Abstract
The freshwater mussel genus Oxynaia Haas, 1911 is thought to be comprised of two geographically disjunct and morphologically variable species groups but the monophyly of this taxon has yet to be tested in any modern cladistic sense. This generic hypothesis has important systematic and biogeographic implications as Oxynaia is the type genus of the currently recognized tribe Oxynaiini (Parreysiinae) and is one of the few genera thought to cross several biogeographically important barriers in Southeast Asia. Morphological and molecular data clearly demonstrate that Oxynaia is not monophyletic, and the type species and its allies (O. jourdyi group) belong to the Unioninae, and more specifically as members of the genus Nodularia Conrad, 1853. Therefore, neither Oxynaia syn. nov. nor Oxynaiini Starobogatov, 1970 are applicable to the Parreysiinae and in the absence of an available name, Indochinella gen. nov. and Indochinellini trib. nov. are described. Several combinations are proposed as follows: Indochinella pugio (Benson, 1862) gen. et comb. nov., Nodularia jourdyi (Morlet, 1886) comb. res., N. gladiator (Ancey, 1881) comb. res., N. diespiter (Mabille, 1887) comb. res. and N. micheloti (Morlet, 1886) comb. res. Finally, we provide an updated freshwater biogeographic division of Southeast Asia.
Introduction
Integrative taxonomic studies are of substantial practical importance to conservation stakeholders as accurate information on the systematics and distributions of biodiversity forms the foundation of taxon- and habitat-based conservation efforts. The application of basic systematic and phylogenetic research has played a critical role in the conservation of freshwater mussels (Bivalvia: Unionidae), which are among the most threatened groups of animals worldwide1. However, the vast majority of recent systematic research has focused on the North American and European fauna, while the comparatively diverse tropical lineages have received disproportionately less attention2. Although, several recent systematic efforts focused on Asian lineages have dramatically improved our understanding of the classification, morphological evolution, and biogeography of many tropical freshwater mussel clades3–9, many biographically interesting and systematically important taxa remain poorly understood from a phylogenetic perspective. This is particularly true of the genus Oxynaia, which has an unusual disjunct geographic distribution in Myanmar and northern Vietnam and is the type genus of the tribe Oxynaiini Starobogatov, 1970.
Whelan et al.10 recently transferred the tribe Oxynaiini from the subfamily Ambleminae to the Parreysiinae on the basis of recovering Oxynaia pugio (Benson, 1862) among the latter subfamily in a molecular phylogeny. Whereas, traditional morphological classifications consistently consider Oxynaia a member of the subfamily Unioninae (in its various usages11–14). However, these seemingly irreconcilable subfamily-level classifications of Oxynaia (Unioninae vs Parreysiinae) have relied primarily on only one of the two geographically disjunct species groups, suggesting that Oxynaia may not be monophyletic.
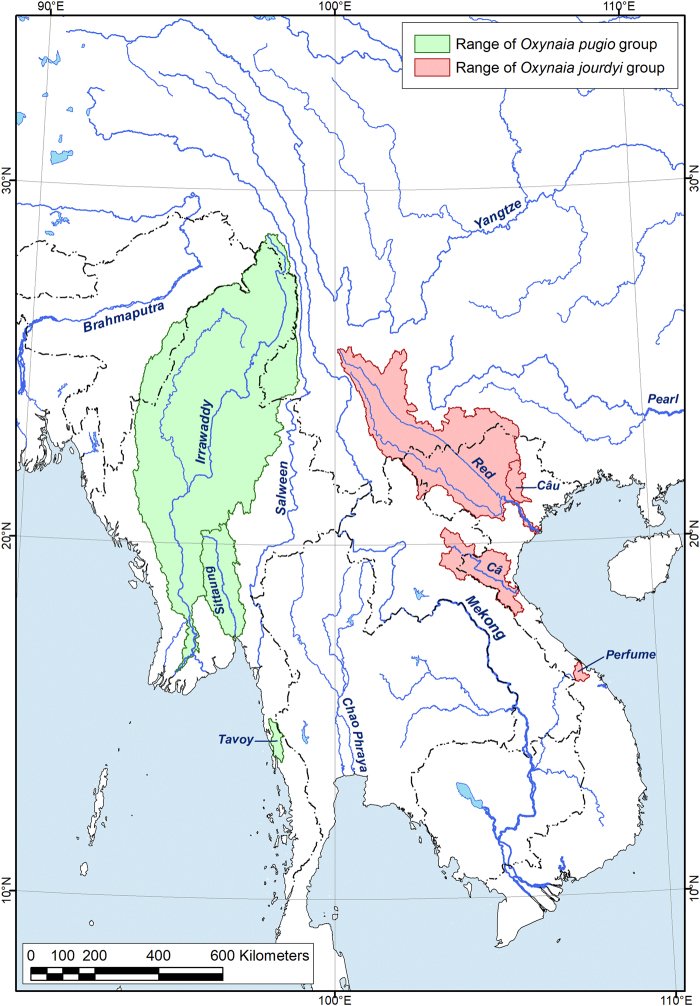
Species in the genus Oxynaia each share the presence of a strongly pointed posterior end (Greek: oxy – ‘sharp’), but can be divided into two distinct geographic species groups, the O. jourdyi group and the O. pugio group15 (Fig. 1). The Oxynaia jourdyi group includes four putative species from northern Vietnam: O. jourdyi (Morlet, 1886) (generic type), O. gladiator (Ancey, 1881), O. diespiter (Mabille, 1887), and O. micheloti (Morlet, 1886). The Oxynaia pugio group contains a single described species (O. pugio) from Myanmar7,10,14.
Figure 1.
Map of distribution ranges of the two Oxynaia species groups in Southeast Asia (see Taxonomic Account for details). The map was created using ESRI ArcGIS 10 software (www.esri.com/arcgis); the topographic base of the map was created with Natural Earth Free Vector and Raster Map Data (www.naturalearthdata.com). (Map: Mikhail Yu. Gofarov).
Our objective herein is to test the monophyly of the genus Oxynaia, evaluate the morphological traits of the resultant suprageneric clades containing Oxynaia species, and to make the appropriate taxonomic changes to more accurately reflect our hypotheses of evolutionary history.
