Abstract
Soon after the National Lung Screening Trial, organizations began to endorse low-dose computed tomography (LCDT) screening for lung cancer in high-risk patients. Concerns about the risks versus benefits of screening, as well as the logistics of identifying and referring eligible patients, remained among physicians. This study aimed to examine primary care physicians' knowledge, attitudes, referral practices, and associated barriers regarding LDCT screening. We administered a national survey of primary care physicians in the United States between September 2016 and April 2017. Physicians received up to 3 mailings, 1 follow-up email, and received varying incentives to complete the survey. Overall, 293 physicians participated, for a response rate of 13%. We used weighted descriptive statistics to characterize participants and their responses. Over half of the respondents correctly reported that the US Preventive Services Task Force recommends LDCT screening for high-risk patients. Screening recommendations for patients not meeting high-risk criteria varied. Although 75% agreed that the benefits of LDCT screening outweigh the risks, fewer agreed that there is substantial evidence that screening reduces mortality (50%). The most commonly reported barriers to ordering screening included prior authorization requirements (57%), lack of insurance coverage (53%), and coverage denials (31%). The most frequently cited barrier to conducting LDCT screening shared decision making was patients' competing health priorities (42%). Given the impact of physician recommendations on cancer screening utilization, further understanding of physicians' LDCT screening attitudes and shared decision-making practices is needed. Clinical practice and policy changes are also needed to engage more patients in screening discussions.
Keywords: Physicians, Mass screening, Computed tomography, Early detection of cancer, Lung cancer
Highlights
-
•
Most physicians had five or less lung cancer screening referrals in the past year
-
•
Recommendation strategies varied, but often aligned with USPSTF or NCCN guidelines
-
•
Physicians were uncertain about the efficacy and cost-effectiveness of screening
-
•
Insurance coverage and costs were commonly cited as barriers to screening referral
-
•
A common barrier to performing SDM was patients' competing health priorities
1. Introduction
In 2017, an estimated 220,000 people will be diagnosed with lung cancer in the United States (U.S.) (Siegel et al., 2017). Most cases are caused by smoking and are diagnosed at late stages, resulting in a mere 28% five-year survival rate for regional stages and a 4% survival rate for distant stages. The five-year survival rate (55%) is substantially better for those diagnosed at a local stage (Siegel et al., 2017). Evidence from the National Lung Screening Trial (NLST) (Aberle et al., 2011) showed that low-dose chest CT (LDCT) performed annually among high-risk patients (i.e., 55–74 years old, current or former smoker who quit <15 years ago, and 30+ pack-year smoking history) for lung cancer screening could not only find cancers earlier but resulted in a 20% relative reduction in mortality from lung cancer and 7% relative reduction in all-cause mortality. If mortality reductions shown in the NLST could be replicated, estimates suggest that low-dose CT (LDCT) screening could avert about 12,000 deaths from lung cancer per year (Ma et al., 2013).
Although nearly all professional societies and cancer-related organizations including the US Preventive Services Task Force endorse LDCT screening for lung cancer in high-risk patients (Moyer, 2014; Smith et al., 2015; Wood et al., 2015), some physicians and associated organizations remain skeptical because of concerns about safety, quality, and generalizability (American Academy of Family Physicians, 2013; Bach, 2013; Centers for Medicare and Medicaid Services, 2014). Specifically, little is known about the quality of LDCT scans and associated image readings performed in community settings, the extent of adherence to follow-up screening or treatment recommendations, and whether patients being referred for LDCT screening meet high-risk criteria. These and other components of lung cancer screening (Mazzone et al., 2015) necessary to ensure high-quality screening remain understudied in the post-NLST era.
As the health care providers for the majority of patients in the U.S., primary care providers play a key role in ensuring the appropriate assessment and referral of patients to radiologic services. In a unique move, the Centers for Medicare and Medicaid Services announced that all Medicare beneficiaries referred for LDCT screening for lung cancer should receive a counseling and shared decision making (SDM) visit with a qualified healthcare provider prior to undergoing screening (Centers for Medicare and Medicaid Services, 2015). This patient-provider discussion is to include: 1) determination of eligibility; 2) counseling on smoking cessation/abstinence; 3) counseling on the importance of adherence to follow-up; and 4) discussion of the benefits and harms of screening. A subsequent Healthcare Common Procedure Coding System (HCPCS) code (G0296) was announced in late 2015 to cover the cost of the SDM visit for primary care providers. The LDCT screening exam is also reimbursed by Medicare and most private insurers (HCPCS code G0297). To counsel patients appropriately, it is vital that providers have a thorough understanding of the eligibility criteria, benefits, and potential harms of LDCT screening, as well as be familiar with SDM principles and associated decision aids.
