Abstract
A diagnosis of perineural invasion (PNI), defined as cancer within or surrounding at least 33% of the nerve, leads to selection of aggressive treatment in squamous cell carcinoma (SCC). Recent mechanistic studies show that cancer and nerves interact prior to physical contact. The purpose of this study was to explore cancer-nerve interactions relative to clinical outcome. Biopsy specimens from 71 patients with oral cavity SCC were stained with hematoxylin and eosin and immunohistochemical (IHC; cytokeratin, S100, GAP43, Tuj1) stains. Using current criteria, PNI detection was increased with IHC. Overall survival (OS) tended to be poor for patients with PNI (P = .098). OS was significantly lower for patients with minimum tumor-nerve distance smaller than 5 μm (P = .011). The estimated relative death rate decreased as the nerve-tumor distance increased; there was a gradual drop off in death rate from distance equal to zero that stabilized around 500 μm. In PNI-negative patients, nerve diameter was significantly related to OS (HR 2.88, 95%CI[1.11,7.49]). Among PNI-negative nerves, larger nerve-tumor distance and smaller nerve diameter were significantly related to better OS, even when adjusting for T-stage and age (HR 0.82, 95% CI[0.72,0.92]; HR 1.27, 95% CI[1.00,1.62], respectively). GAP43, a marker for neuronal outgrowth, stained less than Tuj1 in nerves at greater distances from tumor (OR 0.76, 95% CI[0.73,0.79]); more GAP43 staining was associated with PNI. Findings from a small group of patients suggest that nerve parameters other than presence of PNI can influence outcome and that current criteria of PNI need to be re-evaluated to integrate recent biological discoveries.
Introduction
Head and neck squamous cell carcinoma (SCC) is the sixth most common cancer in the world with ~600,000 new cases a year [1]. Almost half of these patients will die within 5 years of diagnosis making this the fifth most common cause of cancer-related deaths [2]. SCC has a high incidence of neural invasion [3], [4]. Also known as perineural invasion (PNI), this type of invasion is strongly correlated with recurrence and poor survival because nerves are a significant route of tumor spread toward the brain stem and into other nerves [5], [6]. Treatment of SCC is currently based on tumor stage, i.e. tumor size, spread to lymph nodes, and spread to distant sites. Detection of PNI enhances the likelihood of lymph node recurrence and leads to selection of radiation and/or elective lymph node dissection as treatment [7], [8].
PNI occurs in multiple cancers; the definition has evolved extensively from the original description of ‘tumor growing along nerves’ [9] and remains subjective. ‘Clinical PNI’ also known as ‘perineural spread’, refers to neural invasion captured by imaging, while PNI represents microscopic, asymptomatic findings [10]. Batsakis (1985) [11] first described PNI in SCC as “invasion in, around and through” nerves. Currently, PNI is defined as “tumor in close proximity to nerve and involving at least 33% of its circumference or tumor cells within any of the 3 layers of the nerve sheath” [12]. This definition includes perineural and intraneural invasion but could vary in interpretation due to terms such as ‘close proximity’ and the cut-off of 33%. In fact, there is disagreement on the microscopic interpretation of PNI in tissue specimens even among pathologists [13]. The confounding issues and the importance of a diagnosis of PNI in treatment selection highlight the need for establishing an objective, widely acceptable definition for PNI.
Recent evidence supports that PNI is a dynamic process involving mutual tropism between the tumor and nerve. Several groups have shown that nerves and tumor cells communicate prior to physical contact [14], [15], [16]. For example, in SCC we showed that galanin released by nerves induces galanin receptor 2 on SCC cells, to promote release of cytokines [14]. In turn, the cytokines promote invasion and neuritogenesis. Importantly, these findings highlight that nerves and cancer are biochemically committed prior to physical contact. As understanding about biologic mechanisms of neural invasion increases, so does the need for an evolving definition of PNI that encompasses different aspects of progression, from early stages when the tumor and nerve are far apart until physical contact and invasion of the nerve sheaths.
In this study, we comprehensively analyzed nerve-tumor interactions in human SCC tissue specimens to investigate correlations with clinical outcome. We observed that among PNI-negative nerves (using current criteria), smaller nerve-tumor distance and larger nerve diameter were significantly associated with worse patient survival, suggesting a role for nerves in SCC aggressiveness. Findings from a small group of patients suggest that nerve parameters other than presence of PNI can influence outcome and that current criteria of PNI need to be re-evaluated to integrate recent biological discoveries into an objective, reproducible, and clinically-relevant definition.
Methods
Patient Population
De-identified tissue sections of human SCC were obtained from the Tissue Core of the University of Michigan Head and Neck cancer Specialized Program of Research Excellence (HNSPORE). University of Michigan Institutional Review Board approval and patient consent were obtained by the University of Michigan HNSPORE prior to specimen collection. The study population consisted of 71 patients with oral cavity SCC (41 males and 30 females), with a mean age of 60.2 years. The median follow-up time was 56.2 months. All patients were treated with surgery; 29 (40.8%) patients received surgery alone while 20 (28.2%) patients received adjuvant radiotherapy and 22 (31%) patients received adjuvant chemoradiation. SCC recurred in 18 patients (25.3%) and 14 (19.7%) patients died due to disease. Table 1 summarizes the demographic and disease-related characteristics of the patient population.
Table 1.
Demographic and Disease-Related Characteristics of 71 Patients
| Patient Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 41 (57.7) |
| Female | 30 (42.2) |
| Age | Years |
| Mean | 60.2 |
| SD | 12.9 |
| Tumor Characteristics | N (%) |
|---|---|
| Oral Cavity Subsite | |
| Gum | 15 (21.1) |
| Mouth Floor | 10 (14.1) |
| Tongue | 34 (47.9) |
| Retromolar area | 9 (12.7) |
| Other | 3 (4.2) |
| T Stage | |
| 1 | 12 (16.9) |
| 2 | 21 (29.5) |
| 3 | 12 (16.9) |
| 4 | 26 (36.6) |
| N Stage | |
| 0 | 41 (57.7) |
| 1 | 8 (11.2) |
| 2 | 1 (1.4) |
| 2a | 2 (2.8) |
| 2b | 17 (23.9) |
| 2c | 2 (2.8) |
| Histopathologic Characteristics | N (%) |
|---|---|
| Differentiation | |
| Poor | 11 (15.4) |
| Moderate | 27 (38.0) |
| Well | 33 (46.4) |
| Worst Pattern of Invasion (POI)* | |
| POI 1 | 5 (7.0) |
| POI 2 | 19 (26.7) |
| POI 3 | 16 (22.5) |
| POI 4 | 26 (36.6) |
| POI 5 | 5 (7.0) |
| PNI (H&E) | |
| No | 55 (77.4) |
| Yes | 16 (22.5) |
| PNI (H&E + IHC**) | |
| No | 47 (66.2) |
| Yes | 24 (33.8) |
| Expanded N Positivity (H&E + IHC**) | |
| N0, PNI-negative | 30 (42.2) |
| N0, PNI | 11 (15.5) |
| N-positive | 30 (42.2) |
Worst POI assessed as in Brandwein-Gensler et al. 2005.
PNI assessed using H&E and IHC stains.
