Abstract
There is a growing body of evidence to support the involvement of proinflammatory cytokines in the pathophysiology of depression; however, no previous studies have examined the relationship between cytokines and the brain morphology of patients with major depressive disorder (MDD). We therefore evaluated the relationship between serum cytokine levels and cortical thinning during the first depressive episode in drug-naïve patients with MDD. We measured the serum cytokine levels (IL-1β, IL-6, IFN-γ, and TNFα), and whole-brain cortical thickness and hippocampal subfield volumes on brain magnetic resonance imaging (MRI) using surface-based morphometry in 40 patients with MDD and 47 healthy volunteers (controls). Only the serum IL-6 level was significantly higher in patients with MDD than in controls. The prefrontal cortex (PFC) thickness was significantly reduced in patients with MDD, and showed a significant inverse correlation with the serum IL-6 level. Although high serum IL-6 levels were correlated with reduced left subiculum and right CA1, CA3, CA4, GC-DG, subiculum, and whole hippocampus volumes, the presence or absence of MDD had no effect on the volume of any hippocampal subfields. Our results suggest that IL-6 may play a key role in the morphological changes in the PFC during the early stage of MDD.
Introduction
Although there have many theories proposed regarding the cause of major depressive disorder (MDD), the pathogenesis is only partly understood; genes, the environment, and endocrine dysfunction are all considered to be factors influencing MDD1. Accumulating evidence suggests a role of inflammation in the pathogenesis of MDD. For example, several studies have detected higher levels of inflammation in MDD than in healthy controls; however, the strength of the evidence varies according to the specific inflammatory markers that have been examined (i.e., interleukin [IL]-1β, IL-6, interferon γ [IFN-γ], and tumor necrosis factor α [TNFα])2–4.
In several animal models, increased levels of serum proinflammatory cytokines, such as IL-6, were shown to interfere with long-term potentiation5,6, neurogenesis7, and neural plasticity8. Moreover, transgenic mice with high IL-6 expression levels showed neuropathologic manifestations, including neurodegeneration9. This evidence suggests that cytokines may affect the brain morphology through processes related to neurodegeneration. To the best of our knowledge, only one previous study has evaluated the relationship between brain morphology and the serum cytokine levels in patients with MDD; the results showed that the increased expression of IL-6 predicted a decreased hippocampal volume10. However, the authors of that study only evaluated the hippocampal and amygdala structures using a manual tracing method. Furthermore, the study was limited by the fact that two-thirds of patients were on antidepressant medications—according to a meta-analysis11, treatment with antidepressants reduces the serum IL-6 levels in patients with MDD.
Many investigators have applied PET to examine a marker of neuroinflammation, translocator protein (TSPO) binding, in vivo, to test the neuroinflammatory hypothesis of MDD12–15. Several studies have shown that—in addition to the hippocampus—neuroinflammation was present in various brain regions of patients with MDD12–14. This evidence led us to wonder whether elevated cytokine levels might be associated with a reduced cortical volume not only of the hippocampus, but also extend into other brain regions in patients with MDD. To test this possibility, we employed a surface-based morphology (SBM) analysis, which has been proposed to identify the differences in the thickness of gray matter on the brain’s surface16, as a whole-brain voxel based-morphometry (VBM) analysis procedure. We also added a recently developed hippocampal subfield analysis using the automated hippocampal subfield segmentation method17, because it is not possible to analyze the hippocampus by a conventional SBM analysis. This technique allows for the calculation of the hippocampal subfield volumes. The purpose of the current study was to investigate the relationship between the brain morphology (brain cortical thinning and hippocampal subfield volumes) and the serum cytokine levels during the first depressive episode in drug-naïve patients with MDD using a whole-brain SBM analysis.
Materials and Methods
Participants
Human experiments were carried out in accordance with guidelines provided and approved by the Institutional Review Board of University of Occupational and Environmental Health School of Medicine, Japan (approval number: H25-13). The protocol of this prospective study was approved by the Ethics Committee of the University of Occupational and Environmental Health. All of the participants provided their written informed consent to participate in the study.
In the current study, first-episode and drug-naïve patients with MDD were recruited. A psychiatrist (A.K., with 12 years of experience in psychiatry) diagnosed patients with MDD using a fully Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text revision (DSM-IV-TR) Research Version, Non-Patient Edition (SCID-I/NP). To qualify for the study, patients with MDD must not have previously met the criteria for any DSM-IV-TR Axis I disorder during interviews performed by a psychiatrist. In short, none of the patients with MDD in this study had any past episodes of mood disorders. Moreover, patients with mild cognitive impairment were excluded mainly based on information about their activities of daily living from family members or caregivers, in addition a brief cognitive examination including a serial 7 s test and an assessment of the patient’s short-term memory was performed by an experienced psychiatrist.