Results
Phylogenetic analyses
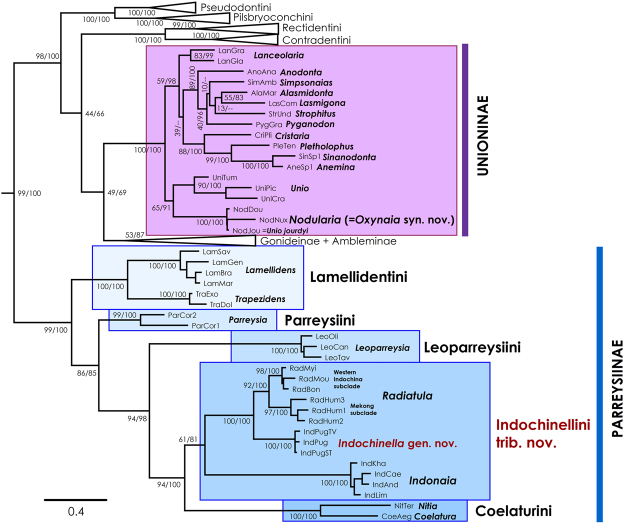
Our family-level phylogenetic analyses based on mitochondrial and nuclear markers (five partitions: three codons of COI + 16S rRNA + 28S rRNA) reveals that Oxynaia is not monophyletic (Fig. 2). Oxynaia jourdyi is well supported as a member of the subfamily Unioninae, whereas O. pugio is recovered in the distantly related subfamily Parreysiinae. Oxynaia jourdyi is resolved in a shallow and strongly supported clade comprised of representatives of the genus Nodularia Conrad, 1853 (BS/BPP = 100). The Oxynaia pugio is recovered in a well-supported clade with the genus Radiatula Simpson, 1900. The genus Indonaia Prashad, 1918 appears to be the closest relative of the Oxynaia pugio + Radiatula clade, but with only moderate support values (BS = 61; BPP = 81).
Figure 2.
Consensus phylogenetic tree of the Unionidae recovered from ML analysis and obtained for the complete data set of mitochondrial and nuclear sequences (five partitions: three codons of COI + 16S rRNA + 28S rRNA). Black numbers near nodes are bootstrap support values/Bayesian posterior probabilities. The names of taxa under revision are in red. The Rectidentinae, Pseudodontinae, Gonideinae and Ambleminae clades are collapsed. Outgroup taxa are not shown.
Morphological analyses
Comparisons of the soft anatomy and larval morphology of representatives of the major freshwater mussel clades in the Oriental Region clearly demonstrate the polyphyly of Oxynaia s. lato (Table 1). The Oxynaia jourdyi group is unambiguously placed in the subfamily Unioninae by the combination of ectobranchous brooding condition and hooked larvae with basal spines, known synapomorphies of the Unioninae3,5,16. The Oxynaia pugio group (=Indochinella gen. nov.) is united with all other members of the subfamily Parreysiinae on the basis of possessing a tetragenous brooding condition (sans Lamellidentini Modell, 1942 = ectobranchous), unhooked glochidia, as well as having the ascending lamella of the inner demibranchs attached to the visceral mass across their entire length (vs. attached only anteriorly in the Unioninae). This combination of traits is a novel method of recognizing the Parreysiinae (sans Lamellidentini). Several shell characters also unite the Oxynaia jourdyi group with representatives of the Unioninae, especially Nodularia, including the position, elevation, and sculpturing of the umbo, as well as several features of the dentition (Table 2 and Fig. 3). Nodularia differs from Indochinella gen. nov. by having a very pronounced and elevated umbo (vs. not pronounced and not elevated), umbo location in the first half of the shell (vs. in the first third), nodulose wrinkles umbo sculpture (vs. v-shaped), and a rectangular and sharp anterior pseudocardinal tooth and a thick and pyramidal posterior pseudocardinal tooth in the left valve (vs. two separated ribbed teeth placed in parallel line with one another).
Table 1.
List of conchological and anatomical characters in Parreysiinae and other selected subfamilies of the Unionidae.
| Taxon | Voucher no. | Inner demibranch ascending lamella fusion to visceral mass | Brooding | Glochidia | Higher classification |
|---|---|---|---|---|---|
| Lamellidens marginalis (Lamarck, 1819) | CAS180833 | Complete | Ectobranchous | Unhooked | Parreysiinae: Lamellidentini |
| Parreysia corrugata (Müller, 1774) | N/A35,36 | Complete | Tetragenous | Unhooked | Parreysiinae: Parreysiini |
| Leoparreysia burmana (Blanford, 1869) | CAS180831 | Complete | Tetragenous | Unhooked | Parreysiinae: Leoparreysiini |
| Coelatura sp. | UF 510905 | Complete | Tetragenous | Unhooked | Parreysiinae: Coelaturini |
| Indonaia caerulea (Lea, 1831) | UF507572 | Complete | Tetragenous | Unhooked | Parreysiinae: Indochinellini |
| Raditula aff. humilis sp.2 | UF 507848 | Complete | Tetragenous | Unhooked | Parreysiinae: Indochinellini |
| Indochinella pugio (Benson, 1862) gen. et comb. nov. | CAS180796, CAS189963 | Complete | Tetragenous | Unhooked | Parreysiinae: Indochinellini |
| Nodularia douglasiae (Griffith & Pidgeon, 1833) | RMBH: biv227_12, biv132, biv134 | Anterior end | Ectobranchous | Triangular, hooked | Unioninae |
| Oxynaia micheloti (Morlet, 1886)* | NCSM 84920, NCSM 84425 | Anterior end | Ectobranchous | Triangular, hooked | Unioninae |
| Pyganodon grandis (Say, 1829) | UF369750 | Anterior end | Ectobranchous | Triangular, hooked | Unioninae: Anodontini |
| Rectidens sumatrensis (Dunker, 1852) | UF410001 | Anterior end | Ectobranchous | n/a | Rectidentinae: Rectidentini |
| Contradens contradens (Lea, 1838) | UF507874, UF507591 | Anterior end | Ectobranchous | Asymmetrical | Rectidentinae: Contradentini |
| Monodontina vondembuschiana (Lea, 1840) | UF507565, UF507438 | Anterior end | n/a | n/a | Pseudodontinae: Pilsbryoconchini |
| Pilsbryoconcha sp. | UF507453 | Anterior end | Tetragenous | Unhooked | Pseudodontinae: Pilsbryoconchini |
| Chamberlainia hainesiana (Lea, 1856) | UF507722, UF507872 | Complete | n/a | n/a | Gonideinae: Chamberlainiini |
n/a – not available. *We used this species as a representative of the Oxynaia jourdyi group, because gravid individuals of the type species of this genus were not available.
Table 2.
