidate for stabilizing super-nucleosomal clusters (chromomeres) within live cells. A considerable amount of research has been performed on the different H1 isotypes (generally, six isotypes in somatic human cells: H1.1, H1.2, H1.3, H1.4, H1.5 and H1.0 in terminally differentiated cells) [50–62]. A few of the conclusions presented in these many research and review articles are as follows. (i) A central highly conserved globular domain with a ‘winged helix fold’ [63], which binds to the nucleosome, is flanked by two unstructured peptide tails. (ii) In vitro, the presence of histone H1 is required to condense polynucleosomal chains at physiological ionic strength. (iii) The H1 C-terminal peptide is more important in the formation of chromatin higher order structure than is the N-terminal peptide (which still plays a role). (iv) There appears to be an insignificant amount of ‘free’ (unbound) H1 in the nucleus. (v) The residence time for H1 on a nucleosome is shorter than the residence time for the inner histones, but longer than for transcription factors or HMG proteins. (vi) H1 exhibits numerous types of post-translational modifications (especially phosphorylation) that are dynamic during the cell cycle and during cell differentiation. (vii) Genetic loss of certain histone isotypes can apparently be compensated by H1 redundancy, until the stoichiometry of H1/nucleosome becomes too low. It is clear that H1 proteins are dynamic within the nucleus, with complex and varying roles in influencing chromatin higher order structure and transcription regulation.
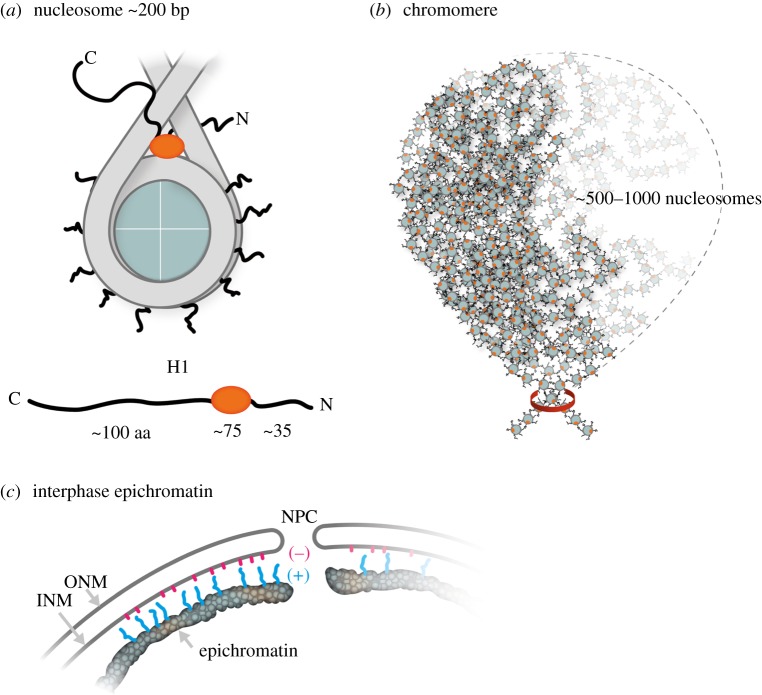
An additional challenge to the concept of highly defined chromatin structures comes from the emerging realization that the constituent histones of the nucleosome are rich in ‘unstructured’ intrinsically disordered peptide region (IDPR) [64–66]. IDPRs are frequently enriched with Lys, Arg, Pro and Ser residues, a clear characteristic of histone tails. IDPRs, by definition, do not exhibit stable peptide conformations in physiological buffers and are generally promiscuous in their interactions with binding partners. IDPR conformations are greatly affected by post-translational modifications [67] and usually acquire more defined peptide conformations as a consequence of binding [68]. Because of the frequently chaotic nature of the IDPR conformations in solution, they have been aptly described as ‘fuzzy’ or forming a peptide ‘cloud’ [69]. Nucleosomes possess 10 inner histone IDPR tails [65] and two IDPR histone H1 tails, becoming multivalent structures with numerous types of binding interactions [43,70] (figure 10a). As an analogue to the multivalent mononucleosome, we suggest considering the multivalent IgM (macro-immunoglobulin) molecule. The IgM molecule is composed of five IgG-like subunits, connected by their Fc regions, generating 10 antibody binding sites on a single macromolecule. The individual binding constants are usually relatively weak; but collectively, based upon the avidity principle, an IgM molecule has among the strongest association constant of any immunoglobulin. As a prototype for inter-nucleosome binding, we also cite the histone H4 tail, which can interact with the acidic patch of an adjacent nucleosome [74]. We suggest that ‘fuzzy’ nucleosomes form the basis for ‘fuzzy’ epichromatin and ‘fuzzy’ chromomeres. The unstructured basic tails extending outward from epichromatin could form electrostatic interactions with nuclear membrane anionic phospholipids (e.g. phosphatidylserine, PS) (figure 10c), analogous to the interaction between PS and the protein MARCKS at the plasma membrane [75,76]. The inner histone and H1 tails could be binding to neighbouring nucleosomes helping to stabilize the chromomeric nucleosome complex, acting as promiscuous ‘sticky tape’ (figure 10b). In this manner, nuclear architecture and chromatin higher order structure can be regarded as being the product of the multivalent mononucleosome, with its binding force derived from the avidity principle.
