Abstract
Batrachochytrium dendrobatidis (Bd) is a pathogen killing amphibians worldwide. Its impact across much of Asia is poorly characterized. This study systematically surveyed amphibians for Bd across rocky plateaus in the northern section of the Western Ghats biodiversity hotspot, India, including the first surveys of the plateaus in the coastal region. These ecosystems offer an epidemiological model system since they are characterized by differing levels of connectivity, edaphic and climatic conditions, and anthropogenic stressors. One hundred and eighteen individuals of 21 species of Anura and Apoda on 13 plateaus ranging from 67 to 1179 m above sea level and 15.89 to 17.92° North latitude were sampled. Using qPCR protocols, 79% of species and 27% of individuals tested were positive for Bd. This is the first record of Bd in caecilians in India, the Critically Endangered Xanthophryne tigerina and Endangered Fejervarya cf. sahyadris. Mean site prevalence was 28.15%. Prevalence below the escarpment was 31.2% and 25.4% above. The intensity of infection (GE) showed the reverse pattern. Infection may be related to elevational temperature changes, thermal exclusion, inter-site connectivity and anthropogenic disturbance. Coastal plateaus may be thermal refuges from Bd. Infected amphibians represented a wide range of ecological traits posing interesting questions about transmission routes.
Keywords: Western Ghats, chytrid, amphibians, caecilians, plateaus
1. Introduction
Batrachochytrium dendrobatidis (Bd) [1] is an aggressive species of chytrid fungus that can cause the lethal amphibian infection chytridiomycosis [2]. Following the emergence of a hypervirulent lineage of Bd, the global panzootic lineage (BdGPL) [3], in the early twentieth century, the pathogen has been responsible for the loss of entire species [4] and is considered a significant threat wherever it is found [5]. The presence of Bd in the Western Ghats (WG) biodiversity hotspot [6] has been known since 2011 [7], with its known range in the WG extended in 2015 [8], and chytridiomycosis was reported from the northern WG in 2013 [9]. It was identified as an endemic Asian strain in 2013 [9]. It remains unclear what factors regulate the distribution of Bd in the WG and its transmission, and what the reasons are for its current generally sub-lethal state in the region. In a peculiar twist of fate, regions that are home to the world's greatest amphibian diversity are also most suitable for Bd [10]. In a global model, Olson et al. [2] found the entire WG to be suitable habitat for the pathogen [2].
The WG in southwest India occupy just 5% of the country's land mass and yet are home to some 42% of its amphibian species (approx. 161 species) [11,12]. Not only are the WG highly specious but many of its amphibian species are rare, with 87% being WG endemics [12]. The amphibians that are endemic to the rocky plateaus (plateaus) face both proximate and ultimate threats including climate change [13], and regional stressors (population growth [14]), along with rapid habitat loss through mining, tourism and wind turbine installations [15–18]. They also face challenges from pathogens such as the fungus Batrachochytrium dendrobatidis (Bd) [7–9]. Systematic studies on Bd in Asia are under-represented in the literature [19], and this study aims to help to address that shortfall.
The three studies that have been published so far examining Bd infection in the Western Ghats biodiversity hotspot cover almost the entire length of the WG and report widely differing levels of infection ranging from 0.6% [7] to 25% [9]. The most geographically extensive study, covering the northern, central and southern WG, reported an infection rate of 1.6% [8]. All three studies excluded the low-lying sites between the coast and the hills. Molur et al. [8] published a predictive model showing higher risk of infection south of approximately 14.5°N in the central section of the WG.
This present study adds considerably to the previous WG publications by surveying low elevation sites for the first time. In addition, this is the first study in the WG to offer data on habitat specific infection rates, and infection patterns across a wide range of elevations. Such data are highly important as the high-level plateaus are becoming recognized as centres of endemism for a number of taxonomic groups and data on all threats are urgently needed for their effective management [17–20].
2. Methods
2.1. Study area
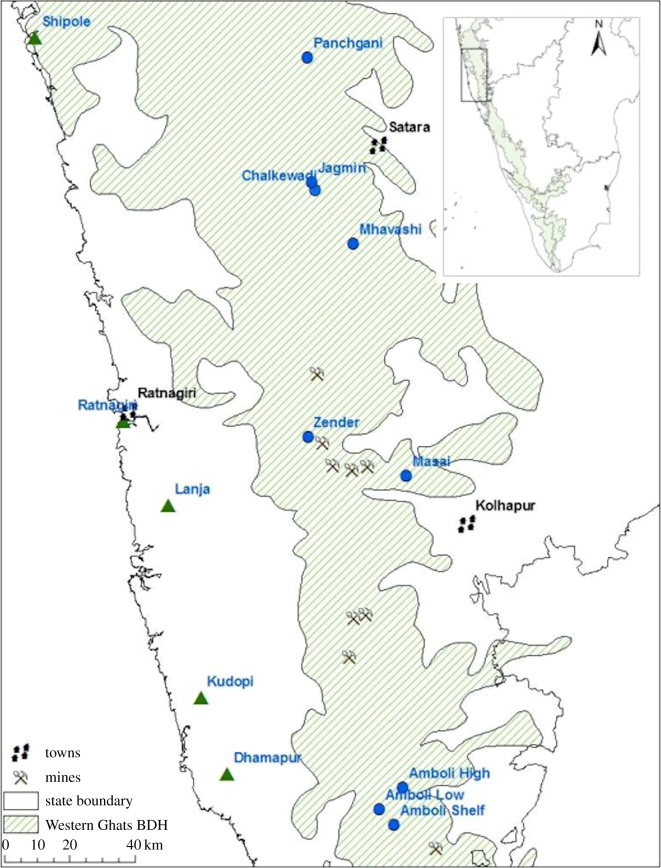
The WG are a chain of hills some 1500 km in length running parallel and slightly inland from the south west coast of India from the Maharashtra/Gujarat state border to the country's southern tip (figure 1). They are part of the Western Ghats--Sri Lanka biodiversity hotspot [6] and the eighth ‘hottest’ hotspot on the planet [21]. Unlike the granitic central and southern sections, the northern section in western Maharashtra, known as the Deccan Traps (DT), is formed from basalt. The plateaus are ferricretes of laterite forming hilltop carapaces above the escarpment on the western edge of the WG rising to 1200 m above sea level (m), with extensive low-lying plateaus below in an area known as the Konkan [22]. Temperatures range from 15°–40°C in the Konkan and 4°–42°C above the escarpment [23]. The higher elevation amphibian populations are exposed to lower temperatures that may be more conducive to Bd infection [24].
