Abstract
To characterize the chemical differences of volatile components between crude and processed Baizhu Shaoyao San (BSS), a classical Chinese herbal formula that is widely applied in the treatment of gastrointestinal diseases, we developed a gas chromatography–mass spectrometry-based needle trap device combined with multivariate data analysis to globally profile volatile components and rapidly identify differentiating chemical markers. Using a triple-bed needle packed with Carbopack X, DVB and Carboxen 1000 sorbents, we identified 121 and 123 compounds, respectively, in crude and processed BSS. According to the results of principal component analysis and orthogonal partial least-squares discriminant analysis, crude and processed BSS were successfully distinguished into two groups with good fitting and predicting parameters. Furthermore, 21 compounds were identified and adopted as potential markers that could be employed to quickly differentiate these two types of samples using S-PLOT and variable importance in projection analyses. The established method can be applied to explain the chemical transformation of Chinese medicine processing in BSS and further control the quality and understand the processing mechanism of Chinese herbal formulae. Besides, the triple-bed needle selected and optimized in this study can provide a valuable reference for other plant researches with similar components. Furthermore, the systematic research on compound identification and marker discrimination of the complex components in crude and processed BSS could work as an example for other similar studies, such as composition changes in one plant during different growth periods, botanical characters of different medicinal parts in same kind of medicinal herbs and quality identification of one species of medicinal herb from different regions.
Keywords: Baizhu Shaoyao San, traditional Chinese medicine processing, needle trap device, GC–MS, multivariate statistical analysis, chemical markers
1. Introduction
Baizhu Shaoyao San (BSS), a classic herbal formula in traditional Chinese medicine (TCM), is generally used for treating gastrointestinal diseases. BSS is composed of Atractylodis Macrocephalae Rhizoma (Asteraceae), Paeoniae Radix Alba (Ranunculaceae), Citri Reticulatae Pericarpium (Rutaceae), and Saposhnikoviae Radix (Umbelliferae) at a ratio of 6 : 4 : 3 : 4. As first recorded in ‘Dan-Xi-Xin-Fa', a comprehensive medical treatise edited by distinguished physician Zhen-heng Zhu (AD 1281–1358) in the Yuan Dynasty, BSS was applied as a typical mediative formula to soften the liver and tonify the spleen, as well as eliminate dampness and relieve diarrhoea. Clinically, in China, BSS has been widely prescribed to treat acute and chronic intestinal inflammation, painful diarrhoea and diarrhoea-predominant irritable bowel syndrome [1,2]. The clinical efficacies of BSS are the synergistic results of some bioactive substances contained in BSS, such as volatile oils, sesquiterpene lactones, monoterpene glycosides, flavonoids and flavonoid glycosides, coumarins, chromones and other ingredients [3–6]. Previous studies have suggested that the volatile components of medicinal herbs contained in BSS exhibit gastrointestinal peristalsis promotion, anti-inflammation, suppression of tumour cell proliferation, anti-oxidation and anti-microbial effects [7–10]. According to the theory of TCM and based on Chinese Pharmacopoeia, three medicinal herbs contained in BSS must be processed in specific processing methods to enhance the curative efficacy of the whole formula and reduce side effects. These medicinal herbs are Atractylodis Macrocephalae Rhizoma (stir-frying with honey-processed wheat bran), Paeoniae Radix Alba (stir-frying with wheat bran) and Citri Reticulatae Pericarpium (stir-frying without any auxiliary materials). The chemical compositions of volatile oils significantly change during stir-frying, causing different treatment effects [11–13]. Previous processing studies have observed that significant transformations occur in the volatile components of Atractylodis Macrocephalae Rhizoma [14], Paeoniae Radix Alba [15] and Citri Reticulatae Pericarpium [16] during processing. However, no study has been performed on the comprehensive mining of global volatile characteristics between crude and processed BSS, causing inconsistencies in the clinical medication. In addition, non-standard prescriptions of mixing crude and processed products also exist in other places, thereby weakening the curative effects or causing toxic reactions. Therefore, a simple and reliable analytical method to evaluate the processing in BSS is necessary for quality control and guaranteed safety in clinical applications.
The purpose of the study is to develop a simple and convenient technique to quickly extract, analyse, and classify the volatile constituents in crude and processed BSS. An efficient approach to extract and determine the overall concentration of volatile compounds is the needle trap device (NTD), which is a powerful tool for preparing semi-volatile and volatile compounds [17,18]. NTD consists of a stainless-steel needle containing various sorbent fillers, a vacuum pumping enrichment system with a heating chamber and a gas-tight syringe. NTD integrates the functions of sample preparation, sampling and sample preconcentration in one step, and can conveniently be coupled with other analytical methods with high sensitivity, such as gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry [19,20]. In addition, NTD reduces the thermal decomposition of compounds and waste of time and resources compared with traditional steam distillation extraction, and increases the detection range and sensitivity of compounds from low to high boiling point compared with conventional static headspace extraction [21]. Moreover, a specific packing material or a combination of several packing materials coating the inside of the extraction needle endows the NTD with superb sampling selectivity and enables it to adsorb the target volatile compounds and reduce the matrix effects [22]. In recent years, NTD was successfully applied to analyse volatile organic components in the chemical industry, food science and environmental monitoring fields [23–25]; however, it has been seldom employed in the study of TCM processing.
Multivariate data analysis enables comprehensive profiling and classification of several samples containing numerous and complicated data; thus, it has been extensively used for the analytical research of TCM, e.g. monitoring the chemical changes in different cultivation areas and ages [26], classifying the quality grades of same species [27] and exploring the marker compounds of variant processing methods [28]. The chemometrics tools for statistical strategy, such as principal component analysis (PCA), partial least-squares-discriminant analysis and orthogonal partial least-squares-discriminant analysis (OPLS-DA), are gradually becoming increasingly important because they supply efficient and stable patterns for analysis, modelling, and interpretation of the extremely complex original dataset of biology and chemistry. Moreover, these kinds of chemometrics methods provide convenient, fast and reliable data processing modes based on data dimensionality reduction while retaining the information of variables as completely as possible.
In this study, NTD coupled with GC–MS was applied to extract and analyse the volatile compounds in crude and processed BSS for the first time. Multivariate data analysis consisting of PCA and OPLS-DA based on the spectra data of chemical information was performed to overview and discriminate the potential marker compounds between crude and processed BSS. This method of study will immensely facilitate the analysis of volatile components that can be used to control the quality and reveal the chemical transformation of processing among other TCM formulae.
2. Experimental
2.1. Samples and materials
Herbal materials of crude Atractylodis Macrocephalae Rhizoma, Paeoniae Radix Alba, Citri Reticulatae Pericarpium and Saposhnikoviae Radix were all purchased from Bozhou Jingwan TCM Pieces Factory in China and were authenticated by Professor Hao Cai. Processed Atractylodis Macrocephalae Rhizoma, processed Paeoniae Radix Alba and processed Citri Reticulatae Pericarpium were prepared according to the processing standards described in Chinese Pharmacopoeia [29]. The voucher specimens were deposited in School of Pharmacy, Nanjing University of Chinese Medicine (Nanjing, China).
The following materials were purchased from PAS Technology (Magdala, Germany): 22-gauge stainless-steel needle (51 mm × 0.40 mm i.d., 0.72 mm o.d.) with a straight cavity through both ends packing with 0.7 cm of Carbopack X (60/80 mesh), 0.7 cm of DVB (60/80 mesh), 0.7 cm of Carboxen 1000 (60/80 mesh) and an NT-Case (42.5 cm × 28.4 cm × 15.5 cm) multifunctional sampling device.