Comparative analysis of the genera Indochinella Bolotov, Pfeiffer, Vikhrev & Konopleva gen. nov., Oxynaia Haas, 1911, and Nodularia Conrad, 1853 on the basis of conchological features.
| Conchological features | Unio pugio Benson, 1862, the type species of the genus Indochinella | Unio jourdyi Morlet, 1886, the type species of the genus Oxynaia* | Unio douglasiae Griffith & Pidgeon, 1833, the type species of the genus Nodularia** |
|---|---|---|---|
| Shell shape | Cuneiform | Somewhat cuneiform, with more height, anteriorly rounded, posteriorly elongated and pointed | Oval-form, slightly narrow in the posterior part |
| Umbo | Not pronounced | Very pronounced, elevated | Very pronounced, elevated |
| Umbo sculpture | V-shaped | Nodulose wrinkles | Nodulose wrinkles |
| Umbo position | In the first third of the shell | In the first half of the shell | In the first half of the shell |
| Pseudocardinal teeth of the left valve | Two separated ribbed teeth placed in parallel line with one another | Anterior tooth rectangular and sharp, posterior tooth thick and pyramidal | Anterior rectangular, sharp and ribbed, posterior tooth small and pyramidal |
| Pseudocardinal teeth of the right valve | Anterior tooth reduced, posterior tooth somewhat pyramidal and wrinkled | Anterior tooth lamella-shaped, posterior tooth rectangular, wrinkled | Anterior tooth reduced, lamella-shaped, posterior tooth somewhat trapeziform and ribbed |
| Lateral teeth | Rather short, two teeth on the left and one tooth on the right valve | Long, two teeth on the left and one tooth on the right valve | Straight, elongate, sharp with small scratches, two teeth on the left and one tooth on the right valve |
*Based on the two syntypes (MNHN-IM-2000-33685, Coll. du Journal de Conchyliologie, ex Coll. Morlet; type locality: Tonkin. Environs de Dang-son (Jourdy) [p. 77]18; Bac-Hat, étangs du bord de la rivière Claire (Jourdy) [p. 290]18. **Based on a sample from Soldatskoe Lake, Razdolnaya River Basin, Russian Far East (RMBH no. biv_227_12).
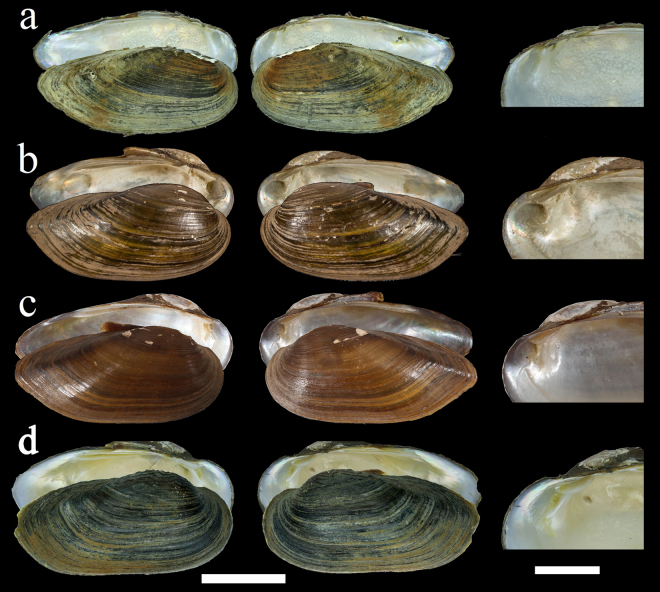
Figure 3.
Shell morphology and hinge plate of species in the genera Indochinella Bolotov, Pfeiffer, Vikhrev & Konopleva gen. nov., Oxynaia Haas, 1911, and Nodularia Conrad, 1853. (a) Indochinella pugio (Benson, 1862) gen. et comb. nov., Nant Phar Lake, Irrawaddy River basin, Myanmar (RMBH no. biv_258_1). (b) Oxynaia jourdyi (Morlet, 1886) (syntype MNHN-IM-2000-33685). (c) O. jourdyi, our sequenced specimen, northern Vietnam (UF 507885). (d) Nodularia douglasiae (Griffith & Pidgeon, 1833), Soldatskoe Lake, Razdolnaya River basin, Russian Far East (RMBH no. biv_227_12). Scale bars: 2 cm (shells) and 1 cm (hinge plates). (Photos: Ekaterina S. Konopleva (a,d), Manuel Caballer (b) MNHN, Program RECOLNAT, no. ANR-11-INBS-0004], and John M. Pfeiffer (c).
The combination of the molecular phylogeny, soft anatomy, larval characters, and shell morphology clearly demonstrate the polyphyly of Oxynaia s. lato. The type species and its allies (i.e. the Oxynaia jourdyi group) are unambiguously placed in the subfamily Unioninae, rendering both Oxynaia and Oxynaiini inapplicable to the Parreysiinae. The tribe Oxynaiini Starobogatov, 1970 is herein recognized as an available family-group level name of the Unioninae Rafinesque, 1820. In the absence of an available name for the Oxynaia pugio species group and the larger Parreysiinae clade including the O. pugio group, Radiatula, and Indonaia (i.e. the former Oxynaiini), the genus Indochinella gen. nov and tribe Indochinellini trib. nov. are described here.
Range disjunction
The two species groups of Oxynaia have clearly distinct ranges (Fig. 1). All the reliable records (mostly type localities, see Taxonomic Account) of the Oxynaia jourdyi species group are concentrated within the Red, Cả and Cầu River drainage basins of northern Vietnam. The Oxynaia pugio species group is known from the Irrawaddy, Sittaung and Tavoy River drainages. Neither species group occurs in the drainages situated between eastern Myanmar and northern Vietnam (i.e., Salween, Mae Klong, Chao Phraya, and Mekong). Zieritz et al.17 listed two species, Oxynaia gladiator and O. micheloti, from the Mekong River but those reports refer to misidentified specimens (MNHN IM-2014-6880) or suspect localities (FMNH 20402 and NCSM 84425).
Taxonomic Account
Family Unionidae Rafinesque, 1820
Subfamily Unioninae Rafinesque, 1820
Type genus: Unio Philipsson in Retzius, 1788
Genus Nodularia Conrad, 1853
Type species: Unio douglasiae Griffith & Pidgeon, 1833 (by original designation)
Type locality: Unknown.
= Oxynaia Haas, 1911 syn. nov. [Type species: Unio jourdyi Morlet, 1886]
Comments: We recognize Oxynaia as a junior synonym of Nodularia, and transfer the four Vietnamese species previously treated as Oxynaia to Nodularia as well. Additionally, we considered Nodularia dorri as the fifth member of this group. The close geographic proximity of the type localities and the conchological similarity of the named taxa raises questions about their validity and deserves further research.
Nodularia jourdyi (Morlet, 1886) comb. res.
Unio jourdyi Morlet (1886): p. 7618.
Nodularia jourdyi Simpson (1900): p. 81619.
Oxynaia jourdyi Haas (1911): Pl. 1620; Haas (1913): p. 15215.
Type locality: Tonkin. Environs de Dang-son [Đặng Sơn] (Jourdy) [p. 77]18; Bac-Hat [Bắc Hà], étangs du bord de la rivière Claire (Jourdy) [p. 290]18.
Distribution: Red and Cả River drainage basins, northern Vietnam.
Nodularia gladiator (Ancey, 1881) comb. res.
Unio gladiator Ancey (1881): p. 46821.
