Abstract
Background
The perivascular adipose tissue (PVAT), surrounding vessels, constitutes a distinct functional integral layer of the vasculature required to preserve vascular tone under physiological conditions. However, there is little information regarding the relationship between PVAT and blood pressure regulation, including its potential contributions to circadian blood pressure variation.
Methods
Using unique brown adipocyte-specific aryl hydrocarbon receptor nuclear translocator-like protein 1 (Bmal1) and angiotensinogen (Agt) knock out mice we determined the vasoactivity of homogenized PVAT in aortic rings and how brown-adipocyte peripheral expression of Bmal1 and Agt in PVAT regulate the amplitude of diurnal change in blood pressure in mice.
Results
We uncovered a peripheral clock in PVAT and demonstrated that loss of Bmal1 in PVAT reduces blood pressure in mice during the resting phase leading to a super-dipper phenotype. PVAT extracts from wild type mice significantly induced contractility of isolated aortic rings in vitro in an endothelium independent manner. This property was impaired in PVAT from brown adipocyte-selective Bmal1 deficient mice (BA-Bmal1-KO). The PVAT contractile properties are mediated by local angiotensin II (Ang II), operating through angiotensin II type 1 receptor-dependent signaling in the isolated vessels and is linked to PVAT circadian regulation of Agt. Indeed, Agt mRNA and Ang II levels in PVAT of BA-Bmal1-KO mice were significantly reduced. Systemic infusion of Ang II, in turn, reduced Bmal1 expression in PVAT while eliminating the hypotensive phenotype during the resting phase in BA-Bmal1-KOmice. Agt, highly expressed in PVAT, shows circadian expression in PVAT and selective deletion of Agt in brown adipocytes recapitulates the phenotype of selective deletion of Bmal1 in brown adipocytes. Furthermore, Agt is a transcriptional target of Bmal1 in PVAT.
Conclusions
These data indicate that local Bmal1 in PVAT regulates Agt expression and the ensuing increase in Ang II, which acts on smooth muscle cells (SMCs) in the vessel walls to regulate vasoactivity and blood pressure on a circadian fashion during the resting phase. These findings will contribute to better understand cardiovascular complications of circadian disorders, alterations in the circadian dipping phenotype and the crosstalk between systemic and peripheral regulation of blood pressure.
Keywords: blood pressure, Angiotensin II, circadian, perivascular adipose tissue
Introduction
Hypertension, a disorder of complex pathophysiology, is a major contributor to cardiovascular and renal diseases. Although the exact intricacies of hypertension etiology still remain unsolved, it is clear that this is a multi-factorial, complex polygenic disorder with many interacting mechanisms contributing to its pathophysiology and involving many organ systems, including the heart, kidney, brain, vessels and the immune system1. Perivascular adipose tissue (PVAT) is de facto a distinct functional vascular layer and plays an active role in the control of vascular (dys)function in (patho)physiological conditions such as hypertension2, 3. Current studies show paradoxical effects of PVAT in the regulation of vascular tone2. On one hand, PVAT exerts anti-contractile effects in various vascular beds, a phenomenon that seems to be mediated by some, still elusive, PVAT-derived relaxing factors (PVRF). This anti-contractile effect of PVAT is impaired in disease conditions such as metabolic syndrome4, 5 and hypertension5–8, further underscoring the physiological role of PVAT in vascular tone. PVRFs might comprise adiponectin9, 10, H2S11,NO12, Ang-(1-7)13, palmitic acid methyl ester14 and prostacyclin15. On the other hand, we previously demonstrated that PVAT dramatically constricted carotid artery rings15 while coronary artery PVAT of obese swine augmented vasoconstriction16. Additionally, PVAT enhanced the mesenteric artery contractile response to perivascular nerve stimulation through the production of superoxide mediated by NAD(P)H oxidase17. Chemerin is expressed in PVAT and induces vasoconstriction18. Thus, apart from the existence of anti-contractile PVRF in PVAT, the existence of PVAT-derived contractile factors (PVCF) must also be highlighted. A delicate balance between those two faces of PVAT on the regulation of vascular tone could contribute to the maintenance of vessel homeostasis and blood pressure (BP) regulation. Currently, direct evidence derived from animal models to support the notion of an anti-contractile or contractile role for PVAT on the regulation of BP is largely missing. We reported that a mouse model lacking PVAT due to peroxisome proliferator-activated receptor gamma (PPARγ) deletion in vascular smooth muscle cells (VSMCs) presents hypotension in the resting period. However, it is not clear whether loss of PVAT or PPARγ deletion in VSMCs in these mice causes the hypotension19.
Circadian variations in BP are among the best recognized circadian rhythms of physiology in humans20 and rodents21. In humans, the highest BP is observed during the morning hours and the lowest during the sleep hours. This diurnal-nocturnal cycle of BP in rodents is reversed as the result of their nocturnal active-phase. It is known that circadian rhythms in BP are controlled by the “master clock” located in the suprachiasmatic nucleus (SCN)22. The positive (Clock,Npas2, Bmal1) and negative (Per1, Per2, Per3, Cry1, and Cry2) arms of the clock genes in the SCN comprise a negative feedback loop, resulting in cyclic activation of the transcriptional machinery of clock genes in an approximate 24-hour cycle of biological rhythm23. Studies from animals and humans suggest that Baml1 is associated with hypertension. The Bmal1 gene is located within hypertension susceptibility loci in rat and maps close to a region genetically divergent between spontaneously hypertensive rats and their normotensive counterparts24. Genetic association studies showed that two BMAL1 haplotypes were associated with hypertension24 and that the rs3816358 SNP in BMAL1 was significantly associated with the non-dipper phenotype in young hypertensive patients, suggesting a genetic association with diurnal BP changes in essential hypertension25. Indeed, disruption of master clock genes has been shown to change BP and alter circadian rhythms of BP. Conventional Cry-knockout mice are hypertensive during the resting phase and on average normotensive during the active period26. Mice with a mutation of Npas2 are hypotensive27. Clock mutant mice exhibit only a subtle dampening of BP upon light-dark conditioning27. Notably, mice with global deletion of Bmal1 showed evidence of abolished circadian rhythms of BP and were hypotensive27. However, the SCN master clock is not solely responsible for the circadian regulation of BP. Indeed, the components of the circadian clock genes are found in peripheral tissues, including blood vessels28. Furthermore, the peripheral circadian clocks can be uncoupled from the central SCN circadian clocks29. A robust diurnal variation in BP and lower BP during the dark phase was apparent in mice with endothelial cell-specific deletion of Bmal130. PPARγ deletion in aortas repressed Bmal1 activity and herein disrupted circadian rhythms of BP31. Also, selective deletion of Bmal1 in VSMCs decreased BP without affecting SCN-controlled locomotor activity in mice32. Together, these studies suggest that peripheral circadian clocks contribute to BP circadian rhythmicity.