Figure 10.
Cartoons depicting locations of intrinsically disordered peptide regions (IDPR) at three levels of chromatin structure, the nucleosome, the chromomere and epichromatin. (a) Cartoon of a mononucleosome with 10 basic inner histone tails and two basic histone H1 tails forming a multivalent macromolecule with possible analogy to the multivalent IgM antibody molecule. Beneath the nucleosome is a drawing of an H1 molecule, showing the central globular region (red) flanked by N- and C-terminal tails. An untethered 100 aa peptide can extend up to approximately 30–35 nm from the globular domain. (b) Cartoon of a chromomere, contained within a membrane-less domain (approx. 200–300 nm diameter), consisting of a highly convoluted polynucleosome chain (possibly, approximately 500–1000 nucleosomes [42,71]) clamped by condensin or cohesin (red ring) [72,73] into a genetic ‘bouquet’, stabilized by the promiscuous binding of histone tails to adjacent nucleosomes, resulting in a spatially coordinated higher order structure. (c) Cartoon of interphase epichromatin adjacent to the NE, containing a nuclear pore complex (NPC), an outer nuclear membrane (ONM) and an inner nuclear membrane (INM). The outermost layer of nucleosomes in epichromatin exhibits ‘waving’ (+) charged histone tails (blue) interacting with the anionic (−) phospholipids (red) emanating from within the INM. This cartoon does not show the lamins nor transmembrane proteins (e.g. LBR, emerin, etc.), but focuses on the epichromatin regions in contact with the INM.
3. Speculations
(1) We suggest that the conformational plasticity of nucleosomal higher order structure is in large measure due to the multiplicity of IDPR histone tail interactions.
(2) Our conception of interphase and mitotic epichromatin is of a chromatin surface covered with unaffiliated histone tails, forming a cloud of (+) amino acid residues. Interphase epichromatin might be electrostatically attracted to inner nuclear membrane anionic phospholipids. During mitotic chromosome condensation, a rise in cellular Mg2+ [77] might weaken the electrostatic interaction of the histone tails to the membrane anionic phospholipids. During telophase, the positively charged chromatin surface may facilitate post-mitotic NE reformation.
(3) We speculate that inner histone and H1 tails act like ‘sticky tape’ holding multivalent nucleosomes in a coordinated bundle (chromomeres). The effectiveness of this ‘sticky tape’ to bind nucleosomes together can be influenced by post-translational modifications. In vitro experimental and computational evidence supports that the histone tails can bind to DNA within chromatin [78–80]. Thus, it is likely that in vivo, at least some of the histone tails are associated with ‘distant’ nucleosomal DNA.
(4) Chromomeres likely represent only one level in the super-nucleosome organization. If chromatin possesses ‘liquid droplet’-like properties [41], phase separation may fuse together these structures into larger structures or cleave them into smaller units.
