Abstract
Multisensory integration is a mechanism that allows organisms to simultaneously sense and understand external stimuli from different modalities. These distinct signals are transduced into neuronal signals that converge into decision-making neuronal entities. Such decision-making centres receive information through neuromodulators regarding the organism's physiological state and accordingly trigger behavioural responses. Despite the importance of multisensory integration for efficient functioning of the nervous system, and also the implication of dysfunctional multisensory integration in the aetiology of neuropsychiatric disease, little is known about the relative molecular mechanisms. Caenorhabditis elegans is an appropriate model system to study such mechanisms and elucidate the molecular ways through which organisms understand external environments in an accurate and coherent fashion.
Keywords: Caenorhabditis elegans, behavioural plasticity, multisensory processing, interneuron, sensory integration
1. Introduction
Organisms must sense and ‘understand’ external stimuli in order to adapt to continuously changing natural conditions. Adaptability is largely dependent on the ability of the nervous system to receive and integrate information regarding physical parameters, such as temperature and humidity, food availability, presence of predators and sex pheromones, so that it can orchestrate proper physiological and behavioural responses to ensure survival and reproduction. Diversity of physical and biological factors that affect organisms has led to the evolution of several neuronal circuits that accomplish perception of various sensory modalities, such as temperature, vision, taste, smell, touch and hearing. Sensory neurons receive external information that is processed and integrated to regulate behaviour and form memories. Each environmental stimulus can trigger multiple sensory neurons and generate various sensory cues, which must be integrated and assessed by the nervous system. Nevertheless, the stimuli that an organism must perceive and process in order to better confront natural challenges can be highly complex, and simultaneous perception of different stimuli is necessary for the construction of a comprehensible depiction of habitats and a fully featured understanding of natural conditions.
Often organisms must choose between opposing sensory signals in nature. An organism with enhanced food-searching activity or copulating behaviour is under an increased risk to become prey of its predators or face adverse physical microenvironments that can kill it. To make the best decision for its survival and efficient reproduction, an organism must receive as much information as possible regarding the relative degree of danger through its sensory neurons. Subsequently, this heterogeneous information must be integrated and processed into decision-making neuronal centres to regulate relative responses [1]. Such decision-making centres must consider the organism's physiological status, e.g. the level of hunger or food shortage, to judge if the enhanced risk for survival is necessary and accordingly regulate the behavioural response [2,3]. This presupposes the capacity of decision-making centres to sense organism's physiological state and initiate behavioural responses through modulation of executive neurons. Hence, decision-making neurons can serve not only as sensors of external and internal stimuli, but also as behavioural modifiers.
Several studies suggest the existence of decision-making centres that accomplish responses to multisensory cues in all animals tested so far. In Drosophila melanogaster, visual and chemosensory inputs converge into the mushroom bodies to potentiate plasticity in courtship [4]. In primates, cerebral cortex integrates and assesses information from sensory inputs to modulate behavioural responses [5]. The above and several more studies suggest the existence of defined neuronal domains that integrate multisensory information and serve as decision-making centres. Whether multisensory convergence occurs within particular brain regions (areal convergence) or within specific neurons (neuronal convergence) is unknown [1]. Instead, other studies suggest the existence of multiple multisensory integration centres in higher organisms [6,7]. To date, the enormous complexity of the nervous system in higher animals makes functional mapping of the brain impossible and the elucidation of mechanisms governing multisensory processing a difficult task.
Recent research on multisensory integration has focused on Caenorhabditis elegans, a well-studied nematode with a simple nervous system, comprising only 302 neurons. With 6393 chemical synapses, 890 gap junctions and 1410 neuromuscular junctions detected and its synaptic wiring fully reconstructed [8–11], research on C. elegans enables the functional and molecular characterization of single neurons. Moreover, a large arsenal of molecular tools facilitates genetic and behavioural manipulations and analysis. Furthermore, novel techniques, such as calcium imaging, can directly link activation of individual neurons to specific sensory stimuli [12–15]. Hence, C. elegans is a proper animal model to dissect mechanisms regulating multisensory integration in complex organisms such as humans.
2. Multisensory perception in Caenorhabditis elegans
2.1. Sensory neurons in Caenorhabditis elegans
Caenorhabditis elegans has a simple sensory system, consisting of 60 ciliated sensory neurons that sense chemical, olfactory, thermal and mechanical stimuli and relative position of the body (proprioception). Three groups of sensory neurons participate in the identification of chemical cues, the amphids and the inner labial neurons in the head and the phasmids in the tail [16,17]. The neurons with the most prominent role in identifying gustatory stimuli are the ASE. ASE neurons together with ASH mainly, and to a lesser extent ASI, ADF, ASG, ASJ, ASK, ADL and IL2 in the head and PHA and PHB in the tail, recognize water soluble attractants and repellents [18]. Chemotaxis to volatile odorants is mediated by the olfactory neurons AWA, AWB and AWC [19] and the polymodal neuron ASH [20]. AFD, BAG and ASE neurons sense CO2, while AQR, PQR and URX neurons are mainly O2 sensors and weak CO2 sensors [21]. The circuit that senses oxygen also includes SDQ, ALN, PLN, ADL and ASH neurons [22,23]. The main sensory neurons that respond to temperature changes are the AFD neurons, though AWC, ASI, FLP and PHC also participate in thermosensation [24,25]. Low noxious temperatures are perceived by PVD neurons [26]. ADL, ASH and AWB neurons respond to several repulsive stimuli to produce avoidance behaviour [27,28]. These stimuli include hyperosmolarity, mechanical stimuli and volatile repellents. By contrast, sensory neurons called AWA, AWC and ASE are involved in responses to an attractant [19,28]. Moreover, ASH together with ASJ, AWB and ASK neurons mediate light avoidance and electrosensory navigation [29,30]. Thirty sensory neurons have been identified in hermaphrodites to respond to mechanical stimuli. These are the ALM, PLM, AVM, PVM, PVD, ADE and PDE touch receptor neurons found at the midbody of C. elegans and the ASH, FLP, OLQ, CEP and IL1 neurons found at the nose tip [26,31–33].