Oxynaia micheloti Haas (1913): p. 15615.
Nodularia gladiator Simpson (1914): p. 99122.
Type locality: Yon-Bag, Tonkin21.
Distribution: Red River drainage basin, northern Vietnam. Record from the Mekong Basin17 is erroneous.
Nodularia diespiter (Mabille, 1887) comb. res.
Unio diespiter Mabille (1887): p. 16223; Simpson (1900): p. 86119.
Nodularia diespiter Simpson (1914): p. 99322.
Oxynaia diespiter Haas (1911): Pl. 1520; Haas (1913): p. 15415.
Type locality: Tonkin23.
Distribution: Red River drainage basin, northern Vietnam.
Nodularia micheloti (Morlet, 1886) comb. res.
Unio micheloti Morlet (1886): p. 7718.
Nodularia micheloti Simpson (1900): p. 81419.
Oxynaia micheloti Haas (1911): Pl. 1420; Haas (1913): p. 15615.
Type locality: Tonkin, environs de Chu18.
Distribution: Cầu River drainage basin, northern Vietnam. Record from the Mekong Basin17 is erroneous.
Nodularia dorri (Wattebled, 1886)
Unio dorri Wattebled (1886): p. 7124.
Nodularia dorri Simpson (1900): p. 80919.
Type locality: Les arroyos des environs de Hué24.
Distribution: Perfume River, central Vietnam. Zieritz et al.17 erroneously listed N. dorri as an endemic species of the Mekong Basin.
Subfamily Parreysiinae Henderson, 1935
Type genus: Parreysia Conrad, 1853
Comments: This subfamily includes five tribes: Parreysiini Henderson, 1935, Coelaturini Modell, 1942, Lamellidentini Modell, 1942, Leoparreysiini Vikhrev, Bolotov & Kondakov, 20177 and Indochinellini trib. nov., a new tribe described here.
Tribe Indochinellini Bolotov, Pfeiffer, Vikhrev & Konopleva trib. nov.
Type genus: Indochinella Bolotov, Pfeiffer, Vikhrev & Konopleva gen. nov.
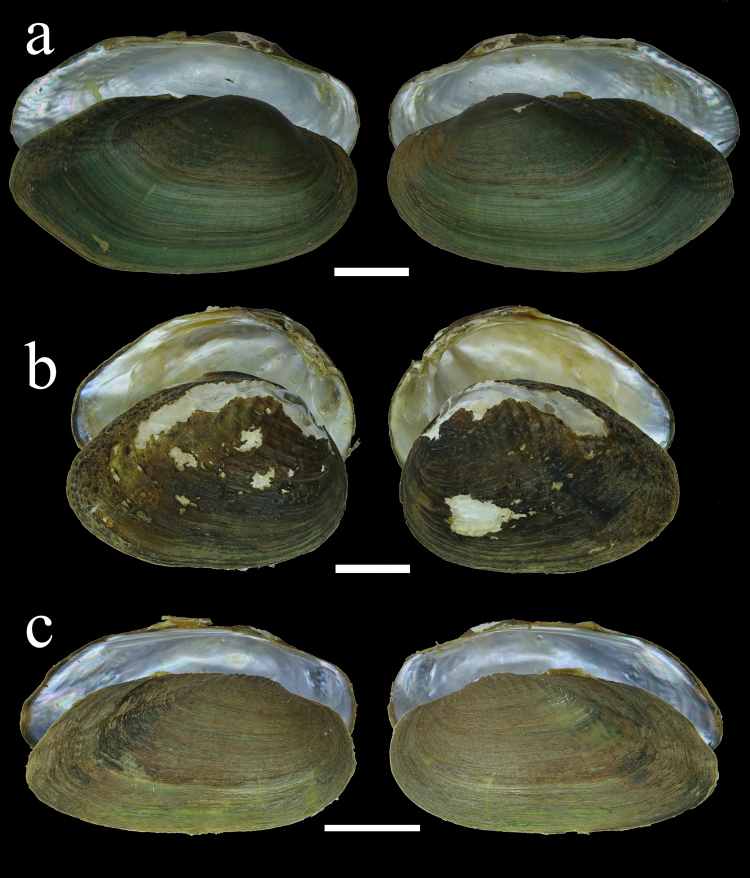
Figure 4.
Shell morphology of additional representatives in the tribe Indochinellini. (a) Indonaia andersoniana (Nevill, 1877), Myaung Lake, Irrawaddy River basin, Myanmar (RMBH no. biv267_1). (b) Radiatula myitkyinae (Prashad, 1930), Indawgyi Lake, Irrawaddy River basin, Myanmar (RMBH no. biv107_2). (c) Radiatula cf. humilis (Lea, 1856), Chi River near Maha Sarakham, Thailand (RMBH no. biv129_1). Scale bars = 1 cm. (Photos: Ekaterina S. Konopleva).
Diagnosis: The Parreysiini and Leoparreysiini are the most morphologically similar family-group level taxa to the Indochinellini. However, the Indochinellini can be distinguished from the Parreysiini and Leoparreysiini by having a much more elongate shell outline (vs. circular) and a nearly straight to convex ventral margin (vs. strongly convex).
Description: Adults small (22 mm) to medium (54 mm) sized for family. Shell outline narrow, elongate, strongly inequilateral, always with a straight or convex ventral margin. Moderately inflated, posterior ridge rounded with moderately to very steep posterior slope. Umbo only slightly elevated above hinge line usually with v-shaped umbo sculpture. Green zigzag sculpturing on shell disc common, but absent in some individuals and taxa, e.g., Indonaia caerulea (Lea, 1831), Radiatula humilis (Lea, 1856), R. pilata (Lea, 1866), and Indochinella pugio gen. et comb. nov. Shells moderately thick. Pseudocardinals erect and stumpy to long and bladelike; two in left valve (may become one in blade-like teeth) and one in right valve occasionally with a second rudimentary anterior tooth. Laterals are moderately short and diverging; two in left, one in right. Unhooked glochidia brooded in all four gills. Mantle margin ventral to incurrent aperture with many prominent simple papillae. Incurrent aperture papilose, excurrent and supra-anal apertures smooth. Ascending lamella of inner demibranch attached to visceral mass for its entire length.
Distribution: Southeast and South Asia6,7.
Comments: This tribe includes at least three valid genera: Indochinella gen. nov., Radiatula Simpson, 1900, and Indonaia Prashad, 1918.
Genus Indochinella Bolotov, Pfeiffer, Vikhrev & Konopleva gen. nov.
Type species: Unio pugio Benson, 1862.
Type locality: Regione Ava [Mandalay]25.
Etymology: The name of this genus is derived from the greater Indochinese Peninsula.