Figure 1.
Map of study area, with study area location within India (inset). Nearest large towns are shown for reference points. Blue circles denote plateaus in the High Region and green triangles those in the Low Region. Mine sites are indicated to reflect one of the risks to these sites. The biodiversity hotspot (BDH) [6] outline was created in ArcGIS based on data downloaded from ESRI (Environmental Systems Research Institute, Redlands, California, USA).
2.2. Amphibian sampling
Amphibians were sampled from 13 representative plateaus situated in the northern WG both above and below the North--South escarpment in western Maharashtra during the early monsoon in 2013 and 2014 (late July–early August; figure 1, electronic supplementary material, table S3). Plateaus were selected to represent the latitudinal and elevation extent of laterite in western Maharashtra together with the range of land uses in each region (electronic supplementary material, table S3). Visual encounter surveys with supplementary refugia searching were performed along four 6 m by 100 m transects on each plateau in each year [25,26]. Anthropogenic disturbance factors assessed at each site were: removal of loose rocks, surfaced road, unsurfaced road, built structures on the plateau, domesticated animal grazing, surfaced road within 200 m of plateau, tourism, part conversion to plantation, adjacent built structures and importation of topsoil. Sites with 0–3 factors were considered to have low levels of disturbance, those with 4–7 medium disturbance and with 8+ high disturbance (electronic supplementary material, table S3).
Sampled amphibians were identified by morphological comparison with the best literature available (including [27–45]). Many WG amphibians are taxonomically cryptic or unstable [43–45]; in cases where species-level identification may be in doubt we use ‘cf.’.
2.3. Field sampling and laboratory techniques
Amphibians were all hand-captured, individually bagged and transported to shelter where skin swabs were taken from ventral surfaces. Swabbing was performed by a pair of surveyors using sterile cotton tip swabs (T/S16-B; Technical Service Consultants Ltd) [46,47]. The ventrum, drink patch, thighs and toe-webbing of each adult anuran and metamorph were swabbed multiple times following published standardized protocols [48]. For caecilians a simpler approach of multiple swab strokes along the whole of the body was used. Swabs were broken off into sterile vials of 99% ethanol. Disposable equipment (latex gloves and polythene bags) were replenished between specimens and sites. Other equipment was sterilized using VirkonS™ solution or dried to minimize cross-contamination.
DNA extraction from the swabs followed the protocol described by Boyle et al. [46,49]. A quantitative real-time polymerase chain reaction (qPCR) diagnostic assay was used with Bd specific probes for the ribosomal gene region ITS-1/5.8S [49]. The qPCR was run on an Applied Biosystems QuantStudio 7 Flex Real-Time PCR System with an additional 10 cycles being added to the Boyle protocol (60 cycles total). Standards of known concentration of Bd DNA (100, 10, 1, 0.1 Bd GE (zoospore genomic equivalents)) were used as positive controls and standards together with no template control (NTC) of molecular grade water as a negative control. The samples were run in duplicate with single positives repeated. A positive result was a sample with a GE greater than 0.1 in both samples in a qPCR pair, a single was a sample for which only one of each qPCR pair had a GE greater than 0.1 and a weak signal was where only one out of four qPCR scores was greater than 0.1.
2.4. Environmental correlates
Bd is sensitive to a range of changes in the abiotic environment. Plateau soils are acidic, ranging as low as pH 4.9 which is below the Bd optimum of pH 6–8 [16,23,50]. In addition, Bd is temperature-sensitive, growing best between 17 and 25°C with an optimum of 23°C [50,51]. Temperature may also regulate the pathogenicity of Bd, with frogs exposed between 17 and 23°C more likely to die than those exposed at 27°C [52]. The fungus is reported to die when the temperature exceeds 30°C, a level exceeded at times on all plateaus [10,16]. Lower seasonal temperatures, such as those during the monsoon at higher elevations in the WG, are known to favour the pathogen [50]. The plateaus are mosaics of microhabitats set in a heterogeneous landscape with unknown degrees of connectivity for Bd [15,16,23,53]. Microhabitats include expanses of exposed rock often with associated loose rocks. Exposed rock absorbs solar radiation giving it a surface temperature higher than the air, perhaps creating microclimates that may reduce the intensity and presence of the pathogen [54]. Other microhabitats include loose rocks and fissures offering refugia from dry areas and excessive temperatures [55–57]. Optimum rainfall for Bd is reported to be between 1500 and 2500 mm a year. Only the low lying plateaus fall within this range, the plateaus along the top of the escarpment receiving between 4000 and 9000 mm, with the exception of Masai which is east of the ridge and may be drier [10,55].
Macro-environment and physiochemical data were recorded for each site: air, soil and water temperature (°C) and pool pH using a calibrated electrical probe (Hanna Instruments™ HI 9064); elevation (m), latitude and longitude for the start and end of each transect using a hand held GPS (Garmin™ 60csx GPS).
3. Permission for fieldwork
Permission for accessing biodiversity in India including the fieldwork was granted by the National Biodiversity Authority, India, to C. J. Thorpe, permit number MC200621. The permit authorizes some other authors to assist with sampling.
4. Data analysis
To help make analytical comparisons at a range of spatial scales, the study area was divided into two regions: above the Western Ghats escarpment (High Region), and below it (Low Region). The dividing line was set at 700 m above sea level. Each region was arbitrarily sub-divided into three latitudinal groups North, Central and South (electronic supplementary material, table S3). Correlations between site elevation, temperature and pH were explored through Pearson product moment correlation for parametric data and Spearman rank correlation for non-parametric data. To assess the impact of the spatial arrangement and disturbance on Bd GE values, general linear model (GLM) and one-way ANOVA analyses were performed to investigate elevation, latitude, region, disturbance type and disturbance intensity. Results are reported with a confidence level (CL) of 95% together with upper and lower bi-nomial confidence bounds (CB), which are the outer values of the confidence interval that are expressed as percentages [58,59].
5. Results
5.1. Overview of Bd infection in the study area
A total of 118 sample swabs were taken from individuals belonging to 2 orders, 6 families, 14 genera and 19 taxa (table 1; electronic supplementary material, tables S4 and S5). Seventy-nine per cent of the taxa tested had individuals positive for Bd (table 1; electronic supplementary material, tables S4 and S5). The study does not provide an inventory of infection for the area as it only covered one ecosystem and complete detection of both species and infection is problematic [60]. Total prevalence in the sample was 11% (95% CL; CB 7–19), 22% (95% CL; CB 15–31) if single positives were included and 27% (95% CL; CB 19–36) including weak positives. As in other WG studies, all GE values recovered were low and all those with a single GE value greater than 0.1 were included in the analysis (table 1; electronic supplementary material, tables S4 and S5 [7–9,61]).