The components of the renin angiotensin system (RAS), including angiotensinogen (Agt) -albeit not renin-are present in PVAT33. In fact, pre-treatment of intact vessel rings with the selective Ang II antagonist, Sari-Ile8-Ang II, significantly attenuated electrical stimulation elicited contraction in a PVAT-dependent fashion34. Chronic treatment with an angiotensin converting enzyme (ACE) inhibitor, quinapril, alleviated the potentiation effects of PVAT in contraction upon electrical stimulation35, further supporting a role for PVAT-derived Ang II in vasoactivity.
In this study, we report a peripheral circadian clock operating in PVAT-dependent circadian regulation of BP. Deletion of Bmal1 or Agt in brown adipocytes including PVAT dramatically reduced BP in the resting phase resulting in a super-dipper phenotype. Furthermore, we found that Bmal1 positively regulates Agt expression at the transcriptional level in PVAT adipocytes. Thus, Bmal1, Agt and Ang II in PVAT coordinately contribute to the regulation of homeostatic circadian rhythmicity of BP during the resting phase.
Methods
Please see detailed experimental methods in online supplemental materials, including the list of primers in supplemental Table I. Requests to access the data, analytic methods and study materials for reproducibility purposes should be addressed to the corresponding authors.
Mice
Brown Adipocyte-specific deletion of Bmal1(BA-Bmal1-KO) or Agt (BA-Agt-KO) mice were generated by crossbreeding Bmal1flox/flox (Jackson Laboratory, stock# 007668)or Agtflox/flox mice (Jackson Laboratory stock # 018388) with UCP-1 driven Cre mice36, respectively (all in theC57BL/6 background). Eight-week-old males were used in this study, and age-matched wild-type littermate mice were used as controls. Mice were housed at the University of Michigan animal facility (∼22°C and 12/12-hour light/dark cycle) and supplied with rodent diet no. 2918 (18% protein, 6% fat and moderate phytoestrogen; Harlan Laboratories) ad libitum. The Animal Research Ethics Committee of the University of Michigan approved the study protocol for animals. Telemetry, Ang II infusion and aortic ring constriction in response to PVAT extracts were described previously19,37 and are further detailed in the supplemental materials.
Statistical analysis
Mean ± SD. values were determined using GraphPad Prism Software (version 7). Statistical comparisons between two groups were performed by Student’s t test (Figure 1C, Figure 2C, Figure 3A, Figure 3D, Figure 4C, Figure 5B Figure 5C, Figure 5D), and more than two groups were performed by one-way ANOVA (Figure 1E, Figure 2B, Figure 2E, Figure 3F, Figure 4F). Groups were considered significantly different if p values were <0.05.
Figure 1. Knockout of Bmal1 in brown adipocytes reduced BP during the light-on resting phase.
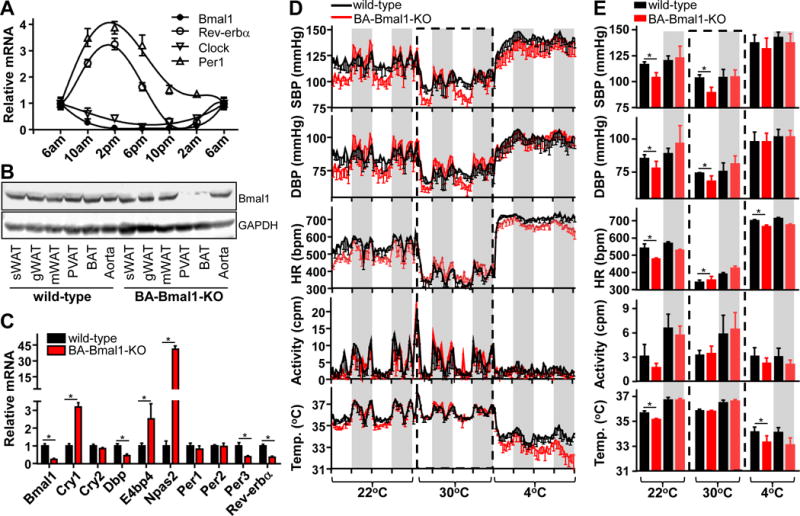
A, Realtime PCR showing mRNA levels of circadian genes in wild-type PVAT, relative to β-actin. Data shown as mean ± SEM, n=5. B, Representative Western blot showing Bmal1 levels in adipose tissues and aorta in wild-type and BA-Bmal1-KOmice.C, Realtime PCR showing mRNA levels of circadian genes in wild-type and BA-Bmal1-KO PVAT, relative to β-actin. Data shown as mean ± SEM, n=5-6, *p<0.05 vs wild-type. D, Systolic (SBP), diastolic BP (DBP), heart rate (HR), locomotor activity and body temperature of wild-type and BA-Bmal1-KO mice was measured by radiotelemetry when the temperature of the environmental chamber was successively set at 22°C, 30°C, or 4°C. Data are shown as mean±SD, n=5/group. The gray shading represents light-off period from 18:00 to 6:00. E, Average of 2 constitutive circadian cycles of systolic, diastolic BP, heart rate, locomotor activity and body temperature data during light-off and light-on periods shown in panel D. Data are shown as mean ± SD, *p<0.05vs wild-type.
Figure 2. PVAT constricts blood vessels.