The severity of depression was evaluated using the 17-item Hamilton Rating Scale for Depression (HAMD-17). Only patients with a total HAMD-17 score of ≥14 were eligible for inclusion in the study. Between March 2009 and January 2017, 50 consecutive patients were recruited. From this initial sample, the psychiatrist excluded patients who met the following criteria: (a) a history of neurological disease or the presence of either Axis I (schizophrenia, other affective disorders, etc.) or Axis II (personality disorders, mental retardation, etc.) psychiatric disorders (n = 5); (b) presence of co-morbid substance use disorders (n = 3); (c) unwillingness to provide informed consent (n = 2). Thus, a total of 40 right-handed, first-episode, drug-naïve patients with MDD were included (Table 1). Thirty of the 40 patients had participated in our previously published studies18,19, which analyzed the relationship between brain structures and the serum cortisol levels in MDD.
Table 1.
The demographic characteristics, cytokine values, and brain volumes of participants.
| Healthy subjects (n = 47) | MDD patients (n = 40) | p-value | |
|---|---|---|---|
| Age, mean, (range, SD) | 40.7 (20-65, 11.4) | 46.6 (20–67, 14.4) | 0.06 |
| Female, numbers | 13 | 20 | 0.05 |
| Obesity, % | 3 (6.4) | 2 (5.0) | 1.00 |
| HAMD-17, mean of total scores (SD) | 21.6 (5.9) | ||
| Duration of depressive episode, month (SD) | 6.3 (10.2) | ||
| ICV, mean (SD) | 1628.2 (160.6) ml | 1551.8 (145.3) ml | 0.58 |
| Inflammatory, mean; pg/ml (SD) | |||
| IL-1β | 0.053 (0.087) | 0.041 (0.048) | 0.23 |
| IL-6 | 0.370 (0.327) | 0.850 (1.808) | 0.04 |
| IFN-γ | 7.459 (13.4) | 9.636 (15.0) | 0.24 |
| TNF-α | 2.041 (5.39) | 1.592 (0.576) | 0.30 |
SD, standard deviation; MDD, Major depression disorder; HAMD, 17-item Hamilton Rating Scale for Depression; ICV, intracranial volume; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor. Obesity was defined by body mass index (>26 kg/m2).
Fifty-seven healthy volunteers (controls) were also recruited from nearby communities via an interview conducted by the same psychiatrist using the full SCID-I/NP. None of the control participants had a history of serious medical or neuropsychiatric illness or a family history of major psychiatric or neurological illness among their first-degree relatives (Table 1).
A radiologist (S.K., 21 years of experience in neuroradiology) who reviewed the conventional magnetic resonance imaging (MRI) data (including T2-weighted images) reported no gross abnormalities such as infarcts, hemorrhages, or brain tumors in any of the study participants.
Cytokine analysis
Forty human blood samples were assayed in singlicate (due to limited sample volumes) using the V-PLEX Human Proinflammatory Panel I (4-Plex), a highly sensitive multiplex enzyme-linked immunosorbent assay used to quantitatively measure cytokines, including IL-1β, IL-6, IFN-γ, and TNFα, from a single small sample volume (25 μL) using an electrochemiluminescent detection method (MesoScale Discovery, Gaithersburg, MD, USA) (Table 1). The mean intra-assay coefficients, based on the standards run in duplicate for each cytokine, were <8.5% for all cytokines. For the purposes of the statistical analysis, any value that was below the lowest limit of detection (LLOD) for the cytokine assay was replaced with half of the LLOD of the assay. This imputation method is robust and well established20.
MRI acquisition
MRI was performed using a 3T MR system (Signa EXCITE 3 T; GE Healthcare, Wankesha, WI, USA) with an 8-channel brain phased-array coil. Original T1 images were acquired by three-dimensional fast-spoiled gradient recalled acquisition with steady state. The acquisition parameters were as follows: repetition time, 10 ms; echo time, 4.1 ms; inversion time, 700 ms; flip angle, 10; field-of view, 24 cm; section thickness, 1.2 mm; and resolution, 0.9 × 0.9 × 1.2 mm. All images were corrected for image distortion due to gradient non-linearity using the Grad Warp software program21 and for intensity inhomogeneity with the “N3” function22.
Image processing
Whole-brain analyses using SBM
The regional cortical thickness was estimated using the FreeSurfer software program (version 6.0, www.freesurfer.net/fswiki/HippocampalSubfields), which has been well documented and which is freely available online. The technical details of the cortical thickness analysis have been described elsewhere23. The entire cortex of each participant was inspected visually; topological defects were corrected manually. Cortical thickness measurements were obtained by reconstructing representations of the gray matter–white matter boundary23,24 and the pial surface. The distance between these surfaces at each point across the cortical mantle was then calculated. For each participant, the regional thickness value at each vertex was mapped to the surface of an average brain template. This allowed for the visualization of data across the entire cortical surface. The data were re-sampled for all participants onto a common spherical coordinate system24. The cortical map of each participant was smoothed with a 10-mm kernel in full width at half-maximum (FWHM) for the cortical analyses.