Table 1.
Taxonomy, risk and infection as prevalence. Where nomenclature is uncertain the rules of the International Code of Zoological Nomenclature (ICZN) have been followed. Where identification is hampered by cryptic species a most likely identity is shown with the prefix ‘cf’. IUCN threat status and known habitat associations accessed 10/02/2017 [51]. NA, not assessed; DD, Data Deficient; LC, Least Concern; EN, Endangered; CR, Critically Endangered. Prevalence is the percentage of the sample tested positive for Bd.
| order | family | taxa | IUCN | N | prevalence (%) | CB (%) |
|---|---|---|---|---|---|---|
| Anura | Bufonidae | Duttaphrynus melanostictus | LC | 7 | 43 | 10–82 |
| Anura | Bufonidae | Xanthophryne tigerina | CR | 15 | 6.7 | 1–32 |
| Anura | Dicroglossidae | Euphlyctis cf. cyanophlyctis | LC | 2 | 0 | 0 |
| Anura | Dicroglossidae | Fejervarya cf. brevipalmata | DD | 9 | 11 | 0.003–0.48 |
| Anura | Dicroglossidae | Fejervarya cf. caperata | DD | 4 | 75 | 19–99 |
| Anura | Dicroglossidae | Fejervarya cf. cepfi | NA | 7 | 14 | 0.01–58 |
| Anura | Dicroglossidae | Fejervarya cf. sahyadris | EN | 14 | 36 | 13–65 |
| Anura | Dicroglossidae | Fejervarya sp. | 10 | 33 | 7–65 | |
| Anura | Dicroglossidae | Hoplobatrachus tigerinus | LC | 9 | 56 | 21–86 |
| Anura | Dicroglossidae | Sphaerotheca dobsonii | LC | 5 | 20 | 1–72 |
| Anura | Micorhylidae | Microhyla ornata | LC | 1 | 0 | 0 |
| Anura | Micorhylidae | Uperodon globulosus | LC | 1 | 0 | 0 |
| Anura | Ranixalidae | Indirana cf. chiravesi | LC | 3 | 33 | 1–91 |
| Anura | Rhacophoridae | Pseudophilautus sp. | 1 | 0 | 0 | |
| Anura | Rhacophoridae | Raorchestes ghatei | NA | 5 | 60 | 15–95 |
| Gymnophiona | Indotyphlidae | Gegeneophis cf. ramaswamii | LC | 5 | 20 | 1–72 |
| Gymnophiona | Indotyphlidae | Gegeneophis seshachari | DD | 9 | 44 | 14–79 |
| Gymnophiona | Indotyphlidae | Indotyphlus cf. battersbyi | DD | 2 | 50 | 1–99 |
| Gymnophiona | Indotyphlidae | Indotyphlus maharashtraensis | DD | 3 | 33 | 1–91 |
All four species of caecilian found in the study were infected (table 1; electronic supplementary material, tables S4 and S5). The critically endangered Amboli toad, Xanthophryne tigerina, was infected with low prevalence (6.7%; 95% CL; CB 1–32) as were 33% of the endangered frog Fejervarya cf. sahyadris in the sample (95% CL; CB 13–65) [51]. One site, Amboli High, returned no positives out of six Xanthophryne tigerina samples. Amboli High was the only site without any positive results. No amphibians were detected with external signs of chytridiomycosis.
5.2. Spatial distribution of Bd infection
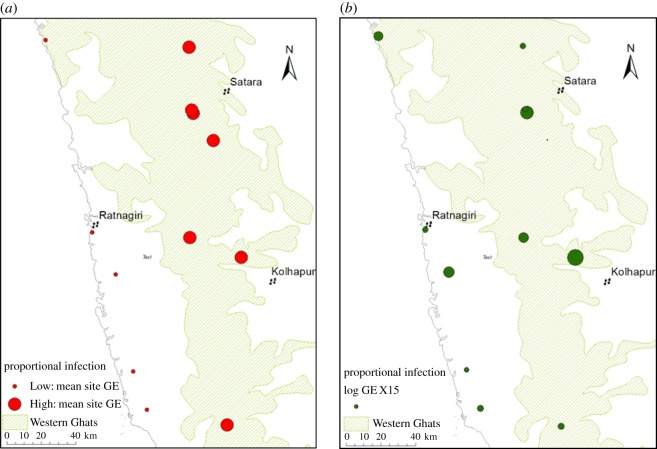
Site prevalence varied between 0 and 50% and species infection rate was 0–60% (table 1; electronic supplementary material, table S4). Macroscale variations in GE values were found with the regions exhibiting a significant 10-fold difference in mean GE (ANOVA, F1,2 = 3.99, p = 0.06) (electronic supplementary material, table S2; figure 2a,b). The Low Region had more infected individuals (56%; 95% CL; CB 26–62) than the High Region (44%; 95% CL; CB 37–56). Individual GE value increased with elevation (ANOVA, F11,20 = 4.85, p < 0.01).
Figure 2.
(a) The proportional difference in regional values of mean site GE values. (b) The individual site mean GE.
5.3. Environmental relationships with Bd infection
As both water temperature and pH covaried with elevation, and pH with water temperature, elevation alone was used to explore spatial relationships (electronic supplementary material, table S1). Temperature in the Low Region had a notable maximum of 36.4°C (mean 30.9°C), much higher than above the escarpment at 28.3°C (mean 22.5°C; minimum 19.3°C) (table 2). Both regions had minimum pH values below the Bd optimum although mean values were within the pathogen's tolerance range (table 2).
Table 2.