Immunohistochemistry
Sections of 5μm thickness were stained with hematoxylin and eosin (H&E) (first and last sections) and sequential sections were stained with the following antibodies (all 1 h, room temperature): S100 purified immunoglobulin fraction of rabbit polyclonal antiserum (Dako, Z0311, 1:1500) to identify nerves, cytokeratin AE1/AE3 mouse monoclonal antibody (EMD Millipore, IHCR2025–6, 1:6) to highlight epithelium, Tuj1 mouse monoclonal antibody (Sigma, T8578, 1:500) to identify β-Tubulin III protein in the axons, and anti-GAP43 rabbit affinity purified polyclonal antibody (Novus Biologicals, NB300–143, 1:2000) to identify regenerating axons and neuronal outgrowth. Mouse (Dako, X0931) or rabbit IgG (Dako, X0936) were used as negative controls at the same concentration as primary antibodies. Antigen retrieval was performed with citrate buffer pH 6.0. Epitope blocking used 1% bovine serum albumin in 1x phosphate buffered saline (1 h, room temperature). The secondary antibody reactions used streptavidin-biotin complex (Biocare Medical), including Biotinylated Goat anti-Rabbit IgG (#GR602H), Biotinylated Goat anti-Mouse IgG (#GM601H), Streptavidin HRP Label (#HP604H) and 3,3′-diaminobenzidine (DAB) chromogen (#DB801R). Slides were rinsed and counterstained with Mayer's hematoxylin. IHC stains, and an IgG control, on sequential slides from a single tissue sample are shown in Supplementary Figure S1.
Data Collection
Slides were digitally scanned with iScan Coreo (Ventana Medical Systems, Roche Diagnostics International Ltd., Switzerland) and images were analyzed using Ventana Image Viewer software v. 3.1.4.
A board certified Oral and Maxillofacial Pathologist used H&E sections to grade tumor differentiation and determine PNI (positive or negative) following current criteria [12]. Since PNI detection may be enhanced by IHC staining [4], [17], using the same criteria, a second investigator, independently scored PNI status of each nerve using all IHC stains, resulting in two independent analyses, PNI (H&E) and PNI (H&E + IHC), for each patient. Other nerve and tumor related parameters were analyzed using IHC as follows: (1) S100 stained-sections were used to locate and measure nerve area and minimum nerve diameter; nerves with minimum diameter <10 μm and nerves more than 2 mm from the tumor margin were not measured (Figure 1A and Supplementary Figure S1A, S1B); (2) Cytokeratin-stained sections were used to determine worst pattern of invasion using previously described criteria [5], to measure distance between each nerve and the nearest tumor island, and to measure the area of the nearest tumor island (Supplementary Figure S1C); (3) Tuj1 stained-sections were used to locate nerves; the proportion of staining in each nerve was assessed relative to S100 and resulted in a ‘score’ of less, equal or more than S100; (4) GAP43-stained sections were used to compare staining of nerves relative to Tuj1 (less, equal or more). Since specimens were de-identified, data were collected without knowledge of demographics, treatment, or clinical outcome.
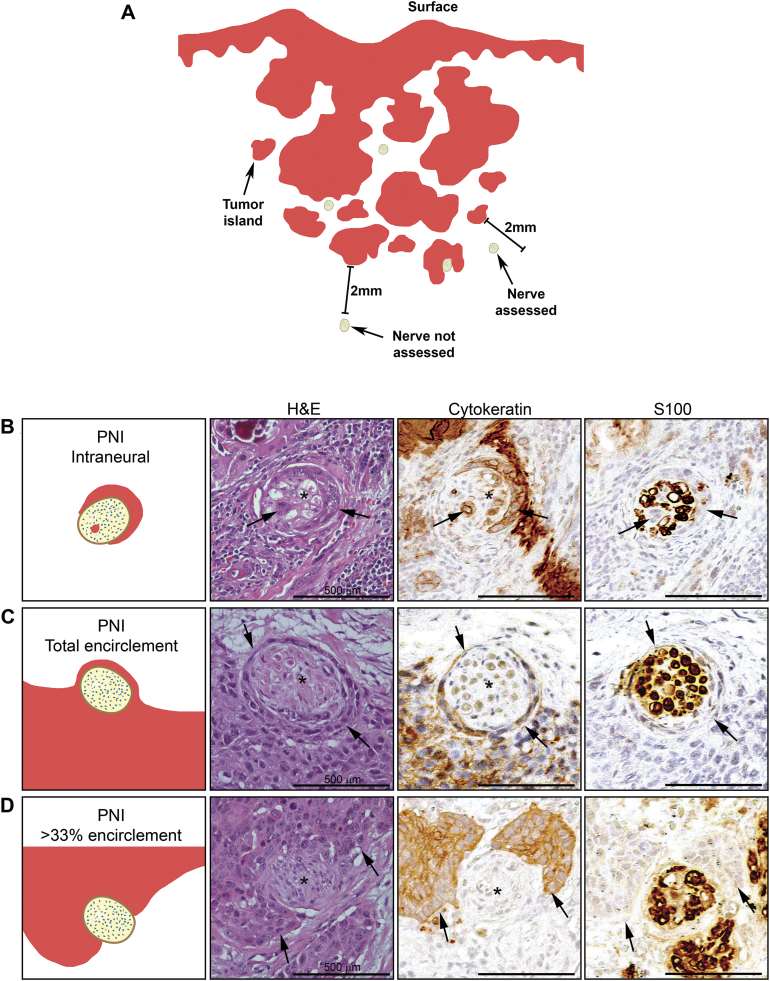
Figure 1.
Microscopic analysis of patients' samples. A: Schematic demonstrating whole tissue analysis. All nerves within 2 mm from the tumor were considered for data collection. B-D: Representation of three different microscopic aspects of PNI observed by H&E, Cytokeratin and S100. B: tumor cells infiltrating the nerve. C: Total encirclement of the nerve by tumor cells. D: At least 33% encirclement of the nerve. Arrows point to tumor cells, asterisks indicate nerves. Scale bars = 500 μm.
Statistics
Data collected were submitted to the cancer biostatistics group of the HNSPORE for analysis. Data were stratified into patient-level and nerve-level data, the first including tumor characteristics for each patient and the second including information from the IHC stains for each nerve. Patient-level data were tumor differentiation, worst pattern of invasion, T stage, N stage, PNI (H&E) and PNI (H&E + IHC) status. Nerve-level data included nerve area measured in μm2, minimum nerve diameter measured in μm, distance between nerve and nearest tumor island or single tumor cell measured in μm, area of the nearest tumor island measured in μm2, information on whether the nerve is stained by Tuj1 and S100, individual nerve expression of GAP43 relative to Tuj1, and PNI status of each nerve as positive or negative analyzed using all stains.
Descriptive statistics were calculated for patient-level and nerve-level characteristics. Kaplan–Meier methods were used to estimate Overall Survival (OS) and Disease-Specific Survival (DSS) probabilities within groups defined by patient-level characteristics. The time of diagnosis was used as the baseline when defining OS and DSS. Regression tree methods based on the log-rank statistic were used to identify potential thresholds of patient-level characteristics related to differences in OS. Cox Regression modeling was used to study the relationship between continuous patient-level characteristics and OS.
