Abstract
The anticancer drug temozolomide is the only drug with proven activity against high-grade gliomas and has therefore become a part of the standard treatment of these tumors. P-glycoprotein (P-gp; ABCB1) and breast cancer resistance protein (BCRP; ABCG2) are transport proteins, which are present at the blood-brain barrier and limit the brain uptake of substrate drugs. We have studied the effect of P-gp and BCRP on the pharmacokinetics and pharmacodynamics of temozolomide, making use of a comprehensive set of in vitro transport experiments and in vivo pharmacokinetic and antitumor efficacy experiments using wild-type, Abcg2−/−, Abcb1a/b−/−, and Abcb1a/b;Abcg2−/− mice. We here show that the combined deletion of Abcb1a/b and Abcg2 increases the brain penetration of temozolomide by 1.5-fold compared to wild-type controls (P < .001) without changing the systemic drug exposure. Moreover, the same increase was achieved when temozolomide was given to wild-type mice in combination with the dual P-gp/BCRP inhibitor elacridar (GF120918). The antitumor efficacy of temozolomide against three different intracranial tumor models was significantly enhanced when Abcb1a/b and Abcg2 were genetically deficient or pharmacologically inhibited in recipient mice. These findings call for further clinical testing of temozolomide in combination with elacridar for the treatment of gliomas, as this offers the perspective of further improving the antitumor efficacy of this already active agent.
Abbreviations: ABC, ATP-binding cassette; AUC, area under the curve; BBB, blood-brain barrier; BCRP, breast cancer resistance protein; GBM, glioblastoma; MGMT, O6-methylguanine-DNA methyltransferase; MRI, magnetic resonance imaging; P-gp, P-glycoprotein; WT, wild-type
Introduction
High-grade gliomas, and in particular glioblastoma (GBM), are refractory to virtually all chemotherapy regimens. Whereas it is possible that the heterogeneity within these tumors favors the presence of innately resistant tumor cells, inadequate drug exposure of tumor cells because of the blood-brain barrier (BBB) is most likely also a major cause of the general lack of efficacy of chemotherapy [1]. The BBB restricts the entry of nearly all commonly used agents. Although this barrier is often disrupted in more central tumor areas, where leakiness can be visualized by contrast-enhanced magnetic resonance imaging (MRI), it is still functional in the more peripheral and surgically unresectable tumor regions that harbor many viable and proliferating tumor cells. Moreover, glioma tumor cells have the propensity to migrate deep into the surrounding normal brain tissue, where the BBB is also fully intact [2], [3].
The principal components of the BBB are the endothelial cells that are linked together by tight junctions, limiting the paracellular movement of substances [4]. Moreover, transcellular routing is further restricted by the absence of fenestrae and the low endocytic activity of brain endothelial cells. Besides these more or less passive restraints, the BBB is also equipped with ATP-binding cassette (ABC) efflux transporters such as P-glycoprotein (P-gp, ABCB1) and breast cancer resistance protein (BCRP, ABCG2) that together limit the brain penetration of almost all classical chemotherapeutics and novel targeted anticancer drugs [5], [6].
For a long time, the standard treatment of GBM consisted of surgical resection followed by local radiotherapy, with or without nitrosourea-based chemotherapy. However, a large number of adjuvant nitrosourea-based chemotherapy trials have been conducted but did not demonstrate significant survival benefit [7], [8]. At the last turn of the century, however, the orally bioavailable alkylating agent temozolomide was reported to have significant activity in the treatment of recurrent grade 3 and grade 4 gliomas, outperforming procarbazine in one study [9], [10], [11]. Then, about a decade ago, a large phase III trial showed a significant survival benefit for radiotherapy in combination with concomitant and adjuvant temozolomide compared to radiotherapy alone [12]. This landmark study set the basis for the new standard treatment of newly diagnosed GBM, where patients start temozolomide (75 mg/m2/day for 42 days) concomitantly with radiotherapy, subsequently followed by six courses of temozolomide monotherapy (150 to 200 mg/m2/day for 5 days with a 23-day rest period). Epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) promoter has been demonstrated to predict benefit to temozolomide chemotherapy [13]. Nevertheless, this drug is currently being prescribed to most GBM patients since it is generally well tolerated, a small survival benefit is seen in patients carrying nonsilenced MGMT promotors, and there is no real alternative [14].
Temozolomide is generally believed to penetrate the BBB relatively well. Indeed, the brain penetration is apparently high enough to improve the median survival of GBM patients from 12.1 to 14.6 months [12]. Nevertheless, we here show that P-gp and BCRP collectively limit the brain penetration of this drug. Using drug transporter knockout mouse models, we show that the temozolomide brain penetration is increased when these two drug transporters are absent or inhibited, while the plasma clearance of the drug remains unaffected. Importantly, we show that the efficacy of temozolomide against three independent experimental intracranial tumor models is significantly improved when P-gp and BCRP are genetically absent or pharmacologically inhibited.
Materials and Methods
Reagents
Temozolomide (Temodal 20 mg hard capsules) originated from Schering Plough BV (Utrecht, The Netherlands). Elacridar (GF120918) was a generous gift from GlaxoSmithKline Inc. (Research Triangle Park, NC). Erlotinib was kindly provided by OSI Pharmaceuticals, Inc., (Melville, NY). Zosuquidar was a generous gift of Eli Lilly (Indianapolis, IN). Gefitinib was purchased from Sequoia Research Products Ltd. (Pangbourne, UK). Bovine Serum Albumin (BSA) fraction V was purchased from Roche Diagnostics GmbH (Mannheim, Germany). All other chemicals were purchased from Merck (Darmstadt, Germany) and were used as supplied.
Preparation of Drug Solutions
The content of a temozolomide capsule containing 20 mg of active substance was dissolved in 0.4 ml ethanol and 3.6 ml saline to yield a solution of 5.0 mg/ml and was used within 60 minutes after preparation. Elacridar was prepared freshly the day before each experiment and suspended at 5 mg/ml in a mixture of hydroxypropyl methylcellulose (0.5 g/l)/1% polysorbate 80 (v/v). The suspension was mixed for 2 minutes using a Polytron PT1200 homogenizer (Kinematica AG, Littau, Switzerland). Additionally, the suspension was kept protected from light and stirred continuously before and during administration. Gefitinib was suspended in 0.5% (v/v) Tween 20 and 0.25% (w/v) carboxymethylcellulose in water at a concentration of 10 mg/ml.
