Abstract
Background and Objectives
Diagnosis of type 1 diabetes often causes a negative psychological impact on families. We examined whether parents and children enrolled in The Environmental Determinants of Diabetes in the Young (TEDDY) study differ in their psychological adjustment to diabetes diagnosis compared to children diagnosed with diabetes in the community.
Subjects and Methods
TEDDY follows 8,676 children at genetic risk for type 1 diabetes from birth. Fifty-four TEDDY children diagnosed with diabetes and 54 age-matched community control children diagnosed with diabetes were enrolled. Participants were aged 3–10 years and study visits occurred at 3, 6, and 12 months post-diagnosis. Psychological measures included an adapted diabetes-specific State Anxiety Inventory, the Pediatric Quality of Life Inventory -Diabetes Module, and the Pediatric Inventory for Parents, which measures frequency and difficulty of parenting stress.
Results
A generalized estimating equation analysis based on a difference score between TEDDY children and community controls found no significant differences between TEDDY parents and community controls on parent diabetes-specific anxiety (p=0.30). However, TEDDY children exhibited better diabetes-specific quality of life (p = 0.03) and TEDDY parents reported lower frequency (p = 0.004) and difficulty (p = 0.008) of parenting stress compared to community controls.
Conclusions
Children diagnosed with at risk for type 1 diabetes who have previously enrolled in research monitoring have improved diabetes quality of life and lower parenting stress post-diagnosis compared to children diagnosed in the community. Families in follow up studies may be more prepared if their child is diagnosed with diabetes.
Keywords: type 1 diabetes, pediatric diabetes, family adjustment, diagnosis, prospective, psychological measures
Introduction
Type 1 diabetes is a chronic, life threatening condition requiring intensive disease management behaviors and family support; therefore, it is not surprising that the diagnosis of type 1 diabetes in children can have a negative psychological impact within the family. Past research has shown that in the period immediately following diagnosis, parents may experience grief1, depression, anxiety2, stress, and post-traumatic stress disorder3. A review of the literature in this area found that some 34% of parents experience psychological distress following diagnosis4. Children have also shown evidence of psychological symptoms at diabetes onset, such as depression2 and other adjustment difficulties5. More than 85% of newly diagnosed patients do not have a family history of type 1 diabetes6. As such, it is not surprising that qualitative studies have suggested that the unexpected nature of the diagnosis is very difficult for families5,7. Furthermore, there is some evidence that parents of children whose diagnosis was delayed due to parents or health care providers overlooking or misattributing diabetes symptoms or those whose child presented with more severe diabetes symptoms (e.g., diabetic ketoacidosis (DKA)) exhibit even more negative emotions, such as guilt, about their child’s condition6.
Parents of children participating in prospective studies such as TrialNet8, the Diabetes Autoimmunity Study in the Young (DAISY)9 and The Environmental Determinants of Diabetes in the Young (TEDDY) study10 are informed about their child’s increased risk of developing diabetes and are regularly followed for progression to the disease (e.g., antibody development, metabolic evaluations including blood glucose levels, HbA1c and/or oral glucose tolerance test). Parents and children are also educated about potential early signs and symptoms of diabetes. As a result, children followed in prospective studies have been shown to have a lower incidence of DKA and diabetes symptoms at onset11–14 compared to children diagnosed in the community. In addition, families participating in these prospective studies have had long-term interactions with research and medical staff, which affords them greater familiarity with medical providers and procedures, and may also lead to improved coping with the diagnosis. This study examines the psychological impact of type 1 diabetes in the year following diagnosis on families participating in a long-term observational study compared to families diagnosed in the community. We hypothesized that parents of children diagnosed in a prospective observational study (TEDDY) would demonstrated lower levels of diabetes-specific anxiety and parenting stress, and TEDDY children would demonstrate better diabetes-specific quality of life compared to community controls, who had no prior knowledge of the child’s increased risk for type 1 diabetes.
Methods
TEDDY is a natural history study designed to identify environmental triggers of type 1 diabetes autoimmunity/onset in genetically at-risk children. TEDDY children were identified at three US centers and three European sites as described previously10. Infants were screened at birth using HLA genotyping, and families of HLA-eligible children enrolled before 4.5 months of age. Children were primarily recruited from the general population (89%) and a small proportion (11%) had a first-degree relative (FDR) with type 1 diabetes. From 2004 to 2010, TEDDY enrolled a total of 8676 infants. Children participate in clinic visits every 3 months during the first 4 years of life and every 6 months thereafter. Children who develop islet autoantibodies continue to be monitored every 3 months. All TEDDY children are followed until 15 years of age or until the development of type 1 diabetes. Local Institutional Review Board approval and parental informed consent was obtained for all children. The study is monitored by an External Evaluation Committee of the US National Institutes of Health.
The purpose of the Juvenile Diabetes Research Foundation (JDRF) Follow-up study is to determine whether children diagnosed through the TEDDY study have improved (metabolic (stimulated C-peptide levels, HbA1c, insulin needs) and psychological outcomes compared with control children diagnosed through the community. The JDRF Follow-up study began recruiting TEDDY children and community controls diagnosed with type 1 diabetes in January 2012 at four TEDDY sites (USA: Colorado, Washington State; Finland, and Sweden). Diabetes was defined according American Diabetes Association criteria15. Participants completed study visits, with HbA1c measurement and a mixed meal tolerance test (MMTT) within 1 month of diagnosis, then at 3, 6, and 12 months after diagnosis and biannually thereafter, until loss of detectable endogenous C-peptide. The primary outcome measure for the JDRF Follow-up study is the area under the curve (AUC) for endogenous C-peptide in response to a 2-hour MMTT. Glycemic control (HbA1c) was measured by a Tosoh G8 HPLC Analyzer (Tosoh Bioscience Inc., San Francisco, CA) at the Diabetes Diagnostic Laboratory at the University of Missouri, Columbia. A previously published report on preliminary findings from the JDRF Follow-up Study showed that TEDDY children had significantly higher C-peptide values and lower HbA1c levels than community controls, in addition to clinical differences at diagnosis including lower incidence of DKA and fewer diabetes symptoms16.