Since the NLST, a handful of studies have examined the knowledge, attitudes, and early referral practices of healthcare providers for LDCT screening for lung cancer (Ersek et al., 2016; Volk and Foxhall, 2015; Raz et al., 2016; Hoffman et al., 2015; Duong et al., 2017; Henderson et al., 2011; Lewis et al., 2015; Rajupet et al., 2017). In these studies, physicians reported low referral rates (12–52% had referred any patients for screening) (Raz et al., 2016; Lewis et al., 2015), and patients were occasionally incorrectly recommended for screening (Duong et al., 2017). Physicians often expressed that better understanding of private and public insurance coverage, more information about screening centers available in their region, and education were necessary to increase their own personal screening recommendation rates (Volk and Foxhall, 2015; Henderson et al., 2011). Multiple barriers were reported, including uncertainty about the benefits of screening, patient exposure to harmful radiation, lack of institutional infrastructure to support screening, the complexity of discussing screening with patients, and concerns about the generalizability of the NLST findings (Raz et al., 2016; Hoffman et al., 2015; Lewis et al., 2015; Rajupet et al., 2017).
Although these studies have been foundational to our understanding of physicians' knowledge, attitudes, and practices regarding LDCT screening, none conducted since the NLST have included a nationally representative sample of primary care providers. To address this limitation, we conducted a mail and online survey of a national sample of practicing U.S. primary care physicians (i.e., family physicians, internists, and general practitioners) between September 2016 and April 2017 to assess physicians' knowledge of current lung cancer screening guidelines and insurance reimbursement, perceptions of screening effectiveness and cost, screening referral practices, and associated barriers.
2. Methods
2.1. Study participants
We surveyed a nationally representative sample of primary care physicians between September 2016 and April 2017 using a sampling frame of 2500 physicians selected from the American Medical Association (AMA) Master File (American Medical Association, 2017). We oversampled females to ensure an adequate representation of female physicians in the respondent pool. The physicians represented all 50 states, the District of Columbia, and Puerto Rico. Eligible candidates were in direct patient care and self-identified their primary specialty as general medicine, family medicine, or internal medicine. The AMA Master File contained extensive demographic information about each participant (e.g., sex, primary medical specialty, age, location of medical training, degree) and his/her practice (e.g., type of employment, office location). Metropolitan/non-metro designation was determined by geocoding and matching the address of each physician office location to the appropriate county-level 2013 Urban Influence Code U.S. Department of Agriculture, Economic Research Service, 2016.
2.2. Survey procedures
Physicians received up to 3 mailings (i.e., advance cover letter and 2 survey mailings) and 1 additional follow-up email invitation if an email address was available. Participants were randomly assigned to one of five incentive groups: no incentive, unconditional $1 incentive (i.e., participants received $1, and the incentive was not tied to completion of the survey), unconditional $5 incentive, lottery for one of ten $50 Amazon gift cards, and lottery for one of ten $100 Amazon gift cards. This ancillary study on various incentive types was intended to explore whether response rates varied significantly across incentive categories of small denominations.
In early September 2016, all 2500 physicians were mailed an advanced notice letter detailing the survey, alerting participants that they would be receiving a complete survey packet in the mail within the next 1–2 weeks, and requesting that the participants complete the survey as soon as possible once it arrived. The incentives were not mentioned in the advance letter. The initial survey packet was sent to all physicians in September 2016 and contained 1) a cover letter that repeated details of the study and included informed consent information); 2) the survey instrument; and 3) a prepaid addressed envelope in which to return the completed survey. All physicians who had not returned a completed survey within three weeks were mailed a second survey packet. We attempted to find correct or updated addresses via internet searches for all first-round surveys that were returned undeliverable because of an incorrect address or the physician changing office locations. The second survey packet was identical to the first except that the $1 and $5 unconditional incentives were not included, and there was no mention of an incentive in the cover letter accompanying the survey. Participants had the opportunity to complete the survey online with each survey mailing, as the cover letter instructed physicians on how to access the survey online with their unique assigned PIN number. Physicians, survey completion, and updated addresses were tracked using a custom Microsoft Access 2010 database to ensure data integrity. The study protocol was reviewed and approved by the University of South Carolina Institutional Review Board.
Of the 2180 physician that were non-responders to the mailed surveys, 1676 were able to be linked to an email address by the data provider (Medical Marking Services, Inc.), and 1572 were ultimately able to receive the final survey invitation via email (i.e., 104 suppressed/bounced back). Before calculating our response rate, 284 physicians were disqualified and removed based on either 1) secondary specialty other than primary care or self-reported retirement status (n = 272) or 2) failed contact in all survey rounds (i.e., undeliverable mailing address, plus no available email address or bounced/suppressed email, n = 12).