2.2. Sampling by needle trap device
Prior to each sample extraction, the stainless-steel needle of NTD was conditioned in the GC injector at 250°C for 10 min with a constant helium flow to eliminate impure substances. For crude BSS, fine powders (sifted through an 80-mesh sieve) of crude Atractylodis Macrocephalae Rhizoma (30 mg), crude Paeoniae Radix Alba (20 mg), crude Citri Reticulatae Pericarpium (15 mg) and crude Saposhnikoviae Radix (20 mg) were accurately weighed, mixed and transferred into a 20 ml headspace vial. Afterward, 15 µl deionized water was added into the headspace vial to promote desorption and vaporization of volatile analytes from the matrix. The vial was tightly sealed with a polytetrafluoroethylene septum cap, placed in the heating chamber mounted on the NTD and heated at 80°C for 20 min. The needle was inserted into the vial septum and was exposed sufficiently to the headspace of the sample powders during the extraction. The needle was then connected to the vacuum pumping enrichment system of NTD to aspirate the headspace gas through the needle, by which the target compounds of the samples were adsorbed into the sorbent fillers. Pump flow rate and aspiration volume were set to 2 ml min−1 and 30 ml, respectively. After sampling, the needle was removed from the NTD, connected to a 1.0 ml gas-tight syringe, and inserted into the GC injector immediately to thermally desorb the trapped analytes at 250°C for 1 min. The schematic representation of the adsorption and desorption process via the triple-bed packed needle is illustrated in figure 1.
Figure 1.
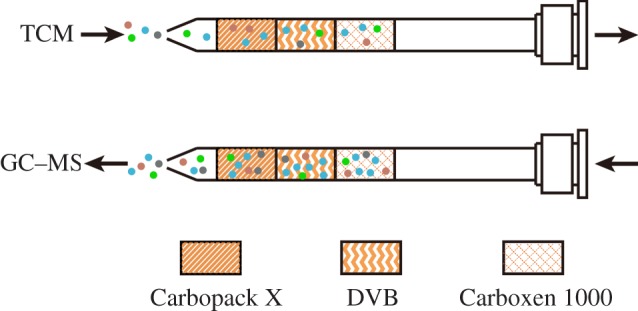
Illustration of adsorption and desorption process via triple-bed packed stainless needle in this experiment.
Similarly, the processed BSS sampling by the NTD was conducted using the same procedure as that of crude BSS using processed Atractylodis Macrocephalae Rhizoma, processed Paeoniae Radix Alba, processed Citri Reticulatae Pericarpium and crude Saposhnikoviae Radix.
2.3. Gas chromatography–mass spectrometry analysis
The analyses of volatile components of crude and processed BSS were performed with an Agilent 7890A-5975C GC-MS system (Agilent Technologies, Santa Clara, CA, USA). The separation was performed on a HP-VOC capillary column (60 m × 0.32 mm i.d.; 1.8 µm film thickness; Agilent Technologies). The injection port temperature was 250°C, GC–MS interface temperature was 250°C, ion source temperature was 230°C, MS quadrupole temperature was 150°C, electron ionization voltage was 70 eV, solvent delay was 5 min and splitless mode was used. Ultra high purity helium (99.999%) was used as the carrier gas at a permanent flow rate of 1 ml min−1. MS analysis was performed in full scan mode with a scan range of m/z 40 to 550. The oven temperature programme was initially held at 50°C for 1 min, then ramped to 200°C at a rate of 15°C min−1, and subsequently ramped to 250°C at a rate of 2°C min−1, and then ramped to 260°C at a rate of 10°C min−1, and finally held at 260°C for 5 min. Raw data were processed using MSD ChemStation E. 02. 02. 1431 (Agilent Technologies), where the peaks of all samples were aligned and integrated. Automatic mass spectral deconvolution and identification system (AMDIS) was employed to deconvolute GC–MS data file for better identifying the target compounds. Identification of the compounds was carried out by matching the experimental spectra with NIST14 MS spectral library and supported by GC Kovats retention index values.
2.4. Multivariate data analysis
The processed three-dimensional data matrices consisting of retention time, peak area and sample name were generated and imported into SIMCA-P 14.1 (Umetrics, Umea, Sweden) for multivariate data analysis. To eliminate the deviation caused by the large range of variable values, all the data underwent linear transformation and Pareto scaling (variables were weighted by 1/√s.d.) before unsupervised PCA and supervised OPLS-DA. To evaluate the quality of the established models, we calculated the goodness of fitting parameter (R2X), the ratio of the variance of the response variable explicated by the model (R2Y) and the ability of predicting new data (Q2) using a seven-round internal cross-validation of the original data using the default settings of SIMCA-P 14.1. In this multivariate data analysis strategy, PCA initially served to cluster all the samples and eliminate the outliers. Afterward, OPLS-DA was subsequently performed to produce visualization results to simplify the procedure for globally classifying and differentiating the targeted profiling obtained from the GC–MS spectra data. The S-plot of OPLS-DA was then employed to better visualize the contribution of chemical components toward the discrimination between crude and processed BSS. The S-plot was a scatter plot consisting of the covariance (p) versus correlation (p(corr)) vectors of the predictive components and helped identify variant markers between classes. This component, which had a high p value, indicated high model contribution, whereas a high p(corr) value indicated a high model reliability. A p(corr) greater than 0.5 combined with p > 0.1 was applied as a cutoff value to acquire the marker compounds in this paper. As a supplement and validation, the variable importance in the projection (VIP) value was applied to evaluate the variable contribution and determine the marker compounds. Variables with VIP values larger than 1 were considered important components that were responsible for model classification.
3. Results and discussion
3.1. Method development and validation
BSS, which consists of four herbal medicines of different families and genera, is an extremely complex matrix that contains numerous volatile components and has a low to relatively high boiling range. Therefore, simultaneous extraction and determination of these various types of volatile compounds in BSS using NTD coupled with GC–MS is challenging. To extract a wide range of volatile compounds in this investigation, we applied and tested different types of sorbents in the needle trap. The extraction efficacies of volatile components in BSS were compared using a double-bed needle packing Tenax and polydimethylsiloxane (PDMS), a triple-bed needle packing PDMS, DVB and Carboxen 1000, and a triple-bed needle packing Carbopack X, DVB and Carboxen 1000 under the same conditions. The results indicated that the triple-bed needle packing Carbopack X, DVB and Carboxen 1000 was highly suitable for the analysis of BSS due to its improved extraction ability for the low-, medium- and high-boiling point compounds, and low peak broadening compared with the other needles. The heating temperature of the NTD method was also a significant parameter that influenced the extraction effect [30]. Heating temperatures ranging from 60°C to 90°C were investigated in this experiment using peak numbers, areas and resolutions as optimization criteria. The amounts and intensities of the peaks were markedly increased when heating temperature increased from 60°C to 90°C. However, the peak intensities of the low-boiling-point compounds and the peak resolutions of all the peaks significantly decreased from 80°C to 90°C, perhaps due to thermal degradation and desorption when temperature was too high. As a result, 80°C was finally selected as the optimal heating temperature of NTD.
Robustness and ruggedness of this instrumental analysis method were evaluated using continuous six-time parallel analysis of one sample. Blank sample was used to investigate the background interference of this method, and little interference was observed according to the result. The profile of the blank sample is shown in electronic supplementary material, figure S1. Relative standard deviation (RSD) values of retention times and peak areas were evaluated to examine the repeatability of the sample NTD method. Resulting data showed that the RSD values of retention times and peak areas of corresponding main peaks in these six samples were less than 0.1% and 10%, which demonstrated that the developed NTD method was robust and had superb stability and repeatability.