4. The ‘unstructured stability’ hypothesis
We propose that various chromatin structures (e.g. epichromatin and chromomeres) are stably maintained by the interactions of the unstructured intrinsically disordered peptide regions (IDPRs) of the inner histones and of histone H1 on multivalent mononucleosomes. This hypothesis stresses the collective contributions of the numerous IDPR–binding partner interactions in establishing these chromatin structures. Furthermore, we propose that the promiscuity of IDPRs will generate many functionally equivalent binding partners (e.g. consisting of DNA, protein or phospholipids), resulting in considerable redundancy, such that mutations in the binding sites can be readily compensated by alternative binding interactions. However, this hypothesis must be consistent with the conception that histone post-translational modifications of IDPRs can modulate (i.e. destabilize or hyper-stabilize) these binding interactions. This hypothesis also does not exclude the importance of specific ‘lock-and-key’ interactions by other chromatin-associated proteins or specific ‘induced fit’ mechanisms by modifying enzymes [81], which may be essential for the specific genetic functions. Rather, this hypothesis focuses attention upon the considerable number of weak and relatively nonspecific interactions, in combination with the avidity principle, that permit chromosomes to fulfil their numerous functions. Testing the hypothesis will require cataloguing and mutating the multitude of IDPR binding interactions, a considerable endeavour. From the point-of-view of Darwinian evolution, if the Unstructured Stability Hypothesis is correct, one could argue that a preservation of some level of IDPRs is advantageous for maximizing evolutionary adaptability.
Supplementary Material
Acknowledgements
The authors wish to express their gratitude to Andreas Ladurner (LMU, Biomedical Center, Planegg-Martinsried) and Wolfgang Baumeister (MPI of Biochemistry, Martinsried) for generously opening their laboratories to our visit and for stimulating discussions. We also wish to thank Peter Becker (LMU, Biomedical Center, Planegg-Martinsried) for stimulating discussions and for striving to make Munich one of the world centres for chromatin research. The authors gratefully acknowledge the University of New England, College of Pharmacy for supporting our research, and Bates College and Travis Gould (Department of Physics and Astronomy) for supporting our joint research. The authors also express their appreciation to Gunnar Knobloch (Postdoctoral Fellow, LMU) for preparing the electronic supplementary material Movie. Our thanks also to Monika Krause (MPI of Biochemistry, Martinsried) for preparing the two cartoon figures. The authors also thank the reviewers for asking thought-provoking questions.
Data accessibility
This article has no additional data.
Authors' contributions
D.E.O. and A.L.O. have contributed equally to the research and writing of this review.
Competing interests
We declare that we have no competing interests.
Funding
The studies upon which this review is based have been funded partially by the University of New England (College of Pharmacy) and partially by an NIH grant (P20GM0103423) to Travis J. Gould (Bates College, Lewiston, Maine).
References
- 1.Olins DE, Olins AL. 2003. Chromatin history: our view from the bridge. Nat. Rev. Mol. Cell Biol. 4, 809–814. (doi:10.1038/nrm1225) [DOI] [PubMed] [Google Scholar]
- 2.Ungricht R, Kutay U. 2017. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 18, 229–245. (doi:10.1038/nrm.2016.153) [DOI] [PubMed] [Google Scholar]
- 3.LaJoie D, Ullman KS. 2017. Coordinated events of nuclear assembly. Curr. Opin. Cell Biol. 46, 39–45. (doi:10.1016/j.ceb.2016.12.008) [DOI] [PubMed] [Google Scholar]
- 4.Vietri M, Stenmark H, Campsteijn C. 2016. Closing a gap in the nuclear envelope. Curr. Opin. Cell Biol. 40, 90–97. (doi:10.1016/j.ceb.2016.03.001) [DOI] [PubMed] [Google Scholar]