Analytical Methods
Based on previous work by Kim et al. [15], we have developed a high-performance liquid chromatographic (HPLC) assay for the determination of temozolomide in medium used for in vitro transport experiments and in mouse plasma and brain tissue homogenates for in vivo pharmacokinetic studies. Separation and quantification were achieved using a Symmetry C18 column (150 × 2.0 mm; ID) together with a mobile phase of 7.5% of methanol in 0.5% acetic acid in water, delivered at a flow rate of 0.2 ml/min and UV detection at 330 nm (PDA996 photodiode array detector; Waters, Milford, MA, or SF 757 detector; Kratos, Ramsey, NJ). Medium from Transwell experiments was diluted 10-fold with 0.2% acetic acid in water, and 50 μl was injected directly into the HPLC system. Temozolomide was extracted from the acidified plasma and brain tissue homogenate samples (200 μl) with 1.0 ml ethyl acetate. The dried extracts were subsequently dissolved in 100 μl of 5% methanol in 0.2% acetic acid in water, and 50 μl was injected into the HPLC system. External calibration was used. The lower and upper limits of quantitation were 0.020 μg/ml and 10.0 μg/ml, respectively. Samples above the upper limit of quantification were first diluted with acidified blank human plasma. All samples from in vivo studies were analyzed twice in two independent analytical series and repeated once more when the duplicates differed by more than 10%.
In Vitro Transport Experiments
The parental LLC porcine kidney (PK1) cell line and its Mdr1a transduced subline [16] and the parental Madine Darby Canine Kidney (MDCKII) cell line and its Bcrp1 transduced subline [17] were used to establish whether temozolomide is a substrate of Mdr1a (P-gp) and Bcrp1, respectively. Cells were seeded on Transwell microporous polycarbonate membrane filters (3.0 μm pore size, 24 mm diameter; Costar Corning, NY) at a density of 1 × 106 cells per well in 2 ml of MEM medium (Invitrogen Corporation, Carlsbad, CA) containing 10% v/v fetal bovine serum. Cells were incubated at 37°C in 5% CO2 for 3 days with one medium replacement after the first day. Two hours before the start of the experiment, the medium in both compartments was replaced with 2 ml of OptiMEM medium (Invitrogen Corp.). At the start of the experiment, the medium in the apical or basolateral compartment was replaced with 2 ml of freshly prepared OptiMEM medium containing 40 μg/ml of temozolomide. The P-gp and/or BCRP inhibitors elacridar (5 μM), gefitinib (5, 10 μM), erlotinib (5, 10, and 20 μM), or novobiocin (10 and 50 μM) were added to both the apical and basolateral compartment. Zosuquidar (LY335979, 5 μM) was always added to the medium when doing experiments with MDCK cell lines to inhibit endogenous canine P-gp. Samples of 50 μl were collected every 30 minutes for up to 2 hours after start of the experiment. Because temozolomide is instable in medium at 37°C, we replaced 1 ml of the donor compartment by a freshly prepared aliquot every 30 minutes. [3H]-inulin (approximately 7 kBq per well) was added to the same compartment as temozolomide to check the integrity of the cell layer. Wells showing a leakage of more than 1.5% per hour were excluded from analysis.
Animals
Animals used in the pharmacokinetics studies were male wild-type (WT), Abcg2−/−, Abcb1a/b−/−, and Abcb1a/b;Abcg2−/− mice of an FVB genetic background within the age of 8 to 15 weeks as described before [18]. Animals used for efficacy studies were WT or Abcb1a/b;Abcg2−/− athymic nude mice of FVB background or Cre-LoxP conditional p53F/F;p16Ink4a/p19ArfF/F;LoxP-Stop-LoxP (LSL)-K-rasv12;Luc as previously described [19]. Animals were housed and handled according to institutional guidelines complying with Dutch and European law. The mice were kept in a temperature-controlled environment with a 12-hour light-dark cycle and were given a standard diet (AM-II; Hope Farms B.V., Woerden, The Netherlands) and acidified water ad libitum. All experiments involving animals were approved by the local animal ethics committee.
In Vivo Pharmacokinetics Studies
The brain penetration study comprised cohorts of WT, Abcg2−/−, Abcb1a/b−/−, and Abcb1a/b;Abcg2−/− mice receiving temozolomide (50 mg/kg) by intravenous injection in the tail vein. Each cohort consisted of at least 40 animals in which at least 6 animals were used per time point (t = 15 minutes, 1, 2, 4, and 7 hours post temozolomide administration). Separate cohorts of WT and Abcb1a/b;Abcg2−/− mice received temozolomide (50 mg/kg) either as single agent or 2 hours after elacridar (100 mg/kg) or 1 hour after gefitinib (100 mg/kg) administered orally by gavage into the stomach. At the different time points, the mice were anesthetized with methoxyflurane, and blood samples were obtained by cardiac puncture and collected on ice in tubes containing potassium EDTA as anticoagulant. The tubes were immediately cooled in melting ice and centrifuged within 60 minutes (10 minutes, 5000g, 4°C) to separate the plasma fraction, which was transferred into clean vials. Subsequently, plasma fraction was mixed with 1 M hydrochloric acid (10 + 1; v/v) and stored at −20°C until analysis. Immediately after cardiac puncture, the mice were sacrificed by cervical dislocation, and the brains were dissected and placed on ice. Within 60 minutes, they were weighed and homogenized in 3 ml of ice-cold 1% of BSA in 0.05 M phosphate buffer adjusted to pH 2 and stored at −20°C until further analysis.
We next established the drug exposure in WT (n = 18) and Abcb1a/b;Abcg2−/− mice (n = 19) receiving an oral dose of 100 mg/kg temozolomide by gavage. Blood was sampled from the tail at 15 and 30 minutes and at 1, 4, and 7 hours post drug administration to obtain a full curve from each animal.
Intracranial Tumor Models
Three different intracranial tumor models were used in this study. First, Mel57-luc cells (100,000 cells/2 μl) were injected stereotactically in the brains of FVB nude mice as previously described in detail [20]. Next, murine GSC457 glioblastoma cells previously isolated from a Cre-LoxP conditional p53F/F;p16Ink4a/p19ArfF/F;LSL-K-rasv12;Luc high-grade glioma [19] were injected (5000 cells/2 μl) in the brains of recipient FVB nude mice. Lastly, we examined the results in spontaneous murine gliomas induced by injecting lentiviral CMV-Cre (2 μL) in the brains of p53F/F;p16Ink4a/p19ArfF/F;-LSL-K-rasv12;Luc mice [19]. All stereotactic injections (tumor cells and virus) were done at 2 mm lateral and 1 mm anterior to the bregma, 3 mm below the skull.
Magnetic Resonance Imaging
MRI was done using a BioSpec 70/20 USR (Bruker, Billerica, MA) using a sequence of T2-weighted, T1-weighted precontrast and T1-weighted postcontrast. Gadoterate meglumine (Dotarem; 0,5 mmol/ml; Guerbet; Villepinte, France) diluted five-fold with saline was used as a contrasting agent and delivered via an intravenous cannula inserted in the tail vein. Mice were anesthetized using isoflurane (Pharmachemie B.V., Haarlem, the Netherlands) delivered via a customized mouse holder, and heart rate and breathing frequency were monitored throughout the entire procedure. Paravision software (v 6.0.1; Bruker) was used for image acquisition.