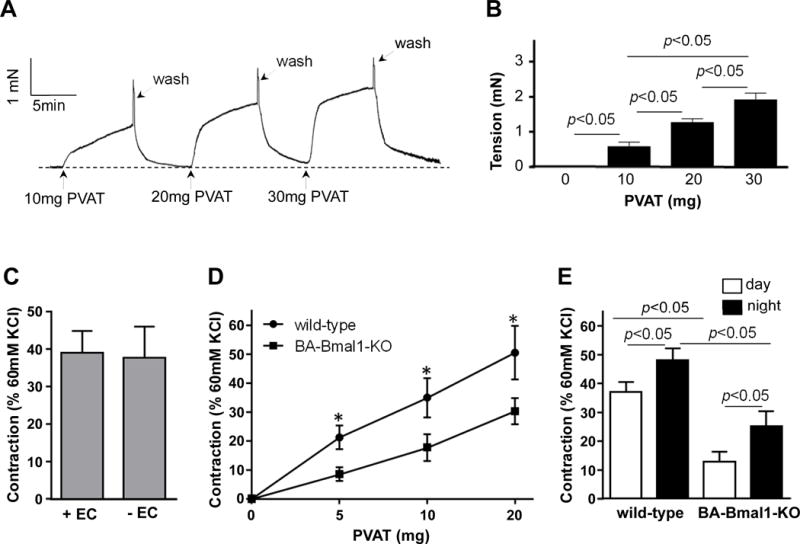
A, Representative trace showing dose-dependent aortic ring constriction in response to extracts from 10–30 mg of thoracic PVAT. The thoracic aortic rings were collected from 8-week old C57BL/6J mice at 10AM. The PVAT adjacent to the rings was cleaned before they were mounted onto the wire myograph. The donor PVAT extracts were collected from 8-week old C57BL/6J mice at 10AM. B, Quantitative analysis of constriction tension of vessel rings in response to PVAT extracts in panel A. The tensions are shown as absolute tension differential between basal level and plateau level and expressed in miliNewton (mN). Data are shown as mean ± SD. n=6. *p<0.05 vs. baseline. C, Vasoconstriction induced by 10mg of C57BL/6J PVAT extracts on vessel rings with the endothelium present (+EC) or removed (-EC). Data shown as mean ± SD. n=6. D, Vasoconstriction of C57BL/6J rings induced by increasing amounts of PVAT extracts collected from C57BL/6J or BA-Bmal1-KOmiceat 10AM (resting phase). Data shown as mean ± SD. n=6, *p<0.05 vs. wild-type. E, Vasoconstriction induced by 10mg PVAT extracts collected from the indicated genotype at 10AM (resting phase) and 10PM (active phase) on vessel rings. Data shown as mean ± SD. n=6.
Figure 3. Ang II in PVAT constricts blood vessels.
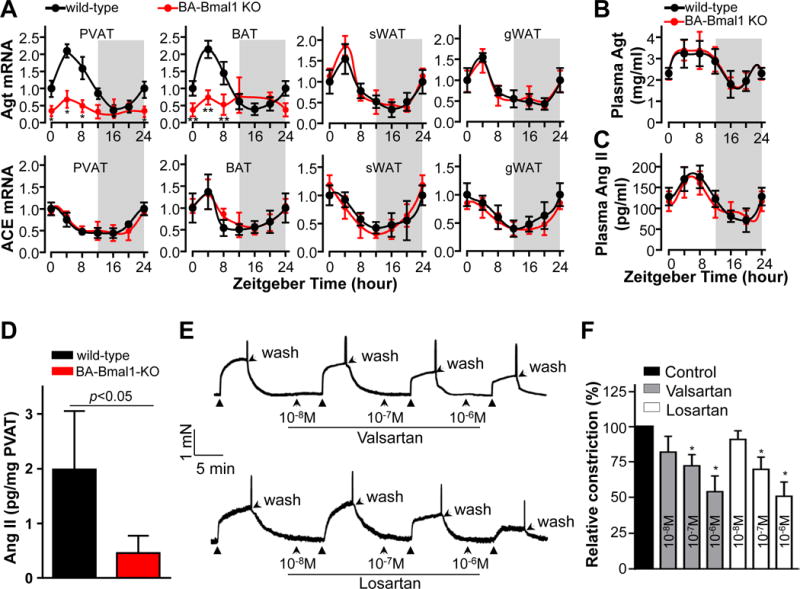
A, Realtime PCR showing the circadian change in mRNA levels (relative to β-actin) of Agt and ACE in PVAT, BAT, sWAT and gWAT collected from the indicated genotype mice every 4 hours. Zeitgebertime“0” indicates the lights-on. Data shown as mean ± SD, n=3. B & C, Plasma of wild-type and BA-Bmal1-KO mice was collected every 4 hours, and the plasma Agt and Ang II were measured by ELISA. Data shown as mean ± SD, n=6/group. D, Ang II levels (ELISA) in thoracic PVAT harvested from wild-type and BA-Bmal1-KO mice at 10AM (resting phase). Data shown as mean ± S.D. n=6. E, Representative traces showing that blocking Ang II type 1receptors (AT1R) with either valsartan (Val) or losartan (Los) at the indicated doses for 5min before stimulation with 10mg of wild-type PVAT extracts blunts the PVAT-induced constriction of wild-type aortic rings. PVAT extracts and rings were collected at 10AM. F, Quantitative data from E expressed as % of PVAT extracts alone (control) in the first trace before addition of either inhibitor. Data shown as mean ± SD. n=6. *p<0.05 vs control.
Figure 4. Agt-deficiency in PVAT impairs PVAT-induced blood vessel contraction.
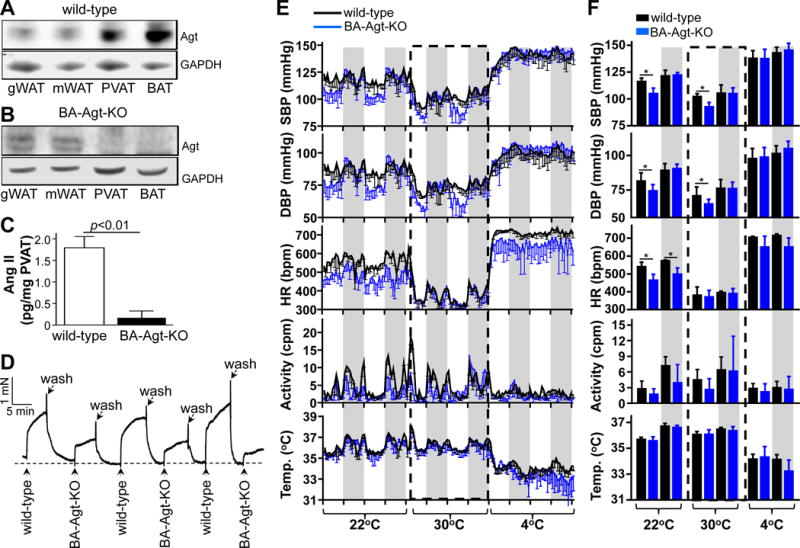
A, Representative Western blots showing Agt protein levels in adipose tissues from wild-type and B, BA-Agt-KO mice. C, Ang II levels in PVAT from wild-type and BA-Agt-KO mice at 10AM. Data shown as mean ± SD. n=6. D, Vasoconstriction of wild-type aortic rings (PVAT removed) induced by 10 mg PVAT extracts collected at 10AM from wild-type and BA-Agt-KO mice. E, Systolic, diastolic BP, heart rate, locomotor activity and body temperature of wild-type and BA-Agt-KO mice as measured by radiotelemetry when the temperature of environmental chamber was successively switched from 22°C, to 30°C and to 4°C. Data shown as mean ± SD, n=5 in each group. The gray shading represents the lights-off period from 18:00 to 6:00. F, Average of the 2 constitutive circadian cycles of systolic, diastolic BP, heart rate, locomotor activity and body temperature data during the lights-off and lights-on periods shown in panel E. Data shown as mean ± S.D, *p<0.05 vs wild-type.