3.2. Gas chromatography–mass spectrometry analysis of volatile components
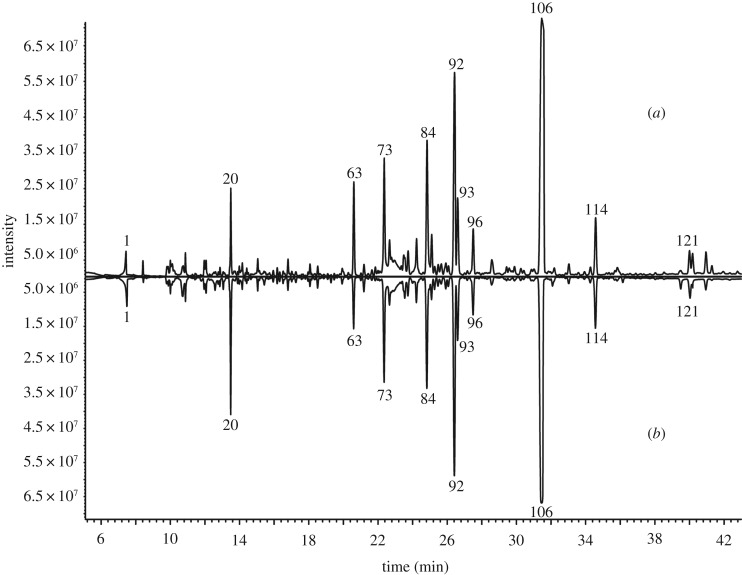
Under the current NTD and GC–MS methods described earlier, 121 and 123 compounds, respectively representing 92.64% and 93.08% of the total volatile composition from crude and processed BSS, were separated and identified with match quality greater than 800 using AMDIS with the simple mode in Agilent MSD ChemStation. The identification of volatiles was confirmed by matching the experimental mass spectral data with NIST14 mass spectral library and was further elucidated using the Wiley 6 library. The chemical composition of volatiles from crude and processed BSS and their corresponding peak areas are summarized in table 1. Figure 2 shows the typical profiles of crude and processed BSS obtained through GC–MS, wherein the peaks with relatively high intensities are marked with serial numbers. On the basis of the structure of the volatiles, the components of crude BSS were divided into five groups: acids and esters (7.31%), aldehydes and ketones (8.15%), terpene hydrocarbons (41.77%), oxygenated terpenes (33.25%) and others (2.16%). Equally, the components of processed BSS were classified into five groups: acids and esters (7.58%), aldehydes and ketones (8.02%), terpene hydrocarbons (42.99%), oxygenated terpenes (31.56%) and others (2.93%).
Table 1.
Identification of volatile compounds in crude and processed BSS by GC–MS (n = 6).
| measured area (×108) |
|||||
|---|---|---|---|---|---|
| no. | TR (min) | compound name | formula | crude BSS | processed BSS |
| 1 | 7.38 | acetic acid | C2H4O2 | 3.33 ± 0.35 | 3.37 ± 0.30 |
| 2 | 9.86 | (R,R)-2,3-butanediol | C4H10O2 | 0.78 ± 0.17 | 0.81 ± 0.13 |
| 3 | 9.99 | (S,S)-2,3-butanediol | C4H10O2 | 1.82 ± 0.24 | 1.97 ± 0.22 |
| 4 | 10.13 | isovaleric acid | C5H10O2 | 3.06 ± 0.84 | 2.86 ± 0.51 |
| 5 | 10.27 | 2-methylbutanoic acid | C5H10O2 | 0.81 ± 0.20 | 0.75 ± 0.22 |
| 6 | 10.70 | furfural | C5H4O2 | 1.06 ± 0.28 | 5.85 ± 0.85 |
| 7 | 10.87 | methyl N-hydroxylbenzenecarboximidoate | C8H9NO2 | 2.43 ± 0.28 | 2.86 ± 0.38 |
| 8 | 11.28 | angelic acid | C5H8O2 | 0.38 ± 0.07 | 0.38 ± 0.09 |
| 9 | 11.44 | heptanal | C7H14O | 0.48 ± 0.04 | 0.50 ± 0.09 |
| 10 | 11.76 | 2,6-dimethylpyrazine | C6H8N2 | 0.33 ± 0.03 | 0.93 ± 0.03 |
| 11 | 12.07 | hexanoic acid | C6H12O2 | 2.24 ± 0.18 | 2.66 ± 0.27 |
| 12 | 12.27 | 1-heptanol | C7H16O | 0.30 ± 0.03 | 0.34 ± 0.04 |
| 13 | 12.52 | phenol | C6H6O | 0.73 ± 0.03 | 0.72 ± 0.08 |
| 14 | 12.59 | 5-methyl-2-furfural | C6H6O2 | — | 0.86 ± 0.15 |
| 15 | 12.62 | 6-methyl-5-hepten-2-ketone | C8H14O | 0.85 ± 0.04 | 0.84 ± 0.03 |
| 16 | 12.78 | benzaldehyde | C7H6O | 0.49 ± 0.03 | 0.52 ± 0.06 |
| 17 | 12.87 | octanal | C8H16O | 0.97 ± 0.08 | 0.98 ± 0.12 |
| 18 | 13.13 | cosmene | C10H14 | 0.50 ± 0.04 | 0.71 ± 0.09 |
| 19 | 13.29 | 4-carene | C10H16 | 0.21 ± 0.02 | 0.20 ± 0.03 |
| 20 | 13.50 | D-limonene | C10H16 | 10.71 ± 1.18 | 15.89 ± 1.15 |
| 21 | 13.70 | benzyl alcohol | C7H8O | 0.75 ± 0.06 | 0.68 ± 0.11 |
| 22 | 13.75 | 1-octanol | C8H18O | 0.56 ± 0.07 | 0.54 ± 0.06 |
| 23 | 13.89 | γ-terpinene | C10H16 | 0.53 ± 0.06 | 0.64 ± 0.03 |
| 24 | 13.99 | benzeneacetaldehyde | C8H8O | 1.05 ± 0.07 | 1.06 ± 0.11 |
| 25 | 14.34 | linalool | C10H18O | 0.31 ± 0.01 | 0.33 ± 0.02 |
| 26 | 14.42 | nonanal | C9H18O | 0.95 ± 0.13 | 0.83 ± 0.11 |
| 27 | 14.50 | p-cymenene | C10H12 | 0.38 ± 0.04 | 0.41 ± 0.05 |
| 28 | 14.66 | guaicolina | C7H8O2 | 0.29 ± 0.05 | 0.33 ± 0.07 |
| 29 | 14.76 | 3-methylbenzaldehyde | C8H8O | 0.33 ± 0.04 | 0.10 ± 0.06 |
| 30 | 14.92 | 1,3,8-p-menthatriene | C10H14 | 0.45 ± 0.15 | 0.18 ± 0.13 |
| 31 | 15.06 | octanoic acid | C8H16O2 | 2.04 ± 0.25 | 1.90 ± 0.41 |
| 32 | 15.43 | benzoic acid | C7H6O2 | 1.43 ± 0.22 | 1.89 ± 0.21 |
| 33 | 15.73 | ethyl octanoate | C10H20O2 | 0.73 ± 0.04 | 0.68 ± 0.05 |
| 34 | 15.95 | (+)-nopinone | C9H14O | 0.78 ± 0.04 | 0.77 ± 0.06 |
| 35 | 16.18 | decanal | C10H20O | 1.04 ± 0.16 | 0.85 ± 0.11 |
| 36 | 16.32 | 4-terpinenol | C10H18O | 0.49 ± 0.02 | 0.61 ± 0.06 |
| 37 | 16.52 | α-terpineol | C10H18O | 0.61 ± 0.06 | 0.90 ± 0.16 |
| 38 | 16.60 | 4-methylacetophenone | C9H10O | 0.38 ± 0.07 | 0.38 ± 0.06 |
| 39 | 16.80 | myrtanal | C10H16O | 2.05 ± 0.23 | 1.88 ± 0.12 |
| 40 | 16.89 | nonanoic acid | C9H18O2 | 0.38 ± 0.04 | 0.45 ± 0.07 |
| 41 | 17.02 | (Z)-carveol | C10H16O | 0.76 ± 0.10 | 0.76 ± 0.08 |
| 42 | 17.45 | (E)-2-decenal | C10H18O | 0.16 ± 0.04 | 0.14 ± 0.02 |