2.2. Sensory transduction
The above sensory receptors are specialized for certain modalities, which are converted to neuronal signals. In C. elegans, the mechanisms facilitating sensory transduction of single stimuli have been studied through genetic and behavioural studies [34,35]. Binding of a chemical ligand or external force on receptor proteins located at the surface of sensory cells provokes conformational changes that, depending on their relative strength, can lead to the induction of intracellular chemical alterations. Such alterations can subsequently lead to the generation of electrical signals, through which sensory information is transferred to the nervous system. Sensory receptor families with chemosensory and mechanosensory functions are the degenerin/epithelial Na+ channel (Deg/ENaC) family, the transmembrane channel-like proteins and ionotropic receptors [18,36–41]. Several sensory receptors are well characterized, such as the odorants-specific G protein-coupled receptors [42,43], the mechanosensory TRP receptors of the NOMPC family [40] and the Deg/ENaC ion channel receptors that are activated by mechanical stimuli [36–38,41,44,45].
2.3. Polymodality of sensory neurons
In C. elegans, avoidance responses require either unimodal or polymodal sensory neurons. In the latter case, single sensory neurons are able to perceive stimuli from various modalities. Such neurons are the nociceptors, sensory neurons that detect intense and putatively harmful mechanic, thermal or chemical stimuli [46]. A well-studied example of avoidance response in C. elegans involves the pair of ASH neurons. They are located at the nose and they are responsible for sensing and conducting avoidance responses against high osmotic strength, low pH, food odours, nose touch, heavy metals and alkaloids [27,33,47]. A reasonable question arising is how ASH neurons coordinate aversive responses to different stimuli. Studies in the previous decade have shown that ASH neurons activate different synaptic pathways to regulate responses against mechanical and osmotic stimuli [35,48,49]. Combined genetic, electrophysiological and behavioural analyses showed that this is achieved through differential activation of postsynaptic NMDA and non-NMDA receptors. Specifically, although mechanical stimulation activates only synaptic non-NMDA receptors, osmotic stimuli induce a much higher secretion of synaptic glutamate that is capable of activating not only non-NMDA but also extrasynaptic NMDA receptors. As a result, the same sensory neurons can sense distinct modalities and adjust behavioural responses through different synaptic outputs. Interestingly, polymodality of sensory neurons also characterizes other organisms. In Drosophila, antennal nerves respond to ammonia, but also to air humidity [50,51]. In mice, olfactory sensory neurons respond to both odours and pressure changes [52]. Hence, polymodality of sensory neurons is a conserved mechanism through which single neurons broaden their sensory capacity and facilitate multisensory integration.
2.4. Co-action of sensory neurons
Sensory neurons can also collaborate to sense external stimuli. A well-studied paradigm is the sense of carbon dioxide [21,53–55]. The main sensory neurons for sensing CO2 are the AFD and BAG neurons. However, their activity is not sufficient to induce a repulsive behaviour. Degree of repulsion is dependent, among others, on ambient oxygen-sensing neurons, the URX neurons. Worms with a mutation reducing expression of the neuropeptide receptor NPR-1 are insensitive to CO2. Carefully designed experiments have shown that NPR-1 receptor inhibits oxygen-sensing URX neurons, which are also activated by increases in ambient oxygen [23,56]. Ablation of the URX neurons in npr-1 mutants restores CO2 avoidance, suggesting that NPR-1 enables CO2 avoidance by inhibiting URX neurons. Moreover, in npr-1 mutants, oxygen-induced activation of URX inhibits CO2 avoidance. Hence, CO2 avoidance requires either low O2 presence or inactivity of URX neurons.
In another example, worms respond to moisture gradient through the combinatorial action of both mechano- and thermosensory neurons. Specifically, the mechanosensory FLP neurons sense the level of hydration-mediated subcuticular stretching via the DEG/ENaC/ASIC mechanoreceptor complex. This information is combined with thermal cues caused by humidity-mediated evaporative cooling that is generated by stimulation of cGMP-gated channels in the thermosensory AFD neuron pair [57]. Thus, hygrosensation in C. elegans requires the integration of both mechanical and thermal cues.
2.5. Crosstalk of sensory neurons
Sensory neurons are also able to cross-modulate their activity. Caenorhabditis elegans senses odours intensity through the combinatorial activity of primary and secondary neurons that crosstalk through neuropeptides signalling. For example, although ASE sensory neurons are responsible for salt detection, dramatic changes in salt concentration are sensed through recruitment of AWC olfactory neurons. This is achieved through the release of INS-6 insulin-like peptide by activated ASE neurons, which, in turn, modulates AWC neurons [58]. Hence, the combined action of ASE and AWC neurons adjusts sensing of high salinity and relative responses. In another example, AWC and AWA neurons sense the food odour benzaldehyde and secrete insulin-like peptides and acetylcholine, to target and sensitize ASEL and AWB neurons [59]. Concerted action of the above neurons is necessary for attraction to benzaldehyde. In conclusion, sensory neurons have the capacity to decode multisensory stimuli through polymodality, simultaneous activity or cross-modulation, and through these mechanisms sensory neurons increase their capacity to fine-tune multisensory integration and provoke relative behavioural responses.