Blood-Brain Barrier Integrity Characterization
Mice bearing orthotopic GSC457 or p53−/−;p16Ink4a/p19Arf−/−;K-rasv12;Luc tumors were intravenously injected with 6 mg/kg TexasRed (Sulforhodamine 101; Invitrogen) in saline. After 30 minutes, the animal was anesthetized with hypnorm/dormicum and perfused with saline. The brain was immediately frozen on dry ice in Tissue-Tek (Sakura Finetek Europe BV, Alphen aan den Rijn, the Netherlands) and kept at −70°C until sectioning. Fluorescence microscopy analysis of the brain slices was done using an Axio Observer Z1 system (Zeiss, Oberkochen, Germany) equipped with an ORCA-AG CCD camera (Hamamatsu Photonics KK, Hamamatsu, Japan).
In Vivo Efficacy Studies
Bioluminescence imaging using an IVIS200 camera (Caliper Life Science, Alameda, CA) was used to establish tumor load in each animal at the start of therapy. Depending on the experiment, the animals were stratified between oral temozolomide (100 mg/kg/day for 5 or 7 days as indicated), no treatment (control), or temozolomide with concomitant oral elacridar (100 mg/kg/day for 5 or 7 days) given 20-30 minutes prior to temozolomide. Bioluminescence imaging was repeated at subsequent days to establish the efficacy of the therapy. The amount of bioluminescence in each animal was calculated relative to the first measurement when therapy was initiated and was logarithmically converted prior to data analysis.
Data Analysis
All statistical analyses in this study were carried out using GraphPad Prism v7.03 (GraphPad Inc., La Jolla, CA) unless otherwise stated. To determine the differences of brain and plasma concentrations among multiple strains or conditions over time, two-way analysis of variance (ANOVA) was performed. All single time point analyses with multiple groups were analyzed by one-way ANOVA. All ANOVA analyses were corrected for multiple comparisons using the post hoc Bonferroni procedure. Plasma and brain AUC0-7h values and standard errors were calculated by the linear trapezoidal rule using standard equations [21]. The plasma half-life of temozolomide was calculated by linear regression analysis after log transformation of the concentration data using Microsoft Excel 2010. The brain-to-plasma ratio was calculated for each individual animal. In vivo tumor growth curves were compared using the General Linear Model repeated measured procedure using SPSS (v22; SPSS Inc., Chicago, IL). Kaplan-Meier curves were drawn using GraphPad Prism v7.03, and the log-rank test was used to determine whether survival curves were significantly different. All differences were considered statistically significant when P < .05.
Results
In Vitro Transport Assays
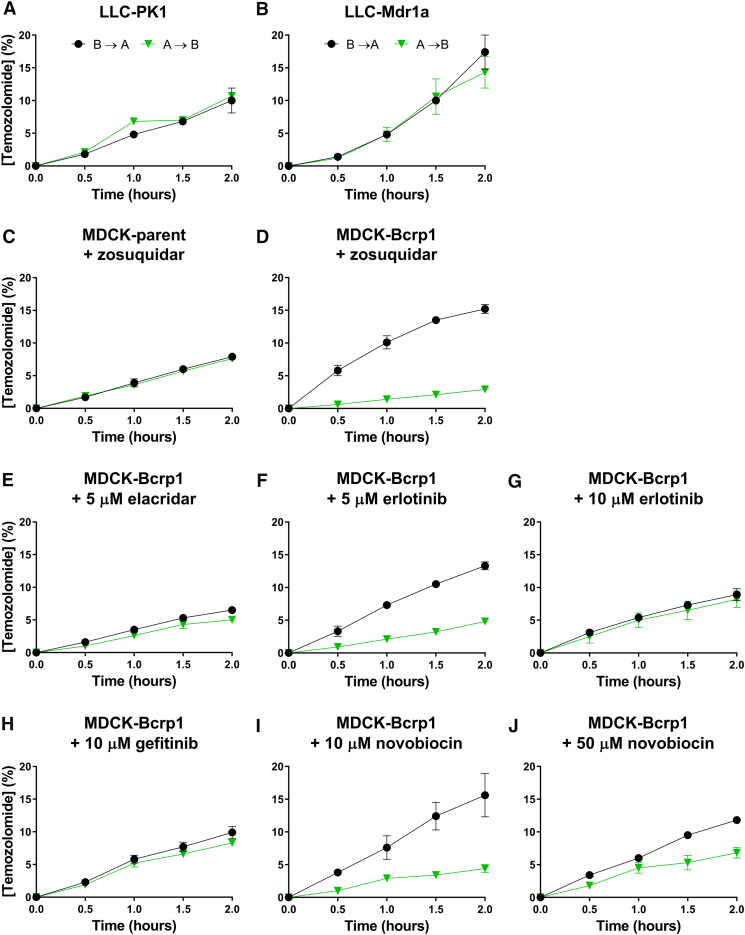
Because the stability of temozolomide in culture medium at 37°C is limited by its short half-life of approximately 30 minutes, in vitro transport experiments were carried out slightly differently than usual for this kind of experiment [22]. First, we took samples every 30 minutes for up to 2 hours instead of every hour for up to 4 hours. Secondly, to maintain adequate temozolomide levels at the donor side throughout the experiment, we replaced 1 ml of the 2 ml of medium at the donor side of the transwell at each sampling time by 1 ml of a freshly prepared temozolomide solution in medium. We have investigated P-gp-mediated transport of temozolomide (40 μg/ml) using polarized monolayers of porcine kidney (LLC-PK1) cells and its murine P-gp transduced subclone (LLC-Mdr1a). Our results show very limited, if any, evidence for transport by P-gp (Figure 1, A and B). Similar experiments were conducted in the parental canine MDCKII cells and its Bcrp1 transduced (MDCKII-Bcrp1) subline and showed clear vectorial transport by Bcrp1 (Figure 1, C and D). This Bcrp1-mediated transport was almost completely abrogated when elacridar (5 μM), gefitinib (10 μM), or erlotinib (10 μM) was present in the medium (Figure 1, E-H). A concentration of 50 μM of novobiocin was not sufficient (Figure 1, I and J). These in vitro results clearly show that whereas temozolomide is a good substrate of Bcrp1, it appears to be transported less efficiently by P-gp.
Figure 1.