Figure 5. Agt is a novel target of Bmal1 in PVAT.
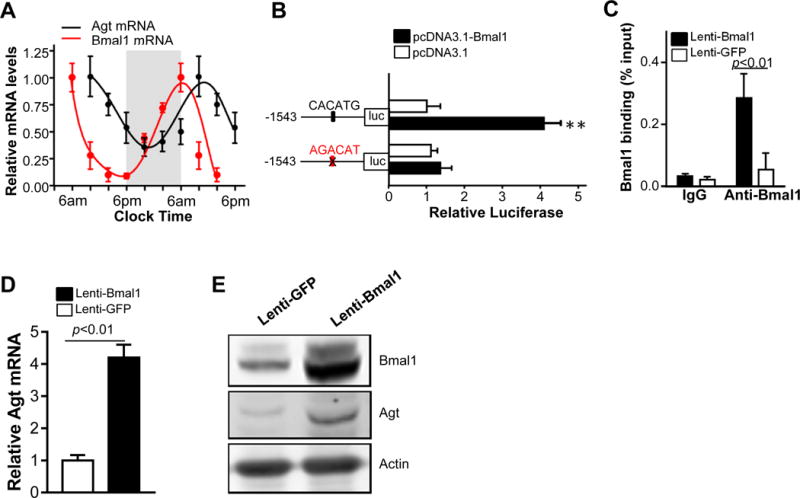
A, QRT-PCR showing the relative circadian variation of Bmal1 and Agt mRNA levels in PVAT from 8-week old male C57BL/6J mice. The Bmal1 and Agt mRNA levels relative to β-actin at 6AM were set as 1. Gray color background shows the lights-off phase. Data are shown as mean ± SEM, n=5. B, Luciferase assay using a reporter containing the putative Bmal1 CACATG E-box (−808−/−814) or a mutated site (AGACAT), as indicated in the structural cartoons on the left. pcDNA3.1 expressing mouse Bmal1 or vector pcDNA 3.1 (control) were co-transfected with the reporters in HEK293 cells. Luciferase readings were normalized by Renilla. Data are shown as mean ± SD, n=6. **p<0.01 vs. control vector. C, ChIP assay showing enrichment of Bmal1 protein on the mouse Agt promoter in brown adipocytes transfected with lenti-Bmal1compared to lenti-GFP. Isotypic IgG was used as negative control. Data are shown as mean ± SD, n=3. D, QRT-PCR showing that overexpression of Bmal1 increases Agt mRNA in differentiated brown adipocytes. Data are shown as mean ± SD, n=3. E, Western blot showing increased Agt protein level in differentiated brown adipocytes with Bmal1 overexpression.
Results
Bmal1 deficiency in brown adipocytes reduces BP during the resting phase
Expression of Bmal1 and Clock mRNA in brown PVAT shows circadian rhythmicity (Figure 1A), higher at 6AM (the onset of resting phase), gradually reduced during the lights-on time (resting phase) to their lowest level at 6PM, and progressively restored during the lights-off time (active phase) to their higher level at 6AM. Per1 and Rev-erbα mRNA show the opposite pattern. Furthermore, at pressor levels (500ng/kg/min) Ang II infusion results in acute and sustained high levels of systolic BP (SBP), albeit with loss of circadian regulation for the first 3 days followed by a progressive restoration of the dipping ability during the resting phase by day 4 (Supplemental Figure I A). Remarkably, the acute loss of circadian dipping in SBP is associated with reduced levels of Bmal1 protein in PVAT when the tissue was collected early in resting phase (Supplemental Figure IB). Subsequent recovery of circadian SBP variation was associated with a progressive restoration of Bmal1 protein levels in PVAT after day 4 of Ang II infusion. These data suggest that the peripheral clock in PVAT might contribute to physiological circadian regulation of BP with Bmal1 expression in PVAT positively associated with the dip in BP during the resting phase. This led to our hypothesis that Bmal1 and its target genes within PVAT may communicate timing information within the vessel beds to regulate BP.
Since adipocytes in mouse PVAT are brown adipocytes15, 38, we generated BA-Bmal1-KO mice to further understand the role of the PVAT peripheral clock on circadian regulation of BP. Bmal1 protein was detected in all types of adipose tissues and in the whole aorta in the wild-type mice while Bmal1 was successfully deleted in PVAT and interscapular brown adipose tissue (BAT) of the BA-Bmal1-KO mice (Figure 1B). During daytime resting phase, Bmal1 mRNA levels and its target circadian related genes such as Dbp, Per3 and Rev-erbα were reduced, while Cry1, E4bp4 and Npas2 were increased in PVAT from BA-Bmal1-KO mice (Figure 1C). As expected, loss of the regulatory circadian feedback loop was observed in the BAT as well, but not in other organs in BA-Bmal1-KO mice (Supplemental Figure II). To determine the effects of loss of Bmal1 in brown adipocytes on BP rhythmicity, we used telemetry to measure the BP in mice kept in a 12/12-hour dark/light cycle. Unlike the global Bmal1 knockout mice in which circadian rhythms of BP are abolished and the mice are hypotensive27, in a 22°C room temperature environment, BA-Bmal1-KO mice are only hypotensive during the resting phase (from 6am to 6pm) and on average normotensive during the active period (from 6pm to 6am) (Figure 1D, 1E). Bmal1-depletion in brown adipocytes results in a ~10% further reduction of BP during the resting phase (SBP 116.8±7.7 mmHg in wild-type mice, and SBP 104.4±4.5 mmHg in BA-Bmal1-KO mice) with comparable BP during the active phase (SBP 132.6±13.3 mmHg in wild-type mice, and SBP 131.4±13.5 mmHg in BA-Bmal1-KO mice), thus indicating that the BA-Bmal1-KO mice present an exacerbated hypotensive (“super-dipper”) phenotype at rest39. Interestingly, the heart rates of the BA-Bmal1-KO mice were reduced during both the resting and active phases without affecting the locomotor activity (Figure 1D, 1E). However, the cardiac function parameters during the resting phase were comparable between wild-type and BA-Bmal1-KO mice (Supplemental Figure I C).