3. Interneurons: the decision-making centres in Caenorhabditis elegans
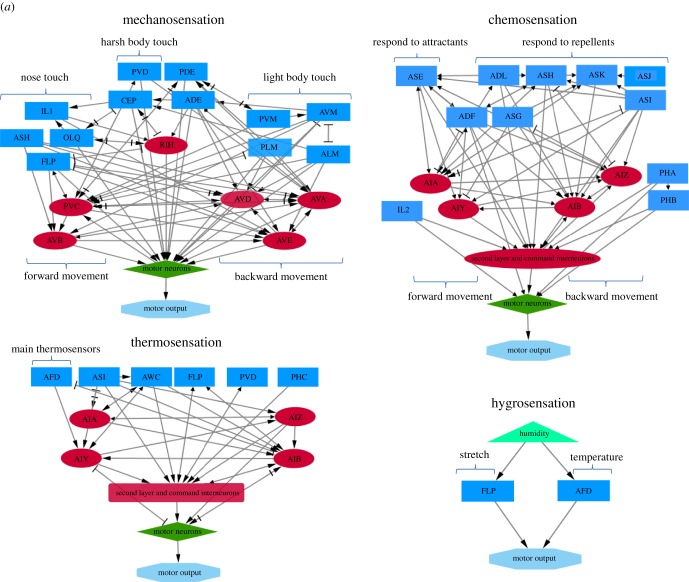
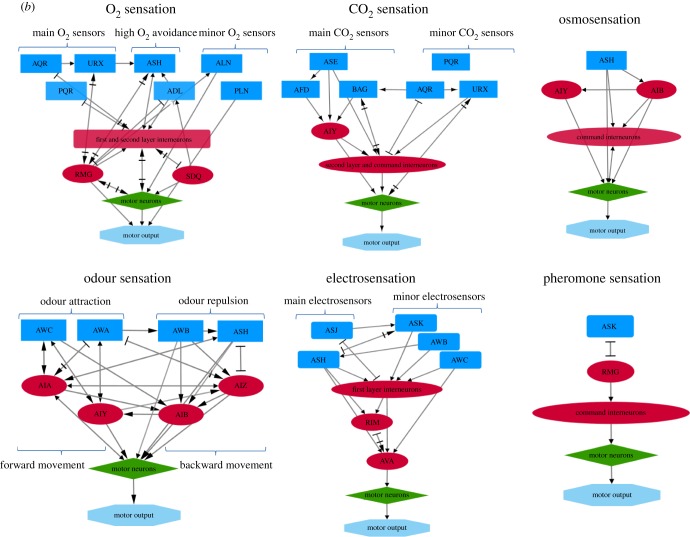
Organisms need to combine information from various sensory modalities to achieve a more coherent and composite understanding of natural environments. This complex flow of information, derived from multiple stimuli, must be integrated into centralized neurons, to be processed and trigger relative behavioural responses. Anatomical but also genetic and behavioural data suggest that information from sensory neurons is transferred and processed into a distinct category of nerve cells, the interneurons (figure 1). A set of five interneurons has been shown to integrate responses to mechanical stimuli and affect the locomotor behaviour, AVB, PVC, AVA, AVD and AVE [8,31,61]. Concerning chemotaxis, activity of AIY interneurons alone is sufficient to mediate chemotactic responses, mainly by promoting forward movement and gradual turnings [62]. However, AIA, AIB and AIZ neurons also participate in the formation of attraction or avoidance behaviours to water soluble attractants [63–65]. Apart from the integration of gustatory stimuli, AIY together with AIZ, AIB, AIA and RIA mediate responses to thermal stimuli [66], while AIY and AIB mediate responses to olfactory stimuli and osmotic changes [67]. AIY and RIA interneurons participate in the regulation of avoidance or attraction by CO2 [68], while RMG interneurons participate in oxygen sensation [69]. RIM and AVA interneurons are implicated in electrosensory detection [30]. In conclusion, several studies support that interneurons are the convergence sites of multisensory inputs from sensory neurons and that they serve as coincidence detectors [70].
Figure 1.
Neural circuits of C. elegans sensory processing. Sensory neurons are indicated with blue rectangles, interneurons with red ellipses, motor neurons with green diamonds and motor output with light blue octagons. Light green triangles indicate sensory stimuli. First layer interneurons are characterized as those that are postsynaptic to sensory neurons, second layer as those that are presynaptic to command interneurons and command interneurons as those that are presynaptic to motor neurons. (a) Neuronal wiring diagrams for mechanosensation, chemosensation, thermosensation and hygrosensation. (b) Neuronal wiring diagrams for O2 and CO2 sensation, osmosensation, electrosensation, pheromone sensation and odour sensation. Arrows denote chemical synapses, while bars denote electrical synapses (gap junctions). Strength and type (excitatory or inhibitory) of the synapse are not indicated. Interactions can be retrieved from http://wormweb.org/neuralnet [60].
A well-studied example in C. elegans is the AIA interneuron, which is the decision centre of behavioural choice between the attractive odorant, diacetyl, and an aversive stimulus, Cu2+ ions. Diacetyl is sensed by the AWA sensory neurons and Cu2+ ions are sensed by the polymodal sensory neurons ASH. The AIA interneuron is postsynaptic of ASH and connected with AWA through gap junctions. Combined genetic and behavioural analyses revealed that integration of the two opposing sensory cues is dependent on AIA neurons and, specifically, on the conflicting pathways GCY-28/CNG-1 and HEN-1/SCD-2, which function in AIA interneurons and modulate their activity [64,71]. According to the proposed model, the AIA interneurons regulate activity of the AIB interneurons through inhibitory synapses. The latter induce avoidance behaviours [67]. Hence, the AIA neurons are likely to promote attraction to odours through inhibition of the AIB neurons. Other studies also indicate a role for the GCY-28/CNG-1 and HEN-1/SCD-2 pathways in multisensory integration of opposing sensory cues [72]. As in the case of salt chemotaxis learning, the GCY-28/CNG-1 and HEN-1/SCD-2 pathways are also shown to modulate food-associated thermotactic behavioural plasticity [64,73,74].
In another example, octanol, an aversive odorant, is sensed by ASH neurons which initially activate AIB interneurons through glutamatergic synapses to promote avoidance behaviour. However, in the presence of food, octanol does not repel worms. AIB interneurons receive synaptic signals from both the ASH and AWC sensory neurons. The food odour-sensing AWC and salt-sensing ASER neurons can activate and deactivate, respectively, AIB through distinct glutamatergic transmissions. Upon the presence of food, worms finally move towards octanol. Food inhibits AWC neurons and their positive effect on AIB activity. Moreover, ASER neurons deactivate AIB. Hence, although octanol initially activates AIB interneurons and avoidance responses, food odours and salt inhibit AIB activation and, consequently, abrogate octanol-evoked avoidance behaviour [75].
