Abstract
Previous murine studies have demonstrated that dietary Aquamin®, a calcium-rich, multi-mineral natural product, suppressed colon polyp formation and transition to invasive tumors more effectively than calcium alone when provided over the lifespan of the animals. In the present study, we compared calcium alone to Aquamin® for modulation of growth and differentiation in human colon adenomas in colonoid culture. Colonoids established from normal colonic tissue were examined in parallel. Both calcium alone at 1.5 mM and Aquamin® (provided at 1.5 mM calcium) fostered differentiation in the adenoma colonoid cultures as compared to control (calcium at 0.15 mM). When Aquamin® was provided at an amount delivering 0.15 mM calcium, adenoma differentiation also occurred, but was not as complete. Characteristic of colonoids undergoing differentiation was a reduction in the number of small, highly-proliferative buds and their replacement by fewer but larger buds with smoother surface. Proliferation marker (Ki67) expression was reduced and markers of differentiation (CK20 and Occludin) were increased along with E-cadherin translocalization to the cell surface. Additional proteins associated with differentiation/growth control (including histone-1 family members, certain keratins, NF2 [merlin], olfactomedin-4 and metallothionines) were altered as assessed by proteomics. Immunohistological expression of NF2 was higher with Aquamin® as compared to calcium at either concentration. These findings support the conclusions that i) calcium (1.5 mM) has the capacity to modulate growth and differentiation in large human colon adenomas and ii) Aquamin® delivering 0.15 mM calcium has effects on proliferation and differentiation not observed when calcium is used alone at this concentration.
Keywords: colonic adenoma, colonoid culture, organoid, colon cancer chemoprevention, differentiation, NF2 (merlin), proteomics
INTRODUCTION
Epidemiological studies have demonstrated a clear (inverse) relationship between calcium intake and incidence of colon polyp formation (1,2). A recent meta-analysis suggests that the relationship between calcium intake and tumor incidence extends to colon cancer itself (3). Animal studies have confirmed the beneficial activity of dietary calcium in colon polyp prevention, demonstrating both reduced incidence and inhibition of disease progression (4,5), and studies with epithelial cells in culture have provided mechanistic insight (6).
Although an adequate supply of dietary calcium is functionally related to protection against the formation of colonic adenomas, the use of calcium supplements to mitigate disease risk has shown only modest success. Reduced colon polyp formation has been shown in some chemoprevention studies (7,8), but others have failed to find significant benefit (9,10). At the same time, a high intake of calcium supplements is associated with increased risk of unwanted consequences including cardiovascular events (11,12).
Recent studies from our laboratory have suggested that combining calcium with additional trace elements may provide a way to enhance the beneficial effects of calcium in the colon. Our studies demonstrated that a natural product (Aquamin®), consisting of the skeletal remains of red marine algae of the Lithothamnion family (13) and containing magnesium as well as detectable amounts of 72 additional trace elements in addition to calcium, suppressed colon polyp formation and transition to invasive tumors in C57BL/6 mice on a high fat diet more effectively than calcium alone when included in the diet over the lifespan of the animals (14,15). In studies with human colon carcinoma cells in monolayer culture, Aquamin® was more effective than calcium alone at suppressing tumor cell growth and inducing differentiation (16,17).
Whether Aquamin® will, ultimately, prove to be more effective than calcium alone as a colon polyp chemopreventive agent in humans remains to be seen. A problem with translating preclinical findings to results in humans is the low incidence of colon polyp formation and the long lag period between initial molecular changes and outgrowth of observable lesions. Furthermore, progression from initial polyp formation to more serious disease is difficult to study experimentally since colonic polyps are removed upon detection. Perhaps most important is the high degree of variability in these premalignant lesions from individual to individual. Colonoid culture (colon epithelial organoids) technology (18), which is now well-developed, provides a way to study human colon polyp responses to potentially useful chemopreventive agents under ex vivo conditions. Using samples from our own bank of human adenoma colonoids (19–22), we have in the present study assessed the effects of calcium (alone) over a range of concentrations on adenoma colonoid growth and differentiation. In parallel, calcium provided in conjunction with additional trace elements as a natural product (i.e., Aquamin®) was evaluated.
MATERIALS AND METHODS
Human colonoid culture: Adenoma-derived and normal colonic mucosa
Colonoid (colon epithelial organoids) cultures were initiated from adenoma tissue obtained by endoscopy. The study was conducted after IRBMED (University of Michigan) approval (HUM 00038437) and all subjects signed written informed consent prior to the procedure. The first adenoma (#584) was a 20 mm (diameter) tumor in the ascending colon of a 61-year-old male. It was characterized by mutations in APC, EP300, KRAS, MET, PMS2, and TP53 (22). The second adenoma (#590) was a 35 mm lesion in the ascending colon of a 58-year-old female. Mutations of interest included BUB1B, CTNNA1, CTNNB1, FLCN, MAP2K4, MLH1, MLH3, MSH3, PALB2, PIK3R1, and TCERG1 (22). The third adenoma (#282) was a 45 mm lesion located in the ascending colon of a 66-year-old female. Mutational analysis revealed mutations in APC, ATM, EP300, MSH2, and R909Q (22). Upon arrival in the laboratory, tissue was established in culture as described in our earlier report (19). Briefly, adenoma tissue specimens were propagated in Matrigel (Corning) which was made to 8mg/mL in growth media, in 6-well tissue culture plates. Culture medium consisted of KGM Gold, a low-calcium (0.15 mM), serum-free formulation containing epidermal growth factor (EGF) and pituitary extract as growth supplements. Growth was at 37oC in an atmosphere of 95% air and 5% CO2. Cultures were passaged every 4-7 days by digesting Matrigel in cold 2mM EDTA and plated on the first day with 10μM Y27632, a Rho-associated protein kinase inhibitor. Short tandem repeat profiling was used throughout the study to confirm colonoid culture identity.
Establishment of colonoid cultures from histologically-normal colon tissue followed the same procedure as that used with adenomas. The normal colon tissue was collected from the sigmoid colon of subjects of an ongoing IRBMED (University of Michigan)-approved study (HUM 00076276) and subjects provided informed written consent prior to their participation. Normal colon tissue-derived colonoids were grown in L-WRN medium (includes a source of Wnt, R-spondin and noggin and is supplemented with 10% fetal bovine serum) (23). For the current study, L-WRN was diluted 1:4 with KGM Gold, bringing the final serum concentration to 2.5% and the final calcium concentration to 0.25 mM. Preliminary experiments demonstrated survival of normal tissue-derived colonoids for up to four weeks in this hybrid culture medium.
Human research ethics statement
These studies involving normal or adenomatous colonic tissue from human subjects were conducted in accordance with the recognized ethical guidelines, e.g., Declaration of Helsinki, International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS), Belmont Report, U.S. Common Rule.
Aquamin®
Aquamin® is a multi-mineral natural product obtained from the skeletal remains of the red marine algae, Lithothamnion sp. (13) and has been used in previous murine studies (14,15,24). Aquamin® contains calcium and magnesium in an approximately (12:1 ratio), along with measurable levels of 72 other trace minerals. Mineral content was established via an independent laboratory (Advanced Laboratories; Salt Lake City, Utah) using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (Supplement Table 1).
Phase-contrast microscopy and quantification
Colonoids were assessed by phase-contrast microscopy (Hoffman Modulation Contrast - Olympus IX70 with a DP71 digital camera) for changes in size and shape over time. Briefly, photographs of individual colonoids were taken at 2-3 day intervals over a 4-week period of growth. Images were scanned and surface area measurements made using Adobe Photoshop CS6 image analysis tool. Changes in surface area between day-8 (1-day after the most recent sub-culture) and day-13 were generated for numerous individual colonoids per treatment group (i.e., in 10-20 fields with 15-20 individual colonoids per field at 200X). From this, growth indices were calculated. The phase-contrast images were also used to assess the percentage of colonoids expressing the differentiated phenotype – i.e., smooth, thick walls and few tiny surface buds.
Histology and immunohistology
Colonoids were isolated from Matrigel using 2mM EDTA and fixed in 10% formalin for 1 hour. Fixed colonoids were suspended in HistoGel (Thermo Scientific) and then processed for histology. Additional details regarding immunohistology processing and antibodies used can be found in Supplement Table 2 and Supplemental Materials and Methods section.
Confocal fluorescence microscopy
Colonoids were isolated from Matrigel as above. Staining was done using a rabbit polyclonal Ki67 antibody for proliferating cells (ab15580; Abcam) overnight at 4C. Prolong Gold containing DAPI to identify nuclei (P36935; Life Technologies Molecular Probes) was used as counterstain. Specimens were visualized and imaged with a Leica Inverted SP5X Confocal Microscope System. Additional methodological details are provided in the Supplemental Methods.
