Abstract
Cancer immunotherapy has experienced remarkable advances in recent years. Striking clinical responses have been achieved for several types of solid cancers (e.g. melanoma, non-small cell lung cancer, bladder cancer and mismatch repair-deficient cancers) after treatment of patients with T-cell checkpoint blockade therapies. These have been shown to be particularly effective in the treatment of cancers with high mutation burden, which places tumour-mutated antigens (neo-antigens) centre stage as targets of tumour immunity and cancer immunotherapy. With current technologies, neo-antigens can be identified in a short period of time, which may support the development of complementary, personalized approaches that increase the number of tumours amenable to immunotherapeutic intervention. In addition to reviewing the state of the art in cancer immunotherapy, we discuss potential avenues that can bring the immunotherapy revolution to a broader patient group including cancers with low mutation burden.
Keywords: neo-antigens, checkpoint blockade, immunotherapy, mutation burden, immunogenicity, combination therapies
1. Introduction
The field of cancer immunotherapy has experienced alternating periods of success and failure in the development of cancer therapies. In the late nineteenth century, William Coley treated cancer patients by local injection with bacterial toxins, which provoked anti-tumour immune responses in some patients [1]. In the 1960s, Thomas and Burnet postulated the cancer immune surveillance theory, where the immune system would specifically eliminate malignant cells, most probably through recognition of tumour-associated antigens [2,3]. This was followed by the elucidation of the role of T cells in anti-tumour immune responses which led to the clinical use of the T-cell growth factor interleukin-2 (IL-2). In 1991, IL-2 was approved by the FDA for the treatment of metastatic renal cell carcinoma and, in 1998, for metastatic melanoma. However, IL-2 treatments produced high toxicity and yielded a relatively low response rate, underlining the need to develop improved immunotherapeutic strategies [4,5].
The transition to targeted immunotherapy was made with the development of the hybridoma technology, in 1975, which supported the production of monoclonal antibodies [6]. Rapidly, monoclonal antibody-based treatments were set up and the first FDA approval was obtained for rituximab in 1997 for the treatment of B-cell lymphomas. Rituximab is a genetically engineered monoclonal antibody directed against the CD20 antigen which is ubiquitously expressed in B cells and triggers cell death by antibody-dependent cell-mediated cytotoxicity, complement activation and direct induction of apoptosis [7,8]. In the same decade, chimaeric antigen receptor (CAR) T cells were developed to combine the antigen-binding properties of antibodies with the cytolytic and self-renewal capacity of T cells [9,10]. CAR T cells are genetically engineered to express an extracellular antigen-recognition domain, such as antibody-derived, single-chain variable fragments, coupled to T-cell activation endodomains. The most significant clinical results have been achieved with CD19-targeting CAR T cells in haematological malignancies [11,12].
More recently, a number of antibodies targeting cellular immune checkpoints (e.g. PD-1/PD-L1 and CTLA-4) have been developed to promote the activation of T cells and subsequent tumour control. This treatment strategy has been shown to be particularly effective in tumours with high mutation burden, putting tumour-mutated antigens (neo-antigens) centre stage in cancer immunotherapy [13–19].
2. Antigen presentation and cancer immunotherapy
Antigen processing and presentation enables the immune system to monitor cellular processes and to act accordingly upon expression of aberrant/foreign proteins. Human leukocyte antigen (HLA) class I molecules present antigens at the surface of most cells throughout the organism. Such antigens can, theoretically, be derived from most cellular proteins as these are processed by the (immuno) proteasome and broken down to peptides [20]. Subsequently, transporter associated with antigen processing (TAP) proteins mediate the intake of these peptides to the endoplasmic reticulum, where they are loaded onto HLA class I molecules with the aid of several chaperones [21,22]. HLA class I/peptide complexes translocate via the Golgi apparatus to the cell surface where they are exposed to CD8+ T cells [23]. Nevertheless, an effective anti-tumour immune response is thought to be initiated by the taking up of tumour antigens by antigen-presenting cells (APCs) which in turn present them, and provide co-stimulatory signals, to both CD4+ and CD8+ T cells [24]. In order to do so, APCs, particularly dendritic cells, process antigens through an exogenous antigen processing pathway where (tumour) cellular material is phagocytosed and converted into HLA class I- and class II-binding peptides that are presented to CD8+ (cross-presentation) and CD4+ T cells, respectively [25]. HLA class II expression is also known to occur in some tumour types although its functional significance and how it can be exploited from an immunotherapeutic point of view require further investigation [26,27].
Antigens that are considered to evoke anti-tumour immune responses and which are therefore suitable as immunotherapeutic targets can be divided into three groups: tumour-mutated antigens (or neo-antigens), tumour-associated antigens and cancer-testis antigens [28]. Viral antigens constitute another class of targetable antigens in the context of viral oncogenesis but will not be discussed here. Tumour-associated and cancer-testis antigens are both self-antigens that are differentially expressed in tumour tissues and rarely expressed (or to lower extent) in normal tissues. The stimulation of endogenous T-cell responses against self-antigens can be challenging as auto-reactive T cells are subjected to negative selection in the thymus [29]. Nevertheless, it has been shown that central tolerance can be broken and that immune responses can be generated against self-antigens, analogous to what is observed in autoimmunity [30]. Positive clinical indications have been described for several tumour-associated antigens (e.g. gp100, MART-1) and cancer-testis antigens (e.g. MAGE-A3 and NY-ESO-1) [31–34]. However, subsequent clinical trials were not always able to confirm patient survival benefits and side-effects were regularly observed due to expression of the targeted antigens in healthy tissues [35–37].
Neo-antigens are by definition tumour-specific as they arise from somatic mutations that are not present in healthy tissue. Theoretically, they constitute ideal targets for immunotherapy because no off-target reactivity and central tolerance of T cells are expected [38]. The accumulation of somatic mutations is a hallmark of tumour progression, but only a minority of mutations is under positive selection and, therefore, recurrently observed in different patients. Hence, individual tumour mutation profiles are dominated by the so-called passenger mutations which are highly variable between cancers and patients [39]. The development of next-generation sequencing (NGS) technologies has made it possible to screen entire (coding) genomes for the detection of potential neo-antigens in a clinically applicable time-frame. In silico tools aiming at identifying neo-antigens more likely to constitute good immunotherapy targets are also under constant development [40–44].