3.1. The hub and spoke circuit
Animals need to respond acutely and accurately to environmental threats and stimuli. Research in C. elegans has revealed a mechanism through which worms respond acutely to multisensory inputs that regulate social behaviour in worms. Specified neuronal circuits underlie social behaviour and facilitate rapid responses to environmental stimuli that affect aggregation and other aspects of social behaviour [76]. In such a circuit, the ASK sensory neurons, among others, sense pheromones and connect to a single pair of interneurons, the RMG neurons. Sensory neurons are also interconnected through electrical synapses and this complex circuit can strengthen coincidence responses through lateral facilitation. Pheromones-sensing neurons and RMG interneurons are connected with gap junctions, thus allowing their direct metabolic and electrical communication. High RMG activity enhances ASK responses in social strains, causing hermaphrodite attraction to pheromones at concentrations that repel solitary hermaphrodites. Also, solitary strains differ from social strains in the activity of the neuropeptides receptor gene npr-1 which mainly acts at the RGM interneurons. Hence, social attraction in C. elegans is mainly regulated by a neuronal circuit that largely resembles a ‘hub and spoke’ circuit, in which RMG interneurons have the role of the ‘hub’ and sensory neurons have the role of the ‘spoke’. Such a system facilitates the integration of multiple sensory cues and the rapid response of worms to population density and the presence of mates [70,76].
Another example of a ‘hub and spoke’ circuit has been described to regulate the nose touch response [33]. Here, three sensory neurons, ASH, FLP and OLQ, sense touch to the nose and activate RIH interneurons through gap junctions. In this case, the three sensory neurons serve as ‘spoke’ neurons and the RIH interneurons serve as the ‘hub’ of the circuit. Sensory neurons interact with each other and this interaction modifies the electric stimulus that gets transferred to the ‘hub’ neuron [77,78]. Hence, the formation of gap junctions between sensory neurons and interneurons and the anatomical pattern of ‘hub’ and ‘spoke’ circuits are common mechanisms for the facilitation of multisensory integration and the relative behavioural response.
4. Biogenic amines and neuropeptides modulate responses to multisensory inputs
Organisms take decisions depending on their internal physiological state. Hunger, stress and health condition are some of the factors that modulate their responses to external stimuli. Internal physiological state affects expression and release of neuromodulators, molecules that can act from a distance on nerve cells and can have a general effect on neuronal circuits. In a previously described multisensory integration circuit, behavioural response to octanol is mediated through activity of AIB interneurons [75]. Food and serotonin modulate this circuit through different modes. Smell of food and serotonin, which is increased upon feeding, deactivate AIB and avoidance behaviour. Several examples show that, except for serotonin, other biogenic amines also regulate neuronal circuits that underlie multisensory integration and relative behavioural responses [70]. Dopamine serves as a signalling molecule that affects avoidance and food-searching behaviours [79,80]. Tyramine, another biogenic amine that represents internal metabolic state of C. elegans, regulates threat tolerance [81]. When worms must cross a hyperosmotic barrier to reach food sources, the choice is made by the RIM interneuron. RIM innervates ASH sensory neurons with tyraminergic inputs. High levels of tyramine represent a well-fed state for worms. When tyramine levels are adequate, ASH neurons are activated and promote avoidance behaviour and backwards movement. Under low tyramine levels, ASH neurons are inactivated and, as a result, osmosensitivity is decreased. This causes the worms to move towards the food source, without being constrained by the hyperosmotic barrier. Biogenic amines levels indicate internal metabolic state in animals and the modulation they exert on multisensory integration is crucial for homeostasis maintenance.
Interestingly, circuits involving different biogenic amines seem to interact to control feeding behaviour. Serotonergic NSM neurons promote feeding in the presence of attractive odours, though tyraminergic RIM interneurons inhibit feeding in the presence of aversive cues. These circuits are shown to interact with each other and the outcome of this interaction determines feeding behaviour [82].
Except for biogenic amines, neuropeptides are also shown to affect multisensory integration and behavioural output. Neuropeptides act as neuromodulators and they can facilitate interaction between distant interneurons and/or sensory neurons. There are several examples showing a regulatory role for neuropeptides on activity of interneurons. AIA interneuron is regulated by HEN-1, which is produced by another interneuron, AIY [64,71]. Chalasani et al. [83] identified a neuropeptide-to-neuropeptide feedback loop that controls sensing ability in primary olfactory neurons. In AWC olfactory neurons, expression of NLP-1 neuropeptide reduces AWC activity. NLP-1 binds the NPR-11 receptor, which is located at the postsynaptic AIA interneurons. The latter, in turn, releases INS-1 neuropeptide that modulates sensitivity to odours in AWC neurons [83]. In another study, insulin and NPR-1 neuropeptides were found to regulate and fine-tune chemosensation through affecting the expression of receptor genes in chemosensory neurons [84]. Hence, neuropeptides play a major regulatory role on multisensory integration through affecting activity of sensory neurons and interneurons, and also through facilitating interaction among interneurons.
5. Deficient multisensory integration and human diseases
Functional multisensory integration has a strong impact on the ability of organisms to understand their complex environment and to sufficiently react against external stimuli. Several findings support that inability to properly integrate environmental cues might lead to neuropsychiatric disorders in humans, such as autism, schizophrenia and attention deficit hyperactivity disorder (ADHD) [85–87]. Interestingly, these disorders are characterized by deficient sensory processing and by common comorbidity [88–92]. Although relative mechanisms are still unknown, several lines of evidence suggest a link between certain neuropsychiatric disorders and dysfunctional sensory integration.