Morphometric analysis
The histological sections were digitized using the Aperio AT2 whole slide scanner (Leica Biosystems) at 40X. Scanned Images were archived in Aperio eSlide Manager (Version 12.3.2.5030). These images were viewed and analyzed using Aperio ImageScope (Version 12.3.3.5048). Complete details can be found in the Supplemental Methods.
Scanning electron microscopy (SEM) and transmission electron microscopy TEM)
Adenoma colonoid specimens were fixed in situ and processed for SEM or TEM as described previously (25). More details are provided in the Supplemental Methods.
Proteomic analyses
Adenoma colonoids were isolated from Matrigel using 2mM EDTA for 15 minutes and then exposed to 8M urea in 0.1M TEAB buffer for protein isolation. Proteomic experiments and analysis were carried out in the Proteomics Resource Facility in the Department of Pathology at the University of Michigan, employing mass spectrometry-based Tandem Mass Tag (TMT, ThermoFisher Scientific) (26,27). Protein names were retrieved using Uniprot.org, and reactome V63 (reactome.org) was used for pathway enrichment analyses (28) based on the abundance ratio as compared to the individual control (calcium 0.15mM) for each adenoma with ≤2% false discovery rate (FDR). Additional methodological details are provided in the Supplement.
Statistical analysis
Means and standard deviations were obtained for discrete morphological and immunohistochemical features as well as for individual proteins. Groups were analyzed by ANOVA followed by student t-test (two-tailed) for unpaired data. Pathways enrichment data reflect Reactome-generated p-values based on the number of entities identified in a given pathway as compared to total proteins responsible for that pathway. Data were considered significant at p<0.05.
RESULTS
Adenoma differentiation in colonoid culture: Morphological and ultrastructural features
Adenoma colonoids from three different individuals were maintained for 30 days under control conditions (0.15 mM calcium) or in medium supplemented with 1.5 mM calcium or with Aquamin® to provide either 0.15 mM or 1.5 mM calcium. Throughout the in-life portion of the study, multiple individual colonoids were examined by phase-contrast microscopy at 2-3 day intervals for changes in size and shape. Individual colonoids increased in size over time. While the rate at which colonoids grew varied with the adenoma (growth indices under control conditions: #584 = 3.9±1.4; #590 = 3.5±2.8; #282 = 2.1±1.0), there was no effect with 1.5mM calcium or with Aquamin® providing calcium up to 1.5 mM (Supplement Figure 1).
Although there was no measurable effect on colonoid size, alterations in adenoma morphology could be seen with intervention. Specifically, individual colonoids maintained under low-calcium (0.15mM) conditions (Figure 1A, panel a) consisted of a central “core” structure with multiple tiny buds growing out from the surface. When Aquamin® providing 0.15 mM calcium was compared to calcium alone at the same concentration, most of the structures were indistinguishable from those maintained under low-calcium conditions alone. However, some of the adenoma colonoids demonstrated a loss of tiny buds on the surface and replacement with fewer, larger buds (Figure 1A, panel b).
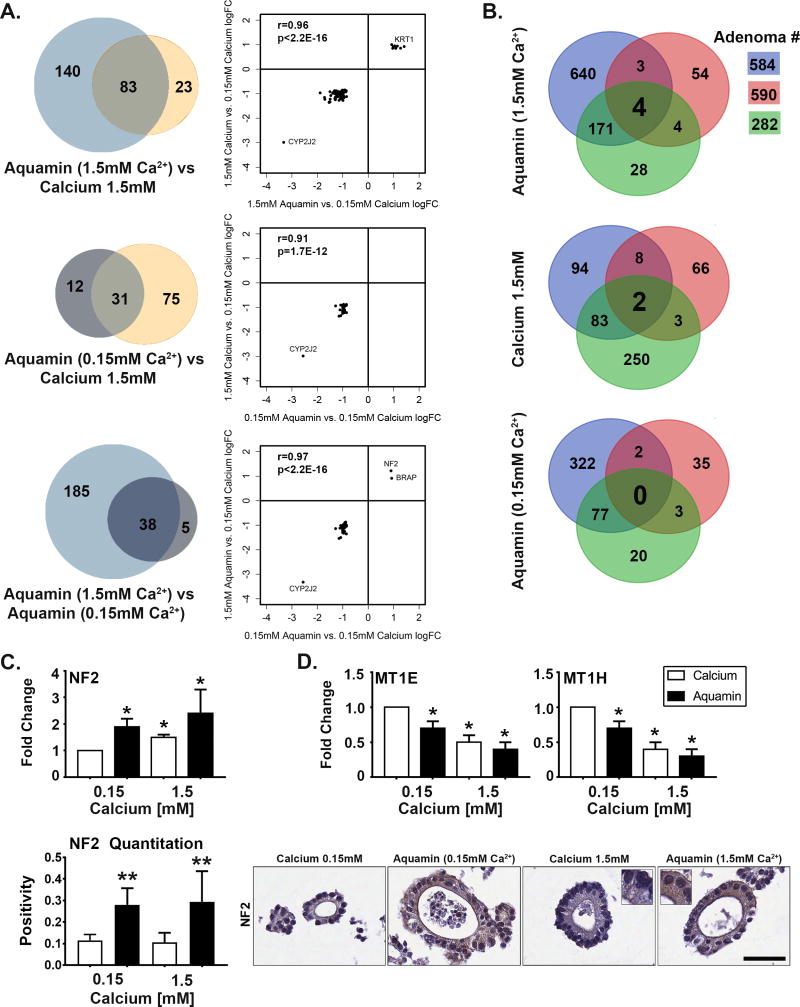
Figure 1. Adenoma colonoid appearance in culture.
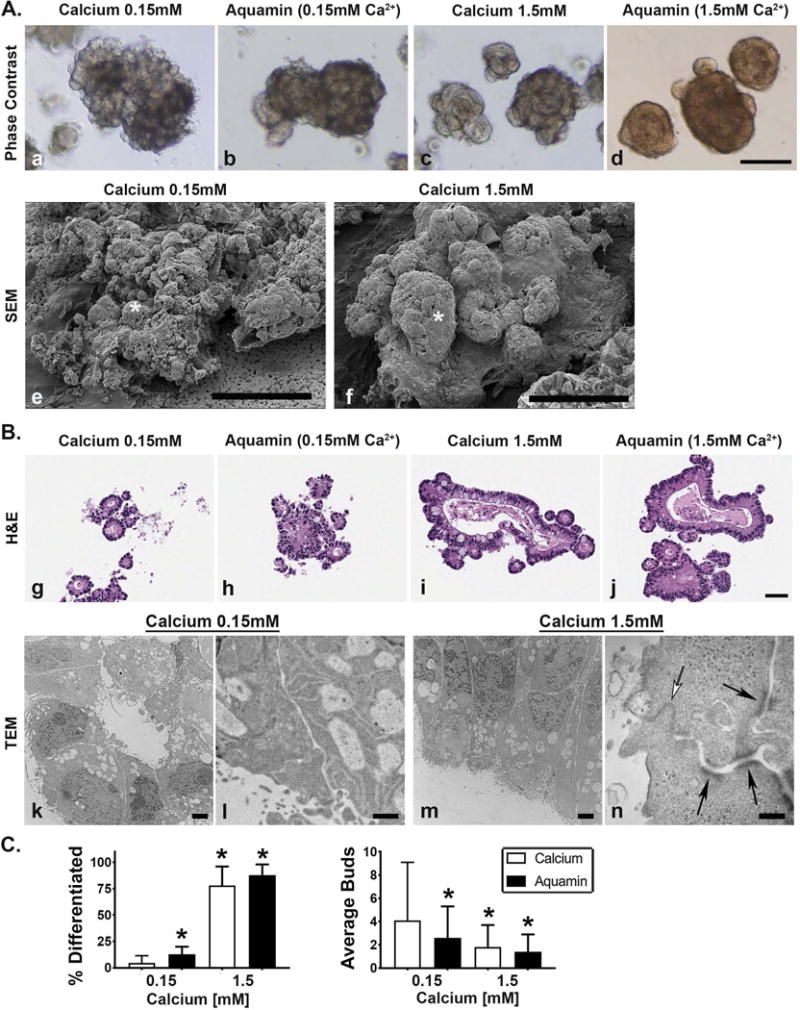
Figure 1A: Phase-contrast and scanning electron microscopy. At the end of the incubation period, virtually all of the adenoma colonoids maintained in 0.15 mM calcium consisted of a core structure with multiple tiny buds on the surface as indicated by phase-contrast microscopy (Figure 1, panel a). Colonoids maintained in 1.5 mM calcium (Figure 1, panel c) or treated with Aquamin® to provide either 0.15 mM (Figure 1, panel b) or 1.5 mM calcium (Figure 1, panel d) had a smooth surface with few buds. Bar=200μm. Scanning electron microscopic images confirmed the presence of multiple tiny buds growing out from the colonoid core structure under low-calcium conditions (asterisk) (Figure 1, panel e) and the presence of fewer but larger buds in the colonoid maintained in 1.5 mM calcium (asterisk) (Figure 1, panel f). Bars=100μm. Figure 1B: Histology and transmission electron microscopy. At the end of the incubation period, colonoids were examined under light-microscopy after sectioning and staining with hematoxylin and eosin. Under control conditions (Figure 1, panel g), tiny crypts of approximately 8-20 cells in cross section were seen. In the presence of 1.5 mM calcium alone (Figure 1, panel i) or with Aquamin® providing 1.5 mM calcium (Figure 1, panel j), larger crypts made up of columnar epithelial cells surrounding a large, often irregular-in-shape lumen were seen. Goblet cells were apparent. Colonoids treated with Aquamin® providing 0.15 mM calcium contained a mix of tiny crypts and larger structures (Figure 1, panel h). Bar= 50μm. When examined by transmission electron microscopy, the tiny crypts in low-calcium medium were found to consist of cuboidal cells surrounding a tiny central lumen. No desmosomes or tight junctures were seen (Figure 1, panels k and l). Under high-calcium conditions, the large crypts were made up of columnar epithelial cells around a larger lumen. Numerous desmosomes (black arrows) along the lateral surface connected by visible intermediate filaments and a tight-juncture at the apical surface (white arrow) were seen (m and n). Bar=2μm in Figure 1, panel k and m; Bar=500nm in Figure 1, panel l and =100nm in Figure 1, panel n. Figure 1C (Left panel): Percentage of individual colonoids with few tiny buds and smooth surface (phase-contrast). Means and standard deviations based on 20 fields per condition with 10-20 colonoids per field at 200X. Asterisks indicate statistical significance from control at p<0.05 level. Figure 1C (Right panel): Number of buds per core structure (hematoxylin and eosin sections). Means and standard deviations based on 5-10 high power fields per condition with 10-20 colonoids per field at 200X. Asterisks indicate statistical significance from control at p<0.05 level.