Since heart rate can be a determinant of diurnal changes in arterial pressure in mice40, we injected 10mg/kg isoproterenol intraperitoneally to increase the heart rate of BA-Bmal1-KO mice to match that of the wild-type mice (Supplemental Figure III A). The BP of wild-type and BA-Bmal1-KO mice was immediately reduced, and restored about 1 hour after injection of isoproterenol during the resting phase. However, recovery of BP in the BA-Bmal1-KO mice was significantly lagged. When injection of isoproterenol was performed in the evening (active phase), recovery of SBP of BA-Bmal1-KO mice was slightly lagged, with comparable DBP levels between wild-type mice and BA-Bmal1-KO mice despite same levels of heart rates in both wild-type and BA-Bmal1-KO mice (Supplemental Figure III B).
Additionally, global loss of Bmal1 leads to compensatory thermogenesis in BAT41, which might be associated with BP and heart rate42. Indeed, selective deletion of Bmal1 in brown adipocytes significantly increased expression of thermogenic genes such as Ucp1, Cidea and Elovl3 in PVAT and BAT (Supplemental Figure IV A). However, the whole body metabolic indexes in BA-Bmal1-KO mice were comparable to those of wild-type when the ambient temperature was set at 22°C or 4°C (Supplemental Figure IV B) despite lower body temperature during the resting phase in BA-Bmal1-KO mice at 22°C (35.6±0.4 °C in wild-type vs 35.1±0.3°C in BA-Bmal1-KO) or 4°C (34.2±0.4 °C in wild-type vs 33.3±0.5°C in BA-Bmal1-KO) (Figure 1D and 1E). This suggests that increasing thermogenesis related genes in PVAT and BAT of BA-Bmal1-KO mice is likely a compensatory response to impaired thermogenesis in these tissues. To determine whether compensatory thermogenesis in brown adipocytes affects the resting phase dipping in BP and bradycardia in BA-Bmal1-KO mice, we further analyzed BP, heart rate and body temperature of the mice housed either in thermoneutral (30°C) or cold (4°C) environments. In a thermoneutral environment, the body temperature of wild-type and BA-Bmal1-KO mice are comparable during resting (35.8±0.2°C vs 35.7±0.3°C) and active phases (36.5±0.2°C vs 36.6±0.1°C) (Figure 1D and 1E). Interestingly, when the temperature was switched from 22°C to 30°C, the BP and heart rate of both wild-type and BA-Bmal1-KO mice significantly dropped. However, compared to wild-type mice, the BP of BA-Bmal1-KO mice was still lower during the resting phase (SBP:100±4 mmHg in wild-type vs 87±6 mmHg in BA-Bmal1-KO), while the heart rate of BA-Bmal1-KO mice was higher than that of wild-type mice (HR:338±32bmp in wild-type vs 365±33bpm in BA-Bmal1-KO). During the active phase, the BP of wild-type and BA-Bmal1-KO mice were comparable (SBP: 108±7 mmHg in wild-type, and 106±9 mmHg in BA-Bmal1-KO), while the heart rate of BA-Bmal1-KO mice was still higher than that of wild-type mice (400±35bpm in wild-type, and 429±38bpm in BA-Bmal1-KO). Next, we switched the temperature from 30°C to 4°C. Both BP and heart rate were markedly increased in all mice, accompanied with a decrease in body temperature and locomotor activity (Figure 1D and 1E). However, the heart rate of BA-Bmal1-KO mice was lower than those of wild-type mice despite comparable BP between the two groups. The steep reduction in body temperature in the BA-Bmal1-KO mice might be due to loss of compensatory thermogenesis in brown adipocytes at 4°C. These data are consistent with prior reports that thermogenesis is associated with the BP and heart rate in mice42. However, the resting phase dipper BP in the BA-Bmal1-KO mice at 22°C and 30°C is independent of thermogenesis in brown adipocytes.
Brown adipocyte-specific Bmal1 deficiency does not alter the intrinsic constriction ability of aortic rings
The constriction levels of aortic rings (PVAT removed) isolated from wild-type and BA-Bmal1-KO mice during the resting phase were comparable in response to stimulation with phenylephrine, serotonin or Ang II (Supplemental Figure VA). Additionally, endothelium-dependent and -independent relaxation was similar in aortic rings of wild-type and BA-Bmal1-KO mice (Supplemental Figure VB), indicating that the aortic rings of the BA-Bmal1-KO mice retain an intact ability to contract. Given these findings, it is likely that the hypotensive phenotype in the BA-Bmal1-KO mice is not caused by changes in the aortic intrinsic ability to constrict but rather arise from differences in factors secreted by PVAT in vivo.
Bmal1-deficient PVAT is less effective in inducing vasoconstriction
PVAT produces and releases large amounts of adipokines and other undetermined or less characterized factors that could target endothelial cells (ECs) and VSMCs, and herein contribute to the maintenance of vessel homeostasis3, 43. We assessed the vasoactivity of thoracic PVAT extracts from wild-type and BA-Bmal1-KO mice on the wild-type aortic rings in which the PVAT was removed15. PVAT extracts from 10, 20 and 30mg of adipose tissue from wild-type mice efficiently constricted recipient thoracic aortic rings harvested from 8-week old C57BL/6J mice in a dose dependent manner (Figure 2A and 2B). Additionally, we asked whether PVAT-induced constriction of vessel rings was dependent or independent of the endothelium. PVAT (10mg) extracts still significantly constricted to the same degree the aortic rings devoid of endothelium (Figure 2C), indicating that PVAT elicits this response in an endothelium-independent manner. Consistent with the hypotensive phenotype in BA-Bmal1-KO mice, PVAT extracts isolated during the resting phase from BA-Bmal1-KO mice show significantly reduced ability to constrict the wild-type rings (Figure 2D), in the same experimental conditions. PVAT isolated from the active phase has 29% higher constriction ability in the wild-type mice (Figure 2E), suggesting an intrinsic circadian association of this PVAT function with the active phase, likely from overall changes in the balance of PVCF and PVRF. Furthermore, this circadian difference is preserved in the BA-Bmal1-KO mice although there is a remarkable overall reduction in the ability of PVAT from BA-Bmal1-KO mice to constrict the wild-type rings at both time points, indicating that the Bmal1-dependent PVAT contractile dysfunction is preserved throughout the day, in spite of the BP being normal during the active phase in the knock out mice. This could be the consequence of systemic factors operating during the active phase and overriding the loss of constriction ability arising from the tissue-specific depletion of Bmal144. In that regard, it is noteworthy that sustained systemic infusion of Ang II at pressor levels abolishes the increased hypotensive phenotype, but not bradycardia during the resting phase in the BA-Bmal1-KO mice (Supplemental Figure VI A and B). In summary, Bmal1 in healthy PVAT contributes to sustain circadian regulation of BP and is required to maintain BP within physiologic levels during the homeostatic dipper phenotype associated with the resting phase.