Physio-chemical parameters described for the survey area as a whole and the two regions. Temperature = water temperature in °C.
| variable | Region | mean | minimum | maximum |
|---|---|---|---|---|
| temperature | Low | 30.9 | 26.2 | 36.4 |
| temperature | High | 22.5 | 19.3 | 28.3 |
| temperature | all | 24.8 | 19.3 | 36.4 |
| pH | Low | 6.7 | 5.0 | 9.6 |
| pH | High | 7.6 | 5.3 | 12.2 |
| pH | all | 7.3 | 5.0 | 12.2 |
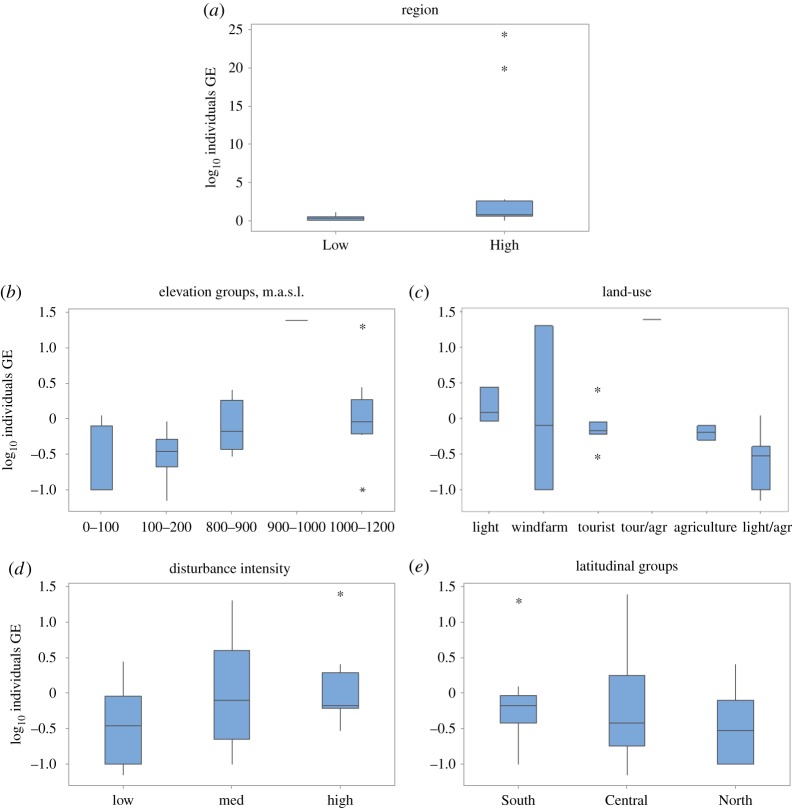
GLM ANOVA analysis was used to assess the factors regulating the distribution of infection intensity (figure 3a–e). Plateau elevation had the greatest impact showing an upward trend with elevation (figure 3b). Land-use was the second most crucial factor: agriculture had a negative impact on sites below the escarpment, and above the escarpment the type of land-use did not have a clear impact (figure 3c). The intensity of disturbance was related to an increasing trend in infection intensity (figure 3d). Latitude, which includes Low and High Region sites in each class, suggests a limited decreasing trend with increasing latitude (figure 3e).
Figure 3.
Log10 transformed GE data for individuals. Quartile 2 and 3 are shaded with the dividing line as the median. The whiskers indicate quartiles 1 and 4. Outliers are indicated by asterisks. (a) Regions (above, High, and below, Low, the Western Ghats escarpment). GLM ANOVA results for the other classes: (b) elevation groups, F = 12.77, d.f.factor = 1, d.f.error = 24, p < 0.01; (c) land-use, F = 10.88, d.f.factor = 1, d.f.error = 24, p < 0.01; (d) disturbance intensity, F = 2.99, d.f.factor = 4, d.f.error = 24, p < 0.05; (e) latitudinal groups, F = 3.33, d.f.factor = 1, d.f.error = 24, p = 0.08.
6. Discussion
There was widespread but low intensity infection of Bd on almost all the plateaus sampled except for one, Amboli High, and in 79% of the amphibian species examined on the rocky plateaus in the northern WG (table 1; electronic supplementary material, tables S3 and S4). These are the first records of infection in the critically endangered Amboli toad, Xanthophryne tigerina; the endangered frog Fejervarya cf. sahyadris; and four species of caecilian, Gegeneophis cf. ramaswamii, Gegeneophis seshachari, Indotyphlus cf. battersbyi and Indotyphlus maharashtraensis [62]. Three of the caecilian species are described by the IUCN as Data Deficient (table 1). Infection was detected in two species yet to be assessed by the IUCN, Fejervarya cf. cepfi and Raorchestes cf. ghatei; and two other Data Deficient species, Fejervarya cf. brevipalmata and Fejervarya cf. caperata (table 1). This is the first study to investigate Bd infection in low lying coastal sites, where there was higher prevalence but lower intensity infection than on sites above the WG escarpment (electronic supplementary material, tables S2 and S3; figure 3a). Site elevation, with its covariables, and disturbance intensity were the most significant explanatory factors in the pattern of Bd distribution (electronic supplementary material, tables S2 and S3; figure 3b,c). However, another explanation, not explored here, for the observed pattern in Bd distribution is that the amphibian populations are relics of ancient dispersals isolated from the pathogen's transmission vectors [63].
6.1. Low elevation plateaus are less conducive to Bd but with greater connectivity
Puschendorf et al. [54] suggest that disease-free amphibian refuges are created in drier areas with temperatures above those tolerated by Bd [54]. We suggest our findings, with lower infection intensities on the Konkan plateaus, support them as a possible refugia for some amphibian species from Bd. However, the higher prevalence on plateaus below the escarpment is more difficult to explain. The plateaus' specific environment derived from their open habitat may have created thermal refugia from Bd in rock pools and surrounding habitats where temperatures exceed the pathogen's upper thermal tolerance, but it should also restrict transmission. The pools are scattered across plateaus with surface temperatures, especially on the exposed rock, greatly more than the pathogen's thermal maximum, which should restrict the pathogen's persistence and transmission [23,64].
Low Region water temperatures were higher than those above the escarpment, on average 30.9°C, with a maximum of 36.4°C, a figure well above published critical zoospore thermal thresholds of 23–28°C (tables 1 and 2; figure 3a; electronic supplementary material, table S2 and S3) [2,10,50,65]. Even more lethal are the rock surface temperatures which can exceed 50°C [23]. The region is also drier, with less rain and fewer wet days than above the escarpment. The annual rainfall falls within the pathogen's preferred rainfall range of 1500–2500 mm but it only rains for five months a year, with the remaining seven months being almost completely dry with very low relative humidity [10,23,65,66]. Infection intensity decreased slightly with increasing latitude possibly reflecting the latitudinal decline in the number of wet days (figure 3e) [56,57,66].