Diagnosis: The genus is distinguished from Nodularia by the presence of tetragenous brooding of unhooked glochidia (vs. ectobranchous brooding of hooked glochidia), as well as complete fusion of the ascending lamella of the inner demibranchs to the visceral mass (vs. only anterior fusion) (Table 1). Indochinella gen. nov. can also be distinguished from Nodularia by the umbo being only slightly elevated above the hinge line (vs. strongly elevated), fine v-shaped beak sculpture (vs. wrinkled and nodular), triangular posterior pseudocardinal (vs. trapezoidal or rectangular), and moderately short lateral teeth (vs. elongate) (Table 2 and Fig. 3). Adult Indochinella (with exception of a lineage from the Tavoy River) can be distinguished from all other representatives of the tribe by the presence of a sharp posterior ridge (vs. rounded) and very steep posterior slope (vs. gradual). Pseudocardinal teeth in Indochinella also tend to be more strongly developed than in other Indochinellini.
Description: Shell moderately thick; elongate with rounded anterior end and strongly pointed posterior end. Posterior ridge sharp. Posterior slope steep. Umbo hardly elevated above hinge line, fine v-shaped umbo sculpture, shell disc smooth to strongly sculptured. Moderately inflated. Pseudocardinal teeth strong, two in the left valve, one in the right. Laterals moderately short, two in left valve, one in right. Tetragenous brooding condition (occasionally ectobranchous), unhooked glochidia.
Distribution: The genus is primarily known from Myanmar in the Irrawaddy, Sittaung, and Tavoy River drainages. The genus may also inhabit several other river basins in Myanmar, e.g., the Great Tenasserim, Salween, and some coastal rivers of the Bay of Bengal14,26–28. A few records from India (e.g., Assam)14 are in need of future studies because these specimens could have been collected within the Irrawaddy Basin.
Comments: We assigned a single described species to the genus, although the divergent molecular lineages from the Sittaung and Tavoy River drainage basins may be worthy of formal taxonomic recognition.
Indochinella pugio (Benson, 1862) gen. et comb. nov.
Unio pugio Benson (1862): p. 19325.
Nodularia pugio Simpson (1900): p. 81419.
Oxynaia pugio Haas (1911): Pl. 1420; Haas (1913): p. 15815.
Figures 1 and 3a, Tables 1 and 2
Material examined: Myanmar: Irrawaddy River basin, Nant Phar Lake, 24.2972°N, 97.2610°E, 29.xi.2016, 4 specimens (RMBH nos. biv_258_1, biv_258_2, biv_258_4, and biv_258_5), Vikhrev leg. Myanmar: Irrawaddy River basin, Myaung Lake, 24.2387°N, 97.1658°E, 01.xii.2016, 4 specimens (RMBH nos. biv_268, biv_268_1, biv_268_2, and biv_268_4), Vikhrev leg. Myanmar: Irrawaddy River basin, Pauk In Lake, 21.81347°N, 95.19746°E, 13.x.2009, 5 specimens (CAS 180788), Pitotrowski et al. leg. Myanmar: Irrawaddy River basin, Irrawaddy River near Myingyan, 21.48187°N, 95.30501°E, 15.x.2009, 5 specimens (CAS 189963), Pitotrowski et al. leg., Myanmar: Irrawaddy River basin, Chindwin River near confluence with Irrawaddy, 21.498445°N, 95.26631°E, 09.x.2009, 5 specimens (CAS 180796), Pitotrowski et al. leg. Myanmar: Sittaung River basin, Myit Kyi Pauk Stream, 18.9613°N, 96.4455°E, 26.xi.2016, 3 specimens (RMBH nos. biv_251_3, biv_251_1, and biv_251_2), Vikhrev leg. Myanmar: Tavoy River, 14.5012°N, 98.1557°E, 26.iv.2015, 26.iv.2015, 38 specimens (RMBH nos. biv_147_10, biv_147_3, biv_147_18, biv_148_4, biv_148_7, and biv_148_15 are sequenced), Bolotov leg.
Redescription: Shell shape cuneiform, elongated, inequilateral, not inflated, rather thick. Posterior ridge sharp, posterior slope steep. Maximum shell length 53.4 mm, height to 24.3 mm, width to 18.7 mm. Fine v-shaped sculpture on umbo, umbo only slightly elevated above hinge line. Periostracum smooth, grey-brown to yellow-green, with dark parts along the radial lines; nacre whitish. Left valve with two parallel rather short lateral teeth and two ribbed parallel pseudocardinal teeth. Right valve with a single slightly curved lateral tooth and two pseudocardinal teeth, anterior tooth reduced, posterior tooth high, ribbed and strong. Umbo cavity not very deep, nacre in umbo cavity commonly tinted peach to golden-brown. Anterior adductor scar well pronounced, funneled; posterior adductor scar rounded. The Sittaung lineage differs from the Irrawaddy lineage in having a shorter and higher shell, more pronounced and curved lateral teeth, and a moderately strong sculpture on shell disc. The Tavoy lineage differs from the two other lineages in having an oval-shaped shell, more rounded posterior ridge, more gradual posterior slope, and distinct zigzag ridges across shell disc.
Distribution: Irrawaddy, Sittaung and Tavoy River basins. In the Irrawaddy River, it is known as far north as Mya Taung and as far south as Hinthada. A few records from India (e.g., Assam)14 are in need of future studies because these specimens could have been collected within the Irrawaddy Basin. The lineages from the Sittaung and Tavoy River catchment areas may represent separate species- or subspecies-level taxa but requires further systematic research.
Habitat and ecology: The species seems to be rather a habitat generalist, and it is known from the mainstream of large, medium-sized and small rivers, as well as from their floodplain lakes.
Comments: Unio digitiformis Sowerby, 1868 from India is not a synonym of Indochinella pugio gen. et comb. nov29. but a separate species, the generic placement of which is unclear. Haas13 noted that the location of this form is certainly not India and that it most likely belong to the Lanceolaria Conrad, 1853.
Discussion
Taxonomic implications
Our integrative molecular and morphological approach has determined that the genus Oxynaia is polyphyletic. This result has clear implications regarding the higher-level classification of the Unionidae, the morphological characteristics of the Parreysiinae, and corroborates broader biogeographic patterns in Southeast Asia. Molecular and morphological data reject the monophyly of Oxynaia with its former constituents being recovered in phylogenetically divergent and morphologically diagnosable clades. The Oxynaia jourdyi group is unambiguously placed in the Unioninae on the basis of our molecular phylogeny and several morphological synapomorphies and is herein considered a junior synonym of Nodularia, rendering the tribe name Oxynaiini inapplicable to the Parreysiinae. This taxonomic rearrangement required the description of a new genus for the “Oxynaia” pugio group (=Indochinella gen. nov.) and a new tribe (=Indochinellini trib. nov.) to recognize these morphologically cohesive clades of Parreysiinae.