The relationships between nerve-level characteristics and GAP43 staining relative to Tuj1 staining were explored using proportional odds modeling. Weighted Cox regression modeling was used to study the association between nerve-level characteristics and OS, where nerves from each subject were weighted by the inverse of the number of nerves observed for that subject. A Cox generalized additive model was fitted using the penalized splines method to explore the shape of the relationship between OS and the distance between the nerve and the nearest tumor island. All analyses were conducted using R package, version 3.3.0 (Vienna, Austria).
Results
PNI and Survival Analysis for Patient-Level Data
Descriptive statistics, including median survival time, OS, and DSS for the 71 patients, are shown in Supplementary Table S1. Recurrence events were not observed after ~20 months.
The histopathologic characteristics of the tumors are shown in Table 1. Most tumors were moderately (38%) or well (46.4%) differentiated and exhibited a worst pattern of invasion grades 2 through 4 (85.8%). Using current criteria (Figure 1, B–D), PNI was observed in 16 (22.5%) patients by H&E. Assessment of PNI with H&E + IHC increased detection to 24 (33.8%) patients (Table 1). Subsequent results focus on PNI in the H&E + IHC group. Of the patients without lymph node involvement, 11 had PNI and 30 did not have PNI (Table 1). Of these 24 patients with PNI, 18 (75%) had multifocal (two or more nerves affected) and 6 had unifocal (one nerve affected) PNI (Supplementary Table S2).
In unadjusted logistic regression of PNI status, the odds of being PNI-positive were greater for Stage III tumors than Stage I tumors (OR 12.5; 95% CI [1.18, 133]). The odds of being PNI-positive in T3 tumors were 7× that of T1 tumors (OR 7.0; 95% CI [1.04, 46.9]) and the odds of being PNI-positive in node-positive tumors were 2× that of node-negative tumors, although not statistically significant (OR 2.08; 95% CI [0.76, 5.66]) (Supplementary Table S3).
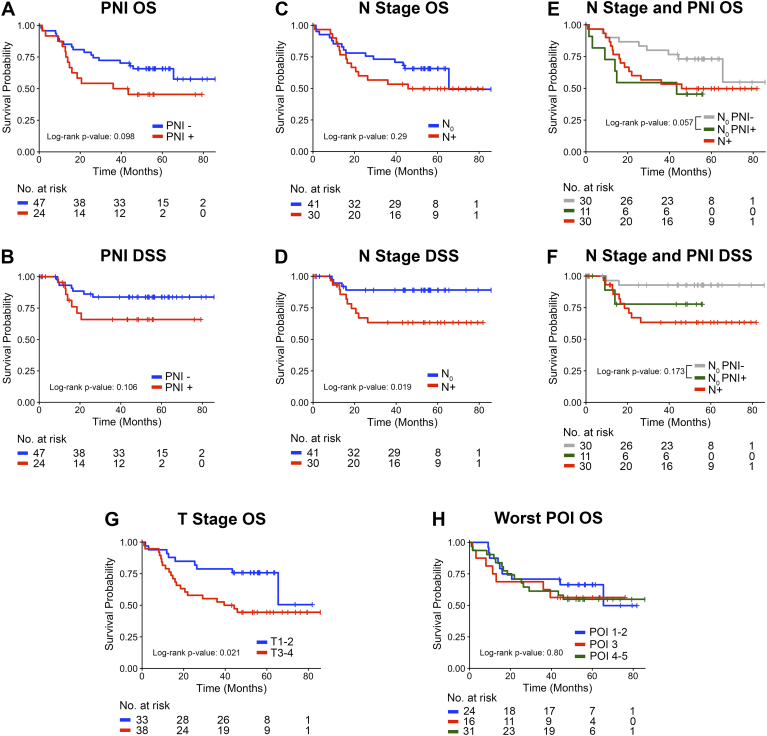
Worse OS was associated with PNI positivity, although not significant (P = .098) (Figure 2A). Kaplan–Meier analysis for DSS showed a similar trend in that the survival curve was lower for patients with PNI than for those without PNI (Figure 2B).
Figure 2.
Kaplan–Meier survival plots for Overall Survival (OS) and Disease Specific Survival (DSS) related to PNI and other tumor characteristics. A-B: PNI status graded by H&E + IHC. C-D: lymph node metastasis clinical status. E-F: Patients with negative lymph node stratified by PNI status. Overall log-rank p-value for E is 0.145 and for F is 0.039. G: Tumor stage with patients stratified as T1 + T2 and T3 + T4. H: Worst pattern of invasion (POI). The number of patients at risk for each group at each time point is shown below the plots.
Thirty of the 71 patients (42.2%) had lymph node involvement. While lymph node metastasis was not associated with OS (P = .29; Figure 2C), it was significantly related to DSS (log-rank P = .019; Figure 2D). We investigated the association between PNI, lymph node involvement and OS. Among patients with negative lymph nodes (n = 41, 57.7%), the occurrence of PNI was associated with poor OS (P = .057) (Figure 2E). The trend for DSS was similar but not significant (P = .173) (Figure 2F), which supports that PNI is a driver of survival among patients without lymph node involvement. As expected, higher T-stage (clinically large tumors) correlated with poor overall survival (Figure 2G, P = .021) whereas the worst pattern of invasion was not associated with survival (Figure 2H, P = .80).
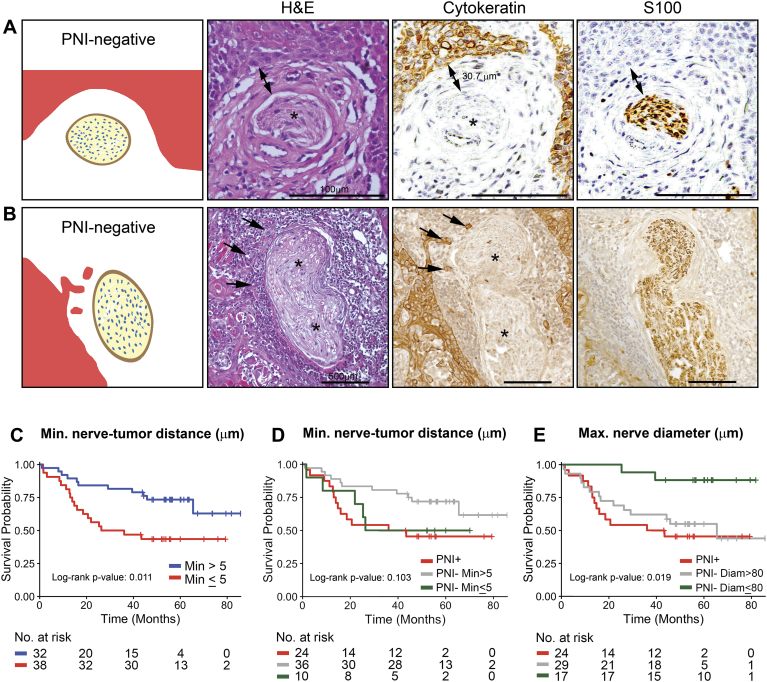
We recently showed that nerves and SCC are biochemically committed to PNI prior to physical contact [14]. Therefore, we investigated the correlation between nerve-cancer distance and patient outcome regardless of the PNI status of the patient (Figure 3, A–B illustrates PNI-negative cases with nerve and tumor in proximity). Regression tree methods were used to break the subjects into two groups that were most different in terms of overall survival with respect to values of a single covariate (based on the log-rank statistic). When this tree was calculated based on minimum nerve-tumor distance, a split was obtained at 5 μm. There were 38 subjects with minimum distance greater than 5 μm and 32 subjects lower than 5 μm. The tissue sections from one subject had no detectable nerves. The Kaplan–Meier plot shows that patients with minimum nerve-tumor distances less than 5 μm have poorer overall survival (Figure 3C, P = .011). Notably, the survival curve for the PNI-negative subjects with minimum nerve-tumor distance ≤5 μm appears similar to the PNI subjects (Figure 3D, red and green lines).