Selection of TEDDY Cases and Community Controls with type 1 diabetes
Families of TEDDY children diagnosed with type 1 diabetes were informed of their eligibility for the JDRF Follow-up study and were asked about their willingness to enroll. As of April 2016, a total of 240 TEDDY children were diagnosed with diabetes. The JDRF follow-up study has been recruiting TEDDY children diagnosed with type 1 diabetes since January 2012; thus, 93 subjects were eligible for the JDRF Follow up study. Of these 93 eligible subjects, 67 subjects enrolled into the JDRF follow-up study whereas 26 did not enroll. The most common reasons for declining participation were concerns about specific study procedures (blood draws, MMTT) and not having enough time. There were no significant differences in participant characteristics at diabetes diagnosis (age, gender, BMI, family history of diabetes, diabetes symptoms at diagnosis, DKA, frequency of hospitalization, hemoglobin A1c (HbA1c), frequency of HLA-DR3/4,DQB1*0302 genotype) between the eligible TEDDY children who enrolled into the JDRF Follow-up study versus those who did not enroll16. For the purposes of this study, 54 TEDDY cases who had matched community controls followed for one year post-diagnosis were included in the analyses.
For each TEDDY case, a matched community control was identified based on the following criteria: age of diabetes diagnosis within one year and clinical center (e.g., a TEDDY case from Sweden was matched with a community control from the same center). Community controls were also required to have at least one positive islet autoantibody. Control participants were identified at the TEDDY clinical centers, were informed of their eligibility for the JDRF Followup study, and were asked about their willingness to enroll. Parents or legal caregivers of all participants provided written informed consent and children provided assent when applicable. For the study period, 112 community controls were approached and 64 agreed to participate. The most common reasons cited by community families for declining participation were equivalent to those reported by TEDDY families: concerns about blood draws, the MMTT, and the study being too time consuming.
Demographic and Clinical Measures
Demographic measures (child age, gender) were collected from caregivers via questionnaire. Clinical measures were collected via direct measurement (BMI), laboratory assays (autoantibodies, HLA genotype, HbA1c), or case report forms completed by staff via parent interview (family history of diabetes, diabetes symptoms at diagnosis, frequency of hospitalization). All diabetes symptoms at diagnosis were collected and then coded into a dichotomous variable (symptoms: yes/no). Full description of data collection is presented in our prior publication.16
Family Adjustment Measures
Caregivers completed questionnaires at 3, 6 and 12 months post diagnosis addressing the child’s diabetes specific quality of life, parent anxiety about the child’s diabetes, and parenting stress. Caregivers also completed questionnaires yearly thereafter, although these data are not presented. In this study, the majority (>95%) of respondents were mothers and this did not differ significantly between TEDDY cases and community controls.
Parent diabetes-specific anxiety
Parents completed a 6-item short form of the state portion of the State-Trait Anxiety Inventory (SAI)17 used to assess parent anxiety about the child’s diabetes at a single point in time. For example, parents were asked how often they feel “worried” specifically when they think about their child’s diabetes. Responses were scored on a 4-point scale and the 6-item score was then converted to a total score comparable to the 20-item State Anxiety Inventory score. Parents with SAI scores > 40 were considered to be highly anxious.18,19 An analogous version of this scale has been used in the TEDDY study with excellent internal consistency20 and in the current study, this abbreviated form showed excellent internal consistency (Cronbach α = 0.87 – 0.95 across study visits).
Pediatric parenting stress
Parents completed the 42-item Pediatric Inventory for Parents (PIP21), which measures stress related to having a child with a chronic illness. The PIP assesses four domains of health-related parenting stress (Communication, Emotional distress, Medical Care, Role function) across 2 scales: Frequency (PIP-F) of stress and Difficulty (PIP-D) of stress. Higher scores indicate more parenting stress and both the Frequency and Difficulty domain scores were used in analyses. The PIP has been used with parents of newly diagnosed children with diabetes22 and has shown good psychometric properties23; internal consistency in the current study was excellent (Cronbach α = 0.91 – 0.97 across study visits for PIP-F and PIP-D domains).
Child diabetes-specific quality of life
Caregivers completed the diabetes module of the Pediatric Quality of Life Inventory (PedsQL 3.2 Type 1 Diabetes;24). The PedsQL 3.2 Diabetes module measures the child’s diabetes-specific health-related quality of life across the domains of Communication, Treatment Barriers, Treatment Adherence, Diabetes Symptoms, and Worry. The PedsQL has parallel forms for parents of children aged 2–4, 5–7, and 8–12 years. Parents responded to items using a 5-point likert scale (0 = never a problem, 4 = almost always a problem). Items are reverse scored and transformed to a 0–100 scale with higher scores indicating better quality of life. The total PedsQL score was used for analyses. The measure showed excellent internal consistency (Cronbach α of 0.86 – 0.94 across study visits).