Regional differences in habitat and micro-habitat availability may also help explain the pattern in Bd distribution, through behavioural mitigation where amphibians move to, or persist on, plateaus with micro-habitats that are refugia from Bd [55,57,67]. Conversely, stream micro-habitats offer one possible transmission route in the Low Region, where streams are more frequent [55]. Sub-tropical stream-breeders are more susceptible to Bd and may be disease vectors with the pathogen spreading from streams into the terrestrial realm [67,68]. Increased landscape connectivity in the Low Region, resulting from lower inter-site variation in elevation (Low Region 103 m, High Region 370 m), may enable greater inter-site transmission through amphibians dispersing between plateaus, explaining the higher prevalence and supporting the findings of Heard et al. [69] (electronic supplementary material, table S2 and S3) [53,70]. The picture is complex though as some refugia such as woody plants and large loose rocks may enable behavioural avoidance of excessive temperatures for both amphibians and Bd [51,69,71]. This idea possibly supported by the results for the four species of caecilian in the study which have similar prevalence to non-fossorial taxa (table 1; electronic supplementary material, tables S4 and S5). They are frequently found under loose rocks where temperatures are tolerable for Bd and are close to streams which may be used by stream-breeding anuran vectors [55,67].
6.2. Bd in the High Region
Despite the High Region offering a more equitable temperature range for Bd with a mean of 22.5°C, within the pathogen's in vitro optimum of 17–25°C, prevalence was less than below the escarpment where the pathogen's upper limit was often exceeded. This suggests the High Region's greater topographical heterogeneity produces barriers to transmission which may explain the lower number of infected individuals (tables 1 and 2; electronic supplementary material, tables S2–S4; [10,50,51]).
Individual GE loadings were greater above the escarpment reflecting the High Region's optimal temperature (table 2 and electronic supplementary material, table S2; [10,51]). While excessive temperatures below the escarpment may regulate the pathogen through mortality the High Region's lower temperature regime may encourage Bd, even when the temperature falls below the organism's lower optimum value (17°C). A temperature of 4°C was used by Voyles et al. [57] as their minimum in a study assessing the impact of temperature regimes on Bd life history; the same minimum temperature was also recorded by Watve [23] on High Region plateaus. Their lower temperature regime resulted in extended zoospore longevity meaning zoospore numbers in water bodies could be expected to be greater than in warmer pools. The trait may lead to greater encounter rates and thus infection prevalence contrary to our findings [57]. However, they also found their low temperature regime increased zoospore production, which offers a plausible explanation for the elevated site GE values in the High Region.
6.3. The impact of anthropogenic disturbance on Bd distribution
Elevated prevalence close to human settlement is to be expected but with unknown causes [72]. The study found that Low Region plateaus, which are less isolated from human settlement, had higher prevalence than their more isolated High Region counterparts [72].
In addition to proximity of human habitation, changes in land-use influences amphibian distribution, and possibly their susceptibility and exposure to disease [55,73,74]. Sites near human habitation are likely to have anthropogenic land-uses. We found land-uses differed either side of the escarpment. Land-use had an impact on mean individual infection intensity with Low Region agricultural sites having higher infection intensity than the nearest sites with limited disturbance (figure 3c). Land-use in the High Region had a negligible impact on mean individual infection levels (figure 3c). Sites with little disturbance, on our arbitrary scale, had lower mean individual GE compared to plateaus with higher disturbance (figure 3d). The actual mechanisms remain unclear, but we can support anthropogenic disturbance as a negative factor in Bd infection. The sites disturbed by tourism had amphibian assemblages dominated by generalist species, but this had no impact on mean individual infection intensity suggesting mobile species may not be pathogen vectors [55].
The impact of disturbance on Bd infection intensity was less than that of elevation (figure 3b–d). Site prevalence did not reflect land-use (electronic supplementary material, table S3; figure 3b–e). As all the sites with tourism were above the escarpment and elevation had the greater explanatory power, we believe spatially driven climate has more effect than land-use. There is a clearer indication that agriculture negatively impacts infection intensity as seen in our Low Region sites, where there is little inter-site variation in elevation but a significant difference in infection intensity (figure 3c).
7. Conclusion
It is clear that the Bd pathogen is very widely distributed in this area and anthropogenic land-use increases the infection risk. The infected plateau amphibians include several threatened and poorly understood species whose infection we record for the first time. None of the individuals that tested positive for Bd showed any external signs of chytridiomycosis. The disease has been reported in Nyctibatrachus humayuni from sites close to the northern edge of this survey [9]. The infection level reported here is well below the mortality threshold of 1–10 000 zoospores [75] and is more indicative of an historical infection, or species that are carriers that do not go on to develop chytridiomycosis [76–78]. The triggers for this low intensity infection to develop into a lethal outbreak of chytridiomycosis are unknown.
Transmission vectors are poorly understood globally as well as in the WG, but we would support the possible explanation of water birds as vectors with lapwing species (Vanellus indicus) being frequently observed on all the plateaus [79]. Proximity to human habitation is a risk factor but the mechanisms of transmission are unknown.
Until there is a better understanding of the mechanisms triggering benign Bd infection to become lethal chytridiomycosis, its presence should be considered in all future conservation policy decisions. Preservation of dispersed populations on sites with refugial properties, good connectivity and preservation of refugia on individual plateaus is essential in offering the best prospect of long-term species persistence [69,80]. The need for further work on modelling infection on a wider scale, especially in the low lying coastal areas, characterized here for the first time for Bd, is a priority. A study into the evolutionary history of Bd in the entire WG area would also help with its management. There is an urgency to determine the routes of transmission and triggers for the pathogen to become lethal. That urgency is illustrated by the 2015 publication of the addition of Duttaphrynus melanostictus to Hoplobatrachus tigerinus as invasive species in another biodiversity hotspot, Madagascar, [81]. Both of these species had high Bd prevalence in this study, 43% and 56% respectively. Resolution of these questions may be helped by a better understanding of the historical lineage of the Bd strain in the WG.
Supplementary Material
Acknowledgements
We thank Dr Neelesh Dahanukar and an anonymous reviewer for their help in refining the manuscript. Many people have helped to bring the project to fruition: Dr Aparna Watve, Sanjay Thakur and Dr Varad Giri for their tireless support; Dr Hemant Ghate and Dr Anand Padhye helped with laboratory space and resolving identification; David Gower aided caecilian identification; Dr N. Dahanukar for freezer space to preserve the swabs; Nikhil Gaitonde for his field assistance; Dr Ramana Athreya kindly provided bench space to A.W. and C.J.T.; Jennifer Shelton and Pria Ghosh for DNA extraction; and Felicity Wynne for help in interpretation. Dr Robert Puschendorf gave invaluable help in many stages of the study.
Ethics
Advice was sought from the University of Plymouth Animal Welfare and Ethics Committee representative who advised that no formal consent was required since the swabs were non-invasive, collected from external swabbing only. Further they advised following strict international handling protocols and these are described under Methods. Sampling was undertaken by kind permission of the National Biodiversity Authority, Chennai, India under permit number: Maharashtra 2014 MC200621.