Figure 3.
Nerve-tumor distance and nerve diameter are associated with survival. A-B: Representation of nerves not classified as PNI by the current definition, but with tumor cells in proximity to the nerve. C-E: Kaplan–Meier survival plots for Overall Survival. C: Patients stratified by the minimum distance between nerve and tumor considering all measured nerves for each patient and using a 5 μm cut-off. D: PNI negative patients split into two groups based on the same nerve-tumor distance cut-off as in C. E: PNI negative patients split into two groups based on the maximum nerve diameter per patient, using a cut-off of 80 μm. The number of patients at risk for each group at each time point is shown below the plots. Single arrows point the tumor cells, asterisks indicate nerves and double arrow indicates distance. Scale bars = 100 μm (A) and 500 μm (B).
PNI of large nerves (>1 mm in diameter) has been correlated with local recurrence and poor OS [5]. Therefore, we investigated the correlation between average nerve diameter and patient outcome. The average nerve diameter regardless of PNI in 71 patients was 41 μm (Supplementary Table S2). Only 7 nerves in 5 of 71 patients (7%) had a maximum nerve diameter greater than 1 mm (all PNI-negative). Notably, in PNI-negative patients, nerve diameter is significantly related to OS (unadjusted Cox modeling; HR 2.88, 95% CI, [1.11, 7.49] P = .029), although it is not significantly associated with OS in the adjusted regression method (adjusted for T stage, age, and nerve-tumor distance). Moreover, PNI-negative patients with maximum nerve diameter >80 μm had worse OS than the subjects with maximum diameter ≤80 μm (Figure 3E, P = .015). As previously, tree-based methods were used to estimate the sample split.
PNI and Survival Analysis For Nerve-Level Data
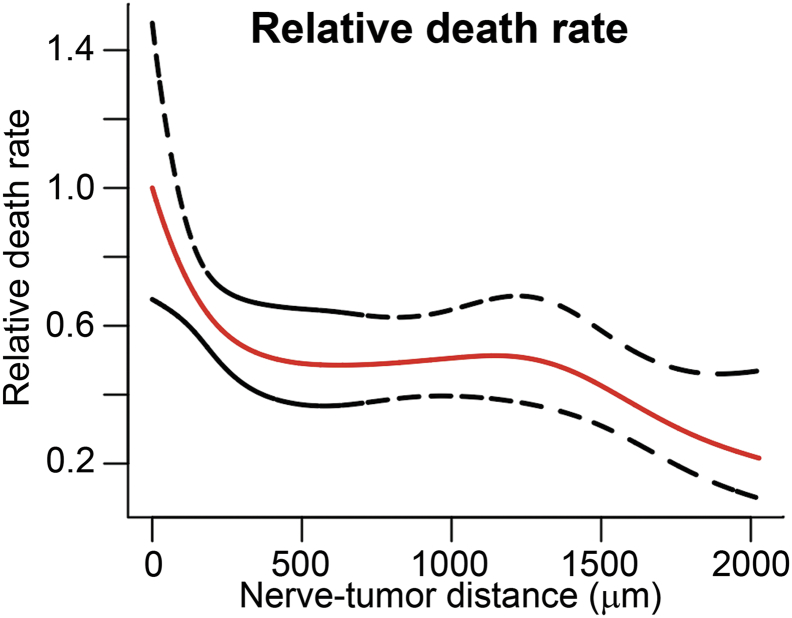
To better understand the impact of nerve characteristics on patients' outcome, we performed a nerve-level set of analyses, considering each nerve individually. The nerve-level characteristics of 2879 nerves (109 of these nerves had PNI) from 71 subjects observed by S100 staining are shown in Supplementary Table S4. A Cox generalized additive model with 4 degrees of freedom was fitted to investigate the relationship between nerve-tumor distances of individual nerves to death rate. The nerves were weighted by the inverse of the number of nerves per patient since larger biopsy specimens were associated with a higher number of observed nerves per patient (positive correlation, R = 0.55). Similarly to the OS patient-level analysis, this method showed that the estimated relative death rate decreases as the nerve-tumor distance increases (relative to distance = zero). The graph shows a gradual drop off in death rate after value equal to zero that stabilizes around distance of 500 μm (Figure 4).
Figure 4.
Nerve-tumor distance associates with patient death rate. Modeling of nerve-tumor distance to relative death rate using nerve-level data assuming individual nerves correlating to outcome (overall survival) and weighting nerves by the inverse of the number of nerves within a patient. The estimates are relative to distance equal to 0.
The Cox regression adjusted modeling of OS using nerve-level data demonstrated that among PNI-negative nerves (N = 2555), nerve-tumor distance and nerve diameter were significantly related to OS (HR 0.82, 95% CI [0.72, 0.92] with P = .001 and HR 1.27, 95% CI, [1.00, 1.62] with P = .048 respectively), even when adjusted for T stage and age (Table 2). This was not observed in the PNI-positive nerves (N = 109).
Table 2.
Nerve-level modeling for Overall Survival (2879 nerves measured)
| Cox Regression Modeling of Overall Survival using nerve-level data, by PNI status* | ||
|---|---|---|
| Among Nerves with PNI HR [95% CI] |
Among Nerves without PNI HR [95% CI] |
|
| Log(nerve-tumor distance) | 0.95 [0.76, 1.21] | 0.82 [0.72, 0.92] |
| Log(nerve diameter) | 1.08 [0.72, 1.60] | 1.27 [1.00, 1.62] |
| T Stage | ||
| T1/2 (reference) | ||
| T3/4 | 1.65 [0.79, 3.46] | 1.42 [0.95, 2.12] |
| Age | 1.02 [0.97, 1.08] | 1.02 [0.98, 1.06] |
Values in bold indicate significance at the 0.05 level.
From an adjusted model weighted by number of nerves per subjects and using a robust variance estimator.