Volumetry of the hippocampal subfields
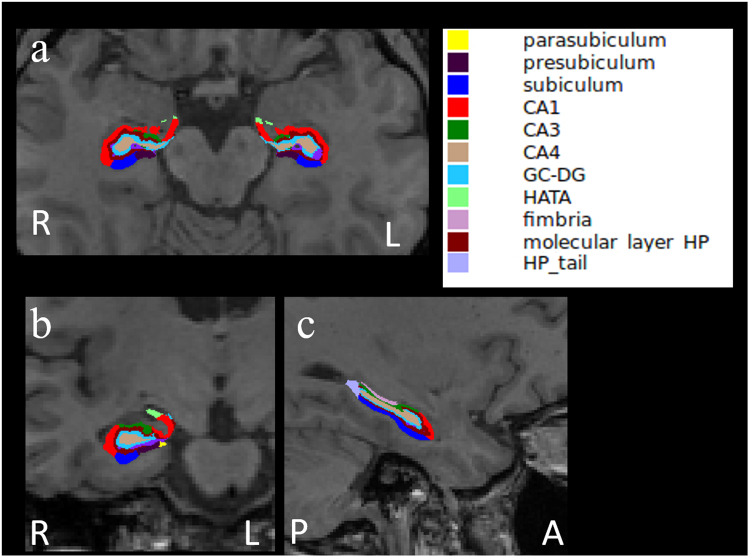
The FreeSurfer software program (version 6.0 www.freesurfer.net/fswiki/ HippocampalSubfields)25 was used to calculate hippocampal subfield volumes. The hippocampal subfields were segmented using a Bayesian inference approach and a novel atlas algorithm of the hippocampal formations built primarily upon ultra-high resolution ex vivo MRI data from autopsy brains26. This presented atlas was more sensitive than the previous version (FreeSurfer 5.3) that used as ab in vivo atlas for segmenting the hippocampal subfields as it allowed for greater accuracy in the delineation of the boundaries within the subfields26. The calculated sub-regions included Cornu Ammonis (CA)1, CA3, CA4, the granule cell (GC) layer of the dentate gyrus (DG) (GC-DG), fimbria, subiculum, presubiculum, parasubiculum, molecular layer, hippocampus-amygdala-transition-area, hippocampal tail, and whole hippocampus (Fig. 1). We calculated the total hippocampal volume in each hemisphere as the sum of the volumes of all subfields except the hippocampal fissure. The technical details of these procedures have been described elsewhere26.
Figure 1.
The representative subdivision of the hippocampal subfields. The mask of each region overlapped on the axial (a), coronal (b), and sagittal (c) T1-weighted images. R, right; L, left; A, anterior; P, posterior. Color classification: parasubiculum, yellow; presubiculum, dark purple; subiculum, blue; CA1, red; CA3, dark green; CA4, brown; granule cell layer of dentate gyrus (GC-DG), sky blue; hippocampus-amygdala-transition-area (HATA), green; fimbria, purple; molecular layer hippocampus (HP), dark brown; hippocampal tail, gray.
Statistical analyses
As age and the serum cytokine levels exhibited Gaussian distributions, we applied independent sample t-tests to assess the differences between healthy participants (“controls”) and patients with MDD (“patients”). The chi-squared test was used for gender comparisons.
To investigate differences in the cortical thickness of patients and controls and to assess the relationship between cortical thickness and serum cytokine levels, we performed SBM using the FreeSurfer QDEC statistical tool after 10-mm FWHM kernel smoothing. A general linear model was then applied at each vertex. The following comparisons were performed in a whole-brain vertex-by-vertex analysis: (a) comparison between controls and patients; (b) correlation between cortical thickness of patients and serum cytokine levels; and (c) correlation between cortical thickness of controls and serum cytokine levels. We set the diagnosis as “discrete” and serum cytokine levels as “continuous.” In addition, age and gender were set as “nuisance factors” to control for confounding variables. It was plausible that the controls and patients included in this study would show different cortical evolution rates; thus, different offsets, different slopes (known as “DODS”) was employed. To correct for multiple comparisons, we used a Monte Carlo simulation for the cluster analysis. The cluster-forming threshold was set at p < 0.05. Clusters were then tested against an empirical null distribution of maximum cluster size built using synthesized Z-distributed data across 10,000 permutations, producing cluster-wise p-values that were fully corrected for multiple comparisons.
We performed a multiple linear regression analysis to evaluate the relationship between the effects of the diagnosis (MDD vs. control) and the hippocampal subfield volumes. A multiple linear regression model was also used to evaluate whether serum proinflammatory cytokines were related to the hippocampal subfield volumes. Age, gender, and intracranial volume were entered as covariates in both analyses.
P values of <0.05 were considered to indicate statistical significance. The statistical analyses were performed using the R software program (version 3.4.0, R Statistical and Computing Software; http://www.r-project.org/).
Results
Baseline demographic data
Table 1 shows participants’ baseline demographic data. There were no significant differences in age, gender, or the presence of obesity between controls and patients. The serum IL-6 levels of the patients were significantly higher in comparison to controls.
None of the serum cytokine levels were associated with the total HAMD-17 score or the duration of the depressive episode in the MDD patients (by Spearman’s rank correlation). The serum cortisol levels were measured in 32 of the 40 MDD patients. Although a previous study showed associations between cytokine levels and the serum cortisol level27, we found no significant correlation between the serum cortisol level and the levels of various cytokines, including IL-6 (IL-6; p = 0.78 by Spearman rank correlation).