Data accessibility
The raw data of infection intensity with species and site localities are available in the electronic supplementary material.
Authors' contributions
C.J.T. designed and coordinated the study, obtained permits, collected field data, processed samples in the laboratory, executed the statistical analysis and drafted and submitted the manuscript. T.R.L. assisted by L.D. and D.P. collected field data, T.R.L. helped edit the manuscript. M.C.F. and C.J.W. carried out the molecular analysis and refined the manuscript. S.K. participated in the data collection and fieldwork logistics. A.W. helped with the permit and logistics and assisted with fieldwork. M.E.K. supervised the project, assisted in some of the fieldwork, and helped to refine the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
C.J.T.: The Royal Geographical Society with IBG through Geographical Fieldwork Grants in 2013 and 2014 and the Monica Cole Award 2012. C.J.T.: The Erasmus Darwin Barlow Expedition Fund, Zoological Society of London grant in 2014. T.R.L.: Percy Sladen Memorial Trust award in 2014. M.C.F.: The Leverhulme Trust grant no. RPG-2014-273.
References
- 1.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227. (doi:10.2307/3761366) [Google Scholar]
- 2.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Fisher MC, The Bd Mapping Group. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 8, e56802 (doi:10.1371/journal.pone.0056802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrer RA, et al. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl Acad. Sci. USA 108, 18 732–18 736. (doi:10.1073/pnas.1111915108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher MC, Garner TW, Walker SF. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 63, 291–310. (doi:10.1146/annurev.micro.091208.073435) [DOI] [PubMed] [Google Scholar]
- 5.Rödder D, et al. 2009. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 1, 52–66. (doi:10.3390/d1010052) [Google Scholar]
- 6.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 7.Nair AS, Daniel O, Gopalan SV, George S, Kumar KS, Merila J, Teacher AGF. 2011. Infectious disease screening of Indirana frogs from the Western Ghats biodiversity hotspot. Herpetol. Rev. 42, 554–557. [Google Scholar]
- 8.Molur S, Krutha K, Paingankar MS, Dahanukar N. 2015. Asian strain of Batrachochytrium dendrobatidis is widespread in the Western Ghats, India. Dis. Aquat. Organ. 112, 251–255. (doi:10.3354/dao02804) [DOI] [PubMed] [Google Scholar]
- 9.Dahanukar N, Krutha K, Paingankar MS, Padhye AD, Modak N, Molur S. 2013. Endemic Asian chytrid strain infection in threatened and endemic anurans of the northern Western Ghats, India. PLoS ONE 8, e77528 (doi:10.1371/journal.pone.0077528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ron SR. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37, 209–221. (doi:10.1111/j.1744-7429.2005.00028.x) [Google Scholar]
- 11.Aravind NA, Gururaja KV. 2011 Theme paper on the amphibians of the Western Ghats. Report submitted to Western Ghats Ecology Expert Panel. Available at: MoEF Electronic database, see http://www.westernghatsindiaorg/sites/default/files/Amphibians .
- 12.Giri V. 2016. Diversity and conservation status of the Western Ghats amphibians. In Threatened amphibians of the world (eds Stuart SN, Hoffman M, Chanson JS, Cox NA, Berridge R, Ramani P, Young BE), pp. 80--82 Barcelona, Spain: Lynx Ediciones. [Google Scholar]
- 13.IPCC. 2014. International Panel on Climate Change, Chapter 24: Asia 2014. See http://www.ipcc.ch/pdf/assessment-report/ar5/wg2/WGIIAR5-Chap24.
- 14.Cincotta RP, Wisnewski J, Engelman R. 2000. Human population in the biodiversity hotspots. Nature 404, 990–992. (doi:10.1038/35010105) [DOI] [PubMed] [Google Scholar]
- 15.Thorpe C, Watve A. 2016. Lateritic plateaus in the Northern Western Ghats, India; a review of bauxite mining restoration practices. J. Ecol. Soc. 28, 25–44. [Google Scholar]
- 16.Watve A. 2013. Status review of rocky plateaus in the northern Western Ghats and Konkan region of Maharashtra, India with recommendations for conservation and management. J. Threat. Taxa 5, 3935–3962. (doi:10.11609/JoTT.o3372.3935-62) [Google Scholar]
- 17.Kasturirangan K, et al. 2013. Report of the higher level working group on Western Ghats. Ministry of Environment and Forests, Government of India.
- 18.Bharucha EK. 2010. Current ecological status and identification of potential ecologically sensitive areas in the Northern Western Ghats. Pune, Maharashtra: Bharti Vidyapeeth Deemed University, Research IoEEa. [Google Scholar]
- 19.Whittaker K, Vredenburg V.2011. An overview of chytridiomycosis. Available from: http://www.amphibiaweb.org/chytrid/chytridiomycosis.html .
- 20.Porembski S, Silveira FAO, Fieldler PL, Watve A, Rabarimanarivo M, Kouame F, Hopper SD. 2016. Worldwide destruction of inselbergs and related rock outcrops threatens a unique ecosystem. Biodiversity Conserv. 25, 2827–2830. (doi:10.1007/s10531-016-1171-1) [Google Scholar]
- 21.Sloan S, Jenkins CN, Joppa LN, Gaveau DLA, Laurance WF. 2014. Remaining natural vegetation in the global biodiversity hotspots. Biol. Conserv. 177, 12–24. (doi:10.1016/j.biocon.2014.05.027) [Google Scholar]
- 22.Widdowson M, Cox K. 1996. Uplift and erosional history of the Deccan Traps, India: evidence from laterites and drainage patterns of the Western Ghats and Konkan Coast. Earth Planet. Sci. Lett. 137, 57–69. (doi:10.1016/0012-821X(95)00211-T) [Google Scholar]
- 23.Watve A.2010. Rocky plateaus (special focus on the Western Ghats and Konkan). Report to ‘ Western Ghats Ecology Expert Panel. Pune, India: BIOME Conservation Foundation.