Autism spectrum disorders (ASDs) are associated with altered multisensory processing and inability to integrate multisensory inputs into a unified percept [93–95]. In mouse models of ASD, multisensory integration is impaired. This is possibly due to impaired integration in the insular cortex, a brain centre where sensory, emotional and cognitive information is converged [96–99]. In support, recent evidence suggests specific neuronal pathways underlying multisensory dysfunction in children with ASD [100,101]. Specifically, a gain-of-function coding variant in the serotonin transporter (SERT) is associated with sensory aversion in humans. Upon its expression in mice, it induces phenotypes reminiscent of ASD, such as deficient social and communicative function and repetitive behaviours. Furthermore, these mice exhibit behavioural deficits in multisensory function that extend beyond changes in unisensory performance [102]. Hence, strong indications suggest that dysfunctional multisensory integration underlies, at least in part, ASDs.
Recent studies show that schizophrenic patients exhibit altered integration of distinct sensory modalities [103,104]. Although we are still far from the elucidation of mechanisms that cause schizophrenia, a role for the NMDA receptor has been suggested [105]. Experiments in rats clearly show that NMDA receptor antagonists can generate a dose-dependent selective impairment in multisensory information processing [106]. In another neuropsychiatric disorder, ADHD, adults with ADHD-like traits have reduced audio-visual integration window compared to those with low levels of ADHD-like traits. The authors suggested that malfunctions in perception of simultaneous stimuli could lead to the increased distractibility that characterizes ADHD [107].
Interestingly, the above neuropsychiatric diseases are all associated with difficulties in sensory processing and sociability. The mechanisms underlying this association are still unknown; however, there is strong evidence that dysfunctional multisensory integration might underlie aetiology and/or symptoms of a spectrum of neuropsychiatric disorders in humans.
6. Conclusion
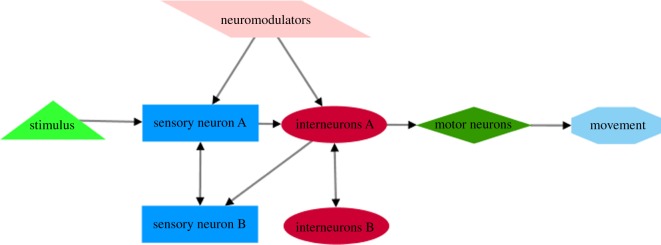
In. this review, we show that multisensory integration is a prominent mechanism through which C. elegans senses external stimuli and fine-tunes relative behavioural responses. In this complex network of interactions, a distinct category of nerve cells, the interneurons, have a distinguished role. Similarly to specific brain domains in mammals, interneurons are the decision-making centres where the flow of information from different modalities is converged and assessed. To initiate the most appropriate behavioural response, interneurons receive information regarding the organism's internal physiological state, through neuromodulators. These internal signals modulate activity of interneurons and, consequently, related responses according to the organism's immediate necessities. In this way, C. elegans takes threat–reward decisions according to its internal physiological conditions. Prior to the flow of information to interneurons, sensory neurons interact with each other and receive modulatory signals from the interior physiological systems. They can even form specific domains with interneurons, which resemble the ‘hub and spoke’ circuits, to ensure acute, automated and accurate responses (figure 2).
Figure 2.
Schematic diagram of information flow during sensory integration in C. elegans. Interneurons integrate signals from multiple sensory neurons to produce appropriate motor output. Sensory neurons are indicated with blue rectangles, interneurons with red ellipses, motor neurons with green diamonds and motor output with light blue octagons. Light green triangle indicates sensory stimulus. Arrows denote flow of information through synapses or extrasynaptic interactions.
Research in C. elegans has the potential to elucidate basic rules governing multisensory integration in higher organisms, including humans. Recent evidence indicates a possible role for dysfunctional multisensory integration in the aetiology of certain neuropsychiatric diseases, such as ASDs. However, dysfunctional multisensory integration might underlie generally bad performance of the nervous system, including dizziness, balance problems and disorientation [108]. Hence, elucidation of mechanisms regulating multisensory integration will lead to a more precise and holistic view of how our nervous system functions and how it reconstructs the physical world in a coherent and unified depiction.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
A.M. is supported by a standard Marie Curie intra-European individual fellowship. D.P. is funded by grants from the European Research Council (ERC—GA695190—MANNA, ERC—GA737599—NeuronAgeScreen).
References
- 1.Meredith MA. 2002. On the neuronal basis for multisensory convergence: a brief overview. Brain Res. Cogn. Brain Res. 14, 31–40. (doi:10.1016/S0926-6410(02)00059-9) [DOI] [PubMed] [Google Scholar]
- 2.Gillette R, Huang RC, Hatcher N, Moroz LL. 2000. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc. Natl Acad. Sci. USA 97, 3585–3590. (doi:10.1073/pnas.97.7.3585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schall JD. 2001. Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2, 33–42. (doi:10.1038/35049054) [DOI] [PubMed] [Google Scholar]
- 4.Joiner MA, Griffith LC. 2000. Visual input regulates circuit configuration in courtship conditioning of Drosophila melanogaster. Learn. Mem. 7, 32–42. (doi:10.1101/lm.7.1.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. (doi:10.1146/annurev.neuro.24.1.167) [DOI] [PubMed] [Google Scholar]
- 6.Sabes PN. 2011. Sensory integration for reaching: models of optimality in the context of behavior and the underlying neural circuits. Prog. Brain Res. 191, 195–209. (doi:10.1016/B978-0-444-53752-2.00004-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang WH, Chen A, Rasch MJ, Wu S. 2016. Decentralized multisensory information integration in neural systems. J. Neurosci. 36, 532–547. (doi:10.1523/JNEUROSCI.0578-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JG, Southgate E, Thomson JN, Brenner S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340. (doi:10.1098/rstb.1986.0056) [DOI] [PubMed] [Google Scholar]