When adenoma colonoids were maintained in 1.5 mM calcium, either alone (Figure 1A, panel c) or in Aquamin® containing 1.5 mM calcium (Figure 1A, panel d), the majority of individual colonoids had a central core structure with few, large surface buds projecting from the core structure. These morphological differences, which were evident in the phase-contrast images, were confirmed by scanning electron microscopy (Figure 1A, panels e and f).
Histological features of adenoma colonoids from the same treatment groups harvested at day-30 are presented in Figure 1B. In 0.15 mM calcium (Figure 1B, panel g), the surface buds seen in phase-contrast and scanning electron microscopic images were found to be tiny spherical crypts (8-20 cells in cross section) surrounding a small central lumen. In some places, there was no lumen at all. Cells were cuboidal in shape. Consistent with phase-contrast findings, colonoids maintained in culture medium containing 0.15 mM Aquamin® (Figure 1B, panel h) demonstrated a mix of morphologies. Tiny crypts similar in morphology to those maintained in 0.15 mM calcium alone could be seen along with large crypts with larger central lumens. When calcium was increased to 1.5 mM (either alone; Figure 1B, panel i) or in Aquamin® (Figure 1B, panel j), most of the tiny crypts were replaced by much larger crypts. The larger crypts (all conditions) consisted of columnar epithelial cells surrounding a large irregularly-shaped central lumen. Some small crypts were also seen, but these were fewer (as a percentage of the total). Ultrastructural features of adenoma colonoids growing under control conditions (0.15 mM calcium) or in medium containing 1.5 mM calcium are also shown. Consistent with histological findings, the electron micrographs demonstrated that under low-calcium (0.15 mM) conditions (Figure 1B, panels k and l), the cells were cuboidal in shape and surrounded a small lumen. Tight junctures and desmosomes were lacking. Upon treatment with 1.5 mM calcium (Figure 1B, panels m and n), colonoids had a very different appearance. The cells were columnar in shape and surrounded a much larger central lumen. Desmosomes (black arrows) and tight junctures (white arrow) could be seen. Both types of junctional complexes were widespread in the colonoids exposed to 1.5 mM calcium.
Phase contrast and histological differences were quantified. The bar graph shown in the left panel of Figure 1C demonstrates the percentage of colonoids in each condition that demonstrated loss of tiny buds and replacement by a smooth surface. With 1.5 mM calcium (either alone or as part of Aquamin®), the majority of individual colonoids expressed this morphological alteration. A lower percentage of colonoids demonstrated this change in 0.15 mM Aquamin® (13±7%) but this was still statistically significant relative to control (2±7%). Quantification of histological features (number of buds per core structure) is presented in the right panel of Figure 1C.
Reversibility of differentiated features in adenoma colonoids
After 30 days of incubation in medium containing 1.5 mM calcium alone or in Aquamin® providing 1.5 mM calcium, adenoma colonoids from all three subjects were washed and then incubated under control conditions (i.e., in medium containing 0.15 mM calcium alone) (Supplement Figure 2). With all three specimens, the morphological features seen under high-calcium conditions (Supplement Figure 2, panel a) demonstrated a rapid reversion (i.e., within 6 days) to the low-calcium morphology (Supplement Figure 2, panel b). With Aquamin® (Supplement Figure 2, panels c and d), similar reversion occurred.
Immunochemical markers of proliferation and differentiation: Effects of calcium alone versus Aquamin®
Calcium (alone or as part of Aquamin®) was evaluated for effects on markers of proliferation and differentiation. Ki67-expressing (proliferating) cells were seen by immunohistology throughout the growing structures in all conditions (Figure 2A, panels a-d). Morphometric analysis (Figure 2B) showed that in the tiny spherical crypts of colonoids maintained in the low-calcium environment, 85±9% of the cells were Ki67-positive. In the larger structures, there was a mix of Ki67-positive and Ki67- negative cells (50±15% positive in 1.5 mM calcium and 41±21% positive in Aquamin® 1.5 mM). With adenoma colonoids maintained in Aquamin® providing 0.15 mM calcium, the percentage of Ki67-positive cells was 64±21%. Confocal fluorescence microscopy was used to obtain a broader perspective on distribution of the proliferation marker. This approach confirmed that Ki67 staining was largely confined to the tiny crypts. Further, it could be seen that where the small crypts were attached to the core structure, virtually all of the staining was in at the outer-facing surface of the colonoid structure (Figure 2C, panels e and f).
Figure 2. Proliferation marker expression.
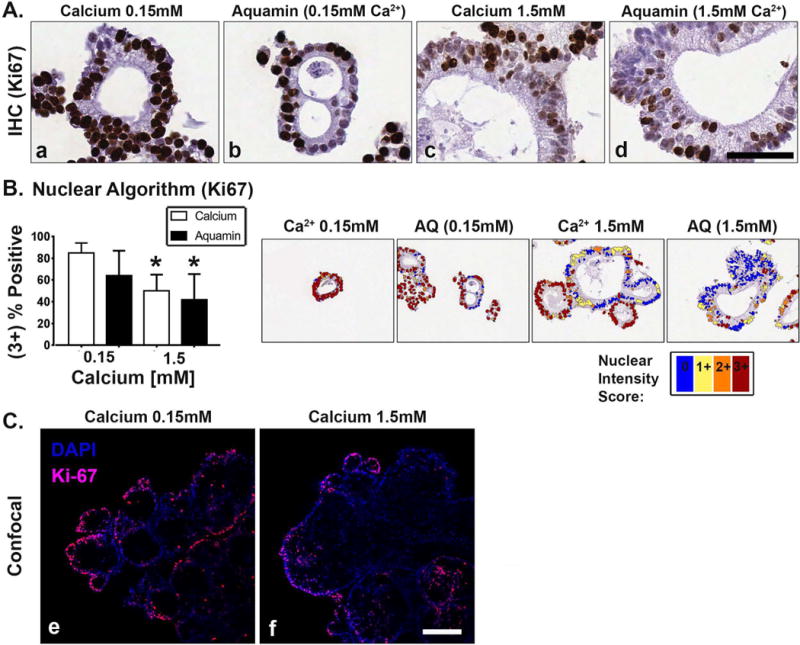
At the end of the 30-day incubation period, adenoma colonoids were examined after immunostaining. Figure 2A: Ki67 expression by immunohistology. The tiny crypts (regardless of condition) were almost universally-positive for Ki67 (Figure 2, panels a-d). The large crypts contained a mix of Ki67-positive and Ki67-negative cells. Bar=50μm. Figure 2B: Percentage of Ki67-positive nuclei. Means and standard deviations based on 15-25 individual crypts per condition. Asterisks indicate statistical significance from control at p<0.05 level. Insert: A markup image exemplifying color coding of Ki67 stained nuclei in all four conditions (nuclear algorithm v9) is shown. Figure 2C: Confocal immunofluorescence microscopy. The majority of Ki67- staining was in the tiny crypts in either 0.15 mM calcium (Figure 2, panel e) or 1.5 mM calcium (Figure 2, panel f). Where the tiny crypts were still visibly attached to the core structure (DAPI counterstain), staining was in outer-facing cells. Bar=100μm.