- 24.Laurance WF, McDonald KR, Speare R. 1996. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv. Biol. 10, 406–413. (doi:10.1046/j.1523-1739.1996.10020406.x) [Google Scholar]
- 25.Crump ML, Scott NJ. 1994. Visual encounter surveys. In Measuring and monitoring biological diversity: standard methods for amphibians (eds Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS), pp. 84–92. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 26.Vonesh JR, Mitchell JC, Howell K, Crawford AJ. 2010. Rapid assessments of amphibian diversity. In Amphibian ecology and conservation: a handbook of techniques (ed Dodd CK., Jnr), pp. 263–280. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Bhatta G. 1998. A field guide to the caecilians of the Western Ghats, India. J. Biosci. 23, 73–85. (doi:10.1007/BF02728526) [Google Scholar]
- 28.Dubois A, Ohler A-M, Biju SD. 2001. A new genus and species of Ranidae (Amphibia, Anura) from south-western India. Alytes 19, 53–79. [Google Scholar]
- 29.Bossuyt F. 2002. A new species of Philautus (Anura: Ranidae) from the Western Ghats of India. J. Herpetol. 36, 656–661. (doi:10.1670/0022-1511(2002)036[0656:ANSOPA]2.0.CO;2) [Google Scholar]
- 30.Biju SD, Bossuyt F.2009. Pseudophilautus amboli (Biju & Bossuyt, 2009) IUCN Red List 2009. See http://www.iucnredlist.org/details/58910/0 .
- 31.Biju SD, Bossuyt F. 2009. Systematics and phylogeny of Philautus gistel, 1848 (Anura, Rhacophoridae) in the Western Ghats of India, with descriptions of 12 new species. Zool. J. Linn. Soc. 155, 374–444. (doi:10.1111/j.1096-3642.2008.00466.x) [Google Scholar]
- 32.Biju SD, Van Bocxlaer I, Giri V, Loader S, Bossuyt F. 2009. Two new endemic genera and a new species of toad (Anura: Bufonidae) from the Western Ghats of India. BMC Res. Notes 2, 241 (doi:10.1186/1756-0500-2-241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel J. 2002. The book of Indian reptiles and amphibians. Oxford, UK: Bombay Natural History Society and Oxford University Press. [Google Scholar]
- 34.Giri V, Wilkinson M, Gower D. 2003. A new species of Gegeneophis Peters (Amphibia: Gymnophiona: Caeciliidae) from the Western Ghats of southern Maharashtra, India, with a key to the species of the genus. Zootaxa 351, 1–10. (doi:10.11646/zootaxa.351.1.1) [Google Scholar]
- 35.Giri V, Gower DJ, Wilkinson M. 2004. A new species of Indotyphlus Taylor (Amphibia: Gymnophiona: Caeciliidae) from the Western Ghats, India. Zootaxa 739, 1–19. [Google Scholar]
- 36.Kuramoto M, Joshy SH. 2003. Two new species of the genus Philautus (Anura: Rhacophoridae) from the Western Ghats, southwestern India. Curr. Herpetol. 22, 51–60. (doi:10.5358/hsj.22.51) [Google Scholar]
- 37.Kuramoto M, Joshy SH, Kurabayashi A, Sumida M. 2007. The genus Fejervarya (Anura: Ranidae) in central Western Ghats, India, with descriptions of four new cryptic species. Curr. Herpetol. 26, 81–105. (doi:10.3105/1881-1019(2007)26[81:TGFARI]2.0.CO;2) [Google Scholar]
- 38.Dinesh K, Radhakrishnan C, Gururaja K, Bhatta G.2009. An annotated checklist of amphibian of India with some insights into the patterns of species discoveries, distribution and endemism. Records of the Zoological Survey of India, Miscellaneous publication ; occasional paper no 302.
- 39.Dinesh K, Radhakrishnan C, Channakeshavamurthy B, Kulkarni NU.2015. Checklist of amphibians of India. See http://mhadeiresearchcenter.org/wpcontent/uploads/2014/01/2017_April_Checklist-of-Amphibians-of-India.pdf .
- 40.Frost DR.2017. Amphibian species of the world: an online reference. Version 6 Electronic database. See http://research.amnh.org/vz/herpetology/amphibia/content/search?taxon&subtree&sub .
- 41.Padhye A, Sayyed A, Jadhav A, Dahanukar N. 2013. Raorchestes ghatei, a new species of shrub frog (Anura: Rhacophoridae) from the Western Ghats of Maharashtra, India. J. Threat. Taxa 5, 4913–4931. (doi:10.11609/JoTT.o3702.4913-31) [Google Scholar]
- 42.Dinesh KP, Vijaykumar SP, Channakeshavamurthy BH, Torsekar VR, Kulkarni NU, Shanker K. 2015. Systematic status of Fejervarya (Amphibia, Anura, Dicroglossidae) from South and SE Asia with the description of a new species from the Western Ghats of Peninsular India. Zootaxa 3999, 16 (doi:10.11646/zootaxa.3999.1.5) [DOI] [PubMed] [Google Scholar]
- 43.Garg S, Biju S. 2017. Description of four new species of burrowing frogs in the Fejervarya rufescens complex (Dicroglossidae) with a notes on morphological affinities of Fejervarya species in Western Ghats. Zootaxa 4277, 451–490. (doi:10.11646/zootaxa.4277.4.1) [DOI] [PubMed] [Google Scholar]
- 44.Garg S, Biju SD. 2016. Molecular and morphological study of leaping frogs (Anura, Ranixalidae) with description of two new species. PLoS ONE 11, e0166326 (doi:10.1371/journal.pone.0166326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahanukar N, Modak N, Krutha K, Nameer P, Padhye AD, Molur S. 2016. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: an integrated taxonomic review. J. Threat. Taxa 8, 9221–9288. (doi:10.11609/jott.2532.8.10.9221-9288) [Google Scholar]
- 46.Hyatt AD, et al. 2007. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 73, 175–192. (doi:10.3354/dao073175) [DOI] [PubMed] [Google Scholar]
- 47.Brem F, Mendelson JR III, Lips KR. 2007. Field-sampling protocol for Batrachochytrium dendrobatidis from living amphibians, using alcohol preserved swabs. Arlington, VA: Conservation International; See http://www.amphibians.org. [Google Scholar]
- 48.Vredenburg V, Briggs C.2009. Chytrid swabbing protocol. Amphibia Web. See https://amphibiaweb.org/chytrid/swab_protocol.html .