| 43 | 17.59 | 4-methyleneisophorone | C10H14O | 0.23 ± 0.02 | 0.33 ± 0.04 |
| 44 | 17.67 | ethyl nonylate | C11H22O2 | 0.29 ± 0.04 | 0.20 ± 0.07 |
| 45 | 17.80 | carvone | C10H14O | 0.15 ± 0.02 | 0.28 ± 0.04 |
| 46 | 17.96 | thymol | C10H14O | 0.50 ± 0.04 | 0.60 ± 0.08 |
| 47 | 18.07 | (−)-myrtanol | C10H18O | 1.38 ± 0.10 | 1.32 ± 0.17 |
| 48 | 18.17 | 4-isopropylanisole | C10H14O | 0.30 ± 0.01 | 0.34 ± 0.03 |
| 49 | 18.28 | undecanal | C11H22O | 0.16 ± 0.01 | 0.17 ± 0.02 |
| 50 | 18.37 | 3-methyl-4-isopropylphenol | C10H14O | 0.22 ± 0.04 | 0.26 ± 0.05 |
| 51 | 18.52 | carvacrol | C10H14O | 1.21 ± 0.06 | 1.60 ± 0.19 |
| 52 | 18.66 | cuminic alcohol | C10H14O | 0.52 ± 0.10 | 0.48 ± 0.09 |
| 53 | 18.87 | perillic alcohol | C10H16O | 0.40 ± 0.02 | 0.41 ± 0.05 |
| 54 | 19.09 | decanoic acid | C10H20O2 | 0.45 ± 0.05 | 0.45 ± 0.14 |
| 55 | 19.29 | 2-methoxy-4-vinylphenol | C9H10O2 | 0.20 ± 0.02 | 0.88 ± 0.13 |
| 56 | 19.47 | nerol acetate | C12H20O2 | 0.31 ± 0.02 | 0.30 ± 0.02 |
| 57 | 19.65 | δ-eIemene | C15H24 | 0.26 ± 0.05 | 0.16 ± 0.03 |
| 58 | 19.80 | silphiperfol-5-ene | C15H24 | 0.49 ± 0.14 | 0.15 ± 0.07 |
| 59 | 19.94 | geranyl acetate | C12H20O2 | 0.92 ± 0.27 | 0.50 ± 0.13 |
| 60 | 19.99 | α-cubebene | C15H24 | 1.12 ± 0.15 | 0.79 ± 0.09 |
| 61 | 20.29 | eugenol | C10H12O2 | 0.63 ± 0.12 | 0.44 ± 0.07 |
| 62 | 20.37 | decyl acetate | C12H24O2 | 0.23 ± 0.05 | 0.13 ± 0.02 |
| 63 | 20.61 | 7,7-dimethyl-1-vinylbicyclo[2.2.1]heptan-2-one | C11H16O | 14.24 ± 1.90 | 7.92 ± 1.45 |
| 64 | 20.76 | lauraldehyde | C12H24O | 0.22 ± 0.03 | 0.13 ± 0.03 |
| 65 | 20.86 | ylangene | C15H24 | 0.13 ± 0.01 | 0.09 ± 0.01 |
| 66 | 21.01 | α-copaene | C15H24 | 0.49 ± 0.08 | 0.46 ± 0.09 |
| 67 | 21.19 | β-elemene | C15H24 | 1.83 ± 0.26 | 1.95 ± 0.22 |
| 68 | 21.65 | modephene | C15H24 | 1.22 ± 0.11 | 0.75 ± 0.18 |
| 69 | 21.85 | berkheyaradulene | C15H24 | 1.66 ± 0.24 | 0.67 ± 0.11 |
| 70 | 22.00 | β-farnesene | C15H24 | 0.87 ± 0.05 | 0.65 ± 0.09 |
| 71 | 22.11 | acoradiene | C15H24 | 0.74 ± 0.03 | 0.62 ± 0.07 |
| 72 | 22.17 | dihydropseudoionone | C13H22O | 0.24 ± 0.01 | 0.47 ± 0.09 |
| 73 | 22.36 | γ-elemene | C15H24 | 21.76 ± 1.78 | 19.08 ± 1.03 |
| 74 | 22.67 | caryophyllene | C15H24 | 6.88 ± 0.64 | 5.28 ± 0.51 |
| 75 | 22.78 | selina-5,11-diene | C15H24 | 3.43 ± 0.42 | 1.88 ± 0.84 |
| 76 | 22.92 | elixene | C15H24 | 7.60 ± 0.60 | 7.18 ± 0.78 |
| 77 | 23.35 | γ-curcumene | C15H24 | 1.60 ± 0.10 | 1.17 ± 0.15 |
| 78 | 23.49 | α-curcumene | C15H22 | 3.05 ± 0.09 | 0.30 ± 0.03 |
| 79 | 23.55 | α-farnesene | C15H24 | 2.42 ± 0.19 | 0.55 ± 0.76 |
| 80 | 23.74 | 1,5,9,9-tetramethyl-1,4,7-cycloundecatriene | C15H24 | 4.47 ± 0.27 | 3.28 ± 0.40 |
| 81 | 24.01 | nootkatene | C15H22 | 1.03 ± 0.06 | 1.63 ± 0.37 |
| 82 | 24.23 | β-bisabolene | C15H24 | 8.18 ± 0.39 | 6.47 ± 1.10 |
| 83 | 24.61 | α-amorphene | C15H24 | 0.78 ± 0.06 | 0.75 ± 0.11 |
| 84 | 24.83 | β-selinene | C15H24 | 25.43 ± 1.33 | 19.85 ± 2.55 |
| 85 | 24.97 | α-selinene | C15H24 | — | 1.60 ± 0.27 |
| 86 | 25.10 | (3E,5E)-7-isopropyl-8-methyl-3,5,7-nonatrien-2-one | C13H20O | 6.62 ± 0.36 | 4.19 ± 0.96 |
| 87 | 25.27 | cadina-1(10),4-diene | C15H24 | 1.60 ± 0.13 | 2.16 ± 0.24 |
| 88 | 25.47 | cuparene | C15H22 | 1.99 ± 0.13 | 1.64 ± 0.17 |
| 89 | 25.66 | 6S,10R-dimethylbicyclo[4.4.0]decan-1-en-3-one | C12H18O | 2.58 ± 0.12 | 1.93 ± 0.20 |
| 90 | 25.92 | guaia-3,9-diene | C15H24 | 2.96 ± 0.12 | 2.22 ± 0.22 |
| 91 | 26.07 | β-cadinene | C15H24 | 1.74 ± 0.05 | 1.70 ± 0.21 |
| 92 | 26.42 | selina-3,7(11)-diene | C15H24 | 36.63 ± 0.97 | 36.61 ± 0.85 |
| 93 | 26.60 | germacrene B | C15H24 | 13.62 ± 0.38 | 12.74 ± 0.97 |
| 94 | 27.01 | β-vatirenene | C15H22 | 0.54 ± 0.02 | 0.38 ± 0.06 |
| 95 | 27.16 | (4aR,8aS)-4a-methyl-1-methylene-7-(propan-2-ylidene)decahydronaphthalene | C15H24 | 0.88 ± 0.06 | 0.71 ± 0.06 |
| 96 | 27.50 | γ-vetivenene | C15H22 | 8.21 ± 0.20 | 7.27 ± 0.29 |
| 97 | 27.84 | 3,5,11-eudesmatriene | C15H22 | 0.48 ± 0.02 | 0.37 ± 0.04 |
| 98 | 28.58 | caryophyllene oxide | C15H24O | 3.82 ± 0.19 | 2.20 ± 0.45 |
| 99 | 29.43 | 2-(1-methylethylidene)octahydro-4H-inden-4-one | C12H18O | 2.07 ± 0.13 | 1.32 ± 0.20 |
| 100 | 29.58 | humulene 6,7-epoxide | C15H24O | 1.68 ± 0.06 | 1.03 ± 0.19 |
| 101 | 29.62 | isospathulenol | C15H24O | — | 0.49 ± 0.13 |
| 102 | 29.88 | spathulenol | C15H24O | 1.79 ± 0.09 | 1.22 ± 0.19 |
| 103 | 30.25 | ledene oxide-(II) | C15H24O | 1.32 ± 0.10 | 0.89 ± 0.15 |