Whole-brain analyses using SBM
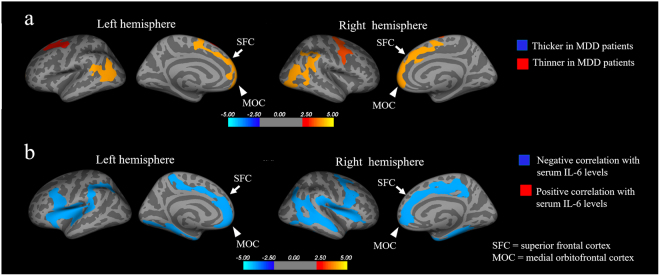
The coordinates of the significantly thinner cortical regions in patients are presented in Table 2. In group comparisons using a whole-brain vertex-by-vertex analysis adjusted for age and gender, the thickness of the bilateral superior frontal and medial orbitofrontal cortices of patients were significantly lower (p < 0.05, Monte Carlo simulation) (Fig. 2a; orange or red clusters). There were no regions in which the cortical thickness of patients was significantly greater in comparison to controls.
Table 2.
Whole brain analyses using surface-based morphometry.
| Cortical regions | Max | VtxMax | Size (mm2) | TalXb | TalYb | TalZb | CWP |
|---|---|---|---|---|---|---|---|
| Thinner in MDD patientsa | |||||||
| Left hemisphere | |||||||
| inferior parietal and lateral occipital cortices | 4.051 | 27613 | 2026.93 | −40.1 | −62.0 | 6.6 | 0.0004 |
| superior frontal, medial orbitofrontal cortices | 2.640 | 152852 | 1948.08 | −8.2 | 2.2 | 50.3 | 0.0013 |
| caudal middle frontal cortices | 3.084 | 105971 | 1511.15 | −27.5 | 5.8 | 46.4 | 0.0095 |
| Right hemisphere | |||||||
| superior frontal and medial orbitofrontal cortices | 3.002 | 92871 | 1978.74 | 8.0 | 19.7 | 41.2 | 0.0012 |
| precentral cortex | 3.376 | 140561 | 1929.42 | 50.4 | 1.2 | 36.7 | 0.0013 |
| inferior parietal and supra-marginal cortices | 3.814 | 33905 | 1868.01 | 52.9 | −45.0 | 27.2 | 0.0015 |
| lateral occipital cortex | 4.195 | 158509 | 1508.89 | 47.1 | −68.8 | 12.4 | 0.0089 |
| Correlation with serum IL-6 level in MDD patientsa | |||||||
| Left hemisphere | |||||||
| triangularis | −4.309 | 16892 | 8536.62 | −29.5 | 22.1 | 9.3 | 0.0001 |
| superior frontal, medial orbitofrontal, and paracentral cortices | −3.701 | 64159 | 3064.38 | −13.5 | 44.6 | 1.0 | 0.0001 |
| parahippocampal cortex | −4.928 | 70540 | 2386.87 | −28.1 | −30.6 | −14.5 | 0.0001 |
| rostral middle frontal cortex | −2.691 | 149979 | 1498.34 | −22.1 | 38.7 | 19.7 | 0.0168 |
| middle temporal cortex | −4.576 | 24712 | 1421.53 | −47.2 | −33.3 | −4.9 | 0.0218 |
| Right hemisphere | |||||||
| caudal middle frontal cortex, insula, and opercularis | −4.132 | 23947 | 5844.73 | 31.1 | 17.1 | 9.8 | 0.0001 |
| superior and middle temporal cortices | −4.777 | 163449 | 5260.70 | 47.2 | −21.7 | −10.2 | 0.0001 |
| superior frontal, medial orbitofrontal, and cingulate cortices | −4.888 | 12712 | 3690.45 | 13.6 | −40.6 | 32.6 | 0.0001 |
| parahippocampal cortex | −4.470 | 9976 | 2465.30 | 32.1 | −29.5 | −13.9 | 0.0001 |
MDD, major depressive disorder; CWP = opercularis clusterwise p value.
aSignificantly thinner cortical regions and IL-6-associated regions in MDD patients were detected using FreeSurfer v 6.0. p < 0.05 (Monte-Carlo simulation).
bBased on Talairach and Toumoux system.
Figure 2.
The whole-brain analysis using surface-based morphology (SBM). (a) Comparison of the cortical thickness in MDD patients vs. healthy controls. Orange or red clusters represent the significantly thinner cortical regions in patients with MDD (p < 0.05 vs. controls, Monte Carlo simulation). The thickness of the bilateral superior frontal (arrows) and medial orbitofrontal (arrow heads) cortices of patients with MDD was significantly lower in comparison to controls (p < 0.05, Monte Carlo simulation). (b) The relationship between the cortical thickness and the serum IL-6 levels in patients with MDD. Blue clusters represent regions in which a significant negative correlation was between the cortical thickness and serum IL-6 levels in patients with MDD (p < 0.05, Monte Carlo simulation). The thickness of the bilateral superior frontal (arrows) and medial orbitofrontal (arrow heads) cortices exhibited a significant negative correlation with the serum IL-6 level (p < 0.05, Monte Carlo simulation).