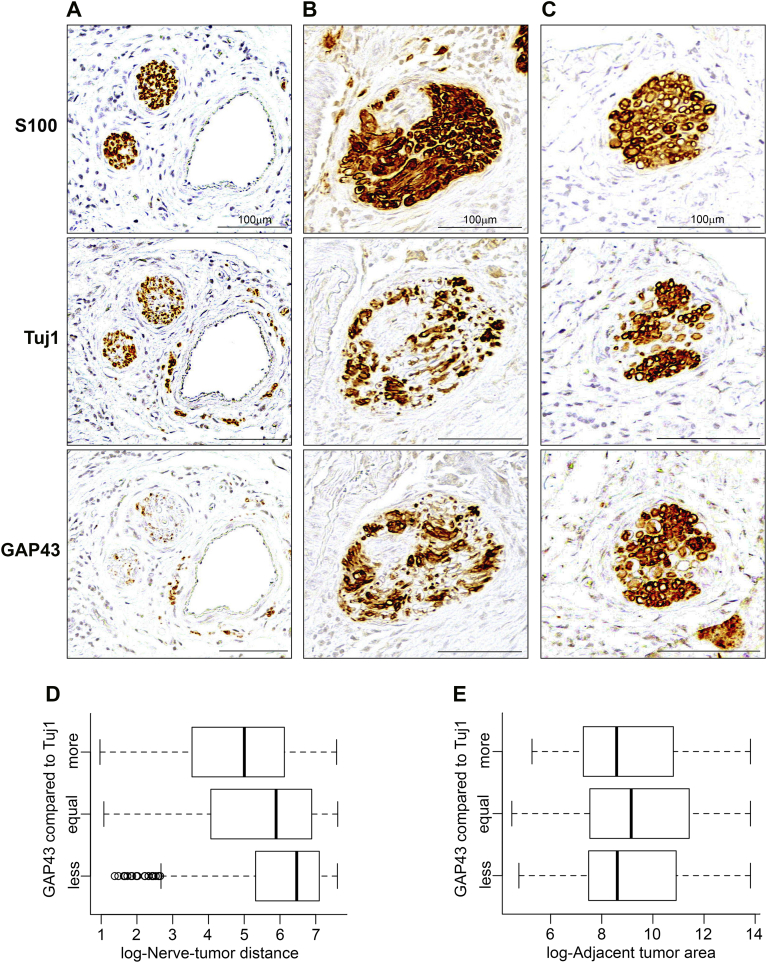
While S100 detects Schwann cells in the nerve sheath, Tuj1 is a specific marker for axons (class III β-tubulin protein) and GAP43 (growth associated protein 43) is a marker for neuronal outgrowth and regeneration [18]. We showed previously that SCC promotes neuritogenesis [14]. Therefore, we compared S100, Tuj1 and GAP43 staining (Table 3). Tuj1 staining was comparable to S100 in highlighting nerves (Figure 5, A–C; top and middle panels). GAP43 staining was graded relative to Tuj1 as less, equal or more stained (Figure 5, A–C; bottom and middle panels). Boxplots showed GAP43 expression was inversely correlated with distance between nerve and tumor (Figure 5D), and was not associated with the area of the adjacent tumor (Figure 5E). Additionally, adjusted proportional odds modeling of GAP43 indicated that higher nerve-tumor distances and larger nerve diameters are associated with lower relative staining of GAP43 compared to Tuj1 (OR 0.76, 95% CI [0.73, 0.79] and (OR 0.56, 95% CI [0.47, 0.66]), respectively) (Table 3). Moreover, PNI positivity was associated with higher relative staining of GAP43 compared to Tuj1 (Unadjusted proportional odds regression, OR 2.15 95% CI [1.58, 2. 91]).
Table 3.
GAP43 expression related to nerve characteristics of 2879 nerves measured
| Proportional Odds Modeling of GAP43 staining relative to Tuj1 staining (more/equal/less) | ||
|---|---|---|
| Unadjusted OR [95% CI] | Adjusted OR [95% CI] | |
| Log(nerve-tumor distance) | 0.79 [0.76, 0.82] | 0.76 [0.73, 0.79] |
| Log(nerve diameter) | 0.64 [0.55, 0.74] | 0.56 [0.47, 0.66] |
| Log(area of adjacent tumor) | 1.01 [0.98, 1.05] | 1.02 [0.99, 1.06] |
Values in bold indicate significance at the 0.05 level.
Figure 5.
GAP43 expression is related to nerve-tumor distance. A-C: Three different examples of Tuj1 staining, relative to S100 and GAP43 from matched tissue sections. A: Tuj1 showing less staining compared to S100 and GAP43 expressing less staining compared to Tuj1. B: Tuj1 showing less staining compared to S100 and GAP43 showing equal staining compared to Tuj1. C: Tuj1 showing less staining compared to S100 and GAP43 showing more staining compared to Tuj1. D: GAP43 expression relative to Tuj1 decreases as the distance between nerve and tumor increases. E: Area of the nearest tumor island has no impact on GAP43 expression relative to Tuj1. Nerves stained in brown. Scale bars = 100 μm.
Discussion
Our findings suggest that nerve characteristics on the initial diagnostic biopsy have an important role in the clinical outcome of patients with oral cavity SCC with or without PNI. In patients without PNI, larger nerve diameter and smaller distances between nerves and tumor are associated with worse patient survival. Nerve-tumor distance related to patient survival in patient-level and nerve-level analyses, suggesting that the poor prognosis of patients with PNI might not be entirely related to the presence of cancer within the nerve, but also influenced by the molecular interactions prior to invasion. This is the first study to explore the characteristics of both PNI-positive and PNI-negative nerves in cancer using patient samples linked to outcome. The findings could change the criteria for histopathologic diagnosis of PNI in oral cavity SCC, and suggest that the definition of PNI should be revisited.
Recent studies show that nerve-tumor crosstalk happens prior to physical contact between tumor and nerve [14], [16], suggesting that the definitions of PNI based on nerve-tumor contact and invasion of the nerve sheath layers do not entirely reflect the biological mechanism. Although the biological mechanisms of PNI are incompletely understood, nerves respond to the presence of tumor both in vitro and in vivo [14], [15], [16], [19], and a gradient of galanin, a neuropeptide, secreted by both nerve and SCC promotes invasion of cancer cells to the nerve and also stimulates neuritogenesis toward the cancer cells [14]. Therefore, we investigated the extent to which proximity rather than direct physical contact between nerves and SCC impact patient outcome. In order to address this question, we performed a comprehensive analysis, measuring every nerve within a 2 mm radius from the nearest tumor island or tumor cell. Our survival data suggest that patients with a minimum nerve-tumor distance lower than 5 μm tend to survive less compared to patients with minimum distance higher than 5 μm (Figure 3C). Patients with PNI were included in the group with minimum distance lower than 5 μm, which may have contributed to poor outcome for the entire group. However, PNI-negative subjects with closer distances behave similarly to patients with PNI (Figure 3D), suggesting that cases not classified as PNI in the current definition (Figure 3, A–B) may have a similar negative association with survival. To verify if differences in treatment were responsible for the poorer survival of PNI-negative patients with closer nerve-tumor distances, we performed an OS analysis separating patients by those who did and did not receive adjuvant therapy (data not shown). Although PNI-negative patients with closer distances had slightly worse survival when not receiving adjuvant therapy, this analysis was not included in the results since the confidence bands were large. Moreover, the number of patients in each group was very small, not allowing for a multivariate analysis to assess the impact of therapy in survival. Further validation with a larger sample would possibly tease out the impact of treatment on survival of PNI-negative patients. The 5 μm cut-off was derived from regression tree analysis of nerve-tumor distances using patient survival as the variable. This number may change with variations in sample size, and should not be considered a definitive cut-off. However, our results suggest an association for nerve-tumor distances greater than zero.