| 104 | 30.42 | caryophylla-4(12),8(13)-dien-5β-ol | C15H24O | 0.99 ± 0.04 | 0.61 ± 0.14 |
| 105 | 31.01 | β-selinenol | C15H26O | 1.62 ± 0.11 | 1.31 ± 0.19 |
| 106 | 31.46 | atractylon | C15H20O | 100.41 ± 2.62 | 81.83 ± 6.35 |
| 107 | 31.96 | aristolone | C15H22O | 0.49 ± 0.05 | 0.62 ± 0.08 |
| 108 | 32.09 | tetradecanoic acid | C14H28O2 | 0.72 ± 0.03 | 0.84 ± 0.39 |
| 109 | 32.18 | juniper camphor | C15H26O | 1.27 ± 0.08 | 1.04 ± 0.25 |
| 110 | 32.85 | 6-isopropenyl-4,8a-dimethyldecahydro-1-naphthalenol | C15H26O | 0.36 ± 0.03 | 0.28 ± 0.03 |
| 111 | 33.03 | isonootkatol | C15H24O | 1.69 ± 0.31 | 1.35 ± 0.16 |
| 112 | 33.93 | 2-(4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydro-octahydro-2-naphthalenyl)-2-propen-1-ol | C15H24O | 0.65 ± 0.05 | 0.49 ± 0.06 |
| 113 | 34.27 | neocnidilide | C12H18O2 | 1.39 ± 0.10 | 1.39 ± 0.26 |
| 114 | 34.57 | dehydrofukinone | C15H22O | 9.42 ± 0.69 | 10.32 ± 0.97 |
| 115 | 35.69 | 7-methyl-4-(1-methylethylidene)bicyclo[5.3.1]undec-1-en-8-ol | C15H24O | 0.63 ± 0.08 | 0.52 ± 0.06 |
| 116 | 35.84 | (1R,7S)-germacra-4(15),5,10(14)-trien-1β-ol | C15H24O | 1.84 ± 0.18 | 1.05 ± 0.17 |
| 117 | 37.35 | ethyl pentadecanoate | C17H34O2 | 0.15 ± 0.02 | — |
| 118 | 37.72 | isovalencenyl formate | C16H24O2 | 0.51 ± 0.72 | 0.16 ± 0.02 |
| 119 | 38.03 | 6-[1-(hydroxymethyl)vinyl]-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-2-naphthalenol | C15H24O2 | 0.34 ± 0.05 | 0.25 ± 0.02 |
| 120 | 39.48 | palmitoleic acid | C16H30O2 | 1.30 ± 0.17 | 1.78 ± 0.49 |
| 121 | 40.00 | hexadecanoic acid | C16H32O2 | 4.95 ± 0.32 | 4.47 ± 0.52 |
| 122 | 40.18 | β-cyclocostunolide | C15H20O2 | 3.51 ± 0.21 | 1.39 ± 0.59 |
| 123 | 40.95 | (E)-valerenyl isovalerate | C20H32O2 | 3.91 ± 0.53 | 2.55 ± 0.44 |
| 124 | 41.29 | ethyl hexadecanoate | C18H36O2 | 1.43 ± 0.15 | 0.33 ± 0.11 |
Figure 2.
Typical profiles of volatiles in crude and processed BSS obtained by GC–MS: (a) crude BSS, (b) processed BSS.
3.3. Differentiation of volatiles in crude and processed Baizhu Shaoyao San
In the present work, some obvious changes of volatile components between crude and processed BSS were observed from the GC–MS chromatogram. A total of 120 compounds were identified as common peaks in both crude and processed BSS samples. Processing might significantly change the volatiles in BSS; thus, one compound that was detected in crude BSS samples disappeared in processed BSS samples, and three compounds were newly generated in BSS after processing.
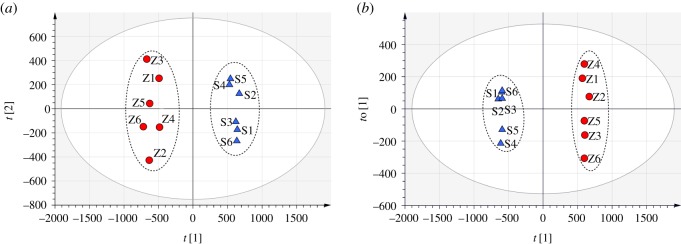
To comprehensively characterize the differences in volatile components between crude and processed BSS, multivariate data analysis was performed for the classification of these two groups. Unsupervised PCA was first employed to investigate if crude and processed BSS could be separated on the basis of their differences in volatile compounds. As shown in figure 3a, a total of six batches of crude and six batches of their corresponding processed BSS samples were distinguished from each other along PC1 in the two-dimensional PCA score scatter plot. Samples of crude BSS were characterized with positive scores, whereas samples of processed BSS showed negative scores, indicating that crude and processed BSS were distinctively different in their chemical patterns. The classification parameters of the PCA model revealed good fitness and predictability, as indicated by R2X (cum) and Q2 (cum) values of 0.777 and 0.608, respectively.
Figure 3.
PCA score scatter plot (a) and OPLS-DA score scatter plot (b) based on global chemical profiling of volatiles from crude and processed BSS. (Triangles) crude BSS; (circles) processed BSS.
To further analyse the distinct components of the volatiles between crude and processed BSS, supervised OPLS-DA was subsequently applied to the GC–MS data matrices. As shown in figure 3b, the score scatter plot of the analysis demonstrated that all the test samples could be unambiguously grouped into two separated clusters along the discriminating t [1]: crude BSS gathered around the negative region, whereas processed BSS gathered around the positive region, suggesting a global difference in the composition of volatile compounds between two kinds of BSS. Moreover, all observation values decreased in the Hotelling T2 (0.95) ellipse, and the fitting parameters of this model were 0.716 of R2X (cum), 0.998 of R2Y (cum) and 0.983 of Q2 (cum), indicating that the OPLS-DA model was remarkably good in fitness and prediction.