Statistical Analysis
A 1:1 case-control matching design was used in the current study. For the comparison of characteristics at diagnosis of diabetes between TEDDY cases and community controls, paired t-tests were used for continuous variables and McNemar’s tests were used for proportions. Differences in TEDDY cases versus community controls on parent anxiety, parenting stress, and child quality of life during the first 12 months were examined using the Generalized Estimating Equation (GEE) method25 with adjustment for visit and for child age at diagnosis. We did not adjust for whether a family member had type 1 diabetes as there was no relationship between this characteristic and psychological functioning. For each measurement, the difference in scores between the TEDDY case and community control in each matching pair was calculated and used as the response in the GEE model. Visit and the difference of child age at diagnosis were covariates in the model. The mean differences of score between TEDDY cases and community controls at 3, 6 and 12 months after diagnosis were calculated. The GEE analysis showed no effect of visit for each measurement. Therefore, the overall mean difference score between TEDDY case and community controls during the first 12 months was also calculated using the GEE method with adjustment for child age at diagnosis. An exchangeable correlation structure was assumed to account for the correlation of repeated measures at multiple follow-up visits for each pair over time and the empirical standard error estimates were used. Ninety five percent confidence limits and p-values from the GEE analyses were based on the Wald test. Data were assumed to be missing at random and observed data were analyzed. Two-tailed p-values less than 0.05 were considered to be statistically significant. Data were analyzed using the Statistical Analysis System software (version 9.4; SAS Institute, Cary, NC).
Results
A total of 54 TEDDY and 54 age-matched community control children were enrolled from the USA (43%), Sweden (33%), and Finland (24%) ranging in age from 3.2 years to 10.5 years. Demographic and disease characteristics at diagnosis of diabetes are presented in Table 1. Although the study protocol matched TEDDY and community controls within one year of age, TEDDY children were slightly younger at diabetes onset than community controls (6.2 vs 6.6 years, p < 0.001). TEDDY children were more likely to have the high-risk HLA-DR3/4, DQA1*05:01-B1*02:01/DQA1*03:01-B1*03:02 genotype (59%, versus 10%, respectively; p<0.001). At diagnosis, TEDDY children (6.8%, 51 mmol/mol) had significantly lower mean HbA1c levels compared to community control children (10.5%, 91mmol/mol; p<0.001). Only half of TEDDY children (51%) had diabetes symptoms at diagnosis compared to 98% of community controls (p <0.001). Further, there were no instances of DKA at diagnosis in TEDDY children while 16% of community controls presented with DKA (p=0.003). There were no differences in family history of type 1 diabetes, body mass index (BMI), or gender between the two groups.
Table 1.
Demographic and disease variables at enrollment.
| TEDDY children N=54 |
Community controls N=54 |
P-value | |
|---|---|---|---|
| Age at diagnosis (years) | 6.2±1.7 | 6.6 ±1.8 | <0.001 |
| Gender (female), N (%) | 25 (46) | 32 (59) | 0.26 |
| Family history of diabetes, N (%) | 10 (19) | 5 (9) | 0.23 |
| HLA DR3/4, DQB1*0302*, N (%) | 32 (59) | 4 (10) | <0.001 |
| Body mass indexa | 16.2 ±2.2 | 15.3±2.4 | 0.09 |
| Diabetes symptoms, N (%) | 25 (51) | 50 (98) | <0.001 |
| HbA1c at diagnosis % (mmol/mol) | 6.8 ±1.2 | 10.5 ±2.2 | <0.001 |
| (51 ± 13) | (91 ± 24) | ||
| Diabetic ketoacidosis, N (%) | 0 (0) | 8 (16%) | 0.003b |
Notes: Paired t-tests were used for continuous variables; McNemar’s test was used for binary variables. Mean +/− standard deviations are shown unless otherwise specified.
Data was missing in some subjects.
McNemar’s test was not feasible, thus Fisher’s exact test was used.
Scores on psychological adjustment measures (parent diabetes specific anxiety, child diabetes quality of life, and pediatric parenting stress) for TEDDY children and community controls at 3-, 6-, and 12-month study visits are shown in Figures 1–4. The estimated score differences between the two groups are presented in Table 2.
Figure 1.
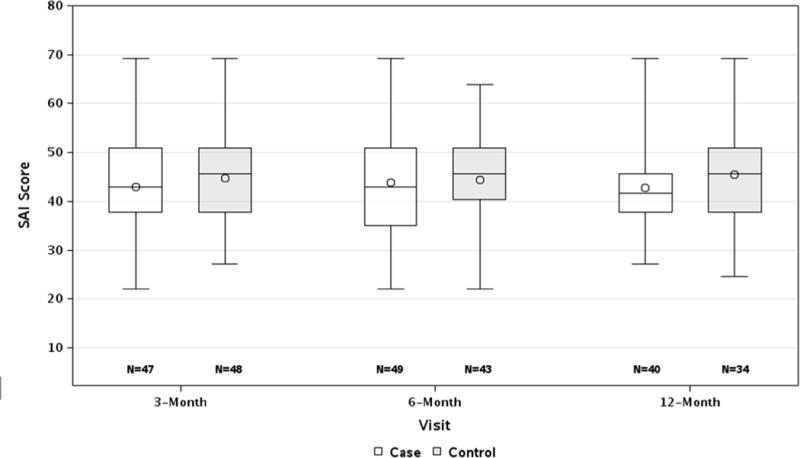
Higher scores suggest more anxiety. A line depicts the median score, a circle depicts the mean score.