The coordinates of the cortical regions, which showed a significant correlation with the serum cytokine levels, are presented in Table 2. A whole-brain vertex-by-vertex correlation analysis of the patients showed that the thickness of the bilateral superior frontal and medial orbitofrontal cortices was significantly negatively correlated with the serum IL-6 level (p < 0.05, Monte Carlo simulation) (Fig. 2b; blue clusters). There were no regions manifesting a significant positive correlation in patients. Furthermore, no brain regions showed significant correlations with the levels of other cytokines (IL-1β, IFN-γ, and TNFα). Finally, we found no regions in which the cortical thickness was significantly correlated with the serum cytokine levels in controls.
There were no brain regions in which the cortical thickness was associated with the total HAMD-17 score or the duration of the depressive episode.
Volumetry of the hippocampal subfields
In multiple linear regression models adjusted for age, gender, and intracranial volume, high serum IL-6 levels were significantly negatively correlated with reduced left subiculum and right CA1, CA 3, CA 4, GC-DG, subiculum, and whole hippocampus volumes (Table 3). However, we found no effects of the diagnosis (patient vs. control) on the volume of any hippocampal subfields.
Table 3.
The relationships among hippocampal subfield volumes and serum proinflammatory cytokines.
| Hippocampal subfields | IL-1β | IL-6 | IFN-γ | TNF-α | Effects of diagnosis (MDD vs. control) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Left | |||||||||||||||
| CA1 | −0.108 | 0.164 | 0.515 | −0.32 | 0.167 | 0.064 | 0.097 | 0.164 | 0.56 | 0.119 | 0.183 | 0.518 | <0.001 | 0.115 | 0.998 |
| CA3 | −0.128 | 0.167 | 0.45 | −0.244 | 0.172 | 0.164 | 0.088 | 0.166 | 0.598 | −0.08 | 0.185 | 0.67 | −0.013 | 0.116 | 0.911 |
| CA4 | −0.071 | 0.161 | 0.66 | −0.262 | 0.164 | 0.119 | 0.082 | 0.159 | 0.609 | 0.04 | 0.178 | 0.823 | −0.009 | 0.115 | 0.941 |
| GC-DG | −0.079 | 0.156 | 0.618 | −0.268 | 0.159 | 0.1 | 0.086 | 0.155 | 0.581 | 0.064 | 0.173 | 0.713 | −0.071 | 0.113 | 0.535 |
| fimbria | 0.087 | 0.145 | 0.556 | 0.169 | 0.149 | 0.262 | 0.221 | 0.137 | 0.116 | 0.139 | 0.157 | 0.381 | −0.163 | 0.105 | 0.125 |
| subiculum | 0.171 | 0.159 | 0.291 | −0.339 | 0.161 | 0.042* | 0.11 | 0.159 | 0.494 | 0.189 | 0.176 | 0.292 | −0.038 | 0.112 | 0.734 |
| presubiculum | 0.066 | 0.162 | 0.687 | −0.106 | 0.167 | 0.529 | −0.191 | 0.155 | 0.225 | 0.167 | 0.175 | 0.346 | −0.178 | 0.113 | 0.118 |
| parasubiculum | 0.154 | 0.171 | 0.376 | −0.038 | 0.181 | 0.833 | −0.294 | 0.163 | 0.08 | 0.052 | 0.19 | 0.788 | −0.116 | 0.115 | 0.319 |
| molecular layer | <0.001 | 0.156 | 0.998 | −0.253 | 0.16 | 0.122 | 0.015 | 0.156 | 0.923 | 0.102 | 0.173 | 0.562 | −0.054 | 0.113 | 0.635 |
| HATA | −0.062 | 0.167 | 0.714 | 0.033 | 0.174 | 0.852 | −0.011 | 0.163 | 0.947 | −0.212 | 0.179 | 0.244 | −0.2 | 0.112 | 0.079 |
| hippocampal tail | 0.011 | 0.157 | 0.947 | −0.186 | 0.161 | 0.254 | −0.041 | 0.154 | 0.789 | 0.109 | 0.171 | 0.528 | 0.029 | 0.11 | 0.794 |
| whole hippocampus | 0.006 | 0.153 | 0.97 | −0.288 | 0.155 | 0.071 | 0.03 | 0.152 | 0.843 | 0.136 | 0.169 | 0.426 | −0.059 | 0.113 | 0.599 |
| Right | |||||||||||||||
| CA1 | −0.026 | 0.162 | 0.871 | −0.347 | 0.162 | 0.039* | 0.098 | 0.161 | 0.549 | 0.054 | 0.181 | 0.765 | 0.058 | 0.114 | 0.612 |
| CA3 | −0.081 | 0.159 | 0.613 | −0.329 | 0.158 | 0.045* | −0.032 | 0.157 | 0.841 | −0.127 | 0.174 | 0.471 | 0.139 | 0.113 | 0.223 |
| CA4 | 0.057 | 0.156 | 0.717 | −0.323 | 0.156 | 0.046* | 0.006 | 0.155 | 0.968 | −0.046 | 0.173 | 0.793 | 0.042 | 0.114 | 0.712 |
| GC-DG | 0.051 | 0.154 | 0.744 | −0.355 | 0.152 | 0.025* | 0.012 | 0.153 | 0.94 | −0.036 | 0.171 | 0.832 | −0.008 | 0.113 | 0.943 |
| fimbria | −0.031 | 0.155 | 0.842 | 0.149 | 0.158 | 0.35 | 0.073 | 0.15 | 0.627 | −0.134 | 0.166 | 0.425 | −0.114 | 0.103 | 0.272 |
| subiculum | 0.11 | 0.171 | 0.525 | −0.261 | 0.175 | 0.144 | −0.056 | 0.169 | 0.741 | 0.152 | 0.187 | 0.422 | −0.048 | 0.115 | 0.674 |