The requirement of a personalized approach to target neo-antigens can be a time-consuming and onerous procedure. While this limitation could be circumvented by the targeting of recurrent mutations at driver genes such as BRAF and KRAS, accumulated evidence suggests that such mutations are seldom immunogenic [45,46]. In fact, this might be expected, as it would be unlikely that immunogenic mutations would be so often favoured by clonal selection during tumour progression. Another aspect complicating the targeting of neo-antigens relates to intra-tumour heterogeneity. The identification of neo-antigens requires that the tumour is sampled and further processed for nucleic acid isolation and sequencing. Several reports have identified sampling issues as a major limitation for a comprehensive characterization of somatic alterations in tumours [47,48]. On the other hand, cancer therapies, including immunotherapies, will probably be the most successful when targeting clonal alterations present in any part of a tumour mass [13]. Another caveat that must be considered is that neo-antigens, particularly the ones derived from point mutations, have very similar sequences to their wild-type counterpart. If amino acid substitutions at anchor residues do not affect the binding affinity to HLA molecules or if substitutions at core residues do not significantly alter the molecular properties of a peptide, the likelihood that high avidity TCRs are present in an autologous T-cell repertoire may be low. This supports a fundamental role for frameshift mutations as these have the potential to generate highly immunogenic peptides [49]. However, frameshifts are notoriously difficult to detect, particularly in NGS data, and the capacity to identify them varies greatly between research groups.
3. The state of the art in cancer immunotherapy
T cells are key players in anti-tumour immunity and, therefore, the bulk of cancer immunotherapy research has focused on inducing T-cell-mediated anti-tumour responses. The current breakthrough in cancer immunotherapy results from the identification and subsequent targeting of checkpoint mechanisms in T cells with antibodies against CTLA-4, PD-1 and PD-L1 [50–53]. CTLA-4 and PD-1 are co-inhibitory receptors found on the cell surface of T cells. Upon binding to their corresponding ligands (CD80/86 and PD-L1/-L2, respectively), T cells become anergic: a physiological mechanism of peripheral tolerance or halting of inflammatory responses [54]. In the context of the tumour microenvironment, the aberrant expression of immune checkpoint ligands (on tumour and immune cells), together with chronic exposure to tumour antigens, can lead to the undesirable suppression of T-cell activity [55]. The blocking of such mechanisms can therefore unleash a renovated anti-tumour immune response. Moreover, checkpoint blockers were found to broaden the target of cytotoxic T-cell responses in cancer patients [56,57].
Treatment with checkpoint blocking antibodies has been approved for a number of cancers including melanoma, urothelial bladder cancer, head and neck squamous cell carcinoma, non-small cell lung cancer and classical Hodgkin lymphoma, while positive indications has been found for many other malignancies [50,58–62]. Immune checkpoint blockade has been shown to be most effective in tumours with high mutation burden that arises either from chronic exposure to DNA-damaging agents (e.g. smoking and ultraviolet radiation) or as a consequence of intrinsic DNA repair defects [16,17,63]. Accordingly, clinical responses have also been correlated with the mutation burden of tumours derived from the same organ [16,17,62]. Notably, pembrolizumab, an anti-PD-1 antibody, constitutes the FDA's first tissue/site-agnostic, molecular-guided approval as it is indicated for advanced mismatch repair-deficient cancers. These findings support the central role of neo-antigens in the therapeutic responses to immune checkpoint blockers. Nevertheless, the majority of patients with the so-called hypermutated tumours do not respond to checkpoint blockade and the ability to predict responses by discovering additional biomarkers is a major focus of research in the field [64]. In order for CD8+ T cells to fulfil their cytotoxic activity, they must infiltrate tumour tissues and subsequently recognize cancer antigens loaded on HLA class I molecules. Therefore, defects in the antigen processing and presentation machinery are often observed as immunoediting phenotypes in tumour cells [65–69]. Additionally, tumour cells can escape cytokine-mediated immune responses by mutating components of the IFN-γ pathway. Metastatic melanoma patients that did not respond to CTLA-4 treatment were found to have tumours with genetic defects in IFNGR1/2, IRF1 and JAK2 [70]. Similarly, melanoma and MMR-deficient colorectal cancer patients were found to be resistant to anti-PD-1 treatment due to inactivating mutations in JAK1/2 [71,72]. Neo-antigen availability can also change in a tumour, due to clonal selection by immunoediting, enforced by neo-antigen-specific T cells [73,74].
Spontaneous, neo-antigen-driven, anti-tumour responses arise in many cancer patients, as demonstrated by the isolation of neo-antigen-reactive tumour-infiltrating lymphocytes (TIL) [75]. Furthermore, the presence of TIL, particularly with a type 1 inflammatory profile (i.e. IFNγ/IL-2-driven immune responses), is generally associated with an improved prognosis in cancer patients [76,77]. One approach to boost an autologous lymphocyte-mediated anti-tumour response is through adoptive T-cell transfer (ACT), which relies on the ex vivo expansion of tumour-reactive T cells and their reinfusion back in the patient [78]. The infusion product can consist of TIL or peripheral blood-derived tumour-specific T cells that are expanded in the presence of tumour cells or tumour antigens [79,80]. ACT-based treatments have produced some encouraging results, particularly for metastatic melanoma patients [73,80–83]. Verdegaal et al. reported on the successful treatment of a metastatic melanoma patient with CD4+ and CD8+ tumour-specific T cells [73,80]. In a fascinating example, the potency of neo-antigen-specific ACT is illustrated by the treatment of a metastatic cholangiocarcinoma patient, treated with a neo-antigen-reactive CD4+ T-cell product derived from TIL, resulting in stable disease [82]. These findings underscore the relevance that ACT might have for some patients, but similar to for checkpoint blockade, there is a need to discover biomarkers that indicate a priori which patients may benefit from it.
Today, many ongoing clinical trials are investigating the clinical effect of combining different immunotherapies. The use of anti-CTLA-4 in addition to anti-PD-1 antibodies resulted in increased overall survival rates in previously untreated melanoma patients [84,85]. Furthermore, other immune regulators, such as LAG-3, TIM-3, ICOS or NKG2D are promising new therapeutic targets [86–90]. Additional research will be important to address resistance to first-generation immune checkpoint blockers as, for instance, LAG-3 and TIM-3 upregulation is observed following anti-PD-1 treatment [86]. Likewise, CD137 co-stimulation is studied for its synergistic effects with ACT [91,92]. Finally, checkpoint blockade therapies may also be used in combination with standard chemo- and radiotherapy interventions which are known to enhance tumour immunogenicity [93,94].