- 5.Grigoryev SA, Bascom G, Buckwalter JM, Schubert MB, Woodcock CL, Schlick T. 2016. Hierarchical looping of zigzag nucleosome chains in metaphase chromosomes. Proc. Natl Acad. Sci. USA 113, 1238–1243. (doi:10.1073/pnas.1518280113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth DG, Beckett AJ, Molina O, Samejima I, Masumoto H, Kouprina N, Larionov V, Prior IA, Earnshaw WC. 2016. 3D-CLEM reveals that a major portion of mitotic chromosomes is not chromatin. Mol. Cell 64, 790–802. (doi:10.1016/j.molcel.2016.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samwer M, Schneider MWG, Hoefler R, Schmalhorst PS, Jude JG, Zuber J, Gerlich DW. 2017. DNA cross-bridging shapes a single nucleus from a set of mitotic chromosomes. Cell 170, 956–972.e923. (doi:10.1016/j.cell.2017.07.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellhaus AK, De Magistris P, Antonin W. 2016. Nuclear reformation at the end of mitosis. J. Mol. Biol. 428, 1962–1985. (doi:10.1016/j.jmb.2015.09.016) [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita K, Hirano T. 2017. Dynamic organization of mitotic chromosomes. Curr. Opin. Cell Biol. 46, 46–53. (doi:10.1016/j.ceb.2017.01.006) [DOI] [PubMed] [Google Scholar]
- 10.Kschonsak M, Haering CH. 2015. Shaping mitotic chromosomes: from classical concepts to molecular mechanisms. BioEssays 37, 755–766. (doi:10.1002/bies.201500020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibcus JH, et al. 2018. A pathway for mitotic chromosome formation. Science 359, eaao6135 (doi:10.1126/science.aao6135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisetsky DS. 2014. The complex role of DNA, histones and HMGB1 in the pathogenesis of SLE. Autoimmunity 47, 487–493. (doi:10.3109/08916934.2014.921811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suurmond J, Diamond B. 2015. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202. (doi:10.1172/jci78084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losman MJ, Fasy TM, Novick KE, Monestier M. 1992. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(-)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J. Immunol. 148, 1561–1569. [PubMed] [Google Scholar]
- 15.Olins AL, Langhans M, Monestier M, Schlotterer A, Robinson DG, Viotti C, Zentgraf H, Zwerger M, Olins DE. 2011. An epichromatin epitope: persistence in the cell cycle and conservation in evolution. Nucleus 2, 47–60. (doi:10.4161/nucl.1.6.13271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prudovsky I, Vary CP, Markaki Y, Olins AL, Olins DE. 2012. Phosphatidylserine colocalizes with epichromatin in interphase nuclei and mitotic chromosomes. Nucleus 3, 200–210. (doi:10.4161/nucl.19662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olins AL, Moyer BA, Kim SH, Allison DP. 1989. Synthesis of a more stable osmium ammine electron-dense DNA stain. J. Histochem. Cytochem. 37, 395–398. (doi:10.1177/37.3.2465337) [DOI] [PubMed] [Google Scholar]
- 18.Olins AL, Buendia B, Herrmann H, Lichter P, Olins DE. 1998. Retinoic acid induction of nuclear envelope-limited chromatin sheets in HL-60. Exp. Cell Res. 245, 91–104. (doi:10.1006/excr.1998.4210) [DOI] [PubMed] [Google Scholar]
- 19.Derenzini M, Olins AL, Olins DE. 2014. Chromatin structure in situ: the contribution of DNA ultrastructural cytochemistry. Eur. J. Histochem. 58, 2307 (doi:10.4081/ejh.2014.2307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould TJ, Toth K, Mucke N, Langowski J, Hakusui AS, Olins AL, Olins DE. 2017. Defining the epichromatin epitope. Nucleus 8, 625–640. (doi:10.1080/19491034.2017.1380141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fol H. 1891. Le quadrille des centres: un épisode nouveau dans l'histoire de la fécondation. Arch. Sci. Phys. Nat. XXV, 393–420. [Google Scholar]
- 22.Fol H. 1896. Die Teilung der Zelle. In Lehrbuch der vergleichenden mikroskopischen Anatomie mit Einschluss der vergleichenden Histologie und Histogenie, pp. 255–281. Leipzig, Germany: Engelmann. [Google Scholar]
- 23.Wilson EB. 1896. The cell in development and inheritance. New York, NY: The Macmillan Company. [Google Scholar]