| presubiculum | 0.106 | 0.167 | 0.53 | −0.034 | 0.175 | 0.849 | −0.289 | 0.157 | 0.075 | 0.074 | 0.184 | 0.69 | −0.174 | 0.113 | 0.129 |
| parasubiculum | 0.073 | 0.166 | 0.664 | 0.073 | 0.175 | 0.677 | 0.072 | 0.164 | 0.663 | −0.072 | 0.184 | 0.699 | −0.025 | 0.115 | 0.826 |
| molecular layer | 0.022 | 0.161 | 0.891 | −0.297 | 0.162 | 0.075 | −0.012 | 0.159 | 0.942 | 0.024 | 0.178 | 0.893 | −0.019 | 0.113 | 0.87 |
| HATA | −0.006 | 0.156 | 0.968 | −0.092 | 0.166 | 0.583 | 0.178 | 0.153 | 0.253 | −0.116 | 0.174 | 0.509 | −0.072 | 0.114 | 0.526 |
| hippocampal tail | 0.097 | 0.154 | 0.534 | −0.332 | 0.152 | 0.036* | −0.031 | 0.152 | 0.84 | 0.142 | 0.169 | 0.407 | −0.12 | 0.104 | 0.252 |
| whole hippocampus | 0.051 | 0.156 | 0.746 | −0.326 | 0.155 | 0.043* | −0.017 | 0.155 | 0.912 | 0.045 | 0.173 | 0.797 | −0.042 | 0.111 | 0.705 |
B (SE) 5 b value (standard error of the mean). MDD = Major depression disorders, IL = Interleukin, IFN = interferon, TNF = Tumor necrosis factor. CA = cornu ammonis, GC-DG = granule cell layer of dentate gyrus, HATA = hippocampus-amygdala-transition-area, hippocampal tail, and whole hippocampus. Age, sex, and intracranial volume are entered as covariates in analyses. *Significant correlation between the hippocampal subfield volumes and serum cytokine levels in MDD patients are detected (p < 0.05).
Discussion
A strength of this study lies in recruitment of first depressive episode and drug-naïve patients with MDD. To our knowledge, this is the first study to investigate which brain region is related to serum cytokines levels by using the whole-brain SBM analysis. We found that the cortical thicknesses in most regions of the superior frontal and medial orbitofrontal cortices were significantly negatively correlated with the serum IL-6 level. Moreover, the thickness of the superior frontal and medial orbitofrontal cortices in MDD patients was significantly decreased in comparison to healthy controls. Thus, our results suggest that the neuroinflammatory status in the early stage of MDD is associated with changes in the gray matter.
The serum IL-6 level was significantly higher in patients with MDD than in controls; this was the only cytokine among the cytokines that we tested that differed between patients and controls. A previous meta-analysis demonstrated that the concentrations of the serum TNF-α and IL-6 levels in patients with MDD were significantly higher in comparison controls2. A more recent meta-analysis also demonstrated that the cerebrospinal fluid levels of IL-6 in patients with MDD were higher than those in controls28. Finally, the third meta-analysis demonstrated that treatment with antidepressants reduced the serum IL-6 levels in patients with MDD11. Thus, the presence of increased circulating concentrations of IL-6 in MDD is well established and was confirmed in the current study.
IL-6 is a multifunctional cytokine that regulates the growth and differentiation of various tissues, and which plays an important role in the immune response and acute-phase reactions29. IL-6 has been proposed to be involved in the pathology of MDD30. In particular, it has been suggested that IL-6 is involved in multiple physiological systems, including the hypothalamic-pituitary-adrenal axis, corticotrophin-releasing hormone activity at limbic sites, noradrenaline utilization, the induction of oxidative stress, apoptotic pathways, and kinase signaling31–34, all of which have very close relationships with the pathophysiology of MDD. Moreover, a previous study demonstrated that IL-6 directly controlled the serotonin transporter (SERT) level and consequently serotonin reuptake35. The SERT activity shapes serotonergic neurotransmission, which is implicated in the behavioral features and pathophysiology of MDD36. More recently, Igata et al. reported that the serotonin transporter genotype was associated with the volume of the gray matter in MDD patients37. Thus, further studies are required to explain the physiological systems in which IL-6 might be related to gray matter changes.