Other avenues like therapeutic vaccination with synthetic peptides corresponding to neo-antigens are being explored. This strategy aims to prime autologous T cells from cancer patients against tumour-specific antigens to unleash anti-tumour immune responses. In addition to providing neo-antigens as immunotherapy products, several co-stimulatory factors are needed to induce an effective anti-tumour T-cell response [95], including provision of danger signals by adjuvants and/or homing of cellular-based vaccines [96–98]. Encouraging clinical responses were obtained with neo-antigen-based peptides plus polyICLC vaccinations in previously untreated metastatic melanoma patients [99]. This intervention was shown to induce CD4+ and CD8+ anti-tumour T-cell responses against several epitopes. Four out of six patients had no recurrence after 25 months; two patients with tumour recurrence received subsequent anti-PD1 therapy leading to complete tumour regression [99]. In another phase I study, stage III melanoma patients pre-treated with ipilimumab and by surgical resection received a vaccine consisting of autologous dendritic cells presenting neo-antigens that were determined by sequencing [100]. Both vaccination strategies induced tumour-directed immune responses with concomitant broadening of the targeted antigen repertoire without inducing side-effects [99,100]. Nevertheless, to date, the number of vaccination studies involving neo-antigens that reported positive clinical outcomes is limited. This might be explained by the fact that the bulk of this research, in previous decades, has focused on targeting oncogenes and tumour suppressors (e.g. TP53) with recurrent mutations [101]. Therefore, these studies did not consider the largest source of neo-antigens in tumour—passenger mutations.
The requirement that neo-antigens are presented in complex with HLA class I hinders the widespread application of neo-antigen-targeted therapies in the form of peptide vaccination or ACT. Therefore, CAR T cells were designed to enable the targeting of any cell surface molecule, in an HLA non-restricted fashion [9]. This strategy has been particularly successful for treating haematological malignancies, because highly tissue/cell-restricted antigens are present on their easily accessible cells of origin [10–12]. In 2010, the first successful CAR T-cell therapy was reported in a lymphoma patient who was pre-treated with chemotherapy [10]. The infusion product consisted of autologous T cells transduced with retroviruses encoding the variable region of the anti-CD19, B-cell antigen, which was joined to part of the co-stimulatory CD28 molecule and CD3ζ signalling domain for T-cell activation. Investigations in larger cohorts showed clinical responses [102,103], but severe side-effects arose, including treatment-related deaths [104–106]. These side-effects derive from high cytokine concentrations (cytokine storm), produced by the infused engineered T cells that become hyper-activated as a result of high affinity of their receptor to the target molecules. Recently, two second generation CAR therapies targeting CD19 have been approved by the FDA for treatment of patients with relapsed/refractory diffuse large B-cell lymphoma and relapsed/refractory B-cell precursor acute lymphoblastic leukaemia [107,108]. In search for optimal effectivity and specificity, third generation CARs are currently being developed, which contain two co-stimulatory domains [109–112]. Furthermore, investigations are ongoing to improve the treatment of haematological diseases while limiting the severity of side-effects, as well as investigations on the clinical efficacy of genetically engineered T cells in solid tumours [109,113,114]. The targeting of the latter has proved to be particularly challenging and complicating factors include the identification of specific, targetable antigens and the homing of CAR T cells to the tumour tissues where in turn they are exposed to a complex tumour microenvironment [115]. On the other hand, CAR T cells are a very attractive tool to treat cancers arising in non-vital organs where specific antigens are expressed (e.g. thyroid and ovaries).
4. The immune landscape of low mutation burden tumours
As discussed, neo-antigens constitute attractive targets for immunotherapy and clinical responses with checkpoint blockers have been correlated to the mutation burden of tumours [16,62]. Cancers with 10 mutations/Mb or more have been proposed as susceptible for checkpoint blockade, indicating the importance of neo-antigen presence for a potent immune response [116]. However, not all patients with high mutation burden tumours benefit from these therapies, and the precise determinants of response are undefined at the moment. Furthermore, the division between tumours with high, moderate and low mutation burden is somewhat arbitrary. In theory, tumours with low/moderate mutation burden that present neo-antigens in complex with HLA class I could still be eligible for T-cell-mediated immunotherapy. However, several questions remain unanswered: does the low number of neo-antigens translate to the improbability that a neo-antigen ‘survives’ the antigen processing machinery? On the other hand, if a small number of neo-antigens is indeed presented by a tumour cell, is it enough to provoke an inflammatory response that is required for tumour elimination?
Medulloblastoma, the most common brain tumour in children, has a low mutation burden, but was found to upregulate IDO1 expression [117]. IDO1 enhances immunosuppressive effects leading to an increase of Tregs and dampened activity of effector T cells [118]. Therefore, upregulation of IDO1 can be classified as an immune escape mechanism, indicating a role for the immune system in the control of medulloblastoma progression. Additionally, acute myeloid leukaemia (AML) cells are known to overexpress PD-L1 [119] and IDO1 [120], and AML blasts can secrete arginase II in order to promote immune escape by suppressing T-cell proliferation and polarizing monocyte differentiation towards an M2 phenotype [121]. Another tumour with low/moderate mutation burden, Hodgkin lymphoma, is characterized by few tumour cells and many immune cells that are attracted by the tumour-secreted cytokines [122]. However, these tumour-infiltrating immune cells display an immunosuppressive rather than anti-tumourigenic phenotype [122]. Immunotherapies are regularly employed to treat this disease, including antibodies targeting CD20, CD30 and checkpoint inhibitors targeting PD-1 [7,61,123]. Effectiveness of the latter may reside in the genetic overexpression of PD-L1 by the tumour cells [122]. TILs in Hodgkin lymphoma were found to express low levels of PD-1, but the blockade of this co-inhibitory mechanism was shown to result in an enhanced anti-tumour activity [61]. This finding underlines the existence of a T-cell-mediated anti-tumour response, which might be circumvented by the tumour through PD-L1 expression. Nevertheless, the immune evasive mechanisms observed in AML and Hodgkin lymphoma are probably closely connected to the function of their precursor cells and the persistent interaction of these pathologies with the immune system. A last example of a tumour type with low/moderate mutation burden that has potential for treament with immunotherapeutic strategies is renal cell carcinoma (RCC). Sensitivity to immunotherapeutic intervention in this tumour type was already known from the clinical responses of some RCC patients to IL-2 treatment [124]. Recently, patient overall survival was shown to increase from 19.6 to 25 months with anti-PD-1 therapy compared to standard care with the mTOR inhibitor everolimus [125]. The underlying mechanisms making this tumour susceptible for immunotherapeutics are not understood yet, but the composition of the tumour microenvironment might play an important role. High lymphocyte infiltration was found to correlate with high risk for disease progression, which is a paradox characteristic of RCC. This might relate to the exhausted phenotype of infiltrating lymphocytes which contributes to an immunosuppressive microenvironment [126]. Furthermore, neo-antigen depletion due to immune selection was demonstrated to occur in RCC and a positive correlation was observed between mutations in the antigen-presenting machinery and cytotoxic activity by immune cells, suggesting the presence of ongoing anti-tumour immune reactions [67]. Finally, RCC was found to have the highest number of frameshift mutations out of 19 different cancer types, which might explain the immunogenicity observed in these tumours despite their moderate total mutation burden [49]. These examples of tumours with low mutation burden presenting susceptibility to immunotherapeutic strategies indicate the existence of autologous tumour-specific T cells with the potential to recognize (neo-) antigens, even when present in small numbers.