In vitro temozolomide transport experiments. Temozolomide was added to the basolateral or apical compartment of the Transwell to measure basolateral-to-apical (B → A) or apical-to-basolateral (A → B) transport, respectively, using LLC-PK1 (parent) versus the Mdr1a subline or using MDCKII (parent) versus the Bcrp1 transduced sublines. The transport of temozolomide is depicted as percentage of temozolomide initially present at the donor compartment (mean ± SD). Because degradation will also take place in the acceptor compartment during the experiment, this value will be an underestimation of the fraction that is actually translocated. Temozolomide transport by Mdr1a was not readily detected by this assay, whereas transport by Bcrp1 was evident. The dual P-gp-BCRP inhibitor elacridar and the presumed Bcrp1 inhibitors gefitinib, erlotinib, and novobiocin were added at the depicted concentrations.
In Vivo Pharmacokinetics
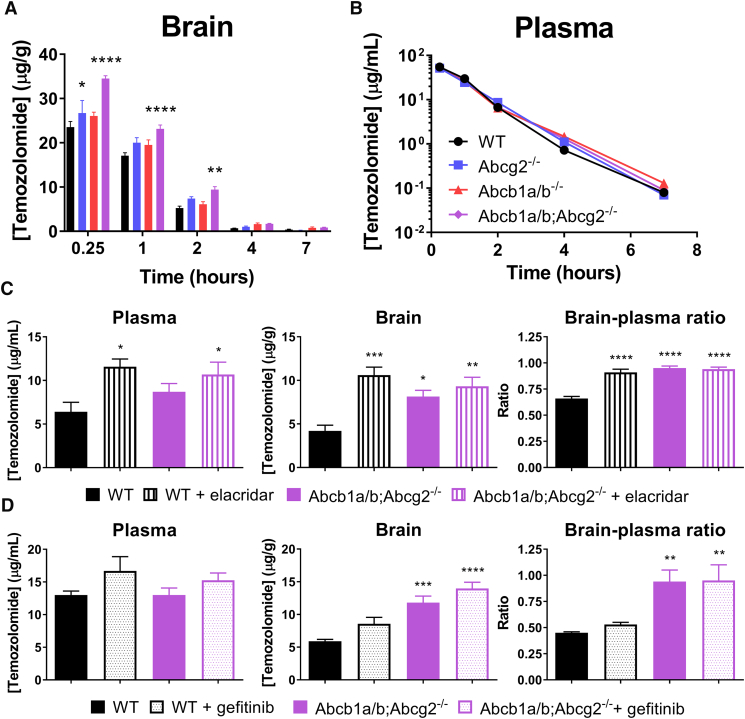
To investigate the impact of BCRP and P-gp on the disposition of temozolomide in vivo, we have performed experiments in WT, Abcg2−/−, Abcb1a/b−/− and Abcb1a/b;Abcg2−/− mice. All animals received temozolomide by i.v. injection in the tail vein. This route was chosen to minimize the interanimal variability that might be higher after oral dosing and because the systemic exposure may be higher in ABC-transporter knockout mice. Interestingly, the brain penetration of temozolomide was significantly (P < .01) higher in both of the single knockout mouse strains compared to the WT control group (Table 1). Although the in vitro results suggested that P-gp (Abcb1a/b) does not transport temozolomide, the brains of Abcb1a/b−/− mice accumulated about 20% more temozolomide than those of WT mice. The same enhancement was seen in Abcg2−/− mice, whereas Abcb1a/b;Abcg2−/− mice accumulated 50% more (P < .001) drug in the brain (Figure 2A). Although a 1.5-fold increase for temozolomide is less than observed previously with several other substrate drugs, temozolomide already has proven activity against high-grade glioma. We therefore expect that this 1.5-fold gain in the brain penetration will be highly relevant for treatment of brain cancer patients. The absence of drug transporters did not affect the plasma clearance of this drug (Table 1 and Figure 2B), nullifying the chance that the higher brain level is due to higher plasma levels. In fact, the decline of temozolomide from plasma follows first-order elimination kinetics with a half-life of 0.7 hours, in line with the fact that temozolomide is unstable at physiological pH due to nonenzymatic degradation into its metabolite 3-methyl-(triazen-1-yl)imidazole-4-carboximide [23].
Table 1.
Pharmacokinetic Parameters of Temozolomide after Intravenous Administration of 50 mg/kg
| Genotype | AUC 0–7 h, brain (μg/g.h) | AUC 0–7 h plasma (μg/ml.h) | T½ plasma (h) |
|---|---|---|---|
| WT | 36.81 ± 1.11 | 65.40 ± 2.00 | 0.689 ± 0.019 |
| Abcg2−/− | 44.78 ± 1.88* | 62.96 ± 2.14 | 0.719 ± 0.017 |
| Abcb1a/b−/− | 44.51 ± 1.56* | 62.16 ± 2.05 | 0.757 ± 0.021 |
| Abcb1a/b;Abcg2−/− | 57.09 ± 1.32**/# | 64.47 ± 1.29 | 0.705 ± 0.014 |
AUC, area under the plasma concentration-time curve; T1/2, elimination half-life. Data are represented as mean ± SE; n ≥ 7; * P < .01 relative to WT mice, ** P < .001 relative to WT mice, #P < .01 relative to Abcb1a/b;Abcg2−/− mice.
Figure 2.
The impact of Abcb1a/b and Abcg2 on the brain and plasma concentration of temozolomide after intravenous administration. (A) Brain and (B) plasma concentrations of temozolomide in WT, Abcg2−/−, Abcb1a/b−/− and Abcb1a/b;Abcg2−/− FVB mice that received 50 mg/kg of temozolomide by intravenous injection. Both Abcg2−/− and Abcb1a/b−/− mice accumulated 20% more temozolomide in the brain compared to WT mice, whereas Abcb1a/b;Abcg2−/− mice even accumulated 50% (P < .001) more drug in the brain. The absence of the drug transporters Abcb1a/b and Abcg2 did not affect the plasma levels (see also Table 1). Data are depicted as mean ± SE; n ≥ 7. (C) Elacridar (100 mg/kg) and (D) gefitinib (100 mg/kg) were orally administered to WT and Abcb1a/b;Abcg2−/− mice 2 hours (elacridar) or 1 hour (gefitinib) prior to intravenous temozolomide, and blood and brain samples were collected 2 hours after temozolomide administration. Elacridar significantly enhanced the brain penetration (brain-to-plasma ratio) in WT mice (P = .001) to levels that were similar to those observed in Abcb1a/b;Abcg2−/− mice, whereas the brain-to-plasma ratio was not significantly different between Abcb1a/b;Abcg2−/− mice with or without elacridar. Administration of gefitinib did not result in a significantly enhanced brain penetration of temozolomide in WT mice. Data are depicted as mean ± SE; n = 5; * P < .05, ** P < .01, *** P < .001, **** P < .0001.