2.3. Survey content
A 19-item survey was designed to determine participants' opinions, knowledge, and recommendation practices regarding LDCT screening for lung cancer (see Supplementary file). Survey items assessing participants' opinions included questions on the perceived risks and benefits of LDCT lung cancer screening and follow-up care. Items assessing knowledge included presenting the participants with various patient vignettes and assessing whether they correctly identified which patients should be screened using low-dose CT, as well as a question assessing whether participants could correctly identify which professional organizations recommend LDCT screening (i.e., USPSTF, American Cancer Society/ACS, National Comprehensive Cancer Network/NCCN, and American Academy of Family Physicians/AAFP). The survey additionally asked about the physicians' perceptions regarding patient interest in low-dose CT lung cancer screening, participants' low-dose CT screening practices, and barriers to discussing and ordering low-dose CT lung cancer screening. Items in the questionnaire were based upon a conceptual framework developed by Cabana et al. (1999) and previously reported physician surveys (Ersek et al., 2016; Klabunde et al., 2012; Klabunde et al., 2010) including a national survey of primary care physicians' cancer screening knowledge, attitudes, and behaviors conducted by the National Cancer Institute in 2006–2007 (Klabunde et al., 2012; Klabunde et al., 2010).
2.4. Data analysis
We used descriptive statistics to characterize the survey respondents and their reported perceptions and practices regarding low-dose CT lung cancer screening. Inverse probability survey weights adjusting for the primary medical specialty and gender of physician respondents compared to the overall distribution of active U.S. physicians were applied in the analyses. SAS Version 9.4 survey procedures were used in the analysis to account for the calculated sample weights. We present the unweighted frequencies, along with their respective weighted proportions. Pearson's chi-square test was used to evaluate differences in response rates across the five incentive groups, and responders vs. non-responders.
3. Results
After excluding physicians with a specialty outside the scope of primary care, those who were retired, and those who failed to receive any survey invitation mailings or emails, 2211 physicians remained in the final denominator. In total, 293 eligible physicians completed the survey, for a final response rate of 13%.
Survey respondents (n = 293) were geographically dispersed across the 4 U.S. Census regions and Puerto Rico (see Table 1). The vast majority (88%) reported practicing in metropolitan areas, had a medical degree (88%) versus an osteopathic degree, and were trained in the United States (77%). The most common employment setting was group practice (53%). Responders were more likely than non-responders to be US trained (77% vs. 68%; p < 0.01), family/general practitioners (49% vs. 37%; p < 0.01), and have an osteopathic degree (16% vs. 9%; p = 0.01); see Table 2. No significant differences were noted for physician age or gender. Response rates also differed significantly by incentive group (p < 0.01). The response rates were 9% for the no-incentive group, 8% for the $50 Amazon gift card drawing, 9% for the $100 Amazon gift card drawing, 14% for the unconditional $1 group, and 25% for the unconditional $5 group.
Table 1.
Demographic and practice characteristics of physician respondents, 2016–2017 (n = 293).
| Characteristics | Unweighted no. | Weighted % |
|---|---|---|
| Census regiona | ||
| Puerto Rico | 4 | (1.07) |
| Northeast | 65 | (21.72) |
| Midwest | 85 | (29.71) |
| South | 67 | (23.58) |
| West | 71 | (23.93) |
| Practice location | ||
| Metro | 253 | (87.85) |
| Non-metro | 40 | (12.15) |
| Degree type | ||
| MD | 251 | (84.39) |
| DO | 42 | (15.61) |
| Sex | ||
| Male | 107 | (62.38) |
| Female | 186 | (37.62) |
| Present employment | ||
| Self-employed solo practice | 36 | (13.98) |
| Two-physician practice (full or part owner) | 9 | (3.18) |
| Group practice | 164 | (52.93) |
| Non-government hospital | 5 | (2.13) |
| City/county/state/government hospital | 19 | (6.88) |
| Veterans affairs | 4 | (2.071) |
| Federal government hospitals | 3 | (1.39) |
| Other/not classified | 53 | (17.43) |
| Primary specialty | ||
| Family/general medicine | 183 | (49.39) |
| Internal medicine | 110 | (50.61) |
| US trained | ||
| Yes | 232 | (77.13) |
| No | 61 | (22.87) |
| Age | ||
| ≤40 | 47 | (14.84) |
| 41–50 | 80 | (27.97) |
| 51–60 | 105 | (30.91) |
| >60 | 61 | (26.28) |
Census region missing, n = 1.
Table 2.
Comparison of survey responders to non-responders' demographics and practice characteristics, 2016–2017, n = 2211.