- 9.Hall DH, Russell RL. 1991. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J. Neurosci. 11, 1–22. (doi:10.1523/JNEUROSCI.11-01-00001.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. 2011. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 7, e1001066 (doi:10.1371/journal.pcbi.1001066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcedo J, Zhang Y. 2013. Molecular and cellular circuits underlying Caenorhabditis elegans olfactory plasticity. In Invertebrate learning and memory (eds Menzel R, Benjamin P), pp. 112–123. San Diego, CA: Elsevier. [Google Scholar]
- 12.Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. 2003. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39, 1005–1017. (doi:10.1016/j.neuron.2003.08.015) [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR. 2008. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454, 114–117. (doi:10.1038/nature06927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura KD, Miyawaki A, Matsumoto K, Mori I. 2004. The C. elegans thermosensory neuron AFD responds to warming. Curr. Biol. 14, 1291–1295. (doi:10.1016/j.cub.2004.06.060) [DOI] [PubMed] [Google Scholar]
- 15.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24, 63–72. (doi:10.1038/sj.emboj.7600493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward S, Thomson N, White JG, Brenner S. 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160, 313–337. (doi:10.1002/cne.901600305) [DOI] [PubMed] [Google Scholar]
- 17.Ware RW, Clark D, Crossland K, Russell RL. 1975. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J. Comp. Neurol. 162, 71–110. (doi:10.1002/cne.901620106) [Google Scholar]
- 18.Bargmann CI. 2006. Chemosensation in C. elegans. WormBook 1–29. (doi:10.1895/wormbook.1.123.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bargmann CI, Hartwieg E, Horvitz HR. 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. (doi:10.1016/0092-8674(93)80053-H) [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Hirotsu T, Tagawa T, Oda S, Wakabayashi T, Iino Y, Ishihara T. 2012. Odour concentration-dependent olfactory preference change in C. elegans. Nat. Commun. 3, 739 (doi:10.1038/ncomms1750) [DOI] [PubMed] [Google Scholar]
- 21.Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, de Bono M. 2011. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron 69, 1099–1113. (doi:10.1016/j.neuron.2011.02.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. 2006. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 4, e274 (doi:10.1371/journal.pbio.0040274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmer M, et al. 2009. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61, 865–879. (doi:10.1016/j.neuron.2009.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beverly M, Anbil S, Sengupta P. 2011. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. 31, 11 718–11 727. (doi:10.1523/JNEUROSCI.1098-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Schulze E, Baumeister R. 2012. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS ONE 7, e32360 (doi:10.1371/journal.pone.0032360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatzigeorgiou M, et al. 2010. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 13, 861–868. (doi:10.1038/nn.2581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M. 1999. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10, 753–757. (doi:10.1097/00001756-199903170-00017) [DOI] [PubMed] [Google Scholar]
- 28.Troemel ER, Kimmel BE, Bargmann CI. 1997. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169. (doi:10.1016/S0092-8674(00)80399-2) [DOI] [PubMed] [Google Scholar]
- 29.Ward A, Liu J, Feng Z, Xu XZ. 2008. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 11, 916–922. (doi:10.1038/nn.2155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabel CV, Gabel H, Pavlichin D, Kao A, Clark DA, Samuel AD. 2007. Neural circuits mediate electrosensory behavior in Caenorhabditis elegans. J. Neurosci. 27, 7586–7596. (doi:10.1523/JNEUROSCI.0775-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5, 956–964. (doi:10.1523/JNEUROSCI.05-04-00956.1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawin ER, Ranganathan R, Horvitz HR. 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631. (doi:10.1016/S0896-6273(00)81199-X) [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JM, Horvitz HR. 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 90, 2227–2231. (doi:10.1073/pnas.90.6.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bargmann CI. 1993. Genetic and cellular analysis of behavior in C. elegans. Annu. Rev. Neurosci. 16, 47–71. (doi:10.1146/annurev.ne.16.030193.000403) [DOI] [PubMed] [Google Scholar]
- 35.Bargmann CI, Kaplan JM. 1998. Signal transduction in the Caenorhabditis elegans nervous system. Annu. Rev. Neurosci. 21, 279–308. (doi:10.1146/annurev.neuro.21.1.279) [DOI] [PubMed] [Google Scholar]
- 36.Chalfie M, Wolinsky E. 1990. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345, 410–416. (doi:10.1038/345410a0) [DOI] [PubMed] [Google Scholar]
- 37.Driscoll M, Chalfie M. 1991. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349, 588–593. (doi:10.1038/349588a0) [DOI] [PubMed] [Google Scholar]
- 38.O'Hagan R, Chalfie M, Goodman MB. 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8, 43–50. (doi:10.1038/nn1362) [DOI] [PubMed] [Google Scholar]
- 39.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. 2006. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172, 2239–2252. (doi:10.1534/genetics.105.050013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Feng Z, Sternberg PW, Xu XZ. 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687. (doi:10.1038/nature04538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bounoutas A, Chalfie M. 2007. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 454, 691–702. (doi:10.1007/s00424-006-0187-x) [DOI] [PubMed] [Google Scholar]
- 42.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218. (doi:10.1016/0092-8674(95)90162-0) [DOI] [PubMed] [Google Scholar]
- 43.Sengupta P, Bargmann CI. 1996. Cell fate specification and differentiation in the nervous system of Caenorhabditis elegans. Dev. Genet. 18, 73–80. (doi:10.1002/(SICI)1520-6408(1996)18:1<73::AID-DVG8>3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 44.Tavernarakis N, Everett JK, Kyrpides NC, Driscoll M. 2001. Structural and functional features of the intracellular amino terminus of DEG/ENaC ion channels. Curr. Biol. 11, R205–R208. (doi:10.1016/S0960-9822(01)00106-3) [DOI] [PubMed] [Google Scholar]
- 45.Syntichaki P, Tavernarakis N. 2004. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol. Rev. 84, 1097–1153. (doi:10.1152/physrev.00043.2003) [DOI] [PubMed] [Google Scholar]
- 46.Woolf CJ, Ma Q. 2007. Nociceptors—noxious stimulus detectors. Neuron 55, 353–364. (doi:10.1016/j.neuron.2007.07.016) [DOI] [PubMed] [Google Scholar]