Ang II in PVAT contributes to vasoconstriction
PVAT-derived adipokines such as adiponectin, leptin and chermerin regulate vasoactivity although our expression data suggests that their contribution may be negligible since they slightly change but in a direction opposing the observed phenotype (Supplemental Figure VII A). Additionally, the levels of PVAT norepinephrine (a vasoconstrictor45), and PVAT-derived prostacyclin (a vasorelaxant15) in BA-Bmal1-KO mice were indistinguishable from those in wild-type mice (Supplemental Figure VII B and C). These data suggest that these PVAT-derived factors do not likely contribute to the hypotensive phenotype in BA-Bmal1-KO mice. The local RAS in adipose tissues at large is one of the critical elements in the development of hypertension46–49. The majority of the local Agt in the vessel wall is located in PVAT, as opposed to the arterial muscle layers49. Although renin mRNA was undetectable in mouse PVAT (data not shown), the Agt mRNA and the Ang II converting enzyme (ACE) mRNA in adipose tissues, including PVAT, are expressed in a circadian manner (Figure 3A), higher during the resting phase and lower during the active phase. The Agt circadian variation is lost in PVAT and BAT of the BA-Bmal1-KO mice showing consistent lower levels (Figure 3A) reflected in the reduced total Ang II in the Bmal1-deficient PVAT extracts (Figure 3D). Plasma Agt and Ang II levels also showed a circadian variation (Figure 3B and 3C), although it was not affected in the BA-Bmal1-KO mice. Subcutaneous WAT is recognized as a beige adipose tissue containing some Ucp1-positive adipocytes50 and hence potentially affected by our knockout strategy. However, in this context, sWAT was more similar to gWAT in that the Agt mRNA circadian variation is comparable between wild-type and BA-Bmal1-KO mice housed at 22°C (Figure 3A). Even though the Agt mRNA in BAT of BA-Bmal1-KO mice was reduced, the plasma concentration of Agt and Ang II were comparable between wild-type and BA-Bmal1-KO mice (Figure 3B and C), indicating that brown adipocyte-derived Agt has negligible contribution to the circulating Agt and Ang II pools. Thus, the local RAS-derived components in PVAT might contribute to circadian rhythmicity of BP in a Bmal1-dependent fashion. We investigated whether RAS in PVAT is involved in vessel ring constriction induced by PVAT extracts. The recipient vessel rings that were pretreated in a dose dependent manner with type 1 Ang II receptor blockers (valsartan or losartan, 10−8M to 10−6M), showed significantly lower response to PVAT extracts from wild-type mice (Figure 3E and 3F), further supporting that local PVAT-derived Ang II may be associated with vessel constriction induced by PVAT extracts. These findings are consistent with the observed hypotensive phenotype during the resting phase and the reduced vasoconstriction ability of PVAT from theBA-Bmal1-KO mice, suggesting a direct relationship between Bmal1 and Agt/AngII expression in the peripheral clock in this tissue.
Deletion of Agt in brown adipocytes leads to hypotension during the resting phase in mice
To further establish that RAS in PVAT contributes to circadian BP regulation, we generated brown adipocyte-selective Agt knockout mice (BA-Agt-KO). Agt protein was present in all types of adipose tissues (Figure 4A) from the wild-type mice, but was undetectable in PVAT and BAT from BA-Agt-KO mice (Figure 4B). The PVAT Ang II levels in BA-Agt-KO mice were reduced more than 90% compared to wild-type PVAT (Figure 4C). Consistently, the PVAT extracts from BA-Agt-KO mice showed a significantly reduced ability to constrict wild-type vessel rings (Figure 4D), comparable to theBA-Bmal1-KOmice. The daytime resting phase BP at 22°C is significantly reduced in the BA-Agt-KO mice when compared to the wild-type mice while the nighttime active phase BP is preserved and comparable to that of the wild-type mice (Figure 4E, 4F), indicating that local deletion of Agt in PVAT recapitulates the BA-Bmal1-KO phenotype of exacerbated resting phase dipping in BP. Interestingly, and as in the BA-Bmal1-KO mice, the heart rate in the BA-Agt-KO mice are lower during both daytime and nighttime with preserved and comparable locomotor activities (Figure 4E, 4F). The response to intraperitoneal injection of isoproterenol during the resting phase parallels that of the BA-Bmal1-KO mice (Supplemental Figure VIII). Additionally, the heart rate in the mice was changed by switching the environmental temperature from 22°C to 30°C or from 30°C to 4°C (Figure 4E and F). At 30°C, the heart rates of the BA-Agt-KO mice were reduced to the same level as those of wild-type mice. However, the resting phase BP was still lower in BA-Agt-KO mice than in wild-type mice. On the other hand, 4°C environmental stimulus significantly elevated both the BP and heart rate in BA-Agt-KO mice to levels comparable of those of wild-type mice. The body temperature and locomotor activity are comparable between wild-type and BA-Agt-KO mice in all experimental conditions (Figure 4E and F). Combined, these results suggest that bradycardia of BA-Agt-KO mice in the22°C environment is unlikely to drive the super-dipper phenotype in BP. Additionally, despite the lower heart rate, the cardiac function parameters of BA-Agt-KO mice are similar to those of wild-type mice (Supplemental Figure IX A). Furthermore, the aortic rings from BA-Agt-KO mice isolated during the resting phase (10AM) and devoid from PVAT show intrinsic constriction properties comparable to those of wild-type mice when vasoactivity is induced by phenylepherine, serotonin and Ang II and are indistinguishable from wild-type rings in endothelium-dependent and -independent relaxation assays (Supplemental Figure IX B and C). This suggests that the hypotensive phenotype during the resting phase in the BA-Agt-KO mice is caused by the Agt deficiency in PVAT. Additionally, the fact that BA-Agt-KO recapitulates the BA-Bmal1-KO phenotypes is consistent with a Bmal1-Agt-AngII axis accounting for the hypotensive phenotype in the resting phase.