| Characteristics | Responders |
Non-responders |
p value⁎ | ||
|---|---|---|---|---|---|
| Unweighted no. | Weighted % | Unweighted no. | Weighted % | ||
| n | 293 | 1918 | |||
| Census region⁎ | |||||
| Northeast | 65 | (21.72) | 347 | (19.95) | <0.01 |
| Midwest | 85 | (29.71) | 415 | (20.54) | |
| South | 67 | (23.58) | 646 | (34.60) | |
| West | 71 | (23.93) | 478 | (24.92) | |
| Practice location | |||||
| Metro | 253 | (87.85) | 1712 | (89.07) | 0.79 |
| Non-metro | 40 | (12.15) | 206 | (10.93) | |
| Degree type | |||||
| MD | 251 | (84.39) | 1737 | (91.31) | 0.01 |
| DO | 42 | (15.61) | 181 | (8.69) | |
| Sex | |||||
| Male | 107 | (62.38) | 760 | (65.58) | 0.29 |
| Female | 186 | (37.62) | 1158 | (34.42) | |
| Present employment | |||||
| Self-employed solo practice | 36 | (13.98) | 234 | (13.80) | 0.01 |
| Two-physician practice (full or part owner) | 9 | (3.18) | 57 | (3.19) | |
| Group practice | 164 | (52.93) | 867 | (43.03) | |
| Non-government hospital | 5 | (2.13) | 45 | (2.79) | |
| City/county/state/government hospital | 19 | (6.88) | 130 | (7.40) | |
| Veterans affairs | 4 | (2.071) | 15 | (0.96) | |
| Federal government hospitals | 3 | (1.39) | 15 | (0.80) | |
| Other/not classified | 53 | (17.43) | 555 | (28.03) | |
| Primary specialty | |||||
| Family/general medicine | 183 | (49.39) | 964 | (36.63) | <0.01 |
| Internal medicine | 110 | (50.61) | 954 | (63.37) | |
| US trained | |||||
| Yes | 232 | (77.13) | 1335 | (67.76) | <0.01 |
| No | 61 | (22.87) | 583 | (32.24) | |
| Age | |||||
| ≤40 | 47 | (14.84) | 350 | (15.88) | 0.10 |
| 41–50 | 80 | (27.97) | 597 | (29.07) | |
| 51–60 | 105 | (30.91) | 539 | (28.24) | |
| >60 | 61 | (26.28) | 432 | (26.80) | |
p-Values based on the Pearson's chi-square statistic.
Forty-nine percent of the survey respondents (n = 144) indicated having had one or more patients ask about LDCT screening in the past 12 months, while 51% reported having no patients ask about LDCT screening. Only 30% of physicians indicated having ordered >5 screening tests in the past 12 months (0 tests: 33%, 1–5 tests: 36%, 6–10 tests: 16%, 11–24 tests: 10%, 25+ tests: 4%, not sure: 2%).
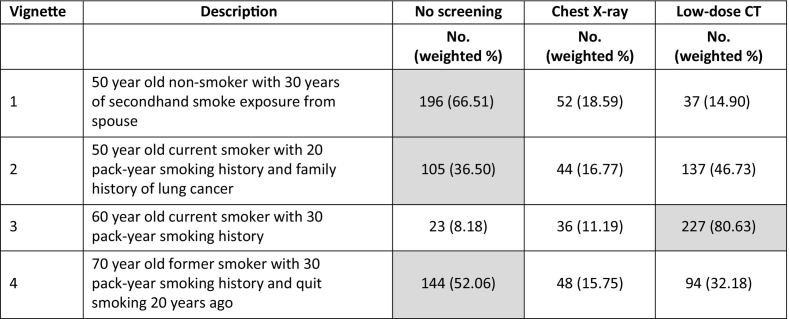
The majority of respondents identified the appropriate screening recommendation in most vignettes (Table 3). Over 80% of respondents indicated they would recommend LDCT screening for a 60-year-old patient with a 30 pack-year smoking history (8% would recommend no screening, and 11% would recommend chest x-ray). There was less consensus about what test to recommend for other scenarios, including a 50-year-old nonsmoker with 30 years of secondhand smoke exposure; in this case, 67% recommended no screening, 19% recommended chest x-ray, and 15% recommended LDCT. In the second vignette describing a 50-year-old patient with a 20 pack-year smoking history and a family history of lung cancer, 36% recommended no screening, 17% recommended chest x-ray, and 47% recommended LDCT.
Table 3.
Primary care physicians' recommended screening strategies for a variety of patient vignettes, 2016–2017, n = 286.
Footnotes: Correct response per USPSTF guidelines highlighted in gray. For vignette #2, LDCT screening is recommended per National Comprehensive Cancer Network guidelines.
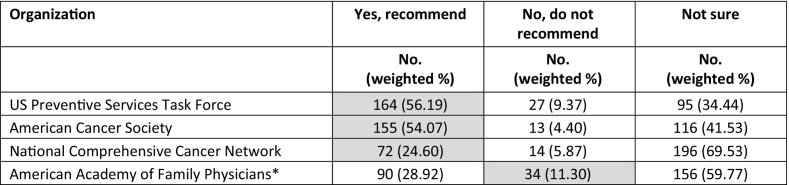
When specifically asked which organizations endorse LDCT screening for asymptomatic high-risk patients, a slight majority correctly noted that the USPSTF and ACS recommend LDCT screening (Table 4). Most were unsure whether the NCCN or AAFP recommended LDCT screening (70% and 60%, respectively). Very few (11%) of surveyed family physicians correctly identified that the AAFP does not recommend LDCT screening.