- 24.Kalashnikova AA, Porter-Goff ME, Muthurajan UM, Luger K, Hansen JC. 2013. The role of the nucleosome acidic patch in modulating higher order chromatin structure. J. R. Soc. Interface 10, 20121022 (doi:10.1098/rsif.2012.1022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olins AL, Ishaque N, Chotewutmontri S, Langowski J, Olins DE. 2014. Retrotransposon Alu is enriched in the epichromatin of HL-60 cells. Nucleus 5, 237–246. (doi:10.4161/nucl.29141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teif VB, Mallm JP, Sharma T, Mark Welch DB, Rippe K, Eils R, Langowski J, Olins AL, Olins DE. 2017. Nucleosome repositioning during differentiation of a human myeloid leukemia cell line. Nucleus 8, 188–204. (doi:10.1080/19491034.2017.1295201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erenpreisa J, Krigerts J, Salmina K, Selga T, Sorokins H, Freivalds T. 2018. Differential staining of peripheral nuclear chromatin with Acridine orange implies an A-form epichromatin conformation of the DNA. Nucleus 9, 171–181. (doi:10.1080/19491034.2018.1431081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeshima K, Kaizu K, Tamura S, Nozaki T, Kokubo T, Takahashi K. 2015. The physical size of transcription factors is key to transcriptional regulation in chromatin domains. J. Phys. Condens. Matter 27, 064116 (doi:10.1088/0953-8984/27/6/064116) [DOI] [PubMed] [Google Scholar]
- 29.Olins AL, Herrmann H, Lichter P, Kratzmeier M, Doenecke D, Olins DE. 2001. Nuclear envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells. Exp. Cell Res. 268, 115–127. (doi:10.1006/excr.2001.5269) [DOI] [PubMed] [Google Scholar]
- 30.De Michele C, De Los Rios P, Foffi G, Piazza F. 2016. Simulation and theory of antibody binding to crowded antigen-covered surfaces. PLoS Comput. Biol. 12, e1004752 (doi:10.1371/journal.pcbi.1004752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeshima K, Hihara S, Eltsov M. 2010. Chromatin structure: does the 30-nm fibre exist in vivo? Curr. Opin. Cell Biol. 22, 291–297. (doi:10.1016/j.ceb.2010.03.001) [DOI] [PubMed] [Google Scholar]
- 32.Fussner E, Ching RW, Bazett-Jones DP. 2011. Living without 30 nm chromatin fibers. Trends Biochem. Sci. 36, 1–6. (doi:10.1016/j.tibs.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 33.Luger K, Dechassa ML, Tremethick DJ. 2012. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 13, 436–447. (doi:10.1038/nrm3382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boule JB, Mozziconacci J, Lavelle C. 2015. The polymorphisms of the chromatin fiber. J. Phys. Condens. Matter 27, 033101 (doi:10.1088/0953-8984/27/3/033101) [DOI] [PubMed] [Google Scholar]
- 35.Even-Faitelson L, Hassan-Zadeh V, Baghestani Z, Bazett-Jones DP. 2016. Coming to terms with chromatin structure. Chromosoma 125, 95–110. (doi:10.1007/s00412-015-0534-9) [DOI] [PubMed] [Google Scholar]
- 36.Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, O'Shea CC. 2017. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357, eaag0025 (doi:10.1126/science.aag0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman-Aiden E, et al. 2009. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293. (doi:10.1126/science.1181369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. 2012. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380. (doi:10.1038/nature11082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao SS, et al. 2014. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680. (doi:10.1016/j.cell.2014.11.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt AD, Hu M, Ren B. 2016. Genome-wide mapping and analysis of chromosome architecture. Nat. Rev. Mol. Cell Biol. 17, 743–755. (doi:10.1038/nrm.2016.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeshima K, Ide S, Hibino K, Sasai M. 2016. Liquid-like behavior of chromatin. Curr. Opin. Genet. Dev. 37, 36–45. (doi:10.1016/j.gde.2015.11.006) [DOI] [PubMed] [Google Scholar]
- 42.Nozaki T, et al. 2017. Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol. Cell 67, 282–293.e287. (doi:10.1016/j.molcel.2017.06.018) [DOI] [PubMed] [Google Scholar]