The nerve-level findings agreed with the patient-level findings relative to an association with poor survival prior to physical contact between the nerve and tumor. There was a decrease in death rate when nerve-tumor distance increased, stabilizing at approximately 500 μm (Figure 4). This model suggests that the 500 μm distance range is where most of the biochemical interactions between nerve and tumor cells occur. Furthermore, the adjusted Cox modeling of nerve level data showed an association between nerve-tumor distances and survival in PNI-negative nerves, indicating that nerve-tumor proximity might be related to worse OS for PNI-negative patients (Table 2).
Nerve diameter was also associated with survival in the present study, both in patient-level and nerve-level analyses. Previously, Brandwein-Gensler et al. [5] and Aivazian et al. [20] showed that PNI in nerves >1 mm in diameter is an important predictor of recurrence and poor survival. In our sample only 7 nerves (5 patients) were larger than 1 mm and all were PNI-negative. Therefore, we were unable to assess nerve diameter using the 1 mm cut-off. However, since our analysis included the diameter of virtually all nerves in the specimens, we were able to investigate the impact of nerve diameter on survival of patients with and without PNI. Notably, the regression model suggests that the presence of larger nerves is associated with poorer OS in PNI-negative subjects. We also showed that among PNI-negative patients, a maximum nerve diameter >80 μm was associated with poor survival. As with minimum nerve-tumor distance, 80 μm is not a definitive cut-off for stratifying patient risk based on nerve diameter; rather, the results can be interpreted as initial evidence of a relationship between nerve diameter and clinical outcome in the absence of PNI. The association between nerve diameter and outcome may be explained by mechanisms of nerve-tumor interaction. For example, larger nerves could produce a steeper gradient of cytokines than smaller nerves, causing a bigger impact on the behavior of cancer cells. Furthermore, Magnon et al. [19] showed the dependence of tumor growth on nerves; larger adjacent nerves may have a bigger impact on aggressive tumor growth and poor survival. Further investigation of the impact of nerve diameter in oral cavity SCC on patient survival in larger patient cohorts would be important due to the impact on diagnostic interpretation of biopsies.
Other PNI variables previously shown in association with worse outcome such as multifocal PNI [20] and extratumoral PNI [21] were also evaluated in this study, with no correlation with worse survival (data not shown).
In SCC, there is large variability in the incidence of PNI reported based on H&E staining; it has ranged from 6.1% when no clear criteria were used to define PNI [22], to 71% using a broad PNI definition [23]. S100 in conjunction with H&E increases PNI detection [4], [17]. Using the Liebig et al. definition [12] in the present study, detection of PNI increased from 22.5% when using H&E alone to 33.8% when adding IHC to the analysis. Other studies that used Liebig's definition reported a similar incidence of 27.4% [8] to 29% [20]. Also using the same PNI criteria, Shen et al. [17] found a stepwise increase in PNI rate in tongue tumors from 22% using medical records, to 39% after re-evaluation of H&E slides, and to 51% after including S100 in the analysis. Regardless of tumor characteristics and oral cavity sub-site, staining with S100 enhances PNI detection possibly because it highlights small nerves embedded in the tumor and also helps differentiate nerves from desmoplastic stroma. Despite increased PNI detection with IHC, it is not clear whether including IHC adds benefit for patient survival i.e., are small nerves detected by IHC associated with poor survival as observed with large nerves detected by H&E alone? Since IHC added only 8 PNI patients, it is difficult to draw conclusions about the association of PNI in small nerves with patients' outcome. Although it is possible that small PNI-positive nerves do not associate with worse outcome in the same manner as large PNI-positive nerves, this possibility is not supported by our observation that nerve-tumor distance associates with worse survival, an analysis that included small nerves. In fact, our sample was mainly comprised of small nerves, suggesting that all nerves are important for assessing prognosis. The other studies looking at PNI by IHC did not address this issue [4], [17].
The presence of PNI has been associated with poor prognosis in oral cavity SCC [7], [20], [24], [25], [26]. However, comparing results from different studies is challenging due to varying criteria for defining PNI. For instance, some studies did not indicate how PNI was defined and evaluated [7], [24], [27], [28], [29], [30] whereas others defined PNI as “infiltration of perineural space by tumor cells” [31]; “carcinoma infiltrating along within a nerve” [32]; or “tumor cells found in the perineural space or epineurium”, excluding cases where tumor cells were not entering the perineural space [25]. Our data with well-defined criteria supports that PNI positivity is associated with poor prognosis, since OS and DSS tended to be poor in the presence of PNI.
Lymph node involvement, particularly extracapsular spread, is associated with poor survival in SCC [33]. Although in the present study, lymph node involvement was associated with OS, this finding was not statistically significant. However, node involvement was significantly associated with DSS. Although an association between PNI and lymph node status is reported in several patient series, this was not verified in our cohort, which assessed the clinical node status. Multiple studies reported PNI as an independent predictor of lymph node recurrence [8], [26], [34], [35], [36], which is a leading cause of treatment failure [37], [38].
We also showed that among patients with no tumor in the lymph nodes, the occurrence of PNI was associated with poor survival (P = .057), suggesting that the presence of PNI is an important risk factor for poor survival among patients with negative nodes. A similar finding was reported previously in a larger cohort (88 patients) [7], supporting that in the absence of lymph node metastasis, PNI status could help predict poor outcome and determine adjuvant treatment choice, such as the use of adjuvant radiation therapy [7]. When assessing the pathological node status using excised lymph node microscopic analysis, many others demonstrated that PNI is an independent predictor of lymph node metastasis for SCC [25], [26], [35], [39] and that PNI patients should undergo elective neck dissection to increase survival rates [26], [29], [35], [40].
Since evaluation of PNI in the surgical specimen is detailed and time-consuming, PNI might be under-reported in clinical practice. Also, complete histological evaluation can only be performed after surgical excision, but detecting PNI in the diagnostic biopsy, before primary surgery, is the preferred situation to improve neck (lymph node) management of patients. Using biopsy samples, our series of multiple oral cavity sub-sites detected PNI in 33.8% of patients with oral cavity SCC, a rate of detection comparable with the studies using surgical specimens and the same definition [20], [35], [40]. This finding suggests that evaluation of PNI using S100 stain on diagnostic biopsies could enhance detection of PNI during the diagnostic phase, which may influence treatment planning for subsequent definitive surgery. Patients with PNI could benefit from more aggressive treatment at the time of the primary surgery. Due to challenges in PNI detection, tumor thickness at the deepest site has been advocated as a surrogate marker of PNI and nodal metastasis in clinical practice [35], also facilitating the decision about elective neck dissection at the time of surgical excision of the tumor excision.
Our approach to detection of PNI involved not only the use of S100 but also Tuj1 antibody to stain nerves. Tuj1 was expressed in nerves in all tumor samples but the staining pattern of the nerves was slightly different from S100. Tuj1 showed a more delicate pattern of expression, which is expected since S100 stains Schwann cells while Tuj1 stains β-tubulin in the axons. However, 99% of nerves expressing S100 were clearly detected by Tuj1, supporting the use of Tuj1 or S100 to detect nerves.