Table 4.
Primary care physicians' knowledge of organizations recommending or not recommending low-dose computed tomography screening for asymptomatic, high-risk patients, 2016–2017.
⁎ Limited to respondents who are family physicians. Correct response highlighted in gray.
Nearly 66% of physicians (n = 193) selected at least one barrier when asked to identify barriers experienced ordering a LDCT screening exam in the past 12 months. Among these 193 physicians, barriers identified included prior authorization requirements by insurance companies (57%), lack of insurance coverage for LDCT screening (53%), coverage denials received (31%), and transportation or financial challenges for the patient (22%). Less commonly experienced barriers included uncertainty about how to document patient eligibility for LDCT screening in the medical record (15%) and not knowing where to refer patients for screening (10%). Other barriers, reported in free text format, included the time to document SDM using decision aids, the requirement of having a separate office visit to discuss LDCT screening, the closest LDCT screening program being located >30 miles away, difficulty ordering screening in the electronic health record, institutional requirements that screening be ordered by a pulmonologist, and—most frequently stated in free text—patients declining/refusing screening (n = 12 physicians).
Over 78% (n = 229) of physicians selected at least one barrier when asked about barriers to discussing LDCT screening with their patients. The most commonly identified barriers included competing health priorities of the patient (42%), discussions taking too much time (22%), forgetting to mention screening to patients (20%), and not yet making screening discussions a routine part of practice (19%). Less common barriers included lacking decision aids (16%), patients' health literacy (15%), the complexity of the topic (14%), and lack of reimbursement to engage in shared decision making (13%). Notably, only 5% of physicians indicated a lack of training in SDM being a barrier. >65% of physicians preferred to make the LDCT screening decision together with their patient, rather than independently or letting the patient decide alone. Most physicians indicated they would be unlikely or very unlikely to engage in SDM with a patient if the discussion took >5 min (<3 min: 10%, 3–5 min: 21%, 6–8 min: 56%, >8 min: 67%). Only 5% of physicians indicated having used the Medicare billing code G0296 for SDM reimbursement, with an additional 11% being aware of the code, but not yet having billed for SDM.
Over 75% of survey respondents agreed or strongly agreed that the benefits of LDCT outweigh the risks for patients at high-risk for lung cancer; however, only half of the respondents agreed or strongly agreed that there is substantial evidence that LDCT screening reduces lung cancer mortality (Table 5). Perceptions of whether the false positive rate is too high varied substantially, with 42% of respondents feeling neutral, 23% in agreement, and 35% in disagreement. Just over 60% of physicians also reported that out-of-pocket costs would be a “real problem for my patients.” Nearly 15% of respondents felt that LDCT screening may undermine smoking cessation efforts with their patients.
Table 5.
Primary care physicians' perceptions about LDCT screening, 2016–2017.
| Strongly agree No. (weighted %) |
Agree No. (weighted %) |
Neutral No. (weighted %) |
Disagree No. (weighted %) |
Strongly disagree No. (weighted %) |
|
|---|---|---|---|---|---|
| The benefits of low-dose CT outweigh the risks for patient at high risk for lung cancer. | 135 (46.52) | 86 (31.79) | 43 (12.56) | 17 (6.89) | 6 (2.24) |
| There is substantial evidence from clinical trials that low-dose CT screening reduces lung cancer mortality. | 58 (19.22) | 87 (30.34) | 112 (38.40) | 27 (10.34) | 4 (1.69) |
| Low-dose CT screening for lung cancer screening is cost-effective. | 49 (15.14) | 93 (36.90) | 104 (33.49) | 34 (12.18) | 8 (2.27) |
| The rate of false positives for low-dose CT is higher than acceptable. | 17 (6.41) | 51 (16.81) | 120 (41.88) | 77 (28.39) | 18 (6.52) |
| Out-of-pocket costs for the low-dose CT and associated follow-up procedures may be a real problem for my patients | 108 (32.69) | 81 (29.83) | 54 (20.19) | 30 (13.53) | 11 (3.76) |
| Lung cancer screening may undermine smoking cessation efforts with my patient population. | 14 (4.57) | 28 (11.15) | 54 (15.80) | 82 (31.10) | 107 (37.38) |
Footnote: sample size varies by item.
4. Discussion
The benefits and potential harms of LDCT screening for high-risk patients, as well as the lack of evidence supporting the use of chest x-rays for screening, has been well described (Moyer, 2014; Bach et al., 2012; Tanoue et al., 2015; Eberth, 2015). While several surveys have assessed physicians' views and referral patterns for lung cancer screening in the early years since the landmark findings of the NLST (Ersek et al., 2016; Volk and Foxhall, 2015; Raz et al., 2016; Duong et al., 2017; Lewis et al., 2015), few have examined a geographically diverse sample of physicians. In this weighted, nationally representative survey, we investigated primary care physicians' perceptions and practices regarding lung cancer screening, in addition to their reported barriers to discussing and ordering LDCT scans for screening.