Figure 3 demonstrates effects of intervention on expression patterns for differentiation markers (CK20, E-cadherin and occludin). With CK20, most of the cells in the small crypts in low-calcium (0.15 mM) culture medium (Figure 3A, panel a) were completely negative or demonstrated weak intracellular staining. However, a few cells in this treatment group were strongly positive. In the other three conditions – including Aquamin® (0.15 mM) (Figure 3A, panels b-d) - numerous strongly-positive cells were seen throughout the crypt.
Figure 3. Differentiation and apoptosis marker expression.
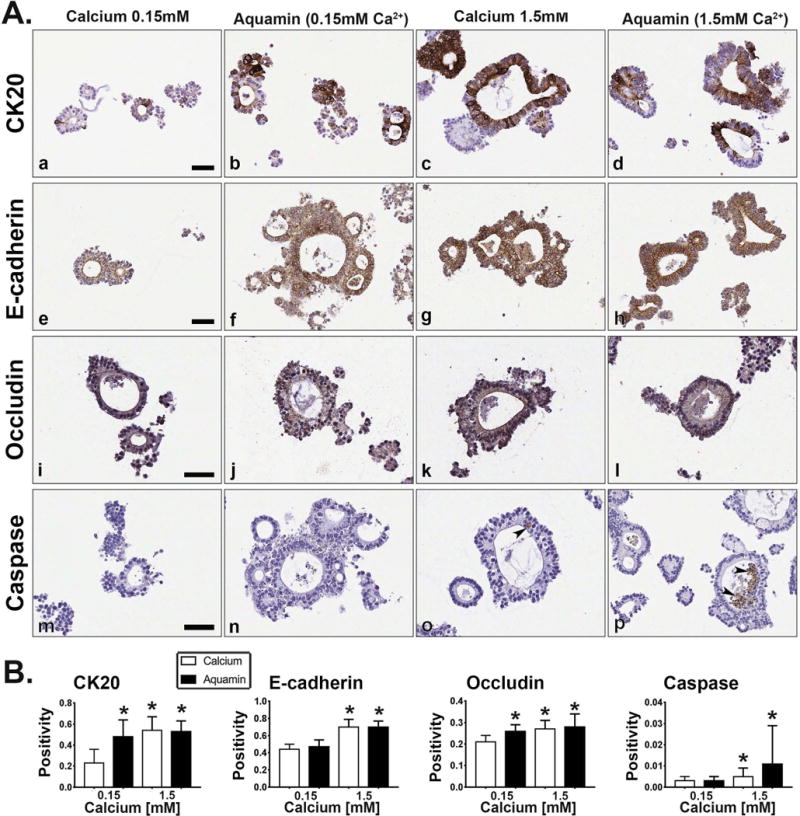
At the end of the 30-day incubation period, adenoma colonoids were examined after immunostaining. Figure 3A. Immunohistology. At the end of the 30-day incubation period, adenoma colonoids were examined under light-microscopy after immunostaining for CK20 (Figure 3, panels a-d), E-cadherin (Figure 3, panels e-h), occludin (Figure 3, panels i-l) and cleaved caspase-3 (Figure 3, panels m-p). CK20 was primarily diffuse and intracellular under low-calcium conditions, though a few cells were strongly positive (Figure 3, panel a). E-cadherin was diffuse and intracellular (Figure 3, panel e) while occludin was barely detectable and limited to the luminal surface (Figure 3, panel i). Cleaved caspase-3 was not detected (Figure 3, panel m). In the large crypts of the other three conditions, intense staining for CK20 was observed in many cells, though negative areas remained (Figure 3, panels b-d). With E-cadherin, both cell surface and intracellular staining were observed. The most intense surface staining was in cells at the lumen (Figure 3, panels f-h). With occludin, staining was present along the lateral surface between cells and at the luminal surface (Figure 3, panels j-l). Cleaved caspase-3 was not detected in colonoids maintained in 0.15 mM Aquamin® (Figure 3, panel n) but was seen in a few cells in the crypt wall (arrows) and in debris present in the lumens of crypts maintained in 1.5 mM calcium (Figure 3, panel o) or with Aquamin® (1.5 mM) (Figure 3, panel p). Bar=50μm. Figure 3B: Positivity (measured using Positive Pixel Value v9). Means and standard deviations based on 25-75 individual crypts per condition. Asterisks indicate statistical significance from control at p<0.05 level.
With E-cadherin, staining was mostly intracellular and diffuse under control conditions (Figure 3A, panel e). With all three interventions (Figure 3A, panels f-h), strong surface staining was observed in the large crypts. An increase in intracellular staining was also seen with 1.5 mM calcium (alone or in Aquamin®) (Figure 3A panels g and h) but we did not see an increase in intracellular staining in the low-Aquamin® treatment group (Figure 3A, panel f).
With occludin, cells from colonoid crypts in low-calcium medium demonstrated a diffuse intracellular staining pattern (Figure 3A, panel i). Colonoids maintained in Aquamin® at 0.15 mM (Figure 3A, panel j) demonstrated a mixed staining pattern. That is, many of the cells in the tiny spherical crypts demonstrated only diffuse intracellular staining, while the larger crypts demonstrated strong staining at cell-cell boundaries and at the apical surface as well as intracellular staining. Under high-calcium conditions (with either calcium alone or with Aquamin®) (Figure 3A, panels k and l), intense surface staining was observed in addition to strong intracellular staining. Strong surface staining was observed in both outer-facing cells and those cells sequestered in the interior of the colonoid structure.
Finally, colonoids were stained with an antibody to cleaved caspase-3 as a marker of apoptosis. While there was little overall staining under any of the conditions (Figure 3A, panels m-p), reactivity was greater in colonoids exposed to calcium alone or Aquamin® (1.5 mM) (Figure 3A, panels o and p). Individual cells within the wall of the large crypts and sloughed cells in the crypt lumen were positive. Higher magnification images of these immuno-markers are shown in Supplement Figure 3A.
Quantification of immunostained markers using positive pixel count v9 is shown in Figure 3B. Of note, Aquamin® at 0.15mM demonstrated enhanced staining with CK20 and occludin as compared to control. The markup images generated during this analysis are shown in Supplement Figure 3B.
Based on the morphologic appearance under phase-contrast microscopy and in histology (and later by confirmation with the immunohistochemical expression pattern of the crypts), adenoma colonoids with a thick-walled and smooth surface appearance along with a lack of small buds were consistent with a differentiated phenotype (Figures 1–3).
Proteomic changes in adenoma colonoids: Comparison of calcium alone versus Aquamin®
Lysates were prepared from each of the three adenoma colonoids following growth for 30 days. Lysates from each tumor were evaluated (separately) for protein expression changes in response to intervention in comparison to control. Then the three sets of data were combined. The Venn plots shown in Figure 4A indicate the total number of proteins demonstrating an average change in expression (increased or decreased) of at least 1.8-fold across the three adenomas with each intervention relative to control, and the overlap between pairs of interventions. The associated scatterplots show the correlation in overlap between the interventions. A total of 223 proteins met this criterion with Aquamin® (1.5 mM) compared to 106 with calcium alone. Of these, 83 were common, with a high concordance (r=0.96; p<2.2×10-16) (4A, upper panel). With Aquamin® (0.15 mM), a total of 43 proteins met the criteria, and of these, 31 overlapped with calcium 1.5mM (concordance, r=0.91; p<1.7×10-12) (4A, middle panel). When the two Aquamin® levels were compared (4A, lower panel), there was a virtual 100% overlap (r=0.97; p<2.2×10-16).
Figure 4. Proteomic profile in adenoma colonoids.