Parent anxiety about diabetes (SAI) in the first year after type 1 diabetes diagnosis.
Figure 4.

Higher scores suggest more parenting stress. A line depicts the median score, a circle depicts the mean score.
Difficulty of pediatric parenting stress (PIP-Difficulty) in the first year after type 1 diabetes diagnosis.
Table 2.
Estimates of score differences between TEDDY cases and community controls on psychological adjustment measures
| Mean difference (95% CI) at visit, post diagnosis1 | Mean difference (95% CI), P-value2 | |||
|---|---|---|---|---|
| 3-month | 6-month | 12-month | Over the first 12 months | |
| Parent anxiety about diabetes (SAI) | −1.9 (−5.6, 1.8) | −0.3 (−4.3, 3.7) | −3.3 (−6.6, 0.1) | −1.7 (–4.9, 1.5), 0.30 |
| Child diabetes quality of life (PedsQL) | 4.6 (0.3, 8.8) | 3.6 (−1.1, 8.3) | 4.7 (−0.7, 10.1) | 4.3 (0. 4, 8.1), 0.03 |
| Parenting stress – Frequency (PIP-F) | −16.2 (−26.2, −6.3) | −6.3 (−17.5, 4.9) | −12.9 (−22.9, −2.9) | −12.3 (−21.4, −3.2), 0.008 |
| Parenting stress – Difficulty (PIP-D) | −15.3 (−23.9, −6.7) | −6.4 (−16.0, 3.2) | −12.5 (−21.8, −3.2) | −11.8 (−19.7, −3.9), 0.004 |
The Generalized Estimating Equation (GEE) method was conducted 25 with adjustment for visit and for child age at diagnosis for each of psychological adjustment measures. The GEE analysis showed no effects of visit or child age at diagnosis on the score differences between the two groups for each of the psychological adjustment measures.
The GEE method was conducted 25 with adjustment for child age at diagnosis for each psychological adjustment measure.
Parent diabetes-specific anxiety as measured by the SAI was high and similar to other reports of parent anxiety at the time of a child’s medical diagnosis. Mean SAI scores for mothers of both TEDDY children and community controls in the current study were higher than those in previously published work for mothers and fathers at TEDDY study enrollment when parents are first informed of their child’s increased genetic risk for type 1 diabetes and also higher than scores following notification within TEDDY that a child has developed islet autoantibodies20,26. Further, the mean SAI score for both groups was above 40, a score that has been suggested as indicative of high levels of anxiety18,19. Although parents of community controls reported slightly more anxiety at each time point, the mean SAI scores between TEDDY children and community controls did not differ during the first year after diabetes diagnosis (p=0.30).
For child diabetes-specific quality of life as measured by the PedsQL-3.2 Diabetes Module, there were no available studies tracking this outcome at the exact time points of our study. However, when compared to available published samples, our data show that parents of TEDDY cases reported scores that were comparable to those of children one year post-diabetes diagnosis. Further, parents of TEDDY cases reported better quality of life for their children than did parents of children from published samples who were three or more years post-diagnosis (using an earlier version of this measure (PedsQL – 3.0 Diabetes Module, Varni24)). In the current study, community controls’ diabetes-specific quality of life was generally below that of previously published reports in children with one or three years diabetes duration27,28.. Overall, parents of TEDDY children reported higher child quality of life at each visit (4.6, 3.6 and 4.7 higher in score at 3, 6 and 12 months respectively) and during the first year post diagnosis (4.3 points higher in score; 95% CI 0.4, 8.1; p=0.03) compared to parents of community controls.
Both the frequency and difficulty of pediatric parenting stress as measured by the PIP was moderately high for TEDDY children and community controls. Compared to previous reports, both TEDDY children and community controls generally reported less parenting stress than parents of children a month or less post-diabetes diagnosis22 but more stress than parents of children with longer diabetes duration23,29. Parents of TEDDY children reported less frequency of parenting stress (PIP-F) compared to community controls at each visit (16.2, 6.3, 12.9 points lower in score at 3, 6 and 12 months, respectively) and across the first year following diagnosis (12.3 points lower in score; 95% CI 3.2, 21.4; p=0.008). Similarly, parents of TEDDY children reported less difficulty with parenting stress (PIP-D) compared to community controls at each visit (15.3, 6.4, 12.5 lower in score at 3, 6 and 12 months, respectively) and during the first year following diagnosis (11.8 lower in score; 95% CI 3.9, 19.7; p=0.004).
Discussion
We have previously reported that at diagnosis, children diagnosed with type 1 diabetes through the TEDDY study have fewer diabetes symptoms, lower rates of DKA, and better glycemic control than community controls16. Importantly, this study suggests that children from the TEDDY study also have better family psychological adjustment after diabetes diagnosis. TEDDY parents reported that their children displayed better diabetes-specific quality of life across the first year following diabetes diagnosis compared to community controls. Our findings also suggest that parents of TEDDY children experience less pediatric parenting stress than do parents of community controls in the first year after diabetes diagnosis. Pediatric parenting stress focuses not only on the stress parents experience in caring for a child with a chronic condition such as type 1 diabetes, but also on the stress related to frequent interactions with the healthcare system (e.g., medical appointments, interactions with healthcare providers). There are several explanations for these findings including both psychological and medical factors.