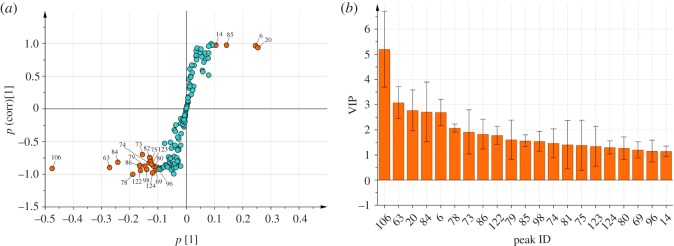
To identify the potential chemical markers that contribute to the difference between crude and processed BSS, the S-plot and VIP analyses were performed following the OPLS-DA. In the S-plot (figure 4a), each round point represented a compound with a peak number corresponding with table 1; the X-axis represented the contribution to the difference, wherein the farther the point is distributed from zero, the more the compound contributed to the difference between crude and processed BSS; the Y-axis represented the confidence to the difference, wherein the farther the point is distributed from zero, the higher the level of confidence that the compound contributed to the difference between crude and processed BSS. Therefore, the points scattering at both ends of ‘S' with p > 0.1 and p(corr) greater than 0.5 are marked red in figure 4a, which were thought to be the potential markers. Moreover, the VIP plot (figure 4b) also supported the result because all the marker compounds obtained from S-plot showed VIP values greater than 1. Finally, 21 volatile compounds with significant difference between crude and processed BSS were found, in which the contents of 4 components (peaks 6, 14, 20 and 85) remarkably increased in BSS after processing, and the contents of 17 components (peaks 63, 69, 73, 74, 75, 78, 79, 80, 82, 84, 86, 96, 98, 106, 122, 123, 124) remarkably decreased in processed BSS compared with crude samples. The results are summarized in table 2.
Figure 4.
S-plot (a) and VIP (b) analyses associated with OPLS-DA score plot scatter. Numbers for peaks used are as given in table 2.
Table 2.
Results of 21 marker compounds that can classify crude and processed BSS (n = 6).
| no. | compound name | p | p(corr) | VIP |
|---|---|---|---|---|
| 6 | furfural | 0.246 | 0.970 | 2.692 |
| 14 | 5-methyl-2-furfural | 0.105 | 0.973 | 1.148 |
| 20 | D-limonene | 0.254 | 0.937 | 2.773 |
| 63 | 7,7-dimethyl-1-vinylbicyclo[2.2.1]heptan-2-one | −0.272 | −0.896 | 3.079 |
| 69 | berkheyaradulene | −0.110 | −0.937 | 1.201 |
| 73 | γ-elemene | −0.156 | −0.700 | 1.915 |
| 74 | caryophyllene | −0.131 | −0.827 | 1.466 |
| 75 | selina-5,11-diene | −0.126 | −0.785 | 1.386 |
| 78 | α-curcumene | −0.189 | −0.998 | 2.069 |
| 79 | α-farnesene | −0.146 | −0.877 | 1.608 |
| 80 | 1,5,9,9-tetramethyl-1,4,7-cycloundecatriene | −0.116 | −0.872 | 1.271 |
| 82 | β-bisabolene | −0.129 | −0.744 | 1.410 |
| 84 | β-selinene | −0.242 | −0.819 | 2.711 |
| 85 | α-selinene | 0.143 | 0.978 | 1.563 |
| 86 | (3E,5E)-7-isopropyl-8-methyl-3,5,7-nonatrien-2-one | −0.164 | −0.865 | 1.830 |
| 96 | γ-vetivenene | −0.104 | −0.893 | 1.154 |
| 98 | caryophyllene oxide | −0.139 | −0.924 | 1.546 |
| 106 | atractylon | −0.475 | −0.916 | 5.202 |
| 122 | β-cyclocostunolide | −0.162 | −0.938 | 1.782 |
| 123 | (E)-valerenyl isovalerate | −0.123 | −0.842 | 1.344 |
| 124 | ethyl hexadecanoate | −0.119 | −0.979 | 1.299 |
3.4. Analysis of proper processing mechanisms of some volatile components in Baizhu Shaoyao San
The contents of furfural and 5-methyl-2-furfural were significantly increased in processed BSS compared with crude BSS. According to the literature, these furfural series compounds can be generated from the Maillard reaction in the presence of amino compounds and reducing sugars in the field of food processing [31]. Similarly, in the processing of Chinese medicinal materials, the plausible mechanisms underlying these changes are that the amino acids and sugars found in medicinal and auxiliary materials may induce the Maillard reaction at high temperatures during stir-frying. Furthermore, these Maillard reaction products could produce a special aroma to stimulate appetite and modulate gut microbiota composition to promote digestion, which is consistent with the theory of TCM that processing BSS could invigorate and enhance the functions of spleen and stomach [32]. Atractylon, which is the main component of the dryness property that can cause impairments to the ‘yin' from the perspective of TCM [33], has the highest content in the volatiles of BSS and shows substantial decrease after processing. The mechanism, as summarized from literature, is that processing reduces the side effects of atractylon by oxidation reaction during stir-frying and transforms it into atractylenolide I, atractylenolide II and atractylenolide III (not detected in this experiment because of their non-volatile property), which are effective in tonifying the spleen, protecting the gastric mucosa and inhibiting inflammation [8,34,35]. Given the high contents of furfural, 5-methyl-2-furfural, atractylenolide I, atractylenolide II and atractylenolide III, and the low content of atractylon, the clinical prescription of processed BSS might be highly effective.
3.5. Comparison of needle trap device method with other conventional methods
Because the study of volatile oils of BSS using the NTD and other conventional methods has not been reported before, some relevant studies on the single herbs contained in BSS were employed to make brief comparisons between the NTD method and other conventional methods, which might not be comprehensive but provide some references for further investigation. Firstly, compared with steam distillation and headspace sampling method in the literature, our NTD method could trap a wider range of volatile compositions ranging from low to high boiling point. Specifically, steam distillation exhibited relatively higher concentrations of the high boiling point compounds and relatively lower concentrations of the low boiling point compounds in Atractylodis Macrocephalae Rhizoma, Paeoniae Radix Alba, Citri Reticulatae Pericarpium and Saposhnikoviae Radix, especially those heavy hydrocarbons (Cn > 16) with more oxygens in their molecular formulas and those light hydrocarbons (Cn < 8) [36–40]. A possible explanation for the phenomenon is the loss of volatiles with low boiling point and the occurrence of oxidation reaction during the high temperature extraction. On the contrary, headspace sampling method exhibited almost all peaks of the low and middle boiling point components and very few high boiling point components concluded from the published reports of Atractylodis Macrocephalae Rhizoma and Citri Reticulatae Pericarpium [36,41]. Secondly, the NTD method showed much higher enrichment efficiency and sensitivity compared with other conventional methods. For the NTD method in our study, only a small amount of the sample (0.085 g) was required to achieve the strong response in detection, while steam distillation needed a few hundred grams and headspace sampling needed approximately 1 g to meet the same goal. In addition, the NTD method could enable a good selectivity in sampling by applying a specific packing material or several packing materials in combination to obtain target components in complex matrix, which was almost impossible for other conventional methods.