Agt is a novel transcriptional target of Bmal1 in PVAT
Bmal1 is expressed in PVAT in a circadian rhythmic manner. Interestingly, Agt expression in PVAT appears to follow a circadian rhythm as well, although right-shifted when compared to Bmal1 (Figure 5A), suggesting delayed expression and opening the possibility that Bmal1 may function as a transcriptional regulator for Agt. We found a putative Bmal1 binding site (CACATG box) located between −808 to −814 bp upstream of the Agt transcription start site. In a luciferase reporter assay (Figure 5B), a 1543bp region from the Agt promoter containing that putative Bmal1 E-box displayed a 4-fold increase in reporter activity relative to the control vector in response to overexpression of mouse Bmal1 in HEK293 cells while mutation of the E-box abolished this response. ChIP analysis confirmed Bmal1 protein binding to the Agt promoter region harboring the proximal CACATG motif located between −808 to −814bp upstream of the transcription start site (Figure 5C). A negative control, isotypic IgG antibody, does not pull down the promoter, demonstrating that this is a functional Bmal1-binding site in the Agt promoter. Furthermore, lentivirus-mediated overexpression of Bmal1 significantly upregulated Agt mRNA (Figure 5D) and protein (Figure 5E) levels in brown adipocytes. These data indicate that Bmal1 binds to the Agt promoter and functionally upregulates Agt expression in brown adipocytes and are consistent with the reduced Agt expression we observed in our BA-Bmal1-KO PVAT mice, thus defining Agt as a novel target of Bmal1 of physiological relevance for circadian regulation of BP in PVAT.
Discussion
Adipose tissues in the body are essential for physiological homeostasis. Obesity is positively associated with hypertension and related cardiovascular diseases. Mice lacking all adipose tissues display hypertension and insulin resistance51. Unlike WATs in visceral and subcutaneous regions, the PVAT, which surrounds large arteries, like the aorta, is a BAT-like adipose tissue functionally different from WAT3, 15. However, the nature and extent of functional relationships between PVAT and the other layers of the blood vessels are still largely unknown. PVAT is actively involved in regulation of BP by controlled release of contractile and anti-contractile factors, among a plethora of paracrine mediators ensuring vessel homeostasis3, 52, 53. However, the specific role of PVAT in regulation of BP is unclear. We showed that selective lack of PVAT enhanced atherosclerosis15, and those mice were hypotensive during the resting phase19.
The mechanisms for central circadian systems control of BP rhythmicity have been well reviewed54. However, the peripheral circadian system in blood vessels contributes to BP circadian rhythmicity as well55. Bmal1 is expressed in both ECs and VSMCs. Bmal1 in the vasculature is transcriptionally controlled by PPARγ. Knockout of PPARγ in ECs or VSMCs significantly reduced circadian variation in BP and heart rate31. Bmal1 in VSMCs regulates Rho-kinase 2 transcription, while deletion of Bmal1 abolished myosin phosphorylation, ROCK2 activation and agonist-induced vasoconstriction. Mice deficient for Bmal1 in ECs or VSMCs had compromised BP circadian rhythmicity and decreased SBP during the nighttime active phase and reduced heart rates throughout the day30,32. Ang II regulates circadian clocks in the SCN56 and VSMCs57. Here we demonstrate that components of the peripheral clock show circadian regulation in PVAT. Furthermore, the Bmal1 protein in PVAT of mice is acutely repressed by systemic Ang II infusion. Consistently, the circadian rhythmicity of BP is temporarily abolished with recovery at day 4 of Ang II infusion, when Bmal1 levels are restored, thus linking Bmal1 expression in PVAT to the circadian regulation of BP.
Selective deletion of Bmal1 in brown adipocytes in mice does not affect the intrinsic ability of the ECs and VSMCs to respond to contractile stimuli. Unlike Bmal1 deficiency in ECs or VSMCs30, 32, the BP in BA-Bmal1-KO mice is only reduced during the resting phase and impaired local RAS, evidenced by strikingly lower levels of Ang II in PVAT can account for that phenotype in this novel mouse model.
During active phase, when the BP and heart rate are simultaneously increased together with the SCN-controlled locomotor activity, BP is normal in BA-Bmal1-KO mice. Even though PVAT extracts collected from BA-Bmal1-KO and wild-type mice during the night active phase enhanced vasoconstriction differently, it is possible that during the active phase altered local PVAT-derived vasoconstrictors, such as Ang II, in the BA-Bmal1-KO mice may be overcome by sympathetic nerve mediated elevation of BP observed in vivo58, thus allowing the BA-Bmal1-KO (and BA-Agt-KO) mice present normal BP during the active phase.
Systemic deletion of Agt in adipocytes, including white adipocytes, leads to systemic reduction in Ang II and hypotension59 while our data uncovered that brown adipocyte-selective Agt deficiency is hypotensive only during the resting phase associated with reduced local Ang II production, recapitulating the BA-Bmal1-KO phenotype, without affecting systemic levels of Ang II. Furthermore, we determined that Agt, which depicts circadian expression in PVAT, is a direct transcriptional target of Bmal1 in PVAT through a functional Bmal1 E-box at −808 to −814 in the cognate Agt promoter. These data are consistent with the loss of PVAT-derived local Ang II in both BA-Bmal1-KO and BA-Agt-KO mice associated with reduced vascular tone in the resting phase, suggesting that local Ang II is one of vasoconstrictors contributing to this PVAT phenotype in a paracrine fashion.