The diagnosis of a child with type 1 diabetes is a difficult event for families, often leading to a variety of psychological symptoms such as depression, anxiety, and stress2,4,5. These psychological adjustment difficulties are, in part, a result of the unexpected and overwhelming nature of the diabetes diagnosis1,7. Given that TEDDY children and their parents have prior knowledge of their child’s increased risk for type 1 diabetes, it is likely that families are less surprised and overwhelmed by the diagnosis. In contrast to community families, who generally experience the acute onset of diabetes, TEDDY families have additional time and information that may allow them to adjust to the diagnosis progressively. In fact, TEDDY families not only have information about their child’s increased genetic risk (which is provided at TEDDY study enrollment), but also are informed at regular intervals about changes in their risk when their child develops islet autoantibodies or impaired glucose values over time (e.g., increasing HbA1c, impaired glucose tolerance), which suggest progression towards clinical diabetes. Given this knowledge, TEDDY families have more time to process and prepare for the prospect that their child will likely develop type 1 diabetes. Qualitative studies have found that parents report the unexpected nature of diabetes as being a significant factor contributing to adjustment challenges1 and the larger psychological literature also suggests that unexpected traumatic events cause more psychological symptoms than do expected or predictable ones7. Additionally, illness uncertainty, which refers to the cognitive appraisal process that occurs when an illness and its outcomes are uncertain, unpredictable, or ambiguous, has been shown to predict more negative long term psychological functioning30. TEDDY families may evidence better psychological functioning because disease onset was somewhat predicable because of the information they had received as participants in TEDDY. The TEDDY protocol emphasizes parental education regarding symptoms and signs of diabetes and testing urine for ketones and home blood glucose monitoring using meters provided by the study for children with multiple islet autoantibodies and/or abnormal oral glucose tolerance testing. For families new to type 1 diabetes, this education and monitoring through TEDDY teaches skills that are the foundation of diabetes management. TEDDY centers are also closely aligned (geographically and with the same personnel in many cases) with local pediatric diabetes clinics and thus parents of TEDDY children may be more comfortable in these health care settings given their long-term relationship with the TEDDY study nurses, physicians, and researchers. In combination, it is likely that these factors make transition to routine clinical care much easier for children and parents participating in TEDDY than those in the community.
In addition to the potential psychological buffering effect of TEDDY study participation prior to diabetes diagnosis, it is also possible that treatment and/or disease-related factors play a role in our findings. TEDDY children were much less likely to present with DKA at diagnosis than both community children in our study and in past studies focusing on the general population (0% DKA in TEDDY children, 16% in community controls, and >40% in the general population31). We have also reported that TEDDY children have higher levels of c-peptide and better glycemic control than community controls throughout the first year post-diagnosis15. Further, TEDDY children were prescribed less intensive diabetes regimens more often than community control children. For example, at diagnosis, 35% of TEDDY children were prescribed two or fewer daily injections while 100% of community children utilized 3 or more injections daily15. Given these differences, it is likely that diabetes management is easier for TEDDY families due to less severe metabolic decompensation at diagnosis (i.e., less DKA), higher levels of endogenous insulin, better glycemic control, and fewer daily injections. This may in turn yield less parental stress and a reduced impact on quality of life for TEDDY children.
In addition to parenting stress and child quality of life, we also examined parental anxiety about diabetes. While parents of community controls did express more anxiety about their child’s diabetes than did TEDDY parents, this difference was not statistically significant. This finding suggests that the improvement in child quality of life and parenting stress, which is conferred by participation in the TEDDY study, does not necessarily translate to notable differences in parental anxiety about the child’s diabetes per se.
It is important to note that while there were significant differences between parent-reported psychological adjustment between TEDDY children and community children, the absolute differences were generally small. The diagnosis of diabetes in a child is stressful for the child and parents. While participating in a prospective study like TEDDY may help mitigate the psychological impact of such a diagnosis, it does not eliminate it entirely. It is also important to note that the measure of child quality of life was based on parent report. Future work should examine the impact of participating in a prospective study like TEDDY from the child’s perspective. We are currently collecting psychological adjustment data from children participating in the JDRF Follow-up study who are 8 years and older.
Although this study is preliminary and future work is needed to validate our findings, it is notable that significant group differences were found, even within a relatively small cohort of 54 children in each group. Further, we expect that our findings may actually underestimate the positive effects of participating in a prospective study like TEDDY at the time of the child’s diagnosis. Our first measure of psychological adjustment was collected 3 months post-diagnosis when we would expect some dissipation of the distress experienced at the time of diagnosis.
In conclusion, this is the first study to demonstrate that parents and their children diagnosed with type 1 diabetes after being followed in the TEDDY study evidence better psychological adjustment compared to children diagnosed in the community. We have previously reported that there are medical benefits to being informed of increased diabetes risk via the TEDDY study, such as reduction in DKA incidence, and we have now shown that there are related psychological benefits in the first year post-diagnosis. Although screening and monitoring at the general population level for type 1 diabetes may not be feasible, our findings do suggest that participation in studies of genetically at-risk populations may be beneficial for those families whose child goes on to develop the disease. Ethical concerns – including potential negative psychological impact – have been raised about these studies because they offer no means to prevent the disease32. Our findings suggest that there may be psychological benefit to study participation for those families whose child develops type 1 diabetes. Of course, most children participating in such studies never develop type 1 diabetes and their psychological welfare is of equal importance. Although not the focus of this study, our previous work suggests that learning that your child is genetically at-risk for type 1 diabetes increases parental anxiety but this anxiety rather rapidly declines to normal levels33. Nevertheless, the psychological well-being of all participants in screening and monitoring studies of individuals at genetic risk for type 1 diabetes remains a critically important area of inquiry34.