- 47.Bargmann CI, Horvitz HR. 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729–742. (doi:10.1016/0896-6273(91)90276-6) [DOI] [PubMed] [Google Scholar]
- 48.Hart AC, Sims S, Kaplan JM. 1995. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378, 82–85. (doi:10.1038/378082a0) [DOI] [PubMed] [Google Scholar]
- 49.Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV. 2002. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron 36, 933–944. (doi:10.1016/S0896-6273(02)01088-7) [DOI] [PubMed] [Google Scholar]
- 50.Silbering AF, Bell R, Munch D, Cruchet S, Gomez-Diaz C, Laudes T, Galizia CG, Benton R. 2016. Ir40a neurons are not DEET detectors. Nature 534, E5–E7. (doi:10.1038/nature18321) [DOI] [PubMed] [Google Scholar]
- 51.Knecht ZA, et al. 2016. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. eLife 5, e17879 (doi:10.7554/eLife.17879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. 2007. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 10, 348–354. (doi:10.1038/nn1856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bretscher AJ, Busch KE, de Bono M. 2008. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 105, 8044–8049. (doi:10.1073/pnas.0707607105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodama-Namba E, Fenk LA, Bretscher AJ, Gross E, Busch KE, de Bono M. 2013. Cross-modulation of homeostatic responses to temperature, oxygen and carbon dioxide in C. elegans. PLoS Genet. 9, e1004011 (doi:10.1371/journal.pgen.1004011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrillo MA, Guillermin ML, Rengarajan S, Okubo RP, Hallem EA. 2013. O2-sensing neurons control CO2 response in C. elegans. J. Neurosci. 33, 9675–9683. (doi:10.1523/JNEUROSCI.4541-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persson A, Gross E, Laurent P, Busch KE, Bretes H, de Bono M. 2009. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature 458, 1030–1033. (doi:10.1038/nature07820) [DOI] [PubMed] [Google Scholar]
- 57.Russell J, Vidal-Gadea AG, Makay A, Lanam C, Pierce-Shimomura JT. 2014. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 111, 8269–8274. (doi:10.1073/pnas.1322512111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leinwand SG, Chalasani SH. 2013. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat. Neurosci. 16, 1461–1467. (doi:10.1038/nn.3511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leinwand SG, Yang CJ, Bazopoulou D, Chronis N, Srinivasan J, Chalasani SH. 2015. Circuit mechanisms encoding odors and driving aging-associated behavioral declines in Caenorhabditis elegans. eLife 4, e10181 (doi:10.7554/eLife.10181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatla N. 2009. An interactive visualization of the C. elegans neural network. See http://wormweb.org/neuralnet .
- 61.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. 1999. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron 24, 347–361. (doi:10.1016/S0896-6273(00)80849-1) [DOI] [PubMed] [Google Scholar]
- 62.Kocabas A, Shen CH, Guo ZV, Ramanathan S. 2012. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature 490, 273–277. (doi:10.1038/nature11431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iino Y, Yoshida K. 2009. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J. Neurosci. 29, 5370–5380. (doi:10.1523/JNEUROSCI.3633-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I. 2002. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109, 639–649. (doi:10.1016/S0092-8674(02)00748-1) [DOI] [PubMed] [Google Scholar]
- 65.Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. 2006. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51, 613–625. (doi:10.1016/j.neuron.2006.07.024) [DOI] [PubMed] [Google Scholar]
- 66.Mori I, Ohshima Y. 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376, 344–348. (doi:10.1038/376344a0) [DOI] [PubMed] [Google Scholar]
- 67.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. 2007. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. (doi:10.1038/nature06292) [DOI] [PubMed] [Google Scholar]
- 68.Guillermin ML, Carrillo MA, Hallem EA. 2017. A single set of interneurons drives opposite behaviors in C. elegans. Curr. Biol. 27, 2630–2639.e2636. (doi:10.1016/j.cub.2017.07.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C, Itakura E, Nelson GM, Sheng M, Laurent P, Fenk LA, Butcher RA, Hegde RS, de Bono M. 2017. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48. (doi:10.1038/nature20818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh DD, Nitabach MN, Zhang Y, Harris G. 2017. Multisensory integration in C. elegans. Curr. Opin. Neurobiol. 43, 110–118. (doi:10.1016/j.conb.2017.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinkai Y, et al. 2011. Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J. Neurosci. 31, 3007–3015. (doi:10.1523/JNEUROSCI.4691-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mori I, Sasakura H, Kuhara A. 2007. Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr. Opin. Neurobiol. 17, 712–719. (doi:10.1016/j.conb.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 73.Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, Iino Y, Mori I. 2006. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 20, 2955–2960. (doi:10.1101/gad.1479906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsunozaki M, Chalasani SH, Bargmann CI. 2008. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron 59, 959–971. (doi:10.1016/j.neuron.2008.07.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Summers PJ, Layne RM, Ortega AC, Harris GP, Bamber BA, Komuniecki RW. 2015. Multiple sensory inputs are extensively integrated to modulate nociception in C. elegans. J Neurosci. 35, 10 331–10 342. (doi:10.1523/JNEUROSCI.0225-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. 2009. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175. (doi:10.1038/nature07886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinowitch I, Chatzigeorgiou M, Schafer WR. 2013. A gap junction circuit enhances processing of coincident mechanosensory inputs. Curr. Biol. 23, 963–967. (doi:10.1016/j.cub.2013.04.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chatzigeorgiou M, Schafer WR. 2011. Lateral facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron 70, 299–309. (doi:10.1016/j.neuron.2011.02.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ezcurra M, Walker DS, Beets I, Swoboda P, Schafer WR. 2016. Neuropeptidergic signaling and active feeding state inhibit nociception in Caenorhabditis elegans. J. Neurosci. 36, 3157–3169. (doi:10.1523/JNEUROSCI.1128-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calhoun AJ, Tong A, Pokala N, Fitzpatrick JA, Sharpee TO, Chalasani SH. 2015. Neural mechanisms for evaluating environmental variability in Caenorhabditis elegans. Neuron 86, 428–441. (doi:10.1016/j.neuron.2015.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghosh DD, Sanders T, Hong S, McCurdy LY, Chase DL, Cohen N, Koelle MR, Nitabach MN. 2016. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron 92, 1049–1062. (doi:10.1016/j.neuron.2016.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z, et al. 2012. Dissecting a central flip-flop circuit that integrates contradictory sensory cues in C. elegans feeding regulation. Nat. Commun. 3, 776 (doi:10.1038/ncomms1780) [DOI] [PubMed] [Google Scholar]