Freshwater biogeography of Southeast Asia
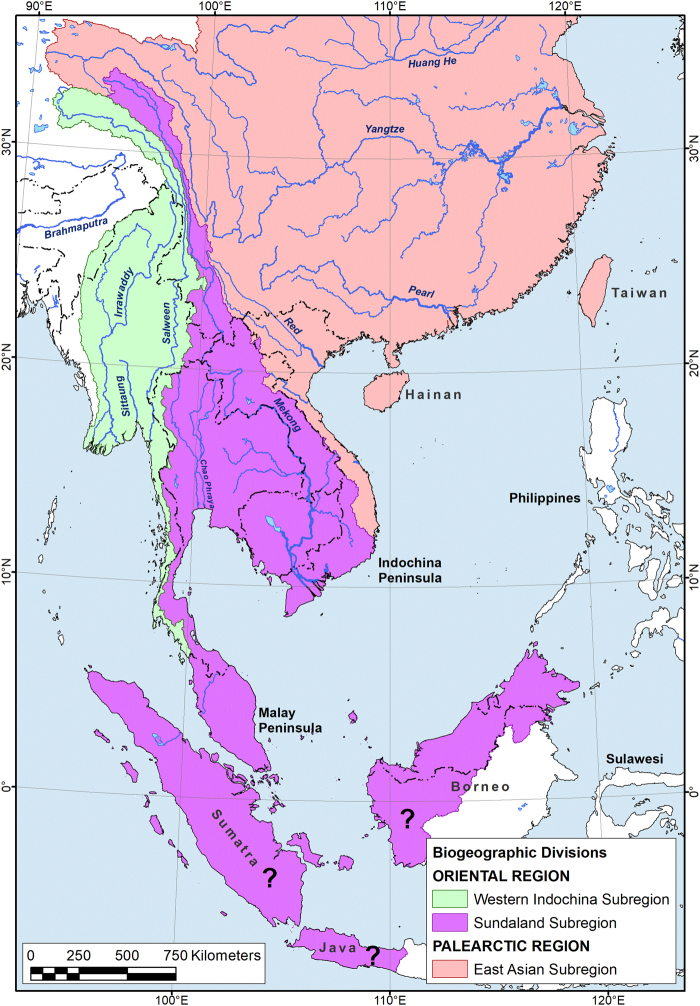
Previous to this study, Oxynaia was thought to be one of the few genera distributed across much of Southeast Asia from central Myanmar to northeastern Vietnam17. This distribution was thought to be unusual in that it crossed two important biogeographic barriers in Southeast Asia: (1) the Salween/Mekong river drainage divide separating the Western Indochinese freshwater mussel assemblage from the Sundaland assemblage, and (2) the Mekong/northern Vietnamese drainage divides separating the Sundaland assemblage from the East Asian fauna. However, the seemingly large distribution of Oxynaia s. lato is discovered here to be spurious and based on incorrect interpretations of common ancestry. The geographic distributions of Nodularia and Indochinella closely follow these two influential biogeographic barriers. These two biogeographic barriers divide eastern Asia into three faunistically distinct subregions (Fig. 5), each of which is briefly discussed below.
Western Indochina Subregion. The drainages of the Arakan coast of Myanmar, the Irrawaddy, Pegu, Sittaung, and Bilin river basins, and east to the Salween River and western drainages of the Kra Isthmus . The subregion currently includes members of three subfamilies (Parreysiinae, Rectidentinae and Pseudodontinae), five tribes and eight genera6,7. Indochinella gen. nov. is the fifth endemic unionid genus of western Indochina, together with Pseudodon Gould, 1844, Trapezoideus Simpson 1900, Leoparreysia Vikhrev, Bolotov & Aksenova, 2017, and Trapezidens Bolotov, Vikhrev & Konopleva, 2017. The other genera in the region, Lamellidens Simpson, 1900, Indonaia, and Radiatula are geographically more widespread, especially to the west into India. Radiatula is the only genus recognized to occur to the west and east of the Salween River drainage basin6,7.
Sundaland Subregion. The Mekong, Chao Phraya, Mae Klong and the drainages of the Malay Peninsula, probably corresponding to the gigantic paleo-Mekong River basin6–8. The fauna of the Greater Sunda Islands (Sumatra, West Java, northern and western Borneo) appears similar to that of mainland Southeast Asia and may also belong to this subregion, as suggested by the molecular data for northern Borneo9 and by the putative connections of paleo-drainages during the Pleistocene30, but further systematic research is necessary to delineate these boundaries. The Sundaland Subregion is comprised at least five subfamilies, i.e. Parreysiinae, Rectidentinae, Pseudodontinae, Gonideinae, and the monotypic Modellnaiinae. A single member of the Unioninae (i.e., Cristaria plicata)31,32 occurs in the region, although, it has likely been introduced. Two large endemic monophyletic radiations of freshwater mussels, i.e., the tribes Pilsbryoconchini and Rectidentini, were recorded in this region6,7. The highest levels of diversity of these clades occur within the Mekong River basin, with a few representatives inhabiting the Chao Phraya River, the Malay Peninsula, and the Greater Sunda Islands6–9. The Indochinellini is the only tribe of the Parreysiinae distributed in the Sundaland Subregion, whose constituents belong to a subclade Radiatula that appears to have significant levels of cryptic diversity (Fig. 2). Several characteristic elements of the fauna of western Indochina are lacking in Sundanese assemblage, such as the members of Lamellidentini, Leoparreysiini, and Pseudodontini.
East Asian Subregion. The Red and Ca River drainage basins, and numerous coastal rivers of Vietnam comprise the East Asian Subregion, whose fauna appears to be more closely allied to the Palearctic Region than to the Oriental Region (Fig. 5). This large biogeographic subregion extends north to Japan and the Far East of Russia. This freshwater mussel fauna is entirely different from those of Western Indochina and Sundaland, and it has strong biogeographic affinities to the Pearl, Yangtze and Huang He river drainage basins33. With respect to available paleontological data34 and phylogenetic modelling6,7, the freshwater bivalve faunas of the Mekong and Yangtze have been developing independently since at least the early Cenozoic epoch. The East Asian Unioninae taxa such as the Nodularia, Cristaria Schumacher, 1817, Sinanodonta Modell, 1945, Lamprotula Simpson, 1900 and Sinohyriopsis Starobogatov, 1970 are the most characteristic elements of the freshwater mussel fauna of eastern Indochina at least since the Eocene34, while the Rectidentinae, Parreysiinae and Pseudodontinae appear completely absent there. Based on the patterns outlined above, we suggest that the East Asian Subregion belongs to the Palearctic Region.
Figure 5.
Freshwater biogeographic division of Southeast Asia based on the phylogeny and phylogeography of the Unionidae5–9. The question marks indicate areas that were tentatively assigned by us to the Sundaland Region but their placement is in need of future research. The map was created using ESRI ArcGIS 10 software (www.esri.com/arcgis); the topographic base of the map was created with Natural Earth Free Vector and Raster Map Data (www.naturalearthdata.com). (Map: Mikhail Yu. Gofarov).