The results of the current study are most relevant to those at highest risk for type 1 diabetes. Our findings show that participation in a study like TEDDY where participants are closely monitored may mitigate a portion of the negative psychological impact of diagnosis. This study also suggests that less intensive monitoring programs, such as TrialNet35 which targets high risk populations of first and second degree relatives with type 1 diabetes, may also have psychological benefits if/when the participants develop diabetes. Further examination is needed to determine whether these benefits are more long lasting. It would also be useful to more fully examine the longitudinal relationship between psychological functioning, glycemic control, and diabetes management behaviors to better understand how the improved psychological functioning of TEDDY children compared to community controls is related to the physiological and treatment-related differences between the two groups.
Figure 2.

Higher scores suggest better quality of life. A line depicts the median score, a circle depicts the mean score.
Parent report of child diabetes-specific quality of life (PedsQL – 3.2 Diabetes Module) in the first year after type 1 diabetes diagnosis.
Figure 3.

Higher scores suggest more parenting stress. A line depicts the median score, a circle depicts the mean score.
Frequency of pediatric parenting stress (PIP-Frequency) in the first year after type 1 diabetes diagnosis.
Acknowledgments
The TEDDY Study Group (See appendix)
Funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Abbreviations
- TEDDY
The Environmental Determinants of Diabetes in the Young
- DAISY
Diabetes Autoimmunity Study in the Young
- DKA
diabetic ketoacidosis
- JDRF
The Juvenile Diabetes Research Foundation
- FDR
first degree relative
- HLA
human leucocyte antigen
- PedsQL
Pediatric Quality of Life Inventory
- SAI
Spielberger State Anxiety Inventory
- PIP
Pediatric Inventory for Parents
- GEE
Generalized Estimating Equation
Appendix
The Teddy Study Group
Colorado Clinical Center: Marian Rewers, M.D., Ph.D., PI1,4,5,6,10,11, Kimberly Bautista12, Judith Baxter9,10,12,15, Ruth Bedoy2, Daniel Felipe-Morales, Brigitte I. Frohnert, M.D.2,14, Patricia Gesualdo2,6,12,14,15, Michelle Hoffman12,13,14, Rachel Karban12, Edwin Liu, M.D.13, Jill Norris, Ph.D.2,3,12, Adela Samper-Imaz, Andrea Steck, M.D.3,14, Kathleen Waugh6,7,12,15, Hali Wright12. University of Colorado, Anschutz Medical Campus, Barbara Davis Center for Childhood Diabetes.
Georgia/Florida Clinical Center: Jin-Xiong She, Ph.D., PI1,3,4,11, Desmond Schatz, M.D.*4,5,7,8, Diane Hopkins12, Leigh Steed12,13,14,15, Jamie Thomas*6,12, Janey Adams*12, Katherine Silvis2, Michael Haller, M.D.*14, Melissa Gardiner, Richard McIndoe, Ph.D., Ashok Sharma, Joshua Williams, Gabriela Young, Stephen W. Anderson, M.D.^, Laura Jacobsen, M.D.*14 Center for Biotechnology and Genomic Medicine, Augusta University. *University of Florida, ^Pediatric Endocrine Associates, Atlanta.
Germany Clinical Center: Anette G. Ziegler, M.D., PI1,3,4,11, Andreas Beyerlein, Ph.D.2, Ezio Bonifacio Ph.D.*5, Michael Hummel, M.D.13, Sandra Hummel, Ph.D.2, Kristina Foterek¥2, Nicole Janz, Mathilde Kersting, Ph.D.¥2, Annette Knopff7, Sibylle Koletzko, M.D.¶13, Claudia Peplow12, Roswith Roth, Ph.D.9, Marlon Scholz, Joanna Stock9,12, Elisabeth Strauss12,14, Katharina Warncke, M.D.14, Lorena Wendel, Christiane Winkler, Ph.D.2,12,15. Forschergruppe Diabetes e.V. and Institute of Diabetes Research, Helmholtz Zentrum München, and Klinikum rechts der Isar, Technische Universität München. *Center for Regenerative Therapies, TU Dresden, ¶Dr. von Hauner Children’s Hospital, Department of Gastroenterology, Ludwig Maximillians University Munich, ¥Research Institute for Child Nutrition, Dortmund.
Finland Clinical Center: Jorma Toppari, M.D., Ph.D., PI¥^1,4,11,14, Olli G. Simell, M.D., Ph.D.¥^1,4,11,13, Annika Adamsson, Ph.D.^12, Suvi Ahonen*±§, Heikki Hyöty, M.D., Ph.D.*±6, Jorma Ilonen, M.D., Ph.D.¥ ¶3, Sanna Jokipuu^, Tiina Kallio^, Leena Karlsson^, Miia Kähönenμ¤, Mikael Knip, M.D., Ph.D.*±5, Lea Kovanen*±§, Mirva Koreasalo*±§2, Kalle Kurppa, M.D., Ph.D.*±13, Tiina Latva-ahoμ¤, Maria Lönnrot, M.D., Ph.D.*±6, Elina Mäntymäki^, Katja Multasuoμ¤, Juha Mykkänen, Ph.D.¥ 3, Tiina Niininen±*12, Sari Niinistö±§, Mia Nyblom*±, Petra Rajala^, Jenna Rautanen±§, Anne Riikonen*±§, Mika Riikonen^, Jenni Rouhiainen^, Minna Romo^, Tuula Simell, Ph.D., Ville Simell^¥13, Maija Sjöberg¥^12,14, Aino Steniusμ¤12, Maria Leppänen^, Sini Vainionpää^, Eeva Varjonen¥^12, Riitta Veijola, M.D., Ph.D.μ¤14, Suvi M. Virtanen, M.D., Ph.D.*±§2, Mari Vähä-Mäkilä^, Mari Åkerlund*±§, Katri Lindfors, Ph.D.*13 ¥University of Turku, *University of Tampere, μUniversity of Oulu, ^Turku University Hospital, Hospital District of Southwest Finland, ±Tampere University Hospital, ¤Oulu University Hospital, §National Institute for Health and Welfare, Finland, ¶University of Kuopio.