GAP43 is a marker for neuronal outgrowth, more often studied in the context of nerve regeneration [18], [41], [42]. Our results demonstrate that the proportion of nerves stained by GAP43 is very high in SCC samples. Nearly all nerves stained by Tuj1 were also detected by GAP43. In the mouse skin, the majority of axons express GAP43 [43], which may be similar to the oral mucosa. Previous reports of GAP43 expression in cancer samples were not identified.
GAP43 in SCC tended to be more prominent in nerves closer to tumors. Furthermore, nerves with PNI had more proportional GAP43 staining than PNI-negative nerves. These findings could be interpreted as more intense nerve stimulation by tumor cells in proximity to nerves, translating the previously described biological mechanisms by which galanin or axon guidance molecules present in the tumor environment appear to drive neuritogenesis [14], [44], [45]. This is the first study to address neuritogenesis in the context of SCC using human clinical samples, linking nerve response to tumor proximity. In prostate cancer, nerve density and the number of ganglion neurons is increased when compared to normal prostate tissue [44], and it is likely that nerves in other tumors might behave in a similar way.
Nerve diameter was inversely related to the staining proportion of GAP43, as larger diameter nerves expressed less GAP43 compared to Tuj1. A subjective finding was that larger nerves consistently showed less GAP43 expression in the inner part of the nerve and more staining at the periphery, while smaller nerves stained evenly throughout the nerve. We believe this pattern is consistent with that expected for a marker of neuronal outgrowth, expressing more activity where the neurites project, that is at the periphery of the nerve.
Pre-treatment incisional biopsy specimens, which are smaller samples than surgical resection specimens, enabled evaluation of all nerves within 2 mm from nearest tumor cells. The study design was robust in that it was limited to oral cavity SCC collected in a similar time frame. Staining for each marker was performed sequentially and slides were scanned upon completion. Digital scanning and analysis enabled comprehensive data collection without concerns about loss of staining intensity, a particular advantage in qualitative comparisons between S100, Tuj1 and GAP43. Additionally, the samples were de-identified allowing for unbiased analysis because statistical analysis was performed by an independent group. However, some limitations such as the relatively small sample size and the use of cut-offs based on regression tree methods might impact the results. Further studies with larger sample size are necessary to establish a risk model exploring the relationship between nerve-tumor distance and nerve diameter with clinical outcomes.
Taken together, our findings support mechanistic studies showing that nerve-tumor crosstalk occurs prior to physical contact between the cancer and nerve, and the hypothesis that nerve parameters in addition to PNI status should be evaluated in histopathological examination of SCC. Most importantly, nerve-tumor proximity and larger nerve diameter seem to be related to worse survival, regardless of the presence of PNI as currently defined. Furthermore, our clinical findings indicate that the current definition of PNI and the dichotomous categorization of patients into PNI-positive and -negative might not be accurate enough to separate patients into categories of more aggressive or indolent tumors, since nerve features appear to be associated with patient outcome. Additional studies would also help elucidate if a new definition of PNI or nerve characteristics could be applied to multiple cancers or if tumor-specific definitions should be developed based on the neurotropism of each cancer.
Acknowledgments
Acknowledgements
The authors thank the many investigators in the University of Michigan Head and Neck Specialized Program of Research Excellence for their contributions to patient recruitment, assistance in data collection and encouragement including Carol R. Bradford, MD, Thomas E. Carey, PhD, Douglas B. Chepeha, MD, Sonia Duffy, PhD, Avraham Eisbruch, MD, Joseph Helman, DDS, Kelly M. Malloy, MD, Jonathan McHugh, MD, Scott A. McLean, MD, Tamara H. Miller, RN, Jeff Moyer, MD, Mark E. Prince, MD, Nancy Rogers, RN, Matthew E. Spector, MD, Nancy E. Wallace, RN, Heather Walline, PhD, Brent Ward, DDS, and Francis Worden, MD. We greatly thank patients and their families who tirelessly participated in survey and specimen collections in the University of Michigan Head and Neck Specialized Program of Research Excellence. The authors also thank Brian Pierchala, PhD for advice on neurobiology, Lisa Peterson, MPH of the University of Michigan Head and Neck Specialized Program of Research Excellence, Ms. Theresa Cody for excellent tissue sectioning, and Christopher Gordon for help with data analysis.
Funding Sources
This work was supported by grants from NIH/NIDCR DE027551, DE022567, DE024384 and a clinical research grant from the department of Periodontics and Oral Medicine (NJD), CA129102 (LJB), and P50CA097248 (GTW).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2018.04.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Singh B. Molecular pathogenesis of head and neck cancers. J Surg Oncol. 2008;97:634–639. doi: 10.1002/jso.21024. [DOI] [PubMed] [Google Scholar]
- 3.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129:354–359. doi: 10.5858/2005-129-354-PAVIIO. [DOI] [PubMed] [Google Scholar]
- 5.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 6.Johnston M, Yu E, Kim J. Perineural invasion and spread in head and neck cancer. Expert Rev Anticancer Ther. 2012;12:359–371. doi: 10.1586/era.12.9. [DOI] [PubMed] [Google Scholar]
- 7.Chinn SB, Spector ME, Bellile EL, McHugh JB, Gernon TJ, Bradford CR, Wolf GT, Eisbruch A, Chepeha DB. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149:893–899. doi: 10.1177/0194599813506867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai SK, Li WY, Yang MH, Chang SY, Chu PY, Tsai TL, Wang YF, Chang PM. Treatment for T1-2 oral squamous cell carcinoma with or without perineural invasion: neck dissection and postoperative adjuvant therapy. Ann Surg Oncol. 2012;19:1995–2002. doi: 10.1245/s10434-011-2182-5. [DOI] [PubMed] [Google Scholar]
- 9.Cruveilhier J. Chez J. B. Baillière; 1835. Anatomie pathologique du corps humain, ou descriptions, avec figures lithographiées et coloriées, des diverses altérations morbides dont le corps humain est susceptible. Vol. [Google Scholar]
- 10.Panizza BJ. An Overview of Head and Neck Malignancy with Perineural Spread. J Neurol Surg B Skull Base. 2016;77:081–085. doi: 10.1055/s-0036-1579778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batsakis J. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94:426–427. [PubMed] [Google Scholar]
- 12.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 13.Chi AC, Katabi N, Chen HS, Cheng YL. Interobserver Variation Among Pathologists in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma. Head Neck Pathol. 2016;10:451–464. doi: 10.1007/s12105-016-0722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanlon CS, Banerjee R, Inglehart RC, Liu M, Russo N, Hariharan A, van Tubergen EA, Corson SL, Asangani IA, Mistretta CM. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. doi: 10.1038/ncomms7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amit M, Na'ara S, Leider-Trejo L, Binenbaum Y, Kulish N, Fridman E, Shabtai-Orbach A, Wong RJ, Gil Z. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene. 2017;36:3232–3239. doi: 10.1038/onc.2016.483. [DOI] [PubMed] [Google Scholar]