- 43.Hansen JC, et al. 2017. The 10-nm chromatin fiber and its relationship to interphase chromosome organization. Biochem. Soc. Trans. 46, 67–76. (doi:10.1042/bst20170101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolbl AC, Weigl D, Mulaw M, Thormeyer T, Bohlander SK, Cremer T, Dietzel S. 2012. The radial nuclear positioning of genes correlates with features of megabase-sized chromatin domains. Chromosome Res. 20, 735–752. (doi:10.1007/s10577-012-9309-9) [DOI] [PubMed] [Google Scholar]
- 45.Macgregor HC. 2012. Chromomeres revisited. Chromosome Res. 20, 911–924. (doi:10.1007/s10577-012-9310-3) [DOI] [PubMed] [Google Scholar]
- 46.Izawa M, Allefrey VG, Mirsky AE. 1963. The relationship between RNA synthesis and loop structure in lampbrush chromosomes. Proc. Natl Acad. Sci. USA 49, 544–551. (doi:10.1073/pnas.49.4.544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spierer A, Spierer P. 1984. Similar level of polyteny in bands and interbands of Drosophila giant chromosomes. Nature 307, 176–178. (doi:10.1038/307176a0) [DOI] [PubMed] [Google Scholar]
- 48.Zhimulev IF, Belyaeva ES, Vatolina TY, Demakov SA. 2012. Banding patterns in Drosophila melanogaster polytene chromosomes correlate with DNA-binding protein occupancy. BioEssays 34, 498–508. (doi:10.1002/bies.201100142) [DOI] [PubMed] [Google Scholar]
- 49.Stormo BM, Fox DT. 2017. Polyteny: still a giant player in chromosome research. Chromosome Res. 25, 201–214. (doi:10.1007/s10577-017-9562-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Happel N, Doenecke D. 2009. Histone H1 and its isoforms: contribution to chromatin structure and function. Gene 431, 1–12. (doi:10.1016/j.gene.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 51.Harshman SW, Young NL, Parthun MR, Freitas MA. 2013. H1 histones: current perspectives and challenges. Nucleic Acids Res. 41, 9593–9609. (doi:10.1093/nar/gkt700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clausell J, Happel N, Hale TK, Doenecke D, Beato M. 2009. Histone H1 subtypes differentially modulate chromatin condensation without preventing ATP-dependent remodeling by SWI/SNF or NURF. PLoS ONE 4, e0007243 (doi:10.1371/journal.pone.0007243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas P, Brown DT. 2012. N- and C-terminal domains determine differential nucleosomal binding geometry and affinity of linker histone isotypes H1(0) and H1c. J. Biol. Chem. 287, 11 778–11 787. (doi:10.1074/jbc.M111.312819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalashnikova AA, Rogge RA, Hansen JC. 2016. Linker histone H1 and protein–protein interactions. Biochim. Biophys. Acta 1859, 455–461. (doi:10.1016/j.bbagrm.2015.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millan-Arino L, Izquierdo-Bouldstridge A, Jordan A. 2016. Specificities and genomic distribution of somatic mammalian histone H1 subtypes. Biochim. Biophys. Acta 1859, 510–519. (doi:10.1016/j.bbagrm.2015.10.013) [DOI] [PubMed] [Google Scholar]
- 56.Bednar J, Hamiche A, Dimitrov S. 2016. H1–nucleosome interactions and their functional implications. Biochim. Biophys. Acta 1859, 436–443. (doi:10.1016/j.bbagrm.2015.10.012) [DOI] [PubMed] [Google Scholar]
- 57.Flanagan TW, Brown DT. 2016. Molecular dynamics of histone H1. Biochim. Biophys. Acta 1859, 468–475. (doi:10.1016/j.bbagrm.2015.10.005) [DOI] [PubMed] [Google Scholar]
- 58.Izzo A, Schneider R. 2016. The role of linker histone H1 modifications in the regulation of gene expression and chromatin dynamics. Biochim. Biophys. Acta 1859, 486–495. (doi:10.1016/j.bbagrm.2015.09.003) [DOI] [PubMed] [Google Scholar]
- 59.Lyubitelev AV, Nikitin DV, Shaytan AK, Studitsky VM, Kirpichnikov MP. 2016. Structure and functions of linker histones. Biochem. Biokhim. 81, 213–223. (doi:10.1134/s0006297916030032) [DOI] [PubMed] [Google Scholar]
- 60.Cutter AR, Hayes JJ. 2017. Linker histones: novel insights into structure-specific recognition of the nucleosome. Biochem. Cell Biol. 95, 171–178. (doi:10.1139/bcb-2016-0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crane-Robinson C. 2016. Linker histones: history and current perspectives. Biochim. Biophys. Acta 1859, 431–435. (doi:10.1016/j.bbagrm.2015.10.008) [DOI] [PubMed] [Google Scholar]