- 49.Boyle AHD, Boyle D, Olsen V, Morgan J, Hyatt A. 2004. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 60, 141–148. (doi:10.3354/dao060141) [DOI] [PubMed] [Google Scholar]
- 50.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9–15. (doi:10.1080/15572536.2005.11832990) [PubMed] [Google Scholar]
- 51.Pounds AJ, et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. (doi:10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 52.Berger L, et al. 2004. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust. Vet. J. 82, 434–439. (doi:10.1111/j.1751-0813.2004.tb11137.x) [DOI] [PubMed] [Google Scholar]
- 53.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 54.Puschendorf R, Hoskin CJ, Cashins SD, McDonald K, Skerratt LF, Vanderwal J, Alford RA. 2011. Environmental refuge from disease-driven amphibian extinction. Conserv. Biol. 25, 956–964. (doi:10.1111/j.1523-1739.2011.01728.x) [DOI] [PubMed] [Google Scholar]
- 55.Thorpe CJ, Lewis TR, Kulkarni S, Watve A, Gaitonde N, Pryce D, Davies L, Bilton DT, Knight ME. 2018. Micro-habitat distribution drives patch quality for sub-tropical rocky plateau amphibians in the northern Western Ghats, India. PLoS ONE 13, e0194810 (10.1371/journal.pone.0194810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puschendorf R, Carnaval AC, VanDerWal J, Zumbado-Ulate H, Chaves G, Bolaños F, Alford RA. 2009. Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: proposing climatic refuges as a conservation tool. Divers. Distrib. 15, 401–408. (doi:10.1111/j.1472-4642.2008.00548.x) [Google Scholar]
- 57.Voyles J, Johnson LR, Briggs CJ, Cashins SD, Alford RA, Berger L, Skerratt LF, Speare R, Rosenblum EB. 2012. Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol. Evol. 2, 2241–2249. (doi:10.1002/ece3.334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogt WP, Johnson RB. 2011. Dictionary of statistics & methodology: a nontechnical guide for the social sciences. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 59.Maestri R, Monteiro LR, Fornel R, de Freitas TRO, Patterson BD. 2018. Geometric morphometrics meets metacommunity ecology: environment and lineage distribution affects spatial variation in shape. Ecography 41, 90–100. (doi:10.1111/ecog.03001) [Google Scholar]
- 60.Guimarães M, Doherty PF Jr, Munguía-Steyer R. 2014. Strengthening population inference in herpetofaunal studies by addressing detection probability. S. Am. J. Herpetol. 9, 1–8. (doi:10.2994/SAJH-D-13-00020.1) [Google Scholar]
- 61.Clare F, Daniel O, Garner T, Fisher M. 2016. Assessing the ability of swab data to determine the true burden of infection for the amphibian pathogen Batrachochytrium dendrobatidis. Ecohealth 13, 360–367. (doi:10.1007/s10393-016-1114-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.IUCN. 2016. The IUCN Red List of Threatened Species 2016-2. See http://www.iucnredlist.org/search.
- 63.Weinstein SB. 2009. An aquatic disease on a terrestrial salamander: individual and population level effects of the amphibian chytrid fungus, Batrachochytrium dendrobatidis, on Batrachoseps attenuatus (Plethodontidae). Copeia 2009, 653–660. (doi:10.1643/CH-08-180) [Google Scholar]
- 64.Retallick RW, McCallum H, Speare R. 2004. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2, e351 (doi:10.1371/journal.pbio.0020351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.India Go. Indiastat, Meteorogical data, rainfall. 2017. See http://www.indiastat.com/meteorologicaldata/22/rainfall/238/stats.aspx .
- 66.IMD, India Meteorological Department. 2016. Onset and withdrawal of southwest monsoon 2016: Ministry of Earth Sciences, Government of India; See http://www.imd.gov.in/pages/monsoon_main.php.
- 67.Lips KR, et al. 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA 103, 3165–3170. (doi:10.1073/pnas.0506889103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherer RD, Muths E, Noon BR, Corn PS. 2005. An evaluation of weather and disease as causes of decline in two populations of boreal toads. Ecol. Appl. 15, 2150–2160. (doi:10.1890/05-0431) [Google Scholar]
- 69.Heard GW, Thomas CD, Hodgson JA, Scroggie MP, Ramsey DS, Clemann N. 2015. Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecol. Lett. 18, 853–863. (doi:10.1111/ele.12463) [DOI] [PubMed] [Google Scholar]
- 70.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. (doi:10.1086/282487) [Google Scholar]
- 71.Scheffers BR, Edwards DP, Diesmos A, Williams SE, Evans TA. 2014. Microhabitats reduce animal's exposure to climate extremes. Glob. Change Biol. 20, 495–503. (doi:10.1111/gcb.12439) [DOI] [PubMed] [Google Scholar]
- 72.Bosch J, Donaire D, El Mouden EH, Fernández-Beaskoetxea S, Fisher MC, Slimani T. 2011. First record of the chytrid fungus Batrachochytrium dendrobatidis in North Africa. Herpetol. Rev. 42, 71–75. [Google Scholar]
- 73.Cortés-Gómez AM, Castro-Herrera F, Urbina-Cardona JN. 2013. Small changes in vegetation structure create great changes in amphibian ensembles in the Colombian Pacific rainforest. Trop. Conserv. Sci. 6, 749–769. (doi:10.1177/194008291300600604) [Google Scholar]
- 74.Newbold T, et al. 2014. A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc. R. Soc. B 281, 20141371 (doi:10.1098/rspb.2014.1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694. (doi:10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daszak P, Cunningham AA, Hyatt AD. 2003. Infectious disease and amphibian population declines. Divers. Distrib. 9, 141–150. (doi:10.1046/j.1472-4642.2003.00016.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padgett-Flohr GE, Hopkins RL II. 2009. Batrachochytrium dendrobatidis, a novel pathogen approaching endemism in central California. Dis. Aquat. Organ. 83, 1–9. (doi:10.3354/dao02003) [DOI] [PubMed] [Google Scholar]
- 78.Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM. 2005. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv. Biol. 19, 1431–1440. (doi:10.1111/j.1523-1739.2005.00108.x) [Google Scholar]
- 79.Garmyn A, Van Rooij P, Pasmans F, Hellebuyck T, Van Den Broeck W, Haesebrouck F, Martel AN. 2012. Waterfowl: potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 7, e35038 (doi:10.1371/journal.pone.0035038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heard GW, Scroggie MP, Ramsey DSL, Clemann N, Hodgson JA, Thomas CD. 2017. Can habitat management mitigate disease impacts on threatened amphibians? Conserv. Lett. 11, e12375 (doi:10.1111/conl.12375) [Google Scholar]
- 81.Moore M, Fidy JFSN, Edmonds D. 2015. The new toad in town: distribution of the Asian toad, Duttaphrynus melanostictus, in the Toamasina area of eastern Madagascar. Trop. Conserv. Sci. 8, 440–455. (doi:10.1177/194008291500800210) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of infection intensity with species and site localities are available in the electronic supplementary material.