5. Immunotherapies for tumours with low mutation burden
Previous works by Tran et al. [82,127] support the idea that most tumours present neo-antigens and that these can be targeted by the immune system, e.g. gastrointestinal cancers with low and moderate mutation burden including a cholangiocarcinoma patient with only 26 non-synonymous mutations. Therefore, the clinical applicability of neo-antigen-targeted ACT or peptide-based vaccination strategies for low mutation burden tumours should be explored. The detection rate of autologous T-cell reactivity to neo-antigens is often described to be approximately 1% of the non-synonymous mutations that are transcribed in a tumour [83,127,128]. Currently, NGS is regularly used to determine neo-antigen presence, but improvements in capture methods for targeted panels (e.g. exome) and mutation detection algorithms might enhance the initial pool of targetable mutations in tumours with low mutation burden. For these, the use of in silico prediction models for antigen processing and HLA binding affinity might not be necessary for a first T-cell reactivity screening using long peptides, because the number of mutations is low and all neo-antigens can be tested for their ability to induce T-cell activation. However, to directly investigate T-cell reactivity against short peptides, in silico tools are still required.
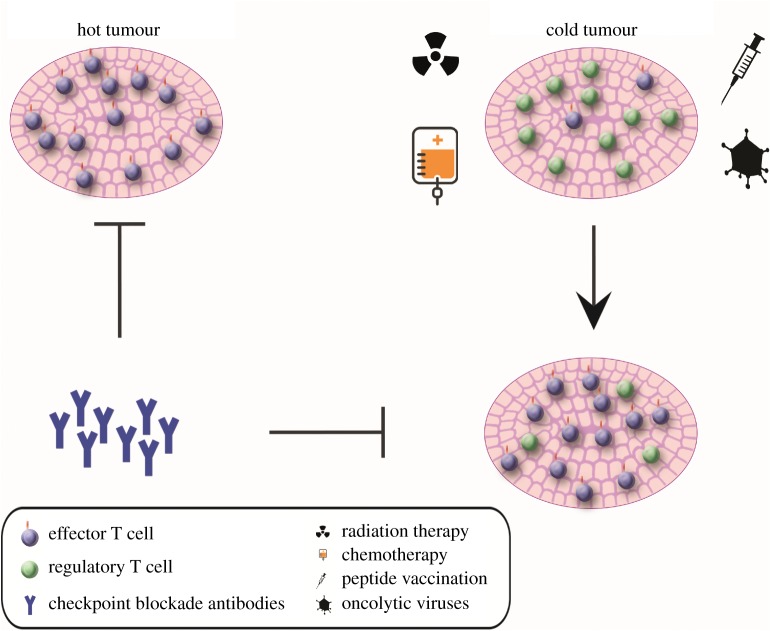
Immunotherapies have a high synergistic potential with standard chemo- and radiotherapies as these are known to induce immunogenic cell death [129,130]. This synergy might be especially valuable for tumours with low mutation burden which do not respond to immunotherapy alone, and which could benefit from the transformation of a ‘cold’ immune microenvironment into a ‘hot’ microenvironment with an inflammatory profile (figure 1) [131,132]. The rationale of classical chemotherapy and radiotherapy encompasses the targeting of fast-dividing tissues by impairing mitosis and inducing DNA damage. This leads to the release of tumour antigens and damage-associated molecular patterns which activate APCs [133]. Macrophages are attracted to consume the damaged tumour cells, which further enhances the anti-tumour response of T cells upon presentation of the tumour antigens [134]. In addition, radiotherapy leads to the release of nuclear DNA in the cytoplasm, activating the stimulator of interferon genes (STING) pathway, which is a direct link between the innate immune system and DNA damage [135,136]. Furthermore, the tumour microenvironment is disrupted by chemoradiation, thereby disturbing the immune suppressive milieu in tumours. This includes increased antigen presentation and expression of co-stimulatory molecules as well as inhibition of regulatory T-cell and myeloid-derived suppressor cell function [129,137–139]. In melanoma patients, an improved clinical response rate was observed upon treatment with a combination of anti-CTLA-4/PD-1 with radiotherapy, compared to treatment without radiation [130]. Moreover, combined radiotherapy with anti-CTLA-4 treatment induced abscopal effects (shrinkage of tumour lesions outside of the target region of radiotherapy), in this case consisting of complete regression of metastases at different sites [140]. Similarly, abscopal effects were observed in a treatment-refractory metastatic lung adenocarcinoma patient after therapy with radiotherapy and ipilimumab [141]. Tumours treated pre-surgically with neo-adjuvant therapy might be particularly interesting for the investigation of the synergistic effect of chemoradiation and immunotherapy in cancers with low mutation burden. Among these, rectal cancers and oesophageal tumours are excellent candidates for clinical trials aiming at reducing mortality and treatment-related morbidity.
Figure 1.
(Immuno) therapeutic strategies in tumours with ‘hot’ and ‘cold’ immune microenvironments. Checkpoint blockade therapies are mostly applicable to ‘hot’ tumours which present an inflammatory profile as a consequence of their high mutation burden. We propose that ‘cold’ tumours might be sensitized to checkpoint blockade if this is used in combination with radiotherapy, chemotherapy, peptide vaccination or oncolytic viruses, to boost anti-tumour immune responses.