To investigate the possibility of pharmacologically enhancing the brain accumulation of temozolomide by inhibition of P-gp and BCRP, we have used the dual P-gp/BCRP inhibitor elacridar. Elacridar significantly (P = .001) enhanced the brain penetration of temozolomide in WT mice when given at a single oral dose of 100 mg/kg, reaching similar levels as those achieved in Abcb1a/b;Abcg2−/− mice (Figure 2C). Moreover, no further significant enhancement in the brain penetration of temozolomide was seen when elacridar was given to Abcb1a/b;Abcg2−/− mice, demonstrating that the interaction by elacridar was selective for BCRP and P-gp. In contrast, gefitinib given at 100 mg/kg, did not significantly enhance the brain-to-plasma ratio of temozolomide (Figure 2D) even though a previous report suggested it to be a potent P-gp/BCRP inhibitor [24].
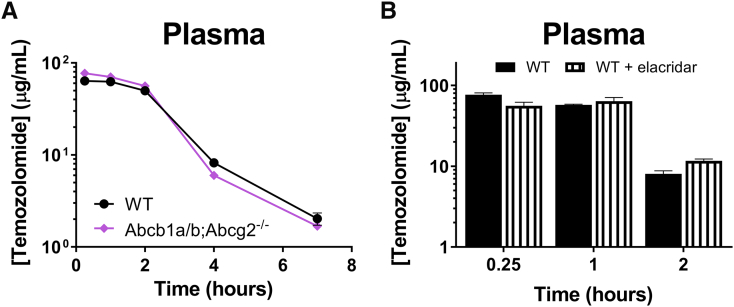
Temozolomide is orally administered to patients. Since both P-gp and BCRP may limit the oral bioavailability of substrate drugs, we have investigated the drug exposure of oral temozolomide in WT versus Abcb1a/b;Abcg2−/− mice using the dose that would be used in the subsequent in vivo efficacy study against intracranial xenografts (Figure 3A). While we used relatively large cohorts of animals (WT n = 18, Abcb1a/b;Abcg2−/− n = 19) for sufficient statistical power, the plasma AUC was not significantly different in Abcb1a/b;Abcg2−/− mice (Table 2). This result is in line with the almost complete oral bioavailability of temozolomide in C57BL × DBA/2 F1 mice that was previously reported by Stevens et al. [25]. Moreover, we did not find any impact of elacridar on the plasma kinetics of oral temozolomide in brain tumor–bearing animals (Figure 3B).
Figure 3.
Plasma concentration-time curves of orally administered temozolomide. (A) Temozolomide (100 mg/kg) was given orally to WT and Abcb1a/b;Abcg2−/− mice, after which serial blood samples were drawn from the tail vein at various time points. The plasma curves of temozolomide were similar in both mouse strains. Depicted are the curves of the mean ± SE concentrations; n ≥ 18. (B) Plasma levels of temozolomide in intracranial Mel57 tumor-bearing mice following oral temozolomide administration (100 mg/kg) with or without orally co-administered elacridar (100 mg/kg). Blood samples were collected from the tail vein of the mice used in the study depicted in Figure 5B. Elacridar did not significantly affect the plasma pharmacokinetics of orally given temozolomide. Data are depicted as mean ± SE; n = 8.
Table 2.
Pharmacokinetic Parameters of Temozolomide after Oral Administration of 100 mg/kg
| Genotype | AUC 0–7 h, plasma (μg/ml.h) |
|---|---|
| WT | 154.2 ± 4.1 |
| Abcb1a/b;Abcg2−/− | 164.5 ± 4.5 |
Data are represented as mean ± SE; n ≥ 18.
In Vivo Efficacy Studies
To investigate the relevance of the higher brain penetration of temozolomide for treatment of intracranial tumors, we performed in vivo efficacy studies in three different intracranial tumor mouse models. The first model mimicked melanoma brain metastases by stereotactically implanting Mel57 cells in the brains of recipient nude mice [20]. As a second model, we injected murine GSC457 glioma stem–cell like cells that were derived from a spontaneous transgenic mouse model [26]. Lastly, we used this transgenic mouse model where tumors were induced by stereotactic injection of a Lenti-Cre vector in the brains of p53F/F;p16Ink4a/p19ArfF/F;LSL-K-rasv12;Luc mice. These mice spontaneously develop high-grade gliomas within a couple of weeks after vector injection.
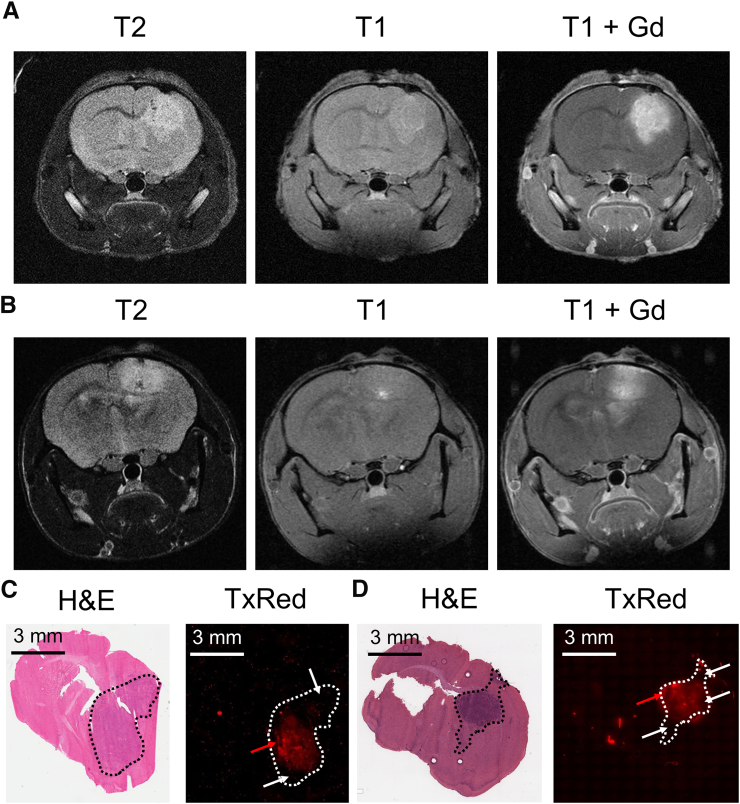
Using gadolinium-DTPA MRI, we have previously shown that the BBB in brain tumors from the Mel57 cell line is relatively intact, rendering this tumor model especially suited to explore the impact of the BBB on antitumor efficacy [20]. In contrast, the BBB integrity of the GSC457 and p53−/−;p16Ink4a/p19Arf−/−;K-rasv12;Luc glioma models appeared to more compromised, as indicated by the moderate contrast enhancement we observed on postgadolinium T1-weighted MRI (Figure 4, A and B). We further characterized the BBB integrity by i.v. injection of tumor-bearing mice with TexasRed just prior to brain tissue collection. TexasRed is a fluorescent small molecule compound with very little penetration into healthy brain tissue. TexasRed extravasation in our orthotopic brain tumor models is quite heterogeneous, with some regions of the tumors showing mild to moderate and other parts little to no extravasation (Figure 4, C and D).