This biogeographic division of Southeast Asia largely corresponds with that of Graf and Cummings33 suggesting four freshwater biogeographic subregions, i.e. (1) Yangtze-Huang, from the Pei south to the Qiantang and Taiwan; (2) Indochina, including southern China and the Mekong west to the Salween; (3) India–Burma, from the Indus to the Irrawaddy; and (4) Sunda Islands–Philippines. However, our new scheme (Fig. 5) reveals that the Salween, Irrawaddy and Sittaung unionid faunas are close to each other and should belong to the separate Western Indochina Subregion and that the Yangtze-Huang (=East Asian) Subregion comprises the drainage basins in northern Vietnam and appears to be a part of the Palearctic Region. Additionally, we suggest that Sumatra, West Java, northern and western Borneo may belong to the Sundaland Subregion, but this preliminary hypothesis is in need of future confirmation based on an expanded molecular dataset.
Zieritz et al.17 distinguished two major hotspots (“epicentres”) of the subfamily-level diversity and endemism of the Unionidae in Asia, i.e. (1) Southeast Asian Hotspot harboring the highest diversity of the Rectidentinae, Gonideinae (+Pseudodontinae), Parreysiinae, and Modellnaiinae, and (2) Chinese Hotspot dominated by the Unioninae (+Anodontini) lineages. Our biogeographic division is largely congruent with this diversity-based model, i.e., the Western Indochinese and Sundaland subregions correspond to the Southeast Asian diversity hotspot and the East Asian Subregion correlates with the Chinese diversity hotspot.
Methods
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org), the online registration system for the ICZN. The LSID for this publication is: urn:lsid:zoobank.org:pub:C585DACD-6AB1-4692-B68A-50ABA47940A4. The electronic edition of this paper was published in a journal with an ISSN, and has been archived and is available from PubMed Central.
Studied museum collections
The shell lots were studied in the malacological collections of the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (NMNH), British Museum of Natural History, London, UK (NHMUK), Muséum National d’Histoire Naturelle, Paris, France (MNHN), Museo Civico di Storia Naturale di Genova, Genoa, Italy (MSNG), California Academy of Natural Sciences (CAS), North Carolina State Museum (NCSM), and Florida Museum of Natural History (UF). Additionally, we accessed the images of the types of several nominal taxa at the MUSSELp Database29.
Morphological methods
Soft anatomy and larval characters were scored for representatives of both Oxynaia species groups (Table 1). These three characters were chosen as they have been previously demonstrated to be useful in diagnosing suprageneric clades of freshwater mussels16. Character states were observed using a Leica M27s dissecting scope and a Leica DM LB2 compound microscope. Representative taxa relevant to previous classifications of Oxynaia were also included, as were other major lineages present in the region (Table 1). Parreysia corrugata was scored using literature sources35,36, as no soft anatomy for this species was available. A more detailed comparison of the shell characters that distinguish the three most commonly confused genera are provided in Table 2. Umbo character states follow Zieritz et al.37.
Phylogenetic analyses
We included novel Oxynaia jourdyi sequences in the recent phylogenetic data set of Bolotov et al.7 to test the monophyly of Oxynaia. This data set was simplified to include only one haplotype of each species, with exception of the Oxynaia pugio sequences (Supplementary Table 1). Additionally, we excluded several taxa that were represented only by sequences of the COI gene, but Parreysia spp. and Indonaia spp. from India were left as the members of the Parreysiinae. The sequence alignment of COI, 16S rRNA and 28S rRNA gene fragments was performed separately using the Muscle algorithm implemented in MEGA638. The alignment data sets were joined in a multi-locus alignment. Lacking sites were treated as missing data. We performed maximum likelihood and Bayesian inference phylogenetic analyses using RAxML v. 8.2.6 HPC Black Box39 and MrBayes v. 3.2.640, respectively. The settings of the analyses were as described in Bolotov et al.7. The phylogenetic models were calculated at the San Diego Supercomputer Center through the CIPRES Science Gateway41.
Data availability
The sequences used in this study are available from GenBank. Accession numbers for each specimen are presented in Supplementary Table 1.
Electronic supplementary material
Acknowledgements
This work was partly funded by grants from the Russian Ministry of Education and Science (project no. 6.2343.2017/4.6), Federal Agency for Scientific Organizations (project no. 0409-2015-0143), Russian Foundation for Basic Research (project no. 16-34-00638), a National Science Foundation Doctoral Dissertation Improvement Grant (DEB-1701901), National Geographic Society (project no. NGS-274R-18), and Northern Arctic Federal University. We are grateful to late Dr. Tony Whitten (Fauna & Flora International – Asia-Pacific), Mr. Frank Momberg, Mr. Zau Lunn, and Mr. Nyein Chan (Fauna & Flora International – Myanmar Program, Myanmar) for their great help during this study. We would like to express our sincerest gratitude to the Department of Fisheries of the Ministry of Agriculture, Livestock and Irrigation (Myanmar) for the permission of the field work and sampling in Myanmar (survey permission no. 5/6000/MOLFRD-3103/2016 and export permission no. Nga La/Nga Tha Hta-Phont Thu/2016-5856). Special thanks go to Dr. Philippe Bouchet, Dr. Virginie Héros and Manuel Caballer (MNHN, Program RECOLNAT, no. ANR-11-INBS-0004, France) for the photographs of the syntypes of Unio jourdyi Morlet, 1886. The authors would also like to thank Christina Piotrowski and Elizabeth Kools (California Academy of Sciences, USA) and Art Bogan and Jamie Smith (North Carolina Museum of Natural History, USA) for assistance with specimen loans.