- 62.Hergeth SP, Schneider R. 2015. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep. 16, 1439–1453. (doi:10.15252/embr.201540749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. 1993. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature 362, 219–223. (doi:10.1038/362219a0) [DOI] [PubMed] [Google Scholar]
- 64.Hansen JC, Lu X, Ross ED, Woody RW. 2006. Intrinsic protein disorder, amino acid composition, and histone terminal domains. J. Biol. Chem. 281, 1853–1856. (doi:10.1074/jbc.R500022200) [DOI] [PubMed] [Google Scholar]
- 65.Pepenella S, Murphy KJ, Hayes JJ. 2014. Intra- and inter-nucleosome interactions of the core histone tail domains in higher-order chromatin structure. Chromosoma 123, 3–13. (doi:10.1007/s00412-013-0435-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frege T, Uversky VN. 2015. Intrinsically disordered proteins in the nucleus of human cells. Biochem. Biophys. Rep. 1, 33–51. (doi:10.1016/j.bbrep.2015.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bah A, Forman-Kay JD. 2016. Modulation of intrinsically disordered protein function by post-translational modifications. J. Biol. Chem. 291, 6696–6705. (doi:10.1074/jbc.R115.695056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burger VM, Nolasco DO, Stultz CM. 2016. Expanding the range of protein function at the far end of the order-structure continuum. J. Biol. Chem. 291, 6706–6713. (doi:10.1074/jbc.R115.692590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uversky VN. 2016. Dancing protein clouds: the strange biology and chaotic physics of intrinsically disordered proteins. J. Biol. Chem. 291, 6681–6688. (doi:10.1074/jbc.R115.685859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roque A, Ponte I, Suau P. 2017. Post-translational modifications of the intrinsically disordered terminal domains of histone H1: effects on secondary structure and chromatin dynamics. Chromosoma 126, 83–91. (doi:10.1007/s00412-016-0591-8) [DOI] [PubMed] [Google Scholar]
- 71.Maeshima K, et al. 2016. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. EMBO J. 35, 1115–1132. (doi:10.15252/embj.201592660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonev B, Cavalli G. 2016. Organization and function of the 3D genome. Nat. Rev. Genet. 17, 661–678. (doi:10.1038/nrg.2016.112) [DOI] [PubMed] [Google Scholar]
- 73.Rao SSP, et al. 2017. Cohesin loss eliminates all loop domains. Cell 171, 305–320.e324. (doi:10.1016/j.cell.2017.09.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorigo B, Schalch T, Bystricky K, Richmond TJ. 2003. Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J. Mol. Biol. 327, 85–96. (doi:10.1016/S0022-2836(03)00025-1) [DOI] [PubMed] [Google Scholar]
- 75.Nakaoka T, Kojima N, Ogita T, Tsuji S. 1995. Characterization of the phosphatidylserine-binding region of rat MARCKS (myristoylated, alanine-rich protein kinase C substrate). Its regulation through phosphorylation of serine 152. J. Biol. Chem. 270, 12 147–12 151. (doi:10.1074/jbc.270.20.12147) [DOI] [PubMed] [Google Scholar]
- 76.Sundaram M, Cook HW, Byers DM. 2004. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem. Cell Biol. 82, 191–200. (doi:10.1139/o03-087) [DOI] [PubMed] [Google Scholar]
- 77.Maeshima K, et al. 2018. A transient rise in free Mg2+ ions released from ATP-Mg hydrolysis contributes to mitotic chromosome condensation. Curr. Biol. 28, 444–451.e446. (doi:10.1016/j.cub.2017.12.035) [DOI] [PubMed] [Google Scholar]
- 78.Peng Z, Mizianty MJ, Xue B, Kurgan L, Uversky VN. 2012. More than just tails: intrinsic disorder in histone proteins. Mol. Biosyst. 8, 1886–1901. (doi:10.1039/c2mb25102g) [DOI] [PubMed] [Google Scholar]
- 79.Erler J, Zhang R, Petridis L, Cheng X, Smith JC, Langowski J. 2014. The role of histone tails in the nucleosome: a computational study. Biophys. J. 107, 2911–2922. (doi:10.1016/j.bpj.2014.10.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang H, Wei S, Lee TH, Hayes JJ. 2016. Chromatin structure-dependent conformations of the H1 CTD. Nucleic Acids Res. 44, 9131–9141. (doi:10.1093/nar/gkw586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koshland DE. 1958. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl Acad. Sci.USA 44, 98–104. (doi:10.1073/pnas.44.2.98) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.