Similar to our previous findings from a survey of family physicians in South Carolina (Ersek et al., 2016), we found that about 80% of physicians correctly identified a low-dose CT as the screening modality for a hypothetical 60-year-old current smoker with a 30 pack-year smoking history. Over half of the respondents correctly reported that the USPSTF and ACS recommend annual LDCT screening for high-risk patients. Whether to recommend LDCT screening to patients at younger ages, those who quit smoking >15 years ago, or presented with other lung cancer risk factors varied considerably. The disagreement on which test to recommend may reflect the differing clinical practice guidelines/eligibility criteria endorsed by the NCCN (i.e., ≥50 years old and ≥20 pack years of smoking and one other additional risk factor other than secondhand smoke) (Wood et al., 2015), USPSTF (i.e., 55–80 years old, current or former smoker who quit <15 years ago, and 30+ pack-year smoking history) (Moyer, 2014), and the American Academy of Family Physicians (i.e., do not recommend for or against annual LDCT screening) (American Academy of Family Physicians, 2013). Additionally, physicians displayed uncertainty about the efficacy of LDCT screening (i.e., agree/strongly agree that screening reduces mortality), as well as whether screening was cost-effective and had an acceptable false positive rate. Over 75% of respondents agreed, however, that the benefits of screening outweigh the risks for high-risk patients.
Our findings are largely consistent with recent survey findings from other regional studies. In a study of primary care physicians in Los Angeles, Raz et al. (2016) found that approximately half of respondents knew that the USPSTF and ACS recommended screening with LDCT, with fewer physicians aware of the NCCN recommendation. Awareness of USPSTF lung cancer screening guidelines was much higher (86–89%) in convenience samples selected from Texas and Stanford, California (Volk and Foxhall, 2015; Duong et al., 2017), but awareness of the NCCN guidelines remained low (Duong et al., 2017). Both Raz et al. (2016)and Duong et al. (2017) reported between 50 and 60% of providers had ordered LDCT screening, and Volk and Foxhall (2015) reported that 56% planned to refer eligible patients for screening. Costs/insurance coverage for LDCT screening, patient co-morbidities, lack of time to discuss screening during patient visits, and concerns about false positives/unnecessary diagnostic tests were cited barriers in these other regional studies (Raz et al., 2016; Duong et al., 2017; Lewis et al., 2015; Rajupet et al., 2017).
Half of all respondents in our sample reported having no patients ask them about LDCT screening in the past 12 months, reflecting a substantial need for more public education about screening. Previous studies seem to indicate a general lack of awareness in the public about LDCT screening (Cardarelli et al., 2017) and its components (Retrouvey et al., 2015). In an awareness campaign in Appalachia Kentucky, Cardarelli et al. (2017) demonstrated that outreach to high-risk populations with tailored LDCT screening information (i.e., website, postcard mailings and newspaper ads) resulted in increased utilization of LDCT screening. In addition to presenting information about the benefits of and eligibility for screening, the campaign's materials reflected a “common message of hope and survival” (Cardarelli et al., 2017). Educational initiatives aiming to increase LDCT screening utilization among eligible patients, and in particular vulnerable populations, should incorporate easy to understand messages that reflect the values and culture of the target audience, encourage rather than stigmatize smokers, and promote an understanding of shared decision making among patients, their significant others and primary care providers (Carter-Harris et al., 2014; Chambers et al., 2012; Carter-Harris et al., 2017; Carter-Harris et al., 2017). Several limitations in our study should be noted. Although we had broad geographic representation of physicians in our sample, the overall sample size and response rate were suboptimal, which limits our generalizability. As indicated by the results of our sub-study on response rate variation across incentive groups, a higher incentive amount would have likely increased our final analytic sample. While we made every effort to eliminate physicians who are not engaged in primary care, it is unlikely that all physicians who received our survey were actively practicing in primary care settings. Many physicians in the AMA Physician Masterfile had ‘Internal Medicine’ listed as their primary specialty and ‘Unspecified’ listed as their secondary specialty, leading to uncertainty regarding the medical specialty of some physicians. Finally, because of the quantitative nature of our survey, we were unable to determine whether the recommended screening strategy associated with the vignettes presented in Table 2 was based on knowledge of the eligibility criteria for LDCT screening (based on explicit screening guidelines) vs. physician preferences or concerns (also noted by studies outside the context of cancer screening (de Ferranti et al., 2017)). Despite these limitations, this study is one of the first assessments of primary care physicians' perceptions, referral practices, and reported barriers to implementation of LDCT screening across a geographically diverse sample of US physicians. Our findings on barriers to performing SDM and ordering LDCT scans is also a unique aspect of this study. We provide evidence for the need for policy and systems changes to make the SDM and referral process more transparent and feasible for providers.