- 83.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. 2010. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat. Neurosci. 13, 615–621. (doi:10.1038/nn.2526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gruner M, Nelson D, Winbush A, Hintz R, Ryu L, Chung SH, Kim K, Gabel CV, van der Linden AM. 2014. Feeding state, insulin and NPR-1 modulate chemoreceptor gene expression via integration of sensory and circuit inputs. PLoS Genet. 10, e1004707 (doi:10.1371/journal.pgen.1004707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uhlhaas PJ, Singer W. 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113. (doi:10.1038/nrn2774) [DOI] [PubMed] [Google Scholar]
- 86.Ebert DH, Greenberg ME. 2013. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493, 327–337. (doi:10.1038/nature11860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panagiotidi M, Overton PG, Stafford T. 2017. Attention-deficit hyperactivity disorder-like traits and distractibility in the visual periphery. Perception 46, 665–678. (doi:10.1177/0301006616681313) [DOI] [PubMed] [Google Scholar]
- 88.Brown C, Cromwell RL, Filion D, Dunn W, Tollefson N. 2002. Sensory processing in schizophrenia: missing and avoiding information. Schizophr. Res. 55, 187–195. (doi:10.1016/S0920-9964(01)00255-9) [DOI] [PubMed] [Google Scholar]
- 89.Posar A, Visconti P. 2017. Sensory abnormalities in children with autism spectrum disorder. J. Pediatr. (doi:10.1016/j.jped.2017.08.008) [DOI] [PubMed] [Google Scholar]
- 90.Miller LJ, Nielsen DM, Schoen SA, Brett-Green BA. 2009. Perspectives on sensory processing disorder: a call for translational research. Front. Integr. Neurosci. 3 (doi:10.3389/neuro.07.022.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mouridsen SE, Rich B, Isager T, Nedergaard NJ. 2008. Pervasive developmental disorders and criminal behaviour: a case control study. Int. J. Offender Ther. Comp. Criminol. 52, 196–205. (doi:10.1177/0306624X07302056) [DOI] [PubMed] [Google Scholar]
- 92.Kas MJ, Fernandes C, Schalkwyk LC, Collier DA. 2007. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol. Psychiatry 12, 324–330. (doi:10.1038/sj.mp.4001979) [DOI] [PubMed] [Google Scholar]
- 93.Kanner L. 1943. Autistic disturbances of affective contact. Nervous Child. 2, 217–250. [PubMed] [Google Scholar]
- 94.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. 2011. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 69, 48R–54R. (doi:10.1203/PDR.0b013e3182130c54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stevenson RA, Segers M, Ferber S, Barense MD, Wallace MT. 2014. The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Front. Psychol. 5, 379 (doi:10.3389/fpsyg.2014.00379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. (doi:10.1007/s00429-010-0255-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamm C, Singer T. 2010. The role of anterior insular cortex in social emotions. Brain Struct. Funct. 214, 579–591. (doi:10.1007/s00429-010-0251-3) [DOI] [PubMed] [Google Scholar]
- 98.Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. 2014. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 83, 894–905. (doi:10.1016/j.neuron.2014.06.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hornix BE, Havekes R, Kas MJH. In press. Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neurosci. Biobehav. Rev. (doi:10.1016/j.neubiorev.2018.02.010) [DOI] [PubMed] [Google Scholar]
- 100.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. 2016. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 321, 24–41. (doi:10.1016/j.neuroscience.2015.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hannant P, Cassidy S, Van de Weyer R, Mooncey S. 2018. Sensory and motor differences in autism spectrum conditions and developmental coordination disorder in children: a cross-syndrome study. Hum. Mov. Sci. 58, 108–118. (doi:10.1016/j.humov.2018.01.010) [DOI] [PubMed] [Google Scholar]
- 102.Siemann JK, Muller CL, Forsberg CG, Blakely RD, Veenstra-VanderWeele J, Wallace MT. 2017. An autism-associated serotonin transporter variant disrupts multisensory processing. Transl. Psychiatry 7, e1067 (doi:10.1038/tp.2017.17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevenson RA, Park S, Cochran C, McIntosh LG, Noel JP, Barense MD, Ferber S, Wallace MT. 2017. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr. Res. 179, 97–103. (doi:10.1016/j.schres.2016.09.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zvyagintsev M, Parisi C, Mathiak K. 2017. Temporal processing deficit leads to impaired multisensory binding in schizophrenia. Cogn. Neuropsychiatry 22, 361–372. (doi:10.1080/13546805.2017.1331160) [DOI] [PubMed] [Google Scholar]
- 105.Lum JS, Millard SJ, Huang XF, Ooi L, Newell KA. 2017. A postmortem analysis of NMDA ionotropic and group 1 metabotropic glutamate receptors in the nucleus accumbens in schizophrenia. J. Psychiatry Neurosci. 42, 170077 (doi:10.1503/jpn.170077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacklin DL, Goel A, Clementino KJ, Hall AW, Talpos JC, Winters BD. 2012. Severe cross-modal object recognition deficits in rats treated sub-chronically with NMDA receptor antagonists are reversed by systemic nicotine: implications for abnormal multisensory integration in schizophrenia. Neuropsychopharmacology 37, 2322–2331. (doi:10.1038/npp.2012.84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panagiotidi M, Overton PG, Stafford T. 2017. Multisensory integration and ADHD-like traits: evidence for an abnormal temporal integration window in ADHD. Acta. Psychol. 181, 10–17. (doi:10.1016/j.actpsy.2017.10.001) [DOI] [PubMed] [Google Scholar]
- 108.Viaud-Delmon I, Venault P, Chapouthier G. 2011. Behavioral models for anxiety and multisensory integration in animals and humans. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1391–1399. (doi:10.1016/j.pnpbp.2010.09.016) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.