Author Contributions
I.N.B. and J.M.P. developed the concept of the study. I.V.V. coordinated field works and sampling. I.V.V., J.M.P. and E.S.K. studied the type series of the nominal taxa. I.N.B., I.V.V., E.S.K. and O.V.A. collected samples. A.V.K. designed and carried out molecular analyses, with contribution from E.S.K., M.Y.G. created the map. I.N.B. performed phylogenetic modeling. I.N.B., J.M.P. and E.S.K. wrote the paper, with input from A.V.K., I.V.V., M.Y.G., O.V.A., T.W. and S.T. All authors discussed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28385-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopes-Lima M, et al. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia. 2018;810:1–14. doi: 10.1007/s10750-017-3486-7. [DOI] [Google Scholar]
- 2.Graf DL. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the Unionoida, Sphaeriidae, and Cyrenidae. American Malacological Bulletin. 2013;31:135–153. doi: 10.4003/006.031.0106. [DOI] [Google Scholar]
- 3.Pfeiffer JM, III, Graf DL. Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae) Zoological Journal of the Linnean Society. 2015;175:307–318. doi: 10.1111/zoj.12282. [DOI] [Google Scholar]
- 4.Konopleva ES, Bolotov IN, Vikhrev IV, Gofarov MY, Kondakov AV. An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae) Systematics and Biodiversity. 2016;15:204–217. doi: 10.1080/14772000.2016.1249530. [DOI] [Google Scholar]
- 5.Lopes-Lima M, et al. Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): Defining modern subfamilies and tribes. Molecular Phylogenetics and Evolution. 2017;106:174–191. doi: 10.1016/j.ympev.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Bolotov IN, et al. Ancient river inference explains exceptional Oriental freshwater mussel radiations. Scientific Reports. 2017;7:2135. doi: 10.1038/s41598-017-02312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolotov IN, et al. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Scientific Reports. 2017;7:11573. doi: 10.1038/s41598-017-11957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zieritz A, et al. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Science of the Total Environment. 2016;571:1069–1078. doi: 10.1016/j.scitotenv.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 9.Zieritz A, et al. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biological Conservation. 2018;219:126–137. doi: 10.1016/j.biocon.2018.01.012. [DOI] [Google Scholar]
- 10.Whelan NV, Geneva AJ, Graf DL. Molecular phylogenetic analysis of tropical freshwater mussels (Mollusca: Bivalvia: Unionoida) resolves the position of Coelatura and supports a monophyletic Unionidae. Molecular Phylogenetics and Evolution. 2011;61:504–514. doi: 10.1016/j.ympev.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Thiele, J. Handbuch der systematischen Weichtierkunde (Vol. 2, Part 3), 779–1022 (Jena, Gustav Fischer, 1934).
- 12.Modell H. The natural system of the naiads. 3. Archiv für Molluskenkunde. 1964;93:71–126. [Google Scholar]
- 13.Haas F. Superfamilia Unionacea. Das Tierreich. 1969;88:1–663. [Google Scholar]
- 14.Subba Rao, N.V. Handbook of freshwater molluscs of India (Calcutta, 1989).
- 15.Haas F. Die Unioniden. Systematisches Conchylien-Cabinet von Martini und Chemnitz. 1913;9:137–160. [Google Scholar]
- 16.Graf DL, Cummings KS. Palaeoheterodont diversity (Mollusca: Trigonioida+ Unionoida): what we know and what we wish we knew about freshwater mussel evolution. Zoological Journal of the Linnean Society. 2006;148:343–394. doi: 10.1111/j.1096-3642.2006.00259.x. [DOI] [Google Scholar]
- 17.Zieritz A, et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia. 2018;810:29–44. doi: 10.1007/s10750-017-3104-8. [DOI] [Google Scholar]
- 18.Morlet L. Diagnoses Molluscorum novorum Tonkini. Journal de Conchyliologie. 1886;34:75–78. [Google Scholar]
- 19.Simpson, C. T. Synopsis of the naiades, or pearly fresh-water mussels. Proceedings of the United States National Museum22, 501–1044 (1900).
- 20.Haas F. Die Unioniden. Systematisches Conchylien-Cabinet von Martini und Chemnitz. 2011;9:41–64. [Google Scholar]
- 21.Ancey CF. Coquilles Nouvelles ou peu Connues. Le Naturaliste. 1881;3:468. [Google Scholar]
- 22.Simpson, C. T. A descriptive catalogue of the naiades, or pearly fresh-water mussels (Parts I-III) (Detroit, 1914).
- 23.Mabille J. Sur quelques mollusques du Tonkin. Bulletins de la Société Malacologique de France. 1887;4:73–164. [Google Scholar]
- 24.Wattebled G. Description de Mollusques inédits de l’ Annam. Récolte du capitaine Dorr aux environs de Hué. Journal de Conchyliologie. 1886;34:54–71. [Google Scholar]
- 25.Benson WH. Descriptions of Indian and Burmese species of the genus Unio, Retz. Annals and Magazine of Natural History (Third Series) 1862;10:184–195. doi: 10.1080/00222936208681307. [DOI] [Google Scholar]
- 26.Tapparone-Canefri C. Viaggio de Leonardo Fea in Birmania e regioni vicine. XVIII. Molluschi terrestri e d’acqua dolce. Annali del Museo Civico di Storia Naturale de Genova (series 2) 1889;27:295–359. [Google Scholar]
- 27.Preston, H. B. Mollusca (Freshwater Gastropoda & Pelecypoda). Fauna of British India, including Ceylon and Burma (London, Taylor & Francis, 1915).
- 28.Prashad B. A revision of the Burmese Unionidae. Records of the Indian Museum. 1922;24:91–111. [Google Scholar]
- 29.Graf, D. L. & Cummings, K. S. The freshwater mussels (Unionoida) of the World (and other less consequential bivalves), updated 5 December 2017. MUSSEL Project Web Site. Available: http://www.mussel-project.net (2017).
- 30.Voris HK. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography. 2000;27:1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x. [DOI] [Google Scholar]
- 31.Brandt RAM. The non-marine aquatic mollusca of Thailand. Archiv für Mollusckenkunde. 1974;105:1–423. [Google Scholar]
- 32.Nahok B, Tumpeesuwan C, Srifa A, Tumpeesuwan S. Freshwater molluscan assemblages in upper part of Choen River Basin, Northeastern Thailand. Tropical Natural History. 2017;17:11–24. [Google Scholar]
- 33.Graf DL, Cummings KS. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida) Journal of Molluscan Studies. 2007;73:291–314. doi: 10.1093/mollus/eym029. [DOI] [Google Scholar]
- 34.Schneider S, Böhme M, Prieto J. Unionidae (Bivalvia; Palaeoheterodonta) from the Palaeogene of northern Vietnam: exploring the origins of the modern East Asian freshwater bivalve fauna. Journal of Systematic Palaeontology. 2013;11:337–357. doi: 10.1080/14772019.2012.665085. [DOI] [Google Scholar]
- 35.Ortmann AE. The systematic position of the unionid genus Parreysia. Nautilus. 1910;23:139–142. [Google Scholar]
- 36.Prashad B. Studies on the anatomy of Indian Mollusca, II. The marsupium and glochidium of some Unionidae and on the Indian species hitherto assigned to the genus Nodularia. Records of the Indian. Museum. 1918;15:143–148. [Google Scholar]
- 37.Zieritz A, Sartori AF, Bogan AE, Aldridge DC. (2015). Reconstructing the evolution of umbonal sculptures in the Unionida. Journal of Zoological Systematics and Evolutionary Research. 2015;53:76–86. doi: 10.1111/jzs.12077. [DOI] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 40.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, M., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE). 1–8 (IEEE, 2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences used in this study are available from GenBank. Accession numbers for each specimen are presented in Supplementary Table 1.