4. Conclusion
In this study, GC–MS based on NTD technique combined with multivariate data analysis was established to characterize the chemical consistency and difference of volatiles in BSS before and after processing. Unlike the conventional approaches of phytochemical analysis, the proposed method could globally and rapidly profile the volatile components of crude and processed BSS and reveal the chemical markers between these two kinds of samples more efficiently and comprehensively. Our results suggested that the levels of volatiles were clearly distinguished between crude and processed BSS using PCA and OPLS-DA models; moreover, 21 compounds were identified as candidate markers that could be employed to quickly differentiate these two types of samples using S-PLOT and VIP analyses. These successful extraction, detection and identification of volatile markers for the discrimination of processed BSS from crude BSS suggested that the strategy might also be applicable for the investigation of chemical transformation underlying herb processing in other herbal formulae of TCM. Furthermore, the methods of composition analysis and statistical processing in this study could shed light on other similar studies on plants or medicinal herbs with geographical disparity, environmental variations and species differences.
Supplementary Material
Acknowledgements
The authors thank the sponsorship of Ministry of Science and Technology of the People's Republic of China.
Data accessibility
The data supporting our paper have been deposited in Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6jg5g) [42].
Authors' contributions
Y.X., H.C., G.C. and Y. D. conceived and designed the experimental, carried out the GC–MS experiment, participated in data analysis and interpretation and drafted the manuscript. K.P., J.Z. and L.X. carried out the sample collection and pretreatment and took part in the optimization of sampling parameters. J.Z. and J.L. helped optimize the analysis model of PCA and OPLS-DA. X.W. and L.S. collected and interpreted the spectrogram data of the medicinal herbs of BSS. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was financially supported by the National Natural Science Foundation of China (nos. 81573602, 81673600, 81673559 and 81503331), the Opening Project of Zhejiang Provincial First-rate Subject (Traditional Chinese Medicine), Zhejiang Chinese Medical University (nos. Ya2017004 and 2017005), the Public Welfare Application Project of Zhejiang Province (no. 2017C33174) and the Traditional Chinese Medicine Key Scientific Research Fund Project of Zhejiang Province (no. 2018ZY004).
References
- 1.Fan H, Qiu MY, Mei JJ, Shen GX, Liu SL, Chen R. 2005. Effects of four regulating-intestine prescriptions on pathology and ultrastructure of colon tissue in rats with ulcerative colitis. World J. Gastroenterol. 11, 4800–4806. (doi:10.3748/wjg.v11.i31.4800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian ZX, Wu TX, Liu LX, Miao JF, Wong H, Song L, Sung JJY. 2006. Effectiveness of the Chinese herbal formula TongXieYaoFang for irritable bowel syndrome: a systematic review. J. Altern. Complement. Med. 12, 401–407. (doi:10.1089/acm.2006.12.401) [DOI] [PubMed] [Google Scholar]
- 3.Xu YY, Cai H, Duan Y, Pei K, Fan KL, Liu X, Cao G. 2017. Research progress of Baizhu Shaoyao powder in treating ulcerative colitis. China J. Chin. Mater. 42, 856–862. [DOI] [PubMed] [Google Scholar]
- 4.Li CQ, He LC, Jin JQ. 2007. Atractylenolide I and atractylenolide III inhibit lipopolysaccharide-induced TNF-alpha and NO production in macrophages. Phytother. Res. 21, 347–353. (doi:10.1002/ptr.2040) [DOI] [PubMed] [Google Scholar]
- 5.Kamino T, Shimokura T, Morita Y, Tezuka Y, Nishizawa M, Tanaka K. 2016. Comparative analysis of the constituents in Saposhnikoviae Radix and Glehniae Radix cum Rhizoma by monitoring inhibitory activity of nitric oxide production. J. Nat. Med. 70, 253–259. (doi:10.1007/s11418-016-0969-1) [DOI] [PubMed] [Google Scholar]
- 6.Bakkali F, Averbeck S, Averbeck D, Waomar M. 2008. Biological effects of essential oils — a review. Food Chem. Toxicol. 46, 446–475. (doi:10.1016/j.fct.2007.09.106) [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Liu X, Dou DQ. 2016. Bidirectional effective components of Atractylodis Macrocephalae Rhizoma on gastrointestinal peristalsis. Int. J. Pharmacol. 12, 108–115. (doi:10.3923/ijp.2016.108.115) [Google Scholar]
- 8.Dong HY, He LC, Huang M, Dong YL. 2008. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat. Prod. Res. 22, 1418–1427. (doi:10.1080/14786410801931629) [DOI] [PubMed] [Google Scholar]
- 9.Kuo YC, Lin YL, Huang CP, Shu JW, Tsai WJ. 2002. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Invest. 20, 955–964. (doi:10.1081/CNV-120005911) [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Chen YL, Zhang MW, Xu YJ, Pan SY. 2011. Chemical composition, antioxidant and antimicrobial activity of pericarpium citri reticulatae essential oil. Molecules 16, 4082–4096. (doi:10.3390/molecules16054082) [Google Scholar]
- 11.Zhong LJ, Hua YL, Ji P, Yao WL, Zhang WQ, Li J, Wei YM. 2016. Evaluation of the anti-inflammatory effects of volatile oils from processed products of Angelica sinensis radix by GC MS-based metabolomics. J. Ethnopharmacol. 191, 195–205. (doi:10.1016/j.jep.2016.06.027) [DOI] [PubMed] [Google Scholar]
- 12.Han YQ, Li YX, Wang YZ, Gao JR, Xia LZ, Hong Y. 2016. Comparison of fresh, dried and stir-frying gingers in decoction with blood stasis syndrome in rats based on a GC-TOF/MS metabolomics approach. J. Pharm. Biomed. Anal. 129, 339–349. (doi:10.1016/j.jpba.2016.07.021) [DOI] [PubMed] [Google Scholar]
- 13.Zhong LY, Zhu J, Gong QF, Zhang DF. 2008. The study on the influence of processing on the effects of diaphoresis and relieving ashtma of Ephedra herb. Pharm. Clin. Chin. Mater. 24, 53–56. [Google Scholar]
- 14.Cao G, Cai H, Jiang JP, Yao LJ, Tu SC, Wang L, Ma XQ, Cai BC. 2014. Chemical differentiation of volatile compounds in crude and processed Atractylodis Macrocephalae Rhizoma by using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with multivariate data analysis. J. Sep. Sci. 37, 1194–1198. (doi:10.1002/jssc.201301376) [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Guo L, Xu L. 2014. The GC-MS analysis of the fatty components of the root of herbaceous peony medicinal materials and the different processed products. Lishizhen Med. Mat. Med. Res. 25, 2151–2153. [Google Scholar]
- 16.Gao M, Xu XF, Cheng K, Wang JY, Huang PX. 2012. Study on the difference between components in volatile oil of citrus reticulata before and after being processed. J. Chin. Med. Mater. 35, 1046–1048. [PubMed] [Google Scholar]
- 17.Alonso M, Castellanos M, Besalu E, Sanchez JM. 2012. A headspace needle-trap method for the analysis of volatile organic compounds in whole blood. J. Chromatogr. A 1252, 23–30. (doi:10.1016/j.chroma.2012.06.083) [DOI] [PubMed] [Google Scholar]