Another avenue that may lead to the sensitization of additional tumours to immunotherapeutic intervention is epigenetic modulation of cancer cells [142]. Epigenetic regulation is fundamental for gene expression and, consequently, for neo-antigen availability. Furthermore, in order to evade the immune system, tumours might acquire epigenetic footprints that change the expression of immunomodulatory genes. For instance, the expression of specific HLA alleles, with affinity to neo-antigens, can be suppressed in tumour cells due to epigenetic changes [143,144]. Such observations are strongly supportive of adopting epigenetic modifiers to restore or improve immunogenicity of some cancers [145]. More specifically, epigenetic modifiers have been shown to increase CD8+ T-cell infiltration in ovarian cancer and the immunogenicity of colorectal cancer cells was increased upon treatment with DNA-demethylating agents [146,147]. Epigenetic drugs could thus tackle the heterogenic expression of, among others, HLA molecules and neo-antigens, thereby enhancing anti-tumour immunity.
Another obstacle to employing immunotherapies for the treatment of tumours with low mutation burden relates to the fact that they are usually poorly infiltrated by immune cells. The initiation of an adaptive anti-tumour immune response probably relies on a robust inflammatory trigger that is absent in poorly immunogenic tumours. On the other hand, such inflammatory threshold in tumours with high mutation burden is most likely reached due to the abundance of mutated antigens. A strategy to artificially induce an inflammatory response that complements immunotherapeutic approaches is oncolytic virotherapy (figure 1). Talimogene laherparepvec, a genetically engineered herpes virus, replicates specifically in cancer cells and induces tumour cell death [148]. It was also shown to induce the expression of GM-CSF in tumours, which attracts dendritic cells that take up tumour antigens after cancer cell death. A phase Ib clinical trial obtained objective response rates (62%) and complete response rates (33%) in advanced melanoma patients, which were treated with a talimogene laherparepvec vaccination combined with pembroluzimab (anti-PD-1 blocker) [149]. The vaccination treatment was shown to induce infiltration of T cells that often expressed PD-1, especially in otherwise non-infiltrated ‘cold’ tumours, explaining the patients' sensitivity to PD-1 blockade. While such combination therapies were mainly performed in immunogenic tumours, their success and rationale supports the investigation of their applicability in tumours with low mutation burden.
6. Concluding remarks
Immunotherapy, particularly checkpoint blockade, can induce robust and durable anti-tumour responses in a significant proportion of patients, predominantly when applied for the treatment of cancers with high mutation burden. Until today, the applicability of these treatments for other cancer types is very limited. During the last decade, different groups have demonstrated the possibility of identifying neo-antigen-targeted immune cell repsonses in tumours with intermediate/low mutation burden. Recent work in our laboratory confirms that neo-antigen-reactive T cells are present in low mutation burden, mismatch repair-proficient colorectal carcinomas (van den Bulk et al. 2018, unpublished data). These findings underscore the relevance of developing neo-antigen targeting immunotherapies for low mutation burden tumours by tuning anti-tumour inflammatory responses. ‘Cold’, poorly immunogenic, tumours will require rationale-based interventions that make use of combinatorial therapies, including radio/chemotherapy or oncolytic viruses, to switch cancer immune microenvironments to a ‘hot’ state.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Alpe d'HuZes/KWF Bas Mulder Award (UL2015-7664), Veni ZonMw grant no. (91617144) and KWF grant no. (10815).
References
- 1.Coley WB. 1893. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Am. J. Med. Sci. 105, 488–511.(doi:10.1097/00000441-189305000-00001) [PubMed] [Google Scholar]
- 2.Burnet FM. 1967. Immunological aspects of malignant disease. Lancet 1, 1171–1174. (doi:10.1016/S0140-6736(67)92837-1) [DOI] [PubMed] [Google Scholar]
- 3.Burnet FM. 1970. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13, 1–27. (doi:10.1159/000386035) [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. 1985. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl. J. Med. 313, 1485–1492. (doi:10.1056/NEJM198512053132327) [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, et al. 1999. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17, 2105–2116. (doi:10.1200/JCO.1999.17.7.2105) [DOI] [PubMed] [Google Scholar]
- 6.Kohler G, Milstein C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497. (doi:10.1038/256495a0) [DOI] [PubMed] [Google Scholar]
- 7.Maloney DG, et al. 1997. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90, 2188–2195. [PubMed] [Google Scholar]
- 8.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. 1994. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83, 435–445. [PubMed] [Google Scholar]
- 9.Eshhar Z, Waks T, Gross G, Schindler DG. 1993. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724. (doi:10.1073/pnas.90.2.720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, et al. 2010. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102. (doi:10.1182/blood-2010-04-281931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brentjens RJ, et al. 2011. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828. (doi:10.1182/blood-2011-04-348540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupp SA, et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl. J. Med. 368, 1509–1518. (doi:10.1056/NEJMoa1215134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGranahan N, et al. 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469. (doi:10.1126/science.aaf1490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Allen EM, et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211. (doi:10.1126/science.aad0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rooij N, et al. 2013. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–e442. (doi:10.1200/JCO.2012.47.7521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder A, et al. 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl. J. Med. 371, 2189–2199. (doi:10.1056/NEJMoa1406498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, et al. 2017. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. (doi:10.1126/science.aan6733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domingo E, et al. 2016. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 1, 207–216. (doi:10.1016/S2468-1253(16)30014-0) [DOI] [PubMed] [Google Scholar]
- 19.Eggink FA, et al. 2017. Immunological profiling of molecularly classified high-risk endometrial cancers identifies POLE-mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology 6, e1264565 (doi:10.1080/2162402X.2016.1264565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yewdell JW, Reits E, Neefjes J. 2003. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat. Rev. Immunol. 3, 952–961. (doi:10.1038/nri1250) [DOI] [PubMed] [Google Scholar]
- 21.Neefjes JJ, Momburg F, Hammerling GJ. 1993. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science 261, 769–771. (doi:10.1126/science.8342042) [DOI] [PubMed] [Google Scholar]
- 22.Wearsch PA, Cresswell P. 2008. The quality control of MHC class I peptide loading. Curr. Opin. Cell Biol. 20, 624–631. (doi:10.1016/j.ceb.2008.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neefjes J, Jongsma ML, Paul P, Bakke O. 2011. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836. (doi:10.1038/nri3084) [DOI] [PubMed] [Google Scholar]