At the end of the 30-day incubation period, lysates were prepared for proteomic analysis. Figure 4A: Venn plots showing proteins altered (increased or decreased) by an average of 1.8-fold or greater across all three adenomas in response to each intervention and the overlap between pairs of interventions. The scatterplots demonstrate quantitative relationships between individual proteins altered by each pair of interventions. Figure 4B. Overlap in proteins altered (increased or decreased) with each intervention by 1.8-fold or greater in each of the three adenomas. Figure 4C. NF2 (merlin): Values in the upper bar graph represent fold-change under each condition relative to control. Values are means and standard deviations based on the proteomic assessment of the three adenomas. Asterisks indicate statistical significance from control at p<0.05 level. Values in the lower bar graph are based on the quantitation of immunostaining (measured using Positive Pixel Value v9) and show strong up-regulation in colonoids exposed to Aquamin® at both 0.15 and 1.5mM but little response to calcium. Two asterisks indicate statistical significance relative to calcium at 1.5 mM and control. Insert: NF2 (merlin) stained colonoids. Bar=50μm. Figure 4D. Metallothionines (MT1E and MT1H). Values represent fold-change and are means and standard deviations based on the proteomic assessment of the three adenomas. Both proteins were down-regulated in colonoids exposed to Aquamin® (at 0.15 and 1.5mM) and calcium at 1.5mM. Asterisks indicate statistical significance from control at p<0.05 level.
Figure 4B demonstrates the distribution of altered proteins (1.8 fold-change up and down by individual tumor) – showing inter-individual variability in response to these interventions. Only a single protein, NF2 (merlin), was found to be up-regulated (at least 1.8-fold) in all three tumors with Aquamin® 1.5mM. Three proteins – two metallothionine isoforms and phospholipid-transporting ATPase 1A - were down-regulated by Aquamin® in all three tumors. With 1.5 mM calcium alone, only two proteins (microsomal glutathione S-transferase 2 and protocadherin fat 1) were down-regulated to the same level in all three tumors. With Aquamin® (0.15 mM), there were none.
Figure 4C highlights changes occurring in NF2 (merlin). The upper bar graph shows the fold-changes with 1.5 mM calcium alone or with Aquamin® at either calcium concentration in proteomic assessment. All were statistically higher than control. Of interest, both Aquamin® concentrations were also higher than 1.5 mM calcium (2.4-fold and 1.9-fold versus 1.5-fold) although the differences were not statistically significant. The lower panel presents immunostaining results. Both concentrations of Aquamin® strongly increased NF2 (merlin) expression while little effect was observed with calcium alone at either concentration. The up-regulation seen with either Aquamin® concentration was significantly higher than seen with calcium at 1.5 mM (as well as calcium at 0.15 mM).
Figure 4D shows changes in metallothionine-1E and -1H. With both isoforms, all three interventions led to a statistically-significant reduction as compared to control expression.
Table 1A provides a list of up-regulated proteins – i.e., proteins that were up-regulated by an average of 1.8-fold or greater (with ≤2%FDR) across the three adenomas in response to any of the three interventions and statistically different from control with at least one intervention. For comparison, corresponding averages from the other interventions (even if corresponding average values were below 1.8 fold-change) are shown. Among proteins of interest in addition to NF2 (merlin) were several keratins, several isoforms of histone H1; i.e., proteins involved in terminal differentiation, and olfactomedin-4. Supplement Table 3 provides a comprehensive list of proteins that were up-regulated by an average of 1.8-fold or greater (with ≤2%FDR) across the three adenomas. In some cases, the most highly up-regulated proteins did not reach statistical significance because of high standard deviations. This attests to the variability among individual tumors. The top 18 pathways associated with (statistically significant) up-regulated proteins are shown in Table 1B. Not surprisingly, up-regulated pathways include those involved in differentiation, growth regulation, cell death and cellular response to stress.
Table 1. Up-regulated proteins and associated pathways: Comparison of response to calcium and Aquamin®.
Relative abundance (Average 1.8 fold-change or above relative to 0.15 mM calcium of 3 Adenomas)
| Average FC (From 3 Adenomas) | |||
|---|---|---|---|
| A: Proteins | Calcium | Aquamin® | Aquamin® |
| (1.5 mM) | (0.15mM) | (1.5mM) | |
| Proteins common with all 3 Interventions; CA 1.5, AQ 0.15 & AQ 1.5 (2 common proteins) | |||
| None significant | |||
| Proteins common with 2 Interventions; AQ 0.15 & AQ 1.5 mM (1 common protein) | |||
| Merlin (NF2) | 1.5±0.1* | 1.9±0.3* | 2.4±0.7* |
| Proteins common with 2 Interventions; CA 1.5 & AQ 1.5 mM (9 significant out of 15 common proteins) | |||
| Keratin-10 (KRT10) | 2.0±0.4* | 1.2±0.2 | 5.3±6.4 |
| Keratin-2 (KRT2) | 1.9±0.4* | 1.0±0.1 | 4.6±5.4 |
| Histone H1.5 (HIST1H1B) | 1.9±0.4* | 1.5±0.8 | 2.8±1.6 |
| Histone H1.3 (HIST1H1D) | 1.8±0.4* | 1.5±0.8 | 2.7±1.5 |
| 60S ribosomal protein L7a (RPL7A) | 1.8±0.3* | 1.6±0.9 | 2.3±1.4 |
| Histone H1.0 (H1F0) | 1.8±0.4* | 1.3±0.6 | 2.3±0.9 |
| Chromatin target of PRMT1 protein (CHTOP) | 1.9±0.2* | 1.5±0.6 | 2.1±0.7 |
| Olfactomedin-4 (OLFM4) | 2.1±0.6* | 1.0±0.0 | 2.0±0.6* |
| Brain acid soluble protein 1 (BASP1) | 1.8±0.7 | 0.6±0.1 | 1.8±0.4* |
| Up-regulated Proteins; CA 1.5 mM (3 common proteins) | |||
| None significant | |||
| Up-regulated Proteins; AQ 0.15 mM (1 significant out of 5 common proteins) | |||
| ATP-dependent RNA helicase (DDX52) | 1.6±0.1* | 2.0±0.3* | 1.6±0.2* |
| Up-regulated Proteins; AQ 1.5 mM (6 significant out of 17 common proteins) | |||
| keratin, type II cytoskeletal 5 (KRT5) | 1.5±0.2* | 1.1±0.3 | 5.8±6.6 |
| Histone H1.2 (HIST1H1C) | 1.7±0.3* | 1.5±0.8 | 2.8±1.7 |
| Histone H1.4 (HIST1H1E) | 1.7±0.4* | 1.1±0.4 | 2.0±0.6* |
| Ribonuclease P protein subunit p38 (RPP38) | 1.3±0.1* | 1.7±0.4* | 1.9±0.7 |
| 60S ribosomal protein L14 (RPL14) | 1.5±0.1* | 1.4±0.6 | 1.8±0.8 |
| Fatty acid-binding protein, liver (FABP1) | 1.5±0.2* | 1.1±0.0 | 1.8±0.4* |
| B. Significant Pathways (Reactome V63) | Entities pValue |
|---|---|
| Activation of DNA fragmentation factor | 1.4×10−11 |
| Apoptosis induced DNA fragmentation | 1.4×10−11 |
| Formation of Senescence-Associated Heterochromatin Foci | 3.9×10−11 |
| Apoptotic execution phase | 1.4×10−8 |
| DNA Damage/Telomere Stress Induced Senescence | 3.0×10−8 |
| Cellular Senescence | 3.9×10−6 |
| Apoptosis | 4.6×10−6 |
| Programmed Cell Death | 5.3×10−6 |
| Major pathway of rRNA processing in the nucleolus and cytosol | 1.5×10−4 |
| rRNA processing in the nucleus and cytosol | 1.9×10−4 |
| rRNA processing | 2.2×10−4 |
| Cellular responses to stress | 2.8×10−4 |
| Cellular responses to external stimuli | 6.3×10−4 |
| Formation of the cornified envelope | 9.6×10−4 |
| Metabolism of RNA | 2.8×10−3 |
| Keratinization | 4.2×10−3 |
| Type I hemidesmosome assembly | 0.02 |
| RHO GTPases activate PAKs | 0.03 |
Values represent average fold-change compared to control conditions (0.15 mM calcium) ±standard deviations. Only statistically significant proteins presented in table 1.
Represents significance in up-regulation as compared to the control at P < 0.05 level.
In a similar manner, we assessed the most down-regulated proteins across the three adenomas with all three interventions (i.e., >1.8-fold with ≤2%FDR). Proteins down-regulated by an average of 1.8-fold or greater across the three adenomas in response to any of the three interventions are shown in Supplement Table 4, along with corresponding averages from the other interventions. Many of the most down-regulated proteins are involved in lipid metabolism, energy production, oxidative stress and metal transport.