- 16.Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, Chernichenko N, Lee SY, Barajas F, Chen CH. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126:1538–1554. doi: 10.1172/JCI82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen WR, Wang YP, Chang JYF, Yu SY, Chen HM, Chiang CP. Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43:258–264. doi: 10.1111/jop.12133. [DOI] [PubMed] [Google Scholar]
- 18.Ceber M, Sener U, Mihmanli A, Kilic U, Topcu B, Karakas M. The relationship between changes in the expression of growth associated protein-43 and functional recovery of the injured inferior alveolar nerve following transection without repair in adult rats. J Craniomaxillofac Surg. 2015;43:1906–1913. doi: 10.1016/j.jcms.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science (New York, NY) 2013;341 doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 20.Aivazian K, Ebrahimi A, Low TH, Gao K, Clifford A, Shannon K, Clark JR, Gupta R. Perineural invasion in oral squamous cell carcinoma: quantitative subcategorisation of perineural invasion and prognostication. J Surg Oncol. 2015;111:352–358. doi: 10.1002/jso.23821. [DOI] [PubMed] [Google Scholar]
- 21.Miller ME, Palla B, Chen Q, Elashoff DA, Abemayor E, St John MA, Lai CK. A novel classification system for perineural invasion in noncutaneous head and neck squamous cell carcinoma: histologic subcategories and patient outcomes. Am J Otolaryngol. 2012;33:212–215. doi: 10.1016/j.amjoto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Huang TY, Hsu LP, Wen YH, Huang TT, Chou YF, Lee CF, Yang MC, Chang YK, Chen PR. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010;46:49–55. doi: 10.1016/j.oraloncology.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Durr ML, van Zante A, Li D, Kezirian EJ, Wang SJ. Oral tongue squamous cell carcinoma in never-smokers: analysis of clinicopathologic characteristics and survival. Otolaryngol Head Neck Surg. 2013;149:89–96. doi: 10.1177/0194599813482876. [DOI] [PubMed] [Google Scholar]
- 24.Jardim JF, Francisco AL, Gondak R, Damascena A, Kowalski LP. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44:23–28. doi: 10.1016/j.ijom.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:423–431. doi: 10.1016/j.tripleo.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Chatzistefanou I, Lubek J, Markou K, Ord RA. The role of neck dissection and postoperative adjuvant radiotherapy in cN0 patients with PNI-positive squamous cell carcinoma of the oral cavity. Oral Oncol. 2014;50:753–758. doi: 10.1016/j.oraloncology.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Lin YT, Chien CY, Lu CT, Lou SD, Lu H, Huang CC, Fang FM, Li SH, Huang TL, Chuang HC. Triple-positive pathologic findings in oral cavity cancer are related to a dismal prognosis. Laryngoscope. 2015;125:E300–305. doi: 10.1002/lary.25463. [DOI] [PubMed] [Google Scholar]
- 28.Ling W, Mijiti A, Moming A. Survival pattern and prognostic factors of patients with squamous cell carcinoma of the tongue: a retrospective analysis of 210 cases. J Oral Maxillofac Surg. 2013;71:775–785. doi: 10.1016/j.joms.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131:472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Chen YW, Yu EH, Wu TH, Lo WL, Li WY, Kao SY. Histopathological factors affecting nodal metastasis in tongue cancer: analysis of 94 patients in Taiwan. Int J Oral Maxillofac Surg. 2008;37:912–916. doi: 10.1016/j.ijom.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Woolgar JA, Scott J. Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck. 1995;17:463–472. doi: 10.1002/hed.2880170603. [DOI] [PubMed] [Google Scholar]
- 32.de Matos FR, Lima E, Queiroz LM, da Silveira EJ. Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. 2012;70:1703–1710. doi: 10.1016/j.joms.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Shaw RJ, Lowe D, Woolgar JA, Brown JS, Vaughan ED, Evans C, Lewis-Jones H, Hanlon R, Hall GL, Rogers SN. Extracapsular spread in oral squamous cell carcinoma. Head Neck. 2010;32:714–722. doi: 10.1002/hed.21244. [DOI] [PubMed] [Google Scholar]
- 34.D'Cruz AK, Siddachari RC, Walvekar RR, Pantvaidya GH, Chaukar DA, Deshpande MS, Pai PS, Chaturvedi P. Elective neck dissection for the management of the N0 neck in early cancer of the oral tongue: need for a randomized controlled trial. Head Neck. 2009;31:618–624. doi: 10.1002/hed.20988. [DOI] [PubMed] [Google Scholar]
- 35.Tai SK, Li WY, Chu PY, Chang SY, Tsai TL, Wang YF, Huang JL. Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck. 2012;34:994–1001. doi: 10.1002/hed.21846. [DOI] [PubMed] [Google Scholar]
- 36.Tarsitano A, Asioli S, Morandi L, Monti V, Righi A, Morselli Labate AM, Nardi E, Foschini MP, Marchetti C. Laminin-5 and insulin-like growth factor-II mRNA binding protein-3 (IMP3) expression in preoperative biopsy specimens from oral cancer patients: Their role in neural spread risk and survival stratification. J Craniomaxillofac Surg. 2016;44:1896–1902. doi: 10.1016/j.jcms.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Le Tourneau C, Velten M, Jung GM, Bronner G, Flesch H, Borel C. Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck. 2005;27:801–808. doi: 10.1002/hed.20254. [DOI] [PubMed] [Google Scholar]
- 38.Lo W-L, Kao S-Y, Chi L-Y, Wong Y-K, Chang RC-S. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg. 2003;61:751–758. doi: 10.1016/s0278-2391(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 39.Nair S, Singh B, Pawar PV, Datta S, Nair D, Kane S, Chaturvedi P. Squamous cell carcinoma of tongue and buccal mucosa: clinico-pathologically different entities. Eur Arch Otorhinolaryngol. 2016;273:3921–3928. doi: 10.1007/s00405-016-4051-0. [DOI] [PubMed] [Google Scholar]
- 40.Tai SK, Li WY, Yang MH, Chu PY, Wang YF. Perineural invasion in T1 oral squamous cell carcinoma indicates the need for aggressive elective neck dissection. Am J Surg Pathol. 2013;37:1164–1172. doi: 10.1097/PAS.0b013e318285f684. [DOI] [PubMed] [Google Scholar]
- 41.Schicho R, Schuligoi R, Sirinathsinghji DJ, Donnerer J. Increased expression of GAP-43 in small sensory neurons after stimulation by NGF indicative of neuroregeneration in capsaicin-treated rats. Regul Pept. 1999;83:87–95. doi: 10.1016/s0167-0115(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 42.Teramoto K, Tsuboi Y, Shinoda M, Hitomi S, Abe K, Kaji K, Tamagawa T, Suzuki A, Noma N, Kobayashi M. Changes in expression of growth-associated protein-43 in trigeminal ganglion neurons and of the jaw opening reflex following inferior alveolar nerve transection in rats. Eur J Oral Sci. 2013;121:86–91. doi: 10.1111/eos.12021. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C, Guo GF, Martinez JA, Singh V, Zochodne DW. Dynamic plasticity of axons within a cutaneous milieu. J Neurosci. 2010;30:14735–14744. doi: 10.1523/JNEUROSCI.2919-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 45.Bapat AA, Munoz RM, Von Hoff DD, Han H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material