Sweden Clinical Center: Åke Lernmark, Ph.D., PI1,3,4,5,6,8,10,11,15, Daniel Agardh, M.D., Ph.D.13, Carin Andrén Aronsson2,13, Maria Ask, Jenny Bremer, Ulla-Marie Carlsson, Corrado Cilio, Ph.D., M.D.5, Emelie Ericson-Hallström, Lina Fransson, Thomas Gard, Joanna Gerardsson, Rasmus Bennet, Monica Hansen, Gertie Hansson12, Cecilia Harmby, Susanne Hyberg, Fredrik Johansen, Berglind Jonsdottir M.D., Helena Elding Larsson M.D., Ph.D. 6,14, Sigrid Lenrick Forss, Markus Lundgren14, Maria Månsson-Martinez, Maria Markan, Jessica Melin12, Zeliha Mestan, Kobra Rahmati, Anita Ramelius, Anna Rosenquist, Falastin Salami, Sara Sibthorpe, Birgitta Sjöberg, Ulrica Swartling, Ph.D.9,12, Evelyn Tekum Amboh, Carina Törn, Ph.D. 3,15, Anne Wallin, Åsa Wimar12,14, Sofie Åberg. Lund University.
Washington Clinical Center: William A. Hagopian, M.D., Ph.D., PI1,3,4, 5, 6,7,11,13, 14, Michael Killian6,7,12,13, Claire Cowen Crouch12,14,15, Jennifer Skidmore2, Josephine Carson, Kayleen Dunson, Rachel Hervey, Corbin Johnson, Rachel Lyons, Arlene Meyer, Denise Mulenga, Allison Schwartz, Joshua Stabbert, Alexander Tarr, Morgan Uland, John Willis. Pacific Northwest Diabetes Research Institute.
Pennsylvania Satellite Center: Dorothy Becker, M.D., Margaret Franciscus, MaryEllen Dalmagro-Elias Smith2, Ashi Daftary, M.D., Mary Beth Klein, Chrystal Yates. Children’s Hospital of Pittsburgh of UPMC.
Data Coordinating Center: Jeffrey P. Krischer, Ph.D.,PI1,4,5,10,11, Michael Abbondondolo, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Rasheedah Brown12,15, Brant Burkhardt, Ph.D.5,6, Martha Butterworth2, Joanna Clasen, David Cuthbertson, Christopher Eberhard, Steven Fiske9, Dena Garcia, Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Francisco Perez Laras, Hye-Seung Lee, Ph.D.1,2,13,15, Shu Liu, Xiang Liu, Ph.D.2,3,9,14, Kristian Lynch, Ph.D. 5,6,9,15, Jamie Malloy, Cristina McCarthy12,15, Steven Meulemans, Hemang Parikh, Ph.D.3, Chris Shaffer, Laura Smith, Ph.D.9,12, Susan Smith12,15, Noah Sulman, Ph.D., Roy Tamura, Ph.D.1,2,13, Ulla Uusitalo, Ph.D.2,15, Kendra Vehik, Ph.D.4,5,6,14,15, Ponni Vijayakandipan, Keith Wood, Jimin Yang, Ph.D., R.D.2,15. Past staff: Lori Ballard, David Hadley, Ph.D., Wendy McLeod. University of South Florida.
Project scientist: Beena Akolkar, Ph.D.1,3,4,5,6,7,10,11. National Institutes of Diabetes and Digestive and Kidney Diseases.
HLA Reference Laboratory: Henry Erlich, Ph.D., Steven J. Mack, Ph.D., Anna Lisa Fear. Center for Genetics, Children’s Hospital Oakland Research Institute.
Repository: Sandra Ke, Niveen Mulholland, Ph.D. NIDDK Biosample Repository at Fisher BioServices.
Other contributors: Kasia Bourcier, Ph.D.5, National Institutes of Allergy and Infectious Diseases. Thomas Briese, Ph.D.6,15, Columbia University. Suzanne Bennett Johnson, Ph.D.9,12, Florida State University. Eric Triplett, Ph.D.6, University of Florida.
Committees:
1Ancillary Studies, 2Diet, 3Genetics, 4Human Subjects/Publicity/Publications, 5Immune Markers, 6Infectious Agents, 7Laboratory Implementation, 8Maternal Studies, 9Psychosocial, 10Quality Assurance, 11Steering, 12Study Coordinators, 13Celiac Disease, 14Clinical Implementation, 15Quality Assurance Subcommittee on Data Quality.