Effects of calcium and Aquamin® on growth and differentiation of colonoids from histologically-normal colon tissue
Colonoid cultures were established from three different histologically-normal colon tissue specimens (representing three individuals) and maintained for a 2-week period. Normal colon tissue colonoids differed from adenoma colonoids in several ways. In addition to their more stringent growth medium requirement and failure to survive long term in culture under the conditions used here (see Materials and Methods Section), normal tissue-derived colonoids were significantly different in appearance. As observed by phase-contrast microscopy (Figure 5), cultures of normal tissue colonoids contained a mix of two morphologically-distinct presentations: i.e., thin-walled, translucent “cystic” structures, and budding structures that resembled those seen in the adenoma cultures. Of interest in regard to the present study, there was little difference between normal tissue-derived colonoids maintained under low-calcium conditions (0.25 mM calcium) (Figure 5, panel a) and those treated with 1.5 mM calcium (Figure 5, panel b) or Aquamin® 1.5 mM (Figure 5, panel c). At the histological level (Figure 5), normal tissue-derived colonoids were made up of structures with large circular lumens surrounded by a single layer of flat, cuboidal cells or columnar epithelial cells. As expected based on phase contrast microscopy, there was little difference in appearance between colonoids grown under low-calcium conditions (Figure 5, panel d) and those exposed to elevated calcium (Figure 5, panel e) or Aquamin® (Figure 5, panel f). As part of the analysis, normal colon tissue-derived colonoids were stained for Ki67 and CK20. There was a mix of Ki67-positive cells and Ki67-negative cells, with no observable difference in staining as a function of calcium concentration (Figure 5, panels g-i). Intense CK20 staining was seen throughout the crypts, independent of calcium level (Figure 5, panels j-l). Finally, we saw no caspase-3 expression under control conditions (Figure 5, panel m) but a few positive cells were present in the normal colon sections treated with calcium (Figure 5, panel n) or Aquamin® (Figure 5, panel o).
Figure 5. Colonoids derived from histologically-normal colon tissue.
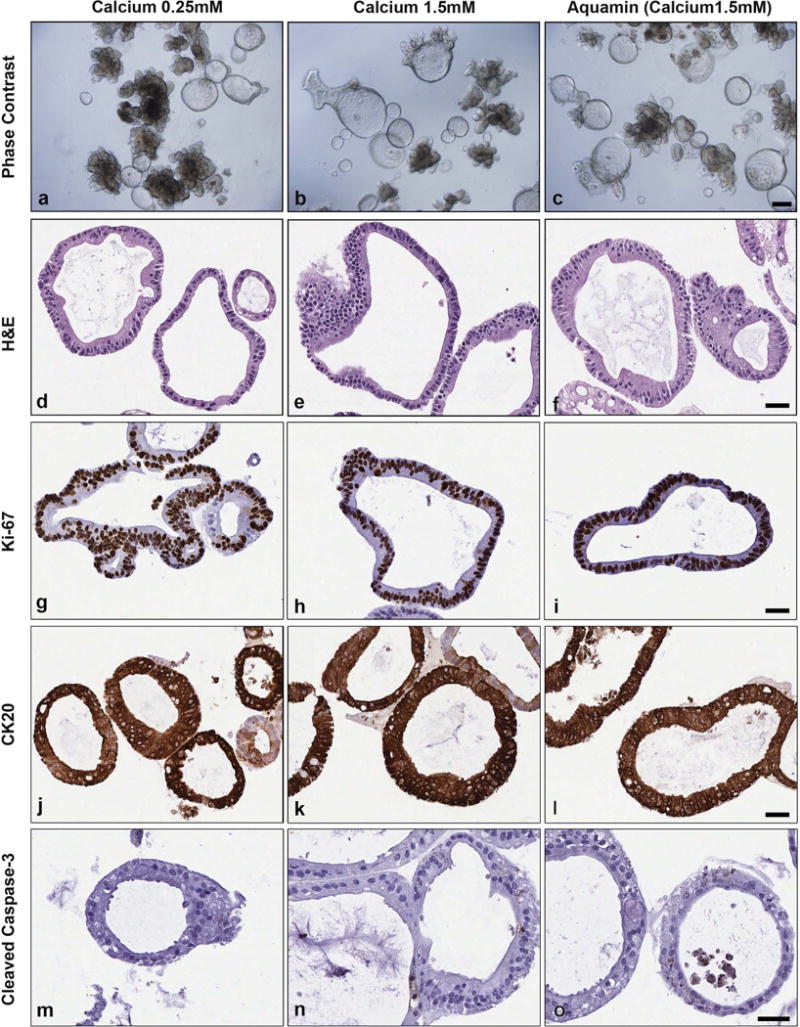
Phase-contrast (Figure 5, panels a-c). Colonoids maintained under all conditions consisted of a mix of thin-walled, translucent “cystic” structures and budding structures that resembled those seen in the adenoma cultures. Bar=200μm. Histology (Figure 5, panels d-f). At the histological level, crypts consisted of a single layer of epithelium surrounding a central lumen after staining with hematoxylin and eosin. Immunohistology (Figure 5, panels g-o). Immunostaining revealed a mix of Ki67-positive and -negative cells. Virtually all were strongly positive for CK20 but there was little or no staining for cleaved caspase-3. Bars=50μm.
DISCUSSION
In the studies described here, we compared responses of three different human colon adenomas in colonoid culture to intervention with calcium alone versus calcium as part of a natural product (Aquamin®) that consists of magnesium and additional trace elements along with calcium. The colonoid cultures were established from tumors with the diagnosis of “large adenoma”. The study had two overall goals. First was to determine if (and to what extent) human colon adenoma tissue in colonoid culture could be used to study the process of differentiation in the colonic epithelium. To address this issue, we utilized calcium, the quintessential epithelial differentiation-inducer (29), as a way to assess phenotypic changes. It is well known from monolayer cell culture studies that optimal epithelial growth occurs at calcium levels of 0.05 to 0.15 mM and that increasing the extracellular calcium level fosters differentiation (29). This was the first study, however, to directly assess calcium-mediated differentiation in actual human colon tumor specimens in colonoid culture. A second goal was to determine if the combination of calcium and additional trace elements (i.e., Aquamin®) would have beneficial activity beyond that seen with calcium alone. Past studies have demonstrated better suppression of colon polyp formation/progression in a mouse model with Aquamin® relative to calcium alone (15), and better growth-regulating activity in human colon carcinoma cell lines in monolayer culture (16,17) but the use of human colon adenoma tissue in colonoid culture provided an opportunity to directly compare the interventions against actual (growing) human colon polyp tissue.
Both study goals were achieved. Calcium alone at 1.5 mM induced differentiation in all three tumor specimens as indicated by morphological, histological and ultrastructural changes as compared to control. This was accompanied by a growth-fraction reduction (reduced Ki67-expression) and by up-regulation of differentiation markers. Several additional differentiation-related proteins were also up-regulated as indicated by proteomic analysis. Thus, in spite of the inherent variability and heterogeneity of human adenomas (30), we were able to document a measurable response to calcium in all three adenomas. Capacity to induce differentiation in large colon adenomas is of interest because the expression of differentiated features is associated with better prognosis in endoscopically-removed large adenomas (31,32), just as it is in fully malignant colon adenocarcinomas (33,34). Development of resistance to the pro-differentiating and growth-regulating activity of calcium has been demonstrated with colon epithelial cells in monolayer culture (16), and resistance of large adenomas to growth-suppression by vitamin D (calcium-regulating) has been suggested as a mechanism allowing for outgrowth and progression of such tumors in the face of calcium (35). The data presented here indicate that, at least in the case of the three specimens studied to date, the tumors retain a measure of calcium-responsiveness even when they have reached the large adenoma stage. Some colon cancer chemoprevention strategies depend on suppression prior to tumor initiation or at the earliest stages of abnormal growth (36). Those strategies would likely have little impact on the tumors that have reached the large stage. In contrast, beneficial effects from calcium might still be seen with polyps that have already reached a detectable size.
In addition to demonstrating adenoma differentiation in response to calcium, we were also able to show additional effects with Aquamin® as compared to calcium alone. Of particular interest, pro-differentiation activity was observed when Aquamin® was included at a concentration providing only 0.15 mM calcium. Even at this low Aquamin® level, some colonoids in each tumor underwent differentiation as detected by morphological alterations and altered biomarker expression (i.e., reduced Ki67 staining and increased CK20 and occludin expression along with membrane localization of E-cadherin). Perhaps of most interest was NF2 (merlin). This protein was significantly up-regulated with both Aquamin® concentrations while there was little change in response to calcium (as shown in immunohistochemical expression). NF2 (merlin) is down-stream of P21, a calcium-regulated tumor growth suppressor (37,38), and is a component of other signaling pathways that are known to have tumor growth-suppressing activity (39,40). These data support earlier findings from animal studies (15) suggesting that the presence of additional trace elements along with calcium has effectiveness as a growth-regulator in the colon over that seen with calcium alone. This is of interest because the use of calcium supplements at high levels is complicated by unwanted side effects including increasing the risk of cardiovascular events (11,12).