- 24.Kurts C, Robinson BW, Knolle PA. 2010. Cross-priming in health and disease. Nat. Rev. Immunol. 10, 403–414. (doi:10.1038/nri2780) [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Banchereau J. 2007. Taking dendritic cells into medicine. Nature 449, 419 (doi:10.1038/nature06175) [DOI] [PubMed] [Google Scholar]
- 26.Van Vreeswijk H, Ruiter DJ, Brocker EB, Welvaart K, Ferrone S. 1988. Differential expression of HLA-DR, DQ, and DP antigens in primary and metastatic melanoma. J. Invest. Dermatol. 90, 755–760. (doi:10.1111/1523-1747.ep12560951) [DOI] [PubMed] [Google Scholar]
- 27.Dengjel J, et al. 2006. Unexpected abundance of HLA class II presented peptides in primary renal cell carcinomas. Clin. Cancer Res. 12, 4163–4170. (doi:10.1158/1078-0432.CCR-05-2470) [DOI] [PubMed] [Google Scholar]
- 28.Ilyas S, Yang JC. 2015. Landscape of tumor antigens in T-cell immunotherapy. J. Immunol. 195, 5117–5122. (doi:10.4049/jimmunol.1501657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takaba H, Takayanagi H. 2017. The mechanisms of T cell selection in the thymus. Trends Immunol. 38, 805–816. (doi:10.1016/j.it.2017.07.010) [DOI] [PubMed] [Google Scholar]
- 30.Abbas AK, Lohr J, Knoechel B, Nagabhushanam V. 2004. T cell tolerance and autoimmunity. Autoimmun. Rev. 3, 471–475. (doi:10.1016/j.autrev.2004.07.004) [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg G, Machlenkin A, Frankenburg S, Mansura A, Pitcovski J, Yefenof E, Peretz T, Lotem M. 2010. Transcutaneous immunization with hydrophilic recombinant gp100 protein induces antigen-specific cellular immune response. Cell. Immunol. 266, 98–103. (doi:10.1016/j.cellimm.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunder NN, et al. 2008. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 358, 2698–2703. (doi:10.1056/NEJMoa0800251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchand M, et al. 1999. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer 80, 219–230. (doi:10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- 34.Skipper JCA, Gulden PH, Hendrickson RC, Harthun N, Caldwell JA, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL. 1999. Mass-spectrometric evaluation of HLA-A*0201-associated peptides identifies dominant naturally processed forms of CTL epitopes from MART-1 and gp100. Int. J. Cancer 82, 669–677. (doi:10.1002/(SICI)1097-0215(19990827)82:5<669::AID-IJC9>3.0.CO;2-#) [DOI] [PubMed] [Google Scholar]
- 35.Sosman JA, et al. 2008. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J. Clin. Oncol. 26, 2292–2298. (doi:10.1200/JCO.2007.13.3165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi P, Mirakhur B. 2009. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin. Lung Cancer 10, 371–374. (doi:10.3816/CLC.2009.n.052) [DOI] [PubMed] [Google Scholar]
- 37.Linette GP, et al. 2013. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871. (doi:10.1182/blood-2013-03-490565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilboa E. 1999. The makings of a tumor rejection antigen. Immunity 11, 263–270. (doi:10.1016/S1074-7613(00)80101-6) [DOI] [PubMed] [Google Scholar]
- 39.Negrini S, Gorgoulis VG, Halazonetis TD. 2010. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228. (doi:10.1038/nrm2858) [DOI] [PubMed] [Google Scholar]
- 40.Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ. 2014. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol. Res. 2, 522–529. (doi:10.1158/2326-6066.CIR-13-0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Buuren MM, Calis JJ, Schumacher TN. 2014. High sensitivity of cancer exome-based CD8T cell neo-antigen identification. Oncoimmunology 3, e28836 (doi:10.4161/onci.28836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreatta M, Nielsen M. 2016. Gapped sequence alignment using artificial neural networks: application to the MHC class I system. Bioinformatics 32, 511–517. (doi:10.1093/bioinformatics/btv639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassani-Sternberg M, Gfeller D. 2016. Unsupervised HLA peptidome deconvolution improves ligand prediction accuracy and predicts cooperative effects in peptide–HLA interactions. J. Immunol. 197, 2492–2499. (doi:10.4049/jimmunol.1600808) [DOI] [PubMed] [Google Scholar]
- 44.Tappeiner E, Finotello F, Charoentong P, Mayer C, Rieder D, Trajanoski Z. 2017. TIminer: NGS data mining pipeline for cancer immunology and immunotherapy. Bioinformatics 33, 3140–3141. (doi:10.1093/bioinformatics/btx377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shono Y, Tanimura H, Iwahashi M, Tsunoda T, Tani M, Tanaka H, Matsuda K, Yamaue H. 2003. Specific T-cell immunity against Ki-ras peptides in patients with pancreatic and colorectal cancers. Br. J. Cancer 88, 530–536. (doi:10.1038/sj.bjc.6600697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharkey MS, Lizee G, Gonzales MI, Patel S, Topalian SL. 2004. CD4(+) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer Res. 64, 1595–1599. (doi:10.1158/0008-5472.CAN-03-3231) [DOI] [PubMed] [Google Scholar]
- 47.Harbst K, et al. 2016. Multiregion whole-exome Sequencing uncovers the genetic evolution and Mutational heterogeneity of early-stage metastatic Melanoma. Cancer Res. 76, 4765–4774. (doi:10.1158/0008-5472.CAN-15-3476) [DOI] [PubMed] [Google Scholar]
- 48.Gerlinger M, et al. 2012. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892. (doi:10.1056/NEJMoa1113205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turajlic S, et al. 2017. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021. (doi:10.1016/S1470-2045(17)30516-8) [DOI] [PubMed] [Google Scholar]
- 50.Hodi FS, et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. (doi:10.1056/NEJMoa1003466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larkin J, Hodi FS, Wolchok JD. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 1270–1271. (doi:10.1056/NEJMc1509660) [DOI] [PubMed] [Google Scholar]
- 52.Brunet J-F, Denizot F, Luciani M-F, Roux-Dosseto M, Suzan M, Mattei M-G, Golstein P.. 1987. A new member of the immunoglobulin superfamily--CTLA-4. Nature 328, 267–270. (doi:10.1038/328267a0) [DOI] [PubMed] [Google Scholar]
- 53.Ishida Y, Agata Y, Shibahara K, Honjo T. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz RH. 2003. T cell anergy. Annu. Rev. Immunol. 21, 305–334. (doi:10.1146/annurev.immunol.21.120601.141110) [DOI] [PubMed] [Google Scholar]
- 55.Schietinger A, Greenberg PD. 2014. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35, 51–60. (doi:10.1016/j.it.2013.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kvistborg P, et al. 2014. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 6, 254ra128 (doi:10.1126/scitranslmed.3008918) [DOI] [PubMed] [Google Scholar]
- 57.Ribas A, et al. 2016. PD-1 blockade expands intratumoral T memory cells. Cancer Immunol. Res. 4, 194–203. (doi:10.1158/2326-6066.CIR-15-0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chow LQM, et al. 2016. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the Phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 34, 3838–3845. (doi:10.1200/JCO.2016.68.1478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powles T, et al. 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562. (doi:10.1038/nature13904) [DOI] [PubMed] [Google Scholar]