Figure 4.
Characterization of the blood-brain barrier integrity of the orthotopic murine glioma models. T2- and T1-weighted MRI of orthotopic (A) GSC457 and (B) p53−/−;p16Ink4a/p19Arf−/−;K-rasv12;Luc tumors. Some contrast enhancement was observed in both tumor models on T1-weighted images after gadolinium (Gd) administration. (C) GSC457 and (D) p53−/−;p16Ink4a/p19Arf−/−;K-rasv12;Luc glioma-bearing WT mice received TexasRed shortly prior to being sacrificed. Comparison of the hematoxylin and eosin (H&E) staining with fluorescence microscopy of coronal brain sections revealed heterogeneous TexasRed (TxRed) extravasation in the tumor regions (dashed circumscribed area) of both models, with some regions showing moderate extravasation (red arrows) and others showing little to no extravasation (white arrows).
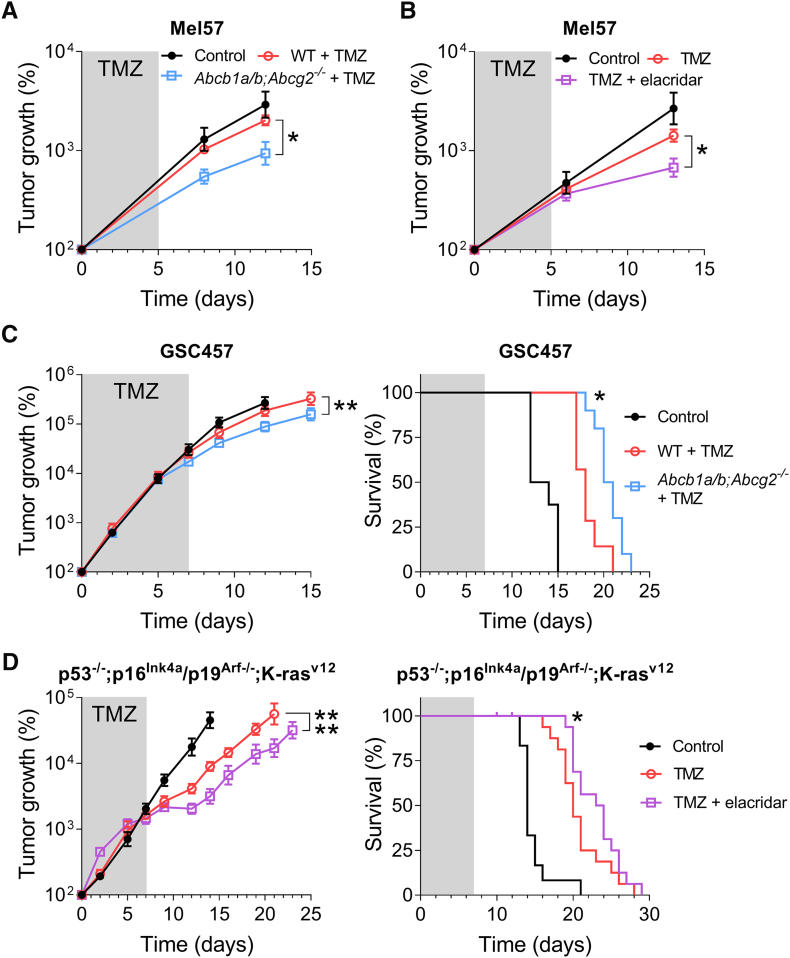
We first used Mel57 and employed two strategies to investigate the impact of P-gp and BCRP activity at the BBB on the efficacy of temozolomide, namely, by administration of temozolomide to WT mice vs. genetic knockouts (Abcb1a/b;Abcg2−/− mice) and by using WT mice receiving temozolomide alone or in combination with elacridar, a pharmacological inhibitor of P-gp and BCRP. Interestingly, the modest efficacy of temozolomide against intracranial Mel57 tumors grafted in WT mice was significantly higher when grafted in Abcb1a/b;Abcg2−/− mice (Figure 5A). Furthermore, Mel57 tumors grafted in WT responded significantly better when concomitantly treated with elacridar compared to temozolomide treatment alone (Figure 5B). These results suggest that P-gp and BCRP at the BBB at least partly attenuate the efficacy of temozolomide in the brain.
Figure 5.
The impact of Abcb1a/b and Abcg2 on the efficacy of temozolomide against intracranial tumors. (A) The efficacy of temozolomide (TMZ) against intracranial Mel57-luc tumors in WT and Abcb1a/b;Abcg2−/− mice. A significantly better response to treatment with oral temozolomide (100 mg/kg/day × 5) was observed in Abcb1a/b;Abcg2−/− mice compared to WT mice. Data are depicted as mean ± SE; n ≥ 6. (B) Intracranial Mel57-luc tumors treated with the combination of temozolomide (100 mg/kg/day × 5) and elacridar (100 mg/kg/day × 5) grew considerably slower in WT mice than tumors treated with temozolomide alone. Data are depicted as mean ± SE; n ≥ 3. (C) WT and Abcb1a/b;Abcg2−/− mice were orthotopically grafted with GSC457 cells and monitored using in vivo bioluminescence imaging. Oral treatment with temozolomide (TMZ; 100 mg/kg/day × 7) reduced tumor growth and significantly improved survival of Abcb1a/b;Abcg2−/− mice compared to WT mice. Data are depicted as mean ± SE; n ≥ 7. (D) Spontaneous p53−/−;p16Ink4a/p19Arf−/−;K-rasv12;Luc gliomas were induced in WT mice using a lenti-Cre vector. As is apparent from previously reported data [19], elacridar and temozolomide co-administration significantly reduced tumor growth and prolonged survival compared to TMZ monotherapy. Data are depicted as mean ± SE; n ≥ 11; * P < .05, ** P < .01, **** P < .0001.
To confirm these findings, we compared the efficacy of temozolomide against the orthotopic GSC457 model grafted in WT and Abcb1a/b;Abcg2−/−mice. In this case, we compared the tumor growth and survival and also found that temozolomide treatment resulted in significantly slower tumor growth and longer survival in Abcb1a/b;Abcg2−/− mice compared to WT mice (Figure 5C).