Additional research is warranted to examine which interventions that target physicians or patients are most effective at increasing LDCT screening utilization in high-risk patients, or at minimum, increasing the prevalence of LDCT screening discussions with potential screening candidates in primary care settings. It is also important to determine the content and length of these screening discussions, as several of our respondents mentioned concerns with the time to document SDM, patients refusing screening, and other issues related to documentation of patient eligibility/referrals. Although the Centers for Medicare & Medicaid requires (and will pay for) a SDM visit prior to obtaining LDCT screening, (Centers for Medicare and Medicaid Services, 2015) we know very little about how health care providers discuss screening with their patients, and if they discuss the critical elements of risks vs. benefits, the importance of annual screening and follow-up diagnostic testing, and smoking cessation or relapse prevention (if applicable). Given the well-known impact of the physicians' recommendation on cancer screening utilization (Pucheril et al., 2015; Hawley et al., 2014; Lafata et al., 2014), further understanding of how physicians discuss LDCT screening with their high-risk patients is needed. Clinical practice and policy changes are also needed to engage more patients in screening discussions, remove barriers to performing SDM and ordering screening tests, and ultimately, improve access to affordable, high-quality LDCT screening.
Conflicts of interests
None to disclose.
Acknowledgments
Acknowledgements
This work was support by Supporting Outstanding Academic Research Seed Grants at USC (SOAR-USC), 124275-IRG-13-043-01-IRG, from the American Cancer Society. The authors wish to thank our graduate assistants and interns, namely Cassie Odahowski, Sazid Khan, Lauren Blew, and Debra Warden, for their assistance with data collection and management. We also thank Dr. Suzanne K. Linder, former Assistant Professor in the Division of Rehabilitation Sciences at the University of Texas Medical Branch at Galveston, for her suggestions and edits to the survey.
Authors contributions
JME conceptualized the study, prepared the original manuscript draft, and provided study oversight. JME is the manuscript guarantor, taking responsibility for the integrity of the work from inception to publication. ES and SK conducted data analysis and prepared manuscript tables. KMM, AD, SK and ES assisted with survey preparation and dissemination. All co-authors (KKM, ES, SK, AD, SMS, RFM, SWV) contributed substantially to the study design, interpretation of study results, and critical editing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2018.05.013.
Contributor Information
Jan M. Eberth, Email: jmeberth@mailbox.sc.edu.
Karen Kane McDonnell, Email: karenkm@mailbox.sc.edu.
Erica Sercy, Email: sercyet@mailbox.sc.edu.
Samira Khan, Email: khans@mailbox.sc.edu.
Scott M. Strayer, Email: strayers@mailbox.sc.edu.
Amy C. Dievendorf, Email: adievend@mailbox.sc.edu.
Reginald F. Munden, Email: rmunden@wakehealth.edu.
Sally W. Vernon, Email: Sally.W.Vernon@uth.tmc.edu.
Appendix A. Survey instrument
Supplementary material
References
- Aberle D.R., Adams A.M., Berg C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Family Physicians Clinical preventive service recommendation: lung dancer. 2013. http://www.aafp.org/patient-care/clinical-recommendations/all/lung-cancer.html Available at:
- American Medical Association AMA physician masterfile. 2017. https://www.ama-assn.org/life-career/ama-physician-masterfile Available at:
- Bach P.B. Perilous potential: the chance to save lives, or lose them, through low dose computed tomography screening for lung cancer. J. Surg. Oncol. 2013;108(5):287–288. doi: 10.1002/jso.23389. [DOI] [PubMed] [Google Scholar]
- Bach P.B., Mirkin J.N., Oliver T.K. Benefits and harms of ct screening for lung cancer: a systematic review. J. Am. Med. Assoc. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana M.D., Rand C.S., Powe N.R. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- Cardarelli R., Reese D., Roper K.L. Terminate lung cancer (TLC) study—a mixed-methods population approach to increase lung cancer screening awareness and low-dose computed tomography in Eastern Kentucky. Cancer Epidemiol. 2017;46:1–8. doi: 10.1016/j.canep.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli R., Roper K.L., Cardarelli K. Identifying community perspectives for a lung cancer screening awareness campaign in Appalachia Kentucky: the terminate lung cancer (TLC) study. J. Cancer Educ. 2017;32(1):125–134. doi: 10.1007/s13187-015-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Harris L., Brandzel S., Wernli K.J., Roth J.A., Buist D.S.M. A qualitative study exploring why individuals opt out of lung cancer screening. Fam. Pract. 2017;34(2):239–244. doi: 10.1093/fampra/cmw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Harris L., Ceppa D.P., Hanna N., Rawl S.M. Lung cancer screening: what do long-term smokers know and believe? Health Expect. 2017;20(1):59–68. doi: 10.1111/hex.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Harris L., Hermann C.P., Schreiber J., Weaver M.T., Rawl S.M. Lung cancer stigma predicts timing of medical help-seeking behavior. Oncol. Nurs. Forum. 2014;41(3):E203–E210. doi: 10.1188/14.ONF.E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services MEDCAC meeting 4/30/2014 — lung cancer screening with low dose computed tomography. 2014. https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=68 Available at:
- Centers for Medicare and Medicaid Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) 2015. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 Available at:
- Chambers S.K., Dunn J., Occhipinti S. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12:184. doi: 10.1186/1471-2407-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong D.K., Shariff-Marco S., Cheng I. Patient and primary care provider attitudes and adherence towards lung cancer screening at an academic medical center. Prev. Med. Rep. Jun 2017;6:17–22. doi: 10.1016/j.pmedr.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberth J.M. Lung cancer screening with low-dose CT in the United States. J. Am. Coll. Radiol. 2015;12(12, Part B):1395–1402. doi: 10.1016/j.jacr.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Ersek J.L., Eberth J.M., Mcdonnell K.K. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- de Ferranti S.D., Rodday A.M., Parsons S.K. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J. Pediatrics. 2017;185:99–105. doi: 10.1016/j.jpeds.2016.12.078. (e102) [DOI] [PubMed] [Google Scholar]
- Hawley S., Lillie S., Cooper G., Elston Lafata J. Managed care patients' preferences, physician recommendations, and colon cancer screening. Am. J. Manag. Care. 2014;20(7):555–561. [PMC free article] [PubMed] [Google Scholar]
- Henderson S., Degroff A., Richards T.B. A qualitative analysis of lung cancer screening practices by primary care physicians. J. Community Health. 2011;36(6):949–956. doi: 10.1007/s10900-011-9394-2. [DOI] [PubMed] [Google Scholar]
- Hoffman R.M., Sussman A.L., Getrich C.M. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev. Chron. Dis. 2015;12:E108. doi: 10.5888/pcd12.150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde C.N., Marcus P.M., Han P.K. Lung cancer screening practices of primary care physicians: results from a national survey. Ann. Fam. Med. 2012;10(2):102–110. doi: 10.1370/afm.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde C.N., Marcus P.M., Silvestri G.A. U.S. primary care physicians' lung cancer screening beliefs and recommendations. Am. J. Prev. Med. 2010;39(5):411–420. doi: 10.1016/j.amepre.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafata J.E., Cooper G., Divine G., Oja-Tebbe N., Flocke S.A. Patient-physician colorectal cancer screening discussion content and patients' use of colorectal cancer screening. Patient Educ. and Couns. 2014;94(1):76–82. doi: 10.1016/j.pec.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.A., Petty W.J., Tooze J.A. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an Academic Medical Center. Cancer Epidem. Biomar. 2015;24(4):664–670. doi: 10.1158/1055-9965.EPI-14-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ward E.M., Smith R., Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- Mazzone P., Powell C.A., Arenberg D. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society policy statement. Chest. 2015;147(2):295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V.A. Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2014;160(5) doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- Pucheril D., Dalela D., Sammon J. The influence of physician recommendation on prostate-specific antigen screening. Urol. Oncol. 2015;33(10) doi: 10.1016/j.urolonc.2015.06.013. (424 e421–427) [DOI] [PubMed] [Google Scholar]
- Rajupet S., Doshi D., Wisnivesky J.P., Lin J.J. Attitudes about lung cancer screening: primary care providers versus specialists. Clin Lung Cancer. 2017;18(6) doi: 10.1016/j.cllc.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz D.J., Wu G.X., Consunji M. Perceptions and utilization of lung cancer screening among primary care physicians. J. Thorac. Oncol. 2016;11(11):1856–1862. doi: 10.1016/j.jtho.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retrouvey M., Patel Z., Shaves S. US preventive services task force CT lung cancer screening recommendations: community awareness and perceptions. J. Am. Coll. Radiol. 2015;12(1):114–115. doi: 10.1016/j.jacr.2014.09.019. [DOI] [PubMed] [Google Scholar]
- SAS 9.4 [computer program]. Cary, NC: SAS Institute Inc.
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. Jan 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Smith R.A., Manassaram-Baptiste D., Brooks D. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA-A Cancer J. Clin. 2015;65(1):30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- Tanoue L.T., Tanner N.T., Gould M.K., Silvestri G.A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 2015;191(1):19–33. doi: 10.1164/rccm.201410-1777CI. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Economic Research Service Urban Influence Codes. 2016. https://www.ers.usda.gov/data-products/urban-influence-codes.aspx Available at:
- Volk R.J., Foxhall L.E. Readiness of primary care clinicians to implement lung cancer screening programs. Prev Med Rep. 2015;2:717–719. doi: 10.1016/j.pmedr.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D.E., Kazerooni E., Baum S.L. Lung cancer screening, version 1.2015: featured updates to the NCCN guidelines. J. Natl. Compr. Canc. Ne. 2015;13(1):23–34. doi: 10.6004/jnccn.2015.0006. (quiz 34) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material