References
- 1.Lowes L, Gregory JW, Lyne P. Newly diagnosed childhood diabetes: a psychosocial transition for parents? J Adv Nurs. 2005;50(3):253–261. doi: 10.1111/j.1365-2648.2005.03388.x. [DOI] [PubMed] [Google Scholar]
- 2.Northam E, Anderson P, Adler R, Werther G, Warne G. Psychosocial and family functioning in children with insulin-dependent diabetes at diagnosis and one year later. J Pediatr Psychol. 1996;21(5):699–717. doi: 10.1093/jpepsy/21.5.699. [DOI] [PubMed] [Google Scholar]
- 3.Landolt MA, Vollrath M, Laimbacher J, Gnehm HE, Sennhauser FH. Prospective study of posttraumatic stress disorder in parents of children with newly diagnosed type 1 diabetes. J Am Acad Child Adolesc Psychiatry. 2005;44(7):682–689. doi: 10.1097/01.chi.0000161645.98022.35. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. The Diabetes Educ. 2012;38(4):562–579. doi: 10.1177/0145721712445216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs M, Feinberg TL, Paulauskas S, Finkelstein R, Pollock M, Crouse-Novak M. Initial coping responses and psychosocial characteristics of children with insulin-dependent diabetes mellitus. J Pediatr. 1985;106(5):827–834. doi: 10.1016/s0022-3476(85)80368-1. [DOI] [PubMed] [Google Scholar]
- 6.Hamalainen AM, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep. 2002;2(4):347–353. doi: 10.1007/s11892-002-0025-2. [DOI] [PubMed] [Google Scholar]
- 7.Rankin D, Harden J, Waugh N, et al. Pathways to diagnosis: a qualitative study of the experiences and emotional reactions of parents of children diagnosed with type 1 diabetes. Pediatric Diabetes. 2014;15(8):591–598. doi: 10.1111/pedi.12124. [DOI] [PubMed] [Google Scholar]
- 8.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 9.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39(7):807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 10.The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 12.Triolo TM, Chase HP, Barker JM. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32(5):769–773. doi: 10.2337/dc08-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–126. doi: 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes A. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37(supp 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 16.Steck AK, Larsson HE, Liu X, et al. Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls [published online January 21 2017] Pediatr Diabetes. 2017 doi: 10.1111/pedi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spielberger CD, Gorsuch RI, Lushene R. Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 18.Grant KA, McMahon C, Austin MP. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord. 2008;108(1–2):101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Dennis CL, Coghlan M, Vigod S. Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory? J Affect Disord. 2013;150(3):1217–1220. doi: 10.1016/j.jad.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Roth R, Lynch K, Lernmark B, et al. Maternal anxiety about a child’s diabetes risk in the TEDDY study: the potential role of life stress, postpartum depression, and risk perception. Pediatr Diabetes. 2015;16(4):287–298. doi: 10.1111/pedi.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streisand R, Braniecki S, Tercyak KP, Kazak AE. Childhood illness-related parenting stress: the pediatric inventory for parents. J Pediatr Psychol. 2001;26(3):155–162. doi: 10.1093/jpepsy/26.3.155. [DOI] [PubMed] [Google Scholar]
- 22.Streisand R, Mackey ER, Elliot BM, et al. Parental anxiety and depression associated with caring for a child newly diagnosed with type 1 diabetes: opportunities for education and counseling. Patient Educ Couns. 2008;73(2):333–338. doi: 10.1016/j.pec.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self-efficacy, responsibility, and fear. J Pediatr Psychol. 2005;30(6):513–521. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26(3):631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 26.Johnson SB, Lynch KF, Roth R, Schatz D, the TEDDY Study Group My Child is Islet Autoantibody Positive: Impact on Parental Anxiety. Diabetes Care. doi: 10.2337/dc17-0166. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi-Frazier JP, Hilliard ME, Fino NF, et al. Whose quality of life is it anyway? Discrepancies between youth and parent health-related quality of life ratings in type 1 and type 2 diabetes. Qual of Life Res. 2016;25(5):1113–1121. doi: 10.1007/s11136-015-1158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonsson L, Lundqvist P, Tiberg I, Hallstrom I. Type 1 diabetes - impact on children and parents at diagnosis and 1 year subsequent to the child’s diagnosis. Scand J Caring Sci. 2015;29(1):126–135. doi: 10.1111/scs.12140. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwesteeg A, Hartman E, Emons W, et al. Paediatric parenting stress in fathers and mothers of young children with Type 1 diabetes: a longitudinal study. Diabet Med. 2017;34(6):821–827. doi: 10.1111/dme.13300. [DOI] [PubMed] [Google Scholar]
- 30.Szulczewski L, Mullins LL, Bidwell SL, Eddington AR, Pai ALH. Meta-Analysis: Caregiver and Youth Uncertainty in Pediatric Chronic Illness. J Pediatr Psychol. 2017;42(4):395–421. doi: 10.1093/jpepsy/jsw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998–2012. JAMA. 2015;313(15):1570–1572. doi: 10.1001/jama.2015.1414. [DOI] [PubMed] [Google Scholar]
- 32.Almond B. Genetic profiling of newborns: ethical and social issues. Genetics. 2006;7:67–71. doi: 10.1038/nrg1745. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SB, Lynch KF, Roth R, Schatz D, TEDDY Study Group My Child Is Islet Autoantibody Positive: Impact on Parental Anxiety. Diabetes Care. 2017;40(9):1167–1172. doi: 10.2337/dc17-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson SB. Psychological impact of screening and prediction in type 1 diabetes. Curr Diabe Rep. 2011;11:454–459. doi: 10.1007/s11892-011-0208-9. [DOI] [PubMed] [Google Scholar]
- 35.Mahon JL, Sosenko JM, Fafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 diabetes: objectives, design, and initial results. Pediatri Diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]