Lastly, we reassessed data from a previously published animal cohort and found further support for the notion that the efficacy of temozolomide is diminished by the BBB [19]. In this earlier study, we demonstrated the negative impact of P-gp and BCRP on the brain penetration and intracranial antitumor efficacy of the PARP inhibitor veliparib. However, the data in this study also demonstrate that chemical inhibition of P-gp and BCRP by elacridar improved the effects of single agent temozolomide on tumor growth and survival of the p53−/−;p16Ink4a/p19Arf−/−;K-rasv12 spontaneous murine glioma model (Figure 5D).
Together, these studies demonstrate that P-gp and BCRP at the BBB diminish the efficacy of temozolomide against intracranial tumors and that concomitant inhibition of these transporters by elacridar can improve survival by counteracting this attenuation of temozolomide efficacy.
Discussion
This study shows that the absence of both P-gp and Bcrp1 enhances the brain penetration of temozolomide by 1.5-fold without reducing the clearance of this drug. A similar effect on the brain penetration was seen in WT mice that received the dual P-gp and BCRP inhibitor elacridar. The increased brain penetration translated into a significantly better antitumor response in experimental intracranial tumor models. As temozolomide has proven activity against high-grade glioma, we expect that this further 1.5-fold gain in the brain penetration of this drug may further enhance the efficacy of temozolomide treatment. Moreover, since the systemic clearance is not altered, this enhanced efficacy may come without enhanced side effects. Consequently, our results provide a basis for further clinical testing of combinations of elacridar and temozolomide in patients suffering from high-grade glioma.
The finding that temozolomide is a substrate of P-gp was not obvious from the in vitro results. In contrast, temozolomide was clearly transported by Bcrp1 in our in vitro transport assay. Most likely, temozolomide is a relatively weak substrate of P-gp, and the in vitro system is not sensitive enough to detect small changes in drug translocation. Moreover, the in vitro transport assay is also complicated by the instability of temozolomide in the transport medium that has a pH of 7.4. Because of this instability of temozolomide in transport medium, it is also not possible to calculate the permeability in moles per cm2. But taking into account this instability, the finding that at least 5% of the donor concentration was recovered in the acceptor compartment after 30 minutes suggests that temozolomide readily permeates membranes, which is an important characteristic for a compound to be able to penetrate the BBB at all [27]. However, despite this relatively weak affinity for P-gp, the combined presence of P-gp and Bcrp1 at the BBB significantly reduces the brain penetration of temozolomide (Figure 2A). In contrast, we could not observe any effect of P-gp and BCRP on the oral bioavailability of temozolomide. As seen previously, drug efflux transporters appear to be much more capable of restricting the entry of substrate drugs into the brain than preventing uptake from the gut. For example, the substrate drug imatinib has an excellent oral bioavailability (>90% [28]) but a poor brain penetration that is significantly enhanced in Abcb1a/b;Abcg2−/− mice [29]. Similar discrepancies are seen for other drugs, including dabrafenib and regorafenib [30], [31], [32].
Many studies have demonstrated that the brain penetration of drugs, classic chemotherapeutics and novel targeted anticancer agents alike, can be substantially reduced by drug transporters at the BBB [5]. For example, we and others found a strikingly higher brain penetration of the chemotherapeutic paclitaxel (5-fold) and the small molecule CDK4/6 inhibitor palbociclib (approximately 15-fold) in Abcb1a/b−/− compared to WT controls [21], [33], [34]. In that perspective, the 1.5-fold (i.e., 50%) enhancement of the brain penetration of temozolomide may appear less impressive. Importantly, however, in contrast to these other agents, temozolomide is an agent with proven activity against high-grade glioma, and in the clinical context, a 50% higher drug level in tumor tissue could signify a clinically meaningful improvement of efficacy. Importantly, our proposed strategy of concomitantly inhibiting P-gp and BCRP would provide a 50% temozolomide dose intensification specifically to the brain without enhancing the systemic exposure to this drug.
The improved BBB penetration of temozolomide translated into a better antitumor response in three different intracranial tumor models. This was the case not only when we used an intracranial Mel57 model that has a very tight BBB [20] but also with tumors that have leakier vasculature based on MRI (Figure 4, A and B). Importantly, the TexasRed extravasation data reported here demonstrate that both the GSC457 and the p53−/−;p16Ink4a/p19Arf−/−;K-rasv12 spontaneous murine glioma models have a heterogeneous BBB integrity (Figure 4, C and D). This heterogeneity is particularly clinically relevant. GBM contains regions where the BBB is disrupted due to VEGF-driven microvascular proliferation, but is also composed of tumor cells that deeply infiltrate into the surrounding brain tissue where the BBB is still functional. Especially this invasive component of the tumor renders this disease incurable by current treatment modalities. In order to target these invasive tumor cells, agents should be capable to penetrate the BBB.
Obviously, the finding that P-gp and BCRP attenuate the brain penetration and intracranial antitumor efficacy of temozolomide can only be exploited in patients when inhibitors of P-gp and BCRP are clinically available. Fortunately, the BBB penetration and antitumor efficacy of temozolomide were improved by using the dual P-gp/BCRP inhibitor elacridar. We have also tested several other proposed inhibitors of P-gp and BCRP, including the EGFR inhibitor gefitinib. However, even at a dose level of 100 mg/kg, which will result in plasma levels of about 10 μM [35], this compound was not able to increase the brain penetration of temozolomide. These results indicate that the capacity of gefitinib to inhibit Abcb1a/b and Abcg2 in vivo at the BBB is insufficient and discount gefitinib as a candidate for clinical testing, in particular because the plasma levels that can be achieved in patients are about 10-fold lower [36].
In general, pharmacological inhibition of P-gp and BCRP could present an interesting potential extra benefit. Whereas this study only focuses on the impact of P-gp and BCRP expression in the blood vessels building the BBB, it is known that GBM tumor cells may themselves also express these transporters, which may further contribute to the multidrug resistant phenotype of GBM [37]. Consequently, inhibition of P-gp and BCRP may also increase the sensitivity of tumor cells to temozolomide chemotherapy. We have previously observed this phenomenon when studying the PARP inhibitor veliparib against intracranial tumors in Abcb1a/b;Abcg2−/− mice [19]. Although these mice lack P-gp and BCRP at the BBB, the grafted tumors expressed these transporters and inhibition by elacridar enhanced the antitumor response.
Temozolomide has a good safety profile, with bone marrow toxicity as main dose-limiting toxicity [38]. Although the clearance of temozolomide was unaffected in mice that received concomitant elacridar, the combined use of temozolomide with P-gp and BCRP inhibitors may have an impact on the toxicity profile. Given that P-gp and BCRP are expressed in bone marrow stem cells [39], [40], their inhibition in combination with the administration of a cytotoxic drug may enhance the myelotoxic effects. Obviously, the effect a P-gp/BCRP inhibitor on the toxicity of temozolomide needs to be monitored closely when such a combination is tested in clinical trial.