- 18.Alonso M, Cerdan L, Godayol A, Antico E, Sanchez JM. 2011. Headspace needle-trap analysis of priority volatile organic compounds from aqueous samples: application to the analysis of natural and waste waters. J. Chromatogr. A 1218, 8131–8139. (doi:10.1016/j.chroma.2011.09.042) [DOI] [PubMed] [Google Scholar]
- 19.Lord HL, Zhang X, Musteata FM, Vuckovic D, Pawliszyn J. 2011. In vivo solid-phase microextraction for monitoring intravenous concentrations of drugs and metabolites. Nat. Protoc. 6, 896–924. (doi:10.1038/nprot.2011.329) [DOI] [PubMed] [Google Scholar]
- 20.Mieth M, Schubert JK, Groeger T, Sabel B, Kischkel S, Fuchs P, Hein D, Zimmermann R, Miekisch W. 2010. Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal. Chem. 82, 2541–2551. (doi:10.1021/ac100061k) [DOI] [PubMed] [Google Scholar]
- 21.Qin Y, Pang YM, Cheng ZH. 2016. Needle trap device as a new sampling and preconcentration approach for volatile organic compounds of herbal medicines and its application to the analysis of volatile components in viola tianschanica. Phytochem. Anal. 27, 364–374. (doi:10.1002/pca.2636) [DOI] [PubMed] [Google Scholar]
- 22.Filipiak W, Filipiak A, Ager C, Wiesenhofer H, Amann A. 2012. Optimization of sampling parameters for collection and preconcentration of alveolar air by needle traps. J. Breath. Res. 6, 027107 (doi:10.1088/1752-7155/6/2/027107) [DOI] [PubMed] [Google Scholar]
- 23.Zang XH, Liang WQ, Chang QY, Wu T, Wang C, Wang Z. 2017. Determination of volatile organic compounds in pen inks by a dynamic headspace needle trap device combined with gas chromatography-mass spectrometry. J. Chromatogr. A 1513, 27–34. (doi:10.1016/j.chroma.2017.07.030) [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li YX, Yang JL, Ruan J, Sun CJ. 2016. Microbial volatile organic compounds and their application in microorganism identification in foodstuff. Trends Anal. Chem. 78, 1–16. (doi:10.1016/j.trac.2015.08.010) [Google Scholar]
- 25.Heidari M, Bahrami A, Ghiasvand AR, Shahna FG, Soltanian AR. 2012. A novel needle trap device with single wall carbon nanotubes sol-gel sorbent packed for sampling and analysis of volatile organohalogen compounds in air. Talanta 101, 314–321. (doi:10.1016/j.talanta.2012.09.032) [DOI] [PubMed] [Google Scholar]
- 26.Li ZY, Li J, Zhang ZZ, Mi X, Du GH, Qin XM. 2017. NMR-based metabolomic analyses for the componential differences and the corresponding metabolic responses of three batches of Farfarae Flos. Chemom. Intell. Lab. 165, 1–10. (doi:10.1016/j.chemolab.2017.03.010) [Google Scholar]
- 27.Xiang Z, Wang XQ, Cai XJ, Zeng S. 2011. Metabolomics study on quality control and discrimination of three curcuma species based on gas chromatograph-mass spectrometry. Phytochem. Anal. 22, 411–418. (doi:10.1002/pca.1296) [DOI] [PubMed] [Google Scholar]
- 28.Pei K, et al. 2015. Ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry combined with statistical analysis for rapidly revealing the influence of sulfur-fumigated Paeoniae Radix Alba on the chemical l constituents of Si Wu Tang. Anal. Methods 7, 9442–9451. (doi:10.1039/C5AY02276B) [Google Scholar]
- 29.Chinese Pharmacopoeia Commission. 2015. Pharmacopoeia of the People's Republic of China, Part IV, p. 31. Beijing, China: China Medical Science and Technology Press. [Google Scholar]
- 30.Lou DW, Lee XQ, Pawliszyn J. 2008. Extraction of formic and acetic acids from aqueous solution by dynamic headspace-needle trap extraction — temperature and pH optimization. J. Chromatogr. A 1201, 228–234. (doi:10.1016/j.chroma.2008.02.066) [DOI] [PubMed] [Google Scholar]
- 31.Giordano L, Calabrese R, Davoli E, Rotilio D. 2003. Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry using isotope dilution. J. Chromatogr. A 1017, 141–149. (doi:10.1016/j.chroma.2003.08.029) [DOI] [PubMed] [Google Scholar]
- 32.Seiquer I, Rubio LA, Peinado MJ, Delgado-Andrade C, Navarro MP. 2014. Maillard reaction products modulate gut microbiota composition in adolescents. Mol. Nutr. Food Res. 58, 1552–1560. (doi:10.1002/mnfr.201300847) [DOI] [PubMed] [Google Scholar]
- 33.Zhao WL, Wu H, Shan GS, Jia TZ. 2013. Verification of processing theory of ‘reducing ketone and dryness, and increasing ester and effect ‘for bran-fried atractylodes. China J. Chin. Mater. 38, 3493–3497. [PubMed] [Google Scholar]
- 34.Wang KT, Chen LG, Yang LL, Ke WM, Chang HC, Wang CC. 2007. Analysis of the sesquiterpenoids in processed atractylodis rhizoma. Chem. Pharm. Bull. 55, 50–56. (doi:10.1248/cpb.55.50) [DOI] [PubMed] [Google Scholar]
- 35.Wang KT, Chen LG, Wu CH, Chang CC, Wang CC. 2010. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 62, 381–388. (doi:10.1211/jpp.62.03.0014) [DOI] [PubMed] [Google Scholar]
- 36.Pan HH, Chen MJ, Liu YP, Liu F, Zhang X, Chen L, Chen HP. 2017. Analysis of Baizhu volatile components extracted with different methods by GC–MS combing with Kovats Retention Index. Pharm. Clin. Chin. Mater. 8, 21–23, 29. [Google Scholar]
- 37.Shi X, Huang YP. 2011. Analysis on change of chemical compositions before and after Atractylodes macrocephala processing by GC-MS. Food Drug 13, 36–38. [Google Scholar]
- 38.Gong F, Zhang Q, Wang BT. 2009. Chemical characterization of herbal formula Yupingfeng powder and its single herbs (I) volatile components. Anal. Lett. 42, 2610–2624. (doi:10.1080/00032710903243539) [Google Scholar]
- 39.Zhao ZX, Feng R, Fu J, Wang Y, Yao QQ. 2015. Analysis of the volatile components from different origins of Radix Paeoniae Alba and Radix Paeoniae Rubra by GC-MS. Chin. J. Pharm. Anal. 35, 627–634. [Google Scholar]
- 40.Liang CY, Qin JP, Chen YP, Cai Y, Li YH, Li YR. 2012. Analysis of the volatile oil from Saposhnikovia divaricata in different habitats by GC-MS. Chin. J. Exp. Tradit. 18, 80–83. [Google Scholar]
- 41.Qiu ZR, Chen T, He LP, Zheng JX, Sun SW, Cao Y. 2017. Analysis of the volatile compounds in dried tangerine peel of different years by optimized solid phase micro-extraction/gas chromatography-mass spectrometry. Modern Food Sci. Technol. 33, 238–244. [Google Scholar]
- 42.Xu YY, et al. 2018. Data from: Discrimination of volatiles in herbal formula Baizhu Shaoyao San before and after processing using needle trap device with multivariate data analysis Dryad Data Repository. (http://dx.doi.org/10.5061/dryad.6jg5g) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Xu YY, et al. 2018. Data from: Discrimination of volatiles in herbal formula Baizhu Shaoyao San before and after processing using needle trap device with multivariate data analysis Dryad Data Repository. (http://dx.doi.org/10.5061/dryad.6jg5g) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting our paper have been deposited in Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6jg5g) [42].