- 60.Brahmer JR, et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465. (doi:10.1056/NEJMoa1200694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansell SM, et al. 2014. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319. (doi:10.1056/NEJMoa1411087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizvi NA, et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. (doi:10.1126/science.aaa1348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mouw KW, Goldberg MS, Konstantinopoulos PA, D'andrea AD. 2017. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 7, 675–693. (doi:10.1158/2159-8290.CD-17-0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. 2017. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 14, 655–668. (doi:10.1038/nrclinonc.2017.88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jongsma MLM, Guarda G, Spaapen RM. In press. The regulatory network behind MHC class I expression. Mol. Immunol. (doi:10.1016/j.molimm.2017.12.005) [DOI] [PubMed] [Google Scholar]
- 66.Dierssen JWF, de Miranda NFCC, Ferrone S, Van Puijenbroek M, Cornelisse CJ, Fleuren GJ, Van Wezel T, Morreau H.. 2007. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 7, 33 (doi:10.1186/1471-2407-7-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. 2015. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61. (doi:10.1016/j.cell.2014.12.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sade-Feldman M, et al. 2017. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (doi:10.1038/s41467-017-01062-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, Von Knebel Doeberitz M.. 2005. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 65, 6418–6424. (doi:10.1158/0008-5472.CAN-05-0044) [DOI] [PubMed] [Google Scholar]
- 70.Gao J, et al. 2016. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e9. (doi:10.1016/j.cell.2016.08.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin DS, et al. 2017. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7, 188–201. (doi:10.1158/2159-8290.CD-16-1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaretsky JM, et al. 2016. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829. (doi:10.1056/NEJMoa1604958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verdegaal EME, et al. 2016. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 536, 91–95. (doi:10.1038/nature18945) [DOI] [PubMed] [Google Scholar]
- 74.Riaz N, et al. 2017. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949.e15. (doi:10.1016/j.cell.2017.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tran E, Robbins PF, Rosenberg SA. 2017. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18, 255–262. (doi:10.1038/ni.3682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. 2017. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717 (doi:10.1038/nrclinonc.2017.101) [DOI] [PubMed] [Google Scholar]
- 77.De Miranda NFCC, Goudkade D, Jordanova ES, Tops CMJ, Hes FJ, Vasen HFA, Van Wezel T, Morreau H.. 2012. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin. Cancer Res. 18, 1237–1245. (doi:10.1158/1078-0432.CCR-11-1997) [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg SA, et al. 1988. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 319, 1676–1680. (doi:10.1056/NEJM198812223192527) [DOI] [PubMed] [Google Scholar]
- 79.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. 2003. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 26, 332–342. (doi:10.1097/00002371-200307000-00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verdegaal EME, et al. 2011. Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha. Cancer Immunol. Immunother. 60, 953–963. (doi:10.1007/s00262-011-1004-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg SA, et al. 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557. (doi:10.1158/1078-0432.CCR-11-0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran E, et al. 2014. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645. (doi:10.1126/science.1251102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robbins PF, et al. 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752. (doi:10.1038/nm.3161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larkin J, et al. 2015. Comment on ‘Combined nivolumab and ipilimumab or monotherapy in untreated melanoma’. N. Engl. J. Med. 373, 23–34. (doi:10.1056/NEJMoa1504030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Postow MA, et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. (doi:10.1056/NEJMoa1414428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koyama S, et al. 2016. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (doi:10.1038/ncomms10501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houchins JP, Yabe T, McSherry C, Bach FH. 1991. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J. Exp. Med. 173, 1017–1020. (doi:10.1084/jem.173.4.1017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA.. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266. (doi:10.1038/16717) [DOI] [PubMed] [Google Scholar]
- 89.Monney L, et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536 (doi:10.1038/415536a) [DOI] [PubMed] [Google Scholar]
- 90.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171, 1393–1405. (doi:10.1084/jem.171.5.1393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weigelin B, Bolaños E, Rodriguez-Ruiz ME, Martinez-Forero I, Friedl P, Melero I. 2016. Anti-CD137 monoclonal antibodies and adoptive T cell therapy: a perfect marriage? Cancer Immunol. Immunother. 65, 493–497. (doi:10.1007/s00262-016-1818-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, HellströM KE, Mittler RS, Chen L.. 1997. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 3, 682 (doi:10.1038/nm0697-682) [DOI] [PubMed] [Google Scholar]
- 93.Jure-Kunkel M, Masters G, Girit E, Dito G, Lee F, Hunt JT, Humphrey R.. 2013. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol. Immunother. 62, 1533–1545. (doi:10.1007/s00262-013-1451-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chediak AE, Shamseddine A, Bodgi L, Obeid JP, Geara F, Zeidan YH. 2017. Optimizing tumor immune response through combination of radiation and immunotherapy. Med. Oncol. 34, 165 (doi:10.1007/s12032-017-1025-z) [DOI] [PubMed] [Google Scholar]
- 95.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. 1999. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 59, 5800–5807. [PubMed] [Google Scholar]
- 96.Melief CJM, Van Hall T, Arens R, Ossendorp F, Van Der Burg SH. 2015. Therapeutic cancer vaccines. J. Clin. Invest. 125, 3401–3412. (doi:10.1172/JCI80009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S, Nguyen MT. 2015. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 15, 51–57. (doi:10.4110/in.2015.15.2.51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503. (doi:10.1016/j.immuni.2010.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ott PA, et al. 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. (doi:10.1038/nature22991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carreno BM, et al. 2015. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348, 803–808. (doi:10.1126/science.aaa3828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vermeij R, Leffers N, Van Der Burg SH, Melief CJ, Daemen T, Nijman HW. 2011. Immunological and clinical effects of vaccines targeting p53-overexpressing malignancies. J. Biomed. Biotechnol. 2011, 702146 (doi:10.1155/2011/702146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee DW, et al. 2015. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528. (doi:10.1016/S0140-6736(14)61403-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maude SL, et al. 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517. (doi:10.1056/NEJMoa1407222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851. (doi:10.1038/mt.2010.24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Senior M. 2017. CAR-T death strikes Kite. Nat. Biotechnol. 35, 492 (doi:10.1038/nbt0617-492) [DOI] [PubMed] [Google Scholar]