In conclusion, we have demonstrated that the brain penetration and antitumor efficacy of temozolomide are limited by P-gp and BCRP and can be increased by concomitant use of the dual P-gp/BCRP inhibitor elacridar. We expect that this combination may further enhance the efficacy of temozolomide against GBM, which should be explored in subsequent clinical trials.
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Pencheva N, de Gooijer MC, Vis DJ, Wessels LFA, Würdinger T, van Tellingen O, Bernards R. Identification of a druggable pathway controlling glioblastoma invasiveness. Cell Rep. 2017;20(1):48–60. doi: 10.1016/j.celrep.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Durmus S, Hendrikx JJMA, Schinkel AH. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv Cancer Res. 2015;125:1–41. doi: 10.1016/bs.acr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2012;64:138–153. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 7.Glioma Meta-analysis Trialists Group Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 8.Medical Research Council Brain Tumour Working Party Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19(2):509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 9.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12(2):259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- 10.Yung WKA, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yung WKA, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O'Neill AM. Multicenter Phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol. 1999;17(9):2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 12.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 13.Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 14.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Likhari P, Parker D, Statkevich P, Marco A, Lin C-C, Nomeir AA. High-performance liquid chromatographic analysis and stability of anti-tumor agent temozolomide in human plasma. J Pharm Biomed Anal. 2001;24(3):461–468. doi: 10.1016/s0731-7085(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 16.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96(4):1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JHM, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92(20):1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 18.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-Glycoprotein and Breast Cancer Resistance Protein: Two Dominant Transporters Working Together in Limiting the Brain Penetration of Topotecan. Clin Cancer Res. 2007;13(21):6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH, Wurdinger T, Beijnen JH, van Tellingen O. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res. 2014;20(10):2703–2713. doi: 10.1158/1078-0432.CCR-14-0084. [DOI] [PubMed] [Google Scholar]
- 20.Kemper EM, Leenders W, Kusters B, Lyons S, Buckle T, Heerschap A, Boogerd W, Beijnen JH, van Tellingen O. Development of luciferase tagged brain tumour models in mice for chemotherapy intervention studies. Eur J Cancer. 2006;42(18):3294–3303. doi: 10.1016/j.ejca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O. Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin Cancer Res. 2003;9(7):2849–2855. [PubMed] [Google Scholar]
- 22.Zhang P, de Gooijer MC, Buil LC, Beijnen JH, Li G, van Tellingen O. ABCB1 and ABCG2 restrict the brain penetration of a panel of novel EZH2-Inhibitors. Int J Cancer. 2015;137(8):2007–2018. doi: 10.1002/ijc.29566. [DOI] [PubMed] [Google Scholar]
- 23.Tsang LLH, Quarterman CP, Gescher A, Slack JA. Comparison of the cytotoxicity in vitro of temozolomide and dacarbazine, prodrugs of 3-methyl-(triazen-1-yl)imidazole-4-carboxamide. Cancer Chemother Pharmacol. 1991;27(5):342–346. doi: 10.1007/BF00688855. [DOI] [PubMed] [Google Scholar]
- 24.Yang C-H, Huang C-J, Yang C-S, Chu Y-C, Cheng A-L, Whang-Peng J, Yang P-C. Gefitinib reverses chemotherapy resistance in gefitinib-insensitive multidrug resistant cancer cells expressing ATP-binding cassette family protein. Cancer Res. 2005;65(15):6943–6949. doi: 10.1158/0008-5472.CAN-05-0641. [DOI] [PubMed] [Google Scholar]
- 25.Stevens MFG, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47(22):5846–5852. [PubMed] [Google Scholar]
- 26.de Vries NA, Bruggeman SW, Hulsman D, de Vries HI, Zevenhoven J, Buckle T, Hamans BC, Leenders WP, Beijnen JH, van Lohuizen M. Rapid and robust transgenic high-grade glioma mouse models for therapy intervention studies. Clin Cancer Res. 2010;16(13):3431–3441. doi: 10.1158/1078-0432.CCR-09-3414. [DOI] [PubMed] [Google Scholar]
- 27.Seelig A. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J Mol Neurosci. 2007;33(1):32–41. doi: 10.1007/s12031-007-0055-y. [DOI] [PubMed] [Google Scholar]
- 28.Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, Pokorny R, Capdeville R, Lloyd P. Absolute bioavailability of imatinib (Glivec®) orally versus intravenous infusion. J Clin Pharmacol. 2004;44(2):158–162. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- 29.Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs. 2009;27(1):31–40. doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]
- 30.Herbrink M, Nuijen B, Schellens JHM, Beijnen JH. Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat Rev. 2015;41(5):412–422. doi: 10.1016/j.ctrv.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaFV600E inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2012;344(3):655–664. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kort A, Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Brain and testis accumulation of regorafenib is restricted by breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) Pharm Res. 2015;32(7):2205–2216. doi: 10.1007/s11095-014-1609-7. [DOI] [PubMed] [Google Scholar]
- 33.de Gooijer MC, Zhang P, Thota N, Mayayo-Peralta I, Buil LC, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs. 2015;33(5):1012–1019. doi: 10.1007/s10637-015-0266-y. [DOI] [PubMed] [Google Scholar]
- 34.Parrish KE, Pokorny J, Mittapalli RK, Bakken K, Sarkaria JN, Elmquist WF. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther. 2015;355(2):264–271. doi: 10.1124/jpet.115.228213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, Stewart CF. Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res. 2006;66(23):11305–11313. doi: 10.1158/0008-5472.CAN-06-0929. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Maddox A-M, Rothenberg ML, Small EJ, Rubin EH, Baselga J, Rojo F, Hong WK, Swaisland H, Averbuch SD. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non–small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20(18):3815–3825. doi: 10.1200/JCO.2002.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Xavier D, Alexandra A, Jean-Yves D, Jean-Michel S. Role of ABC transporters in the chemoresistance of human gliomas. Curr Cancer Drug Targets. 2006;6(5):433–445. doi: 10.2174/156800906777723930. [DOI] [PubMed] [Google Scholar]
- 38.Mason WP, Cairncross JG. Drug insight: temozolomide as a treatment for malignant glioma—impact of a recent trial. Nat Clin Pract Neurol. 2005;1:88–95. doi: 10.1038/ncpneuro0045. [DOI] [PubMed] [Google Scholar]
- 39.Schinkel AH, Mayer U, Wagenaar E, Mol CAAM, van Deemter L, Smit JJM, van der Valk MA, Voordouw AC, Spits H, van Tellingen O. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci. 1997;94(8):4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou S, Schuetz JD, Bunting KD, Colapietro A-M, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]