How the combination of trace elements and calcium in Aquamin® promotes differentiation at the low overall calcium concentration is not fully understood. Several different trace elements represented in the natural product (including members of the lanthanide family) bind to proteins involved in calcium signaling/mobilization (41–45). One of these, the extracellular calcium sensing receptor, plays a critical role in growth-regulating responses to calcium in the colon (46). Producing a “left-shift” in the response to calcium is one possible mechanism (17,47). At the same time, of course, other elements in the natural product could have growth-regulating activity, as well. It would be unwise at this point to rule out their involvement (either functioning with calcium or independently) as part of the mechanism.
As a control, we assessed calcium alone and Aquamin® for effects on features of differentiation in colonoids derived from histologically-normal colonic tissue. Neither produced a change in growth characteristics or morphological features. The lack of effect on normal tissue structure is largely consistent with observations of Bostic et al (48) who demonstrated only minimal effects of calcium intervention on the intact colonic mucosa during a chemoprevention trial. Thus, it seems unlikely that Aquamin® will produce manifestations of toxicity when used in a chemoprevention regimen. In addition, by reducing the amount of calcium needed for efficacy, this might reduce toxicities associated with high calcium supplement intake.
Finally, while our use of the proteomic platform was, primarily, to identify protein changes that are linked to calcium-mediated differentiation, the proteomic approach produced a massive amount of additional data, and analyzing the information effectively is far beyond the scope of a single paper. For example, there were large alterations in expression of several relevant proteins – but seen in only one or two of the tumors. Among these were cancer suppressors; i.e., BRCA1 – associated protein and olfactomedin-4 (49,50) – (both up-regulated). Interestingly, in light of the fact that all three tumors had mutations in DNA-repair pathway genes, was the finding that several proteins involved in DNA repair (e.g., methylated-DNA - protein methyltransferase, DNA repair protein complementing XP-C and mismatch repair endonuclease PMS2) were increased. Determining what accounts for this heterogeneity will be challenging. Another challenge will be to determine the relationship between biological activity (i.e., ability to undergo differentiation) and down-regulated proteins. Numerous proteins were reduced in response to each of the interventions. Yet, beyond noting that many of these proteins are involved in lipid metabolism and energy cycles, no attempt was made to understand their impact. This will require additional work.
In summary, it is well-known that calcium has growth-regulating activity in the colon. Our past studies have shown that the combination of calcium and additional trace elements has better colon epithelial cell growth regulatory activity than that seen with calcium alone. The studies presented here demonstrate that calcium does, in fact, affect growth in human colon adenomas obtained from large tumor specimens in colonoid culture. The studies show, furthermore, that a multi-mineral approach has the capacity to modulate structure and function in these specimens at calcium concentration that are ineffective with calcium alone. At the same time, there is no evidence of toxicity for normal colonic mucosa in colonoid culture. Thus, this work has a high degree of translational potential. Ultimately, however, fostering the use of Aquamin® as a colon chemopreventative agent is not the primary goal of this work. Rather, our goal is proof-of-concept; i.e., demonstrating that providing additional trace elements along with calcium has efficacy over that seen with calcium alone.
Supplementary Material
Adenoma colonoid expansion. Individual colonoids from each of the three adenomas were examined under phase-contrast microscopy after 8 and 13 days in culture (under each condition) and photographed. Photoshop CS6 image analysis tool was used to assess the surface area encompassed by individual colonoids and the difference in colonoid size between day-8 and day-13 was used to calculate the growth index (net change between the two time-points). Values shown are means and standard deviations. The number of individual colonoids assessed ranged from n=250 to n=424 for adenoma 282. For adenoma 590, colonoids ranged from n=53 to n=77. For adenoma 584, numbers ranged from n=15 to n=111. Statistical significance was assessed by ANOVA followed by student t-test (two-tailed) for unpaired data. Asterisks indicate significance at p<0.01 level. Insert: Phase-contrast images of the same fields on day-8 and day-13. Colonoids were more differentiated (thick walled, smooth surface and lack of small buds) at Day 13 with calcium 1.5mM as compared to colonoids exposed to calcium 0.15mM. Bar=200μm
Reversion of morphological features. At the end of the 30-day incubation period in 1.5 mM calcium (alone or as part of Aquamin®) (a,c), colonoids were re-incubated in medium containing 0.15 mM calcium. Reversion to the undifferentiated phenotype was observed by Day 6 (b,d). Bar=100μm.
Higher magnification images of the immunostained colonoid sections. Immunohistology as shown in Figures 3 and 4. At the end of the 30-day incubation period, colonoids were examined under light-microscopy after immunostaining for CK20, E-cadherin, occluding, cleaved caspase-3 and NF2. Bars=50μm.
Markup images of the immunostained colonoids. These markup images were generated during the quantitation process to measure “Positivity” using Positive Pixel Value v9 on Leica Aperio ImageScope. The data from these studies are presented in Figures 3 and 4. Bars=50μm.
SUPPLEMENT TABLE 1: Aquamin® Mineral Analysis. SUPPLEMENT TABLE 2: ANTIBODIES used in the study.
Up-regulated proteins and associated pathways (by significantly up-regulated proteins).
Down-regulated proteins and associated pathways (by significantly down-regulated proteins)
i) Histology and immunohistology. ii) Confocal fluorescence microscopy. iii) Morphometric analysis. iv) Scanning electron microscopy (SEM) and transmission electron microscopy TEM). v) Differential proteomic analysis.
Acknowledgments
This study was supported by National Institutes of Health grants CA181855, CA201782 (JV), ES028802 (JAC), CA046592 (University of Michigan Comprehensive Cancer Center core grant), and by support from The Rose and Lawrence C. Page Foundation (DKT), the Ravitz Foundation (JAC), MCubed (JAC, JV) and Michigan Institute for Clinical & Health Research (UL1TR002240). Part of the proteomics work was supported by The University of Michigan Center for Gastrointestinal Research (5 P30 DK034933)/Department of Pathology pilot grant (MNA). We thank Marigot LTD (Cork, Ireland) for providing Aquamin® as a gift. We thank the Translational Tissue Modeling Laboratory for providing access to human adenoma colonoid cultures; the Proteomic Core (Alexey Nesvizhskii, Director) for help with proteomic data acquisition (Kevin Conlon) and analysis (Dattatreya Mellacheruvu); the In Vivo Animal Core for preparation of samples for histology; the Histology and Immunohistology laboratory (University of Michigan Comprehensive Cancer Center) for immunostaining (Tina Fields); the Microscopy and Imaging Laboratory for help with confocal fluorescence microscopy, scanning electron microscopy and transmission electron microscopy and the Slide-Scanning Services (Peter Ouillette) of the Pathology Department for help with slide scanning and morphometric analysis.
Footnotes
Disclosure of Conflict of Interest: All the authors have no financial or personal conflict of interest.