Most superior frontal and medial orbitofrontal cortices, which were determined in this study, are occupied by the PFC. The PFC has been implicated in the mediation of emotional and autonomic responses to socially significant or provocative stimuli, and specific PFC abnormalities have been implicated in the pathophysiology of mood disorders, including MDD38,39. Many previous neuroimaging studies have suggested that PFC abnormalities are important in the pathophysiology of MDD. Salvadore et al. showed a reduction in the volume of the PFC by a VBM analysis40, and Koolschijn et al.41 and Bora et al.42 conducted meta-analyses of VBM studies and demonstrated a reduced PFC volume in patients with MDD. A number of positron emission tomography studies have repeatedly identified a decrease in the PFC metabolic activity in patients with MDD43–45. Our findings are consistent with these previous studies, and also indicate that alterations of the PFC are already present in the early stage of MDD.
Setiawan et al. detected inflammation in the brains of patients with MDD, as indicated by increased TSPO VT, and this inflammation was prominent in the PFC, anterior cingulate cortex, and insula14. These results indicate that the PFC seems to be more sensitive and vulnerable to increased inflammatory conditions in comparison to other brain regions. This hypothesis may also be supported by previous studies which showed that IL-6 receptors were concentrated in the PFC46–48.
To our knowledge, this is the first study to investigate the influence of proinflammatory cytokines on the hippocampal subfield volumes in MDD patients. We found that high serum IL-6 levels were correlated with reduced volumes of the CA1-3, subiculum, and DG. A hippocampal subfield volume analysis using 4.7 T MRI showed that MDD patients had a lower CA1-3 and DG volumes in comparison to healthy controls49. Lim et al. who also used the same method as our study, reported that patients with late-life depression show reduced CA 2–3 volumes in comparison to healthy controls50. Several animal studies have reported a massive neuronal loss of CA3 pyramidal cells, dendritic retraction in CA1-3 and the DG, and suppressed DG neurogenesis and glial loss in the hippocampi of severely stressed animals51–53. These studies may support our results because acute or chronic stress led to increased proinflammatory cytokine production54. However, in the present study, there were no significant differences in any of the hippocampal subfield volumes between patients and controls. This finding may suggest that the hippocampal subfield volumes may be maintained in the early stage of first-episode MDD, and the volume reduction may occur after the reduction of the PFC volumes. Another explanation for our negative result may be that the pathological changes in the volumes of the hippocampal subfields may be too subtle to detect by 3 T MRI.
Our study was associated with some limitations. First, the study population was relatively small and all of the patients recruited were recruited from a single institution; thus, there is a risk of sampling bias. However, it was difficult to recruit and retain drug-naïve patients with MDD during their first episode because many patients received antidepressants before they underwent MRI. Second, we did not evaluate environmental stress in the present study. Environmental stress is a major trigger of proinflammatory cytokine production51–54 and affects the brain morphology55,56. Further studies on the relationship between environmental stress, the symptoms and behaviors of patients with MDD, and investigations of gray matter changes are needed. Furthermore, we did not perform cognitive tests such as the Mini-Mental State Examination (MMSE). Thus, we cannot rule out the possibility that cognitive functions affected the results. Moreover, the white matter abnormalities constitute one element of the pathogenesis of MDD. Thus, using diffusion tensor imaging (DTI), investigations of the relationship between the integrity of pathways within relevant neural networks and the serum cytokine levels are currently underway in our laboratory. Third, we focused on a cross-sectional association based on one-time assessments of inflammatory marker levels, although these measurements cannot reliably distinguish between chronic and acute inflammation. The few previous studies that have assessed chronic inflammation have revealed stronger associations with mental health when inflammation is determined using repeated measurements rather than a single measurement57. Fourth, we only evaluated the first depressive episode in drug-naïve patients with MDD. Thus, longitudinal work with a larger sample of patients with MDD under various conditions will be required to determine causal links between the serum IL-6 level and alterations in brain morphology. Finally, the difference in the age of the patients and controls was almost statistically significant (p = 0.06), which might have affected the results. Age-related chronic inflammation and dysregulated immune activation are considered to be mechanisms of immunosenescence. Actually, a previous study of a geriatric population showed that serum IL-6 elevation was associated with aging58.
In conclusion, we found that the serum IL-6 levels of patients with MDD were significantly higher in comparison to controls. Importantly, the PFC thickness of patients with MDD was significantly reduced, and showed a significant inverse correlation with the serum IL-6 level. Since the PFC contains a high concentration of IL-6 receptors, IL-6 receptor-mediated neurotoxicity might occur under the high serum IL-6 levels that are present in the early stage of MDD. Our results suggest that IL-6 may play a key role in the changes in brain morphology that occur in the early stage of MDD.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP16H06280, Grant-in-Aid for Scientific Research on Innovative Areas – Platforms for Advanced Technologies and Research Resources “Advanced Bioimaging Support”.