Diurnal variations in BP are essential to cardiovascular homeostasis. Variations in the physiological drop (“dipping”) in BP during the resting phase are known risk factors for cardiovascular events60. These phenotypes are referred to as “non-dipper” (failure to downregulate BP in the resting phase), or “super-dipper” (exacerbated hypotension during the resting phase) and both are known to be strongly related to individual risk of cardiovascular morbidity and mortality39. The coordinated effects between the central clock and tissue specific peripheral clocks may account for these phenotypes, although their crosstalk is not clearly defined so far. Thus, upregulation of the RAS in rats suffering from severe hypertension results in an inverted BP profile61. It was proposed that circulating Ang II mediates this phenomenon62, 63. Central Bmal1 involvement in that model is evidenced by loss of its rhythmic expression64. In adipocytes of healthy PVAT, during the resting phase, we uncovered a circadian regulated axis defined by Bmal1 transcriptionally upregulating Agt expression to ensure physiological levels of Ang II in PVAT to sustain vascular tone during the normal dip in BP associated with the resting phase. Our brown-adipocyte specific Bmal1 and Agt knockout mice present a dipping profile consistent with a “super-dipper” phenotype during the resting phase in vivo. Super-dippers are at risk for silent myocardial ischemia and non-fatal ischemic stroke, while hypertensive patients with super-dipper BP are likely to have silent cerebral infarct and to be at high-risk for future stroke65. Whether our novel mouse models reported here will recapitulate such cardiovascular risks and adverse outcomes remains to be determined. Additionally, it is possible that a phase shift or change in amplitude in this local PVAT homeostatic control of BP during the resting phase may be associated with adverse cardiovascular outcomes in shift workers and upon jet lag66. Furthermore, the PVAT peripheral clock may be altered in the context of obesity, which is known to cause both PVAT dysfunction67, 68 and overall disruption of systemic circadian cycles69. Future studies should also address the molecular aspects of cross-communication between known drivers of systemic oscillation of BP and the synchronization of the local PVAT peripheral clock. For instance, it is likely that systemic Ang II might be tied in a regulatory loop with PVAT controlling local Bmal1 expression, phosphorylation, subcellular localization and overall activation to synchronize the PVAT peripheral clock and prevent ‘super-dipper’ or cause ‘non-dipper’ phenotypes in disease states. Additionally, and from the perspective of experimental designs to understand the nature of and balance between PVRFs and PVCFs in PVAT, our findings here underscore the need to factor in the time of day when PVAT samples are collected.
Conclusions
This study uncovered a peripheral circadian clock in PVAT that contributes to safeguard optimal vascular tone during the resting phase involving an Bmal1-Agt-AngII axis. The results of this study provide important insights regarding the potential physiological and pathophysiological roles of PVAT in circadian BP regulation and support that targeting PVAT function may prove useful in therapeutic or preventive strategies for cardiovascular disease.
Supplementary Material
Clinical Perspective.
What Is New?
This is the first demonstration of a peripheral clock in PVAT contributing to the homeostatic regulation of circadian BP variation.
This study uses novel brown adipocyte specific Bmal1- and Agt-KO mouse models to demonstrate that the PVAT peripheral clock safeguards optimal vascular tone during the resting phase.
This study uncovered local Agt and ACE circadian expression in PVAT and Bmal1-dependent transcriptional circadian regulation of Agt which controls local levels of Ang II during the resting phase.
This study found that deletion of Bmal1 or Agt in PVAT results in a super-dipper phenotype (exacerbated hypotension) during the resting phase.
What Are the Clinical Implications?
Alterations in the peripheral clock in PVAT regulating BP could contribute to the adverse clinical cardiovascular outcomes observed in shift workers or upon jet lag.
It is possible that obesity could alter the PVAT peripheral clock to promote abnormal dipper phenotypes increasing cardiovascular risk.
These findings are likely relevant to the personalized design of clinical studies, including diagnosis, biopsy collection and chronopharmacology.
The present results can inform the design of novel therapeutic approaches for hypertension by targeting the PVAT peripheral clock.
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL122664-01 (to L. Chang), HL088391 (to Y.E. Chen), HL138094 (to Y. Fan), HL138139 (to J. Zhang), American Heart Association National Scientist Development grants 15SDG24470155 (to Y. Guo) and the National Natural Science Foundation of China 81670429 (to Z.S. Jiang).
Footnotes
Disclosures
None
Affiliations
From Cardiovascular Center, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA (L.C., X.Z., Y.F., Y.G., M.G.B., J.Z., Y.E.C.). Institute of Cardiovascular Disease, Key Laboratory for Atherosclerology of Hunan Province, University of South China, Hengyang, Hunan, China (W.X., Z.J.); and Life Sciences Institute and the Department of Cell & Developmental Biology University of Michigan, USA (J.D.L.).
References
- 1.Santos CX, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR. Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role in hypertension. Antioxid Redox Signal. 2014;20:121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J. 2013;77:11–18. doi: 10.1253/circj.cj-12-1393. [DOI] [PubMed] [Google Scholar]
- 3.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 5.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, Ruiz-Gayo M, Fernandez-Alfonso MS, Somoza B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–3306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 6.Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, Su CJ. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens. 2009;31:355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- 8.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 9.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Xu A, Wang Y, Lam KS, Vanhoutte PM. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv Pharmacol. 2010;60:229–255. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]
- 11.Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous h2s formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: The role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol. 2013;33:1320–1328. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D’Alecy LG, Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Sur SH, Mistlberger RE, Morris M. Circadian blood pressure and heart rate rhythms in mice. Am J Physiol. 1999;276:R500–504. doi: 10.1152/ajpregu.1999.276.2.R500. [DOI] [PubMed] [Google Scholar]
- 22.Lemmer B. Importance of circadian rhythms for regulation of the cardiovascular system–studies in animal and man. Conf Proc IEEE Eng Med Biol Soc. 2006;1:168–170. doi: 10.1109/IEMBS.2006.260857. [DOI] [PubMed] [Google Scholar]
- 23.Schibler U. The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep. 2005;6:S9–13. doi: 10.1038/sj.embor.7400424. Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (bmal1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leu HB, Chung CM, Lin SJ, Chiang KM, Yang HC, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, Chen JH, Yin WH, Chiu TY, Chen CI, Fann CS, Chen YT, Pan WH, Chen JW. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: A genetic association with young-onset hypertension. Hypertension Res. 2015;38:155–162. doi: 10.1038/hr.2014.152. [DOI] [PubMed] [Google Scholar]
- 26.Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in cry-deficient mice lacking a biological clock. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- 29.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular ppargamma controls circadian variation in blood pressure and heart rate through bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle bmal1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–336. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: Role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 35.Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: The role of adipocyte-derived angiotensin ii. Eur J Pharmacol. 2010;634:107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, Cypess AM, Xue R, Kleiner S, Kang S, Spiegelman BM, Rosen ED. Irf4 is a key thermogenic transcriptional partner of pgc-1alpha. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin ii-induced hypertension. Circ Res. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007;8:668–680. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Kurtz TW, Lujan HL, DiCarlo SE. The 24 h pattern of arterial pressure in mice is determined mainly by heart rate-driven variation in cardiac output. Physiol Rep. 2014;2:e12223. doi: 10.14814/phy2.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Yu Q, Wang GX, Lin JD. The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis. PloS One. 2013;8:e70109. doi: 10.1371/journal.pone.0070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience. 2009;164:849–861. doi: 10.1016/j.neuroscience.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–147. doi: 10.1515/hmbci-2014-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeman M, Szantoova K, Stebelova K, Mravec B, Herichova I. Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive tgr(mren2)27 rats. J Hypertens Suppl. 2009;27:S21–26. doi: 10.1097/01.hjh.0000358833.41181.