References
- 1.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 2.Flood A, Peters U, Chatterjee N, Lacey JV, Jr, Schairer C, Schatzkin A. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev. 2005;14:126–132. [PubMed] [Google Scholar]
- 3.Keum NN, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: Dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135:1940–8. doi: 10.1002/ijc.28840. [DOI] [PubMed] [Google Scholar]
- 4.Beaty MM, Lee EY, Giauert HP. Influence of dietary calcium on colon epithelial proliferation and 1,2-dimethyhydrazine-induced colonic cancer in rats fed high fat diets. J Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 5.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57BI/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariadson JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, et al. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 7.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 8.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Aspirin, Calcitriol, and Calcium Do Not Prevent Adenoma Recurrence in a Randomized Controlled Trial. Gastroenterology. 2016;150:114–122. doi: 10.1053/j.gastro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effects of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JJB, Kruszka B, Delaney JAC, He K, Burke GL, Alonso A, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA) J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adey WH, McKibbin DL. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Botanical Marina. 1970;13:100–106. [Google Scholar]
- 14.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther. 2010;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslam MN, Bergin I, Naik M, Paruchuri T, Hampton A, Rehman M, et al. A multimineral natural product from red marine algae reduces colon polyp formation in C57BL/6 mice. Nutr Cancer. 2012;64:1020–1028. doi: 10.1080/01635581.2012.713160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslam MN, Bhagavathula N, Chakrabarty S, Hu X, Chakrabarty S, Varani J. Growth-inhibitory effects of a mineralized extract from the red marine algae, Lithothamnion calcareum, on Ca(2+)-sensitive and Ca(2+)-resistant human colon carcinoma cells. Cancer Lett. 2009;283:186–192. doi: 10.1016/j.canlet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Aslam MN, Varani J, Chakrabarty S. Induction of calcium sensing receptor in human colon cancer cells by calcium, vitamin D and aquamin: promotion of a more differentiated, less malignant and indolent phenotype. Mol Carcinog. 2015;54:543–553. doi: 10.1002/mc.22123. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, Vries RG, Snippert HJ, van deWetering M, Barker N, Strange DE, et al. Single Lgr5 stem cells build crypt–villus structures in vitro. Nature. 2009;14:459, 262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 19.Dame MK, Jiang Y, Appelman HD, Copley KD, McClintock SD, Aslam MN, et al. Human Colonic Crypts in Culture: Segregation of Immunochemical Markers in Normal Versus Adenoma-Derived. Lab Invest. 2014;94:222–234. doi: 10.1038/labinvest.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Ramakrishnan SK, Triner T, Centofanti B, Maitra D, Győrffy B, et al. Tumor-Selective Proteotoxicity of Verteporfin Inhibits Colon Cancer Progression independently of YAP1. Sci Signal. 2015;8:397–411. doi: 10.1126/scisignal.aac5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, Attili D, et al. Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metab. 2016;24:447–461. doi: 10.1016/j.cmet.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dame MK, Attili D, McClintock SD, Dedhia PH, Ouilette P, Hardt O, Chin AM, et al. Identification, Isolation, and Characterization of Human LGR5-positive Colon Adenoma Cells. Development. 2018 doi: 10.1242/dev.153049. (online first) http://dev.biologists.org/lookup/doi/10.1242/dev.153049. [DOI] [PMC free article] [PubMed]
- 23.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells as organoids. Nature Protocol. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, Zernicke RF, Goldstein SA, Varani J. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcified tissue international. 2010;86(4):313–24. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleavins K, Perone P, Naik M, Rehman M, Aslam MN, Dame MK, et al. Stimulation of fibroblast proliferation by insoluble gadolinium salts. Biol Trace Element Res. 2012;145:257–267. doi: 10.1007/s12011-011-9176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAlister GC, Nusinow DP, Jedrychowski MP, Wühr M, Huttlin EL, Erickson BK, et al. MultiNotch MS3 enables accurate, sensitive and multiplexed detection of differential expression across cancer cell line proteomes. Analyt Chem. 2014;86:7150–7158. doi: 10.1021/ac502040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabregat A, Sidiropoulos K, Garapati P, et al. The Reactome pathway Knowledgebase. Nucleic Acids Research. 2016;44:D481–D487. doi: 10.1093/nar/gkv1351. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks PG, Parnes SM, Price JC, Risemberg H, Goldstein JC, Marko M, Parsons DF. In vitro modulation of differentiation by calcium in organ cultures of human and murine epithelial tissue. In Vitro Cell Dev Biol. 1985;21:99–107. doi: 10.1007/BF02620950. [DOI] [PubMed] [Google Scholar]
- 30.Sievers CK, Leystra AA, Clipson L, Dove WF, Halberg RB. Understanding intratumoral heterogeneity: lessons from the analysis of at-risk tissue and pre-malignant lesions in the colon. Cancer prevention research. 2016;9(8):638–641. doi: 10.1158/1940-6207.CAPR-16-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosson BC, Whiteway JE, Jones EA, Macrae FA, Williams CB. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut. 1984;25:437–444. doi: 10.1136/gut.25.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham KN, Mills LR, Schuman BM, Mwakyusa DH. Long-term prognosis of well-differentiated adenocarcinoma in endoscopically removed colorectal adenomas. Digestive Dis Science. 1994;39:2034–2037. doi: 10.1007/BF02088143. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, Yu L, Zhao L-Z, Ma D-W. Prognostic factors in the patients with T2N0M0 colorectal cancer. World J Surg Oncol. 2016;14:76. doi: 10.1186/s12957-016-0826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao H, Yoon YS, Hong S-M, Roh SA, Cho D-H, Yu CS, et al. Poorly differentiated colorectal cancers: Correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140:341–347. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- 35.Gardina C, Madigan JP, Tierney CAG, Brenner BM, Rosenberg DW. Vitamin D resistance and colon cancer prevention. Carcinogenesis. 2012;33:475–482. doi: 10.1093/carcin/bgr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang W-C, Jackson C, Riel S, Cooper SH, Devarajan K, Hensley HH, Zhou Y, vanderveer LA, Nguyen MT, Clapper ML. Differential preventive activity of sulindac and atorvastatin in Apc+/Min-FCCC mice with or without colorectal adenomas. GUT. 2017 doi: 10.1136/gutjnl-2017-313942. pii: gutjnl-2017-313942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10,394–10,399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 38.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cd42 signaling to merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 39.Cooper J, Giancotti FG. Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 2014;588:2743–52. doi: 10.1016/j.febslet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis–the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–77. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrillo-Lopez N, Fernandez-Martin JL, Alvarez-Harnandez D, Gonzalez-Surez I, Castro-Santos P, Roman-Garcia P, et al. Lanthanum activates calcium-sensing receptor and enhances sensitivity to calcium. Nephrol Dial Transplant. doi: 10.193/ndt/gfq124. [DOI] [PubMed] [Google Scholar]
- 43.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiPolo R, Beauge L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Reviews. 2006;86:155–203. doi: 10.1152/physrev.00018.2005. [DOI] [PubMed] [Google Scholar]
- 45.Attili D, Jenkins B, Aslam MN, Dame MK, Varani J. Growth control in colon epithelial cells: Gadolinium enhances calcium-mediated growth regulation. Biological trace element research. 2012;150(1–3):467–76. doi: 10.1007/s12011-012-9503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W, Liu L, Masugi Y, Qian ZR, Nishihara R, Keum N, Wu K, Smith-Warner S, Ma Y, Nowak JA, Momen-Heravi F. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR) Gut. 2017 doi: 10.1136/gutjnl-2017-314163. pii: gutjnl-2017-314163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aslam MN, Varani J. The Western-Style Diet, Calcium Deficiency and Chronic Disease. J Nutr Food Sci. 2016;6(496):2. [Google Scholar]
- 48.Bostic RM. Effects of supplemental vitamin D and calcium on normal colon tissue and circulating biomarkers of risk for colorectal cancer. J Steroid Biochem Molec Biol. 2015;148:86–95. doi: 10.1016/j.jsbmb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventii KH, Devi HS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRACA1-associated protein is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Li H, Hong S-H, Piszczek GP, Chen W, Rodgers GP. Olfactomedin 4 deletion induces colon adenocarcinoma in ApcMin/+ mice. Oncogene. 2016;35:5237–5247. doi: 10.1038/onc.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adenoma colonoid expansion. Individual colonoids from each of the three adenomas were examined under phase-contrast microscopy after 8 and 13 days in culture (under each condition) and photographed. Photoshop CS6 image analysis tool was used to assess the surface area encompassed by individual colonoids and the difference in colonoid size between day-8 and day-13 was used to calculate the growth index (net change between the two time-points). Values shown are means and standard deviations. The number of individual colonoids assessed ranged from n=250 to n=424 for adenoma 282. For adenoma 590, colonoids ranged from n=53 to n=77. For adenoma 584, numbers ranged from n=15 to n=111. Statistical significance was assessed by ANOVA followed by student t-test (two-tailed) for unpaired data. Asterisks indicate significance at p<0.01 level. Insert: Phase-contrast images of the same fields on day-8 and day-13. Colonoids were more differentiated (thick walled, smooth surface and lack of small buds) at Day 13 with calcium 1.5mM as compared to colonoids exposed to calcium 0.15mM. Bar=200μm
Reversion of morphological features. At the end of the 30-day incubation period in 1.5 mM calcium (alone or as part of Aquamin®) (a,c), colonoids were re-incubated in medium containing 0.15 mM calcium. Reversion to the undifferentiated phenotype was observed by Day 6 (b,d). Bar=100μm.
Higher magnification images of the immunostained colonoid sections. Immunohistology as shown in Figures 3 and 4. At the end of the 30-day incubation period, colonoids were examined under light-microscopy after immunostaining for CK20, E-cadherin, occluding, cleaved caspase-3 and NF2. Bars=50μm.
Markup images of the immunostained colonoids. These markup images were generated during the quantitation process to measure “Positivity” using Positive Pixel Value v9 on Leica Aperio ImageScope. The data from these studies are presented in Figures 3 and 4. Bars=50μm.
SUPPLEMENT TABLE 1: Aquamin® Mineral Analysis. SUPPLEMENT TABLE 2: ANTIBODIES used in the study.
Up-regulated proteins and associated pathways (by significantly up-regulated proteins).
Down-regulated proteins and associated pathways (by significantly down-regulated proteins)
i) Histology and immunohistology. ii) Confocal fluorescence microscopy. iii) Morphometric analysis. iv) Scanning electron microscopy (SEM) and transmission electron microscopy TEM). v) Differential proteomic analysis.