Author Contributions
S.K. and R.Y. conceived and designed the experiments. S.K., K.W., A.K., N.I., and R.I. performed the experiments. S.K., K.W., K.S., and I.U. analyzed the data. S.K. composed the manuscript. O.A., R.Y., and Y.K. provided expertise and edited the manuscript. All authors read the manuscript and are solely and jointly responsible for its content.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Northoff GG. Brains, and environment-genetic neuroimaging of depression. Current opinion in neurobiology. 2013;23:133–142. doi: 10.1016/j.conb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biological psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain, behavior, and immunity. 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger F, Madamba S, Campbell I, Siggins G. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neuroscience letters. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- 6.Tancredi V, et al. The inhibitory effects of interleukin‐6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen‐activated protein kinase ERK. Journal of neurochemistry. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 7.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 8.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell IL, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proceedings of the National Academy of Sciences. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frodl T, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Translational psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Sagar AP, Kéri S. Translocator protein (18 kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;83:1–7. doi: 10.1016/j.pnpbp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Setiawan E, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. The Lancet Psychiatry. 2018;5:339–347. doi: 10.1016/S2215-0366(18)30048-8. [DOI] [PubMed] [Google Scholar]
- 14.Setiawan E, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannestad J, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C] PBR28 PET study. Brain, behavior, and immunity. 2013;33:131–138. doi: 10.1016/j.bbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malykhin N, Lebel RM, Coupland N, Wilman AH, Carter R. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage. 2010;49:1224–1230. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, et al. Relationship between the Cortical Thickness and Serum Cortisol Levels in Drug-Naive, First-Episode Patients with Major Depressive Disorder: A Surface-Based Morphometric Study. Depress Anxiety. 2015;32:702–708. doi: 10.1002/da.22401. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, et al. Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. The British Journal of Psychiatry. 2016;208:585–590. doi: 10.1192/bjp.bp.114.155689. [DOI] [PubMed] [Google Scholar]
- 20.Vexler, A., Tao, G. & Chen, X. A Toolkit for Clinical Statisticians to Fix Problems Based on Biomarker Measurements Subject to Instrumental Limitations: From Repeated Measurement Techniques to a Hybrid Pooled–Unpooled Design. Advanced Protocols in Oxidative Stress III, 439–460 (2015). [DOI] [PubMed]
- 21.Jovicich J, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao B, et al. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Scientific reports. 2018;8:5434. doi: 10.1038/s41598-018-23685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias JE, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, A.K. & Miller, B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophrenia Bulletin (2017). [DOI] [PMC free article] [PubMed]
- 29.Kopf M, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. Journal of psychiatric research. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Mastorakos G, Weber JS, Magiakou M, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. The Journal of Clinical Endocrinology & Metabolism. 1994;79:934–939. doi: 10.1210/jcem.79.4.7962300. [DOI] [PubMed] [Google Scholar]
- 32.Chrousos GP. The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. New England Journal of Medicine. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 33.Dentino AN, et al. Association of Interleukin‐6 and Other Biologic Variables with Depression in Older People Living in the Community. Journal of the American Geriatrics Society. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 34.Hayley S, Poulter M, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor-and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Kong E, et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Scientific reports. 2015;5:9009–9009. doi: 10.1038/srep09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 37.Igata N, et al. Voxel-based morphometric brain comparison between healthy subjects and major depressive disorder patients in Japanese with the s/s genotype of 5-HTTLPR. Scientific Reports. 2017;7:3931. doi: 10.1038/s41598-017-04347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J Psychiatr Res. 1997;31:393–432. doi: 10.1016/S0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 39.Soares JC, Mann JJ. The anatomy of mood disorders–review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/S0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 40.Salvadore G, et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011;54:2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koolschijn P, et al. Brain volume abnormalities in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Baxter LR, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of general psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 44.Biver F, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biological psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy SH, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 46.Vitkovic L, et al. Cytokine signals propagate through the brain. Molecular psychiatry. 2000;5:604. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 47.Schöbitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin I, interleukin 6 and tumor necrosis factor in the brain. Progress in neurobiology. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 48.Gadient R, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain research. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biological psychiatry. 2013;74:62–68. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Lim HK, et al. Automated hippocampal subfields segmentation in late life depression. Journal of affective disorders. 2012;143:253–256. doi: 10.1016/j.jad.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? European archives of psychiatry and clinical neuroscience. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Annals of the New York Academy of Sciences. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 53.Leuner B, Gould E. Structural plasticity and hippocampal function. Annual review of psychology. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 57.Kivimäki M, et al. Long-term inflammation increases risk of common mental disorder: a cohort study. Molecular psychiatry. 2014;19:149. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirk, G. D. et al. Differential Relationships among Circulating Inflammatory and Immune Activation Biomediators and Impact of Aging and Human Immunodeficiency Virus Infection in a Cohort of Injection Drug Users. Frontiers in immunology8 (2017). [DOI] [PMC free article] [PubMed]