- 106.Park JH, et al. 2018. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459. (doi:10.1056/NEJMoa1709919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neelapu SS, et al. 2017. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544. (doi:10.1056/NEJMoa1707447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maude SL, et al. 2018. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448. (doi:10.1056/NEJMoa1709866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang XY, Sun Y, Zhang A, Hu GL, Cao W, Wang DH, Zhang B, Chen H. 2016. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 6, e013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Till BG, et al. 2012. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950. (doi:10.1182/blood-2011-10-387969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. 2010. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 18, 413–420. (doi:10.1038/mt.2009.210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carpenito C, et al. 2009. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365. (doi:10.1073/pnas.0813101106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silva D, et al. 2016. Direct comparison of in vivo fate of second and third-generation CD19-specific chimeric antigen receptor (CAR)-T cells in patients with B cell non-Hodgkin lymphoma (B-NHL): reversal of toxicity from tonic signaling. Blood 128, 1851. [Google Scholar]
- 114.Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Dotti G.. 2015. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T-lymphocytes. Nat. Med. 21, 524–529. (doi:10.1038/nm.3833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.D'Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. 2018. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 9, 282 (doi:10.1038/s41419-018-0278-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schumacher TN, Schreiber RD. 2015. Neoantigens in cancer immunotherapy. Science 348, 69–74. (doi:10.1126/science.aaa4971) [DOI] [PubMed] [Google Scholar]
- 117.Folgiero V, et al. 2016. IDO1 involvement in mTOR pathway: a molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget 7, 52 900–52 911. (doi:10.18632/oncotarget.9284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Munn DH, Mellor AL. 2016. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 37, 193–207. (doi:10.1016/j.it.2016.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Gajewski TF, Kline J. 2009. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114, 1545–1552. (doi:10.1182/blood-2009-03-206672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fukuno K, et al. 2015. Expression of indoleamine 2,3-dioxygenase in leukemic cells indicates an unfavorable prognosis in acute myeloid leukemia patients with intermediate-risk cytogenetics. Leuk. Lymphoma 56, 1398–1405. (doi:10.3109/10428194.2014.953150) [DOI] [PubMed] [Google Scholar]
- 121.Mussai F, et al. 2013. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 122, 749–758. (doi:10.1182/blood-2013-01-480129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. 2010. The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J. Pathol. 221, 248–263. (doi:10.1002/path.2711) [DOI] [PubMed] [Google Scholar]
- 123.Younes A, et al. 2016. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 17(Suppl. 1), 1283–1294. (doi:10.1016/S1470-2045(16)30167-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fisher RI, Rosenberg SA, Fyfe G. 2000. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J. Sci. Am. 6, S55–S57. [PubMed] [Google Scholar]
- 125.Motzer RJ, et al. 2015. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813. (doi:10.1056/NEJMoa1510665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Becht E, et al. 2015. Prognostic and theranostic impact of molecular subtypes and immune classifications in renal cell cancer (RCC) and colorectal cancer (CRC). Oncoimmunology 4, e1049804 (doi:10.1080/2162402X.2015.1049804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tran E, et al. 2015. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390. (doi:10.1126/science.aad1253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verdegaal EME, Van Der Burg SH. 2017. The potential and challenges of exploiting the vast but dynamic neoepitope landscape for immunotherapy. Front. Immunol. 8, 1113 (doi:10.3389/fimmu.2017.01113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vincent J, et al. 2010. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061. (doi:10.1158/0008-5472.CAN-09-3690) [DOI] [PubMed] [Google Scholar]
- 130.Aboudaram A, et al. 2017. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 27, 485–491. (doi:10.1097/CMR.0000000000000386) [DOI] [PubMed] [Google Scholar]
- 131.Haanen JBAG. 2017. Converting cold into hot tumors by combining immunotherapies. Cell 170, 1055–1056. (doi:10.1016/j.cell.2017.08.031) [DOI] [PubMed] [Google Scholar]
- 132.Giannakis M, et al. 2016. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 15, 857–865. (doi:10.1016/j.celrep.2016.03.075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hernandez C, Huebener P, Schwabe RF. 2016. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene 35, 5931–5941. (doi:10.1038/onc.2016.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hou W, Zhang Q, Yan Z, Chen R, Zeh Iii HJ, Kang R, Lotze MT, Tang D. 2013. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 4, e966 (doi:10.1038/cddis.2013.493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fu J, et al. 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra52 (doi:10.1126/scitranslmed.aaa4306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deng L, et al. 2014. STING-dependent cytosolic DNA sensing promotes radiation-induced Type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852. (doi:10.1016/j.immuni.2014.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. 2005. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105, 2862–2868. (doi:10.1182/blood-2004-06-2410) [DOI] [PubMed] [Google Scholar]
- 138.Beyer M, et al. 2005. Reduced frequencies and suppressive function of CD4+ CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 106, 2018–2025. (doi:10.1182/blood-2005-02-0642) [DOI] [PubMed] [Google Scholar]
- 139.Reits EA, et al. 2006. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271. (doi:10.1084/jem.20052494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ.. 2012. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 5, 404–407. (doi:10.1593/tlo.12280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. 2013. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 1, 365–372. (doi:10.1158/2326-6066.CIR-13-0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weintraub K. 2016. Take two: combining immunotherapy with epigenetic drugs to tackle cancer. Nat. Med. 22, 8–10. (doi:10.1038/nm0116-8) [DOI] [PubMed] [Google Scholar]
- 143.Nie Y, Yang G, Song Y, Zhao X, So C, Liao J, Wang LD, Yang CS. 2001. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis 22, 1615–1623. (doi:10.1093/carcin/22.10.1615) [DOI] [PubMed] [Google Scholar]
- 144.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F.. 2001. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int. J. Cancer 94, 243–251. (doi:10.1002/ijc.1452) [DOI] [PubMed] [Google Scholar]
- 145.Dunn J, Rao S. 2017. Epigenetics and immunotherapy: the current state of play. Mol. Immunol. 87, 227–239. (doi:10.1016/j.molimm.2017.04.012) [DOI] [PubMed] [Google Scholar]
- 146.Peng D, et al. 2015. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253. (doi:10.1038/nature15520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roulois D, et al. 2015. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973. (doi:10.1016/j.cell.2015.07.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu BL, et al. 2003. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292 (doi:10.1038/sj.gt.3301885) [DOI] [PubMed] [Google Scholar]
- 149.Ribas A, et al. 2017. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–19.e10. (doi:10.1016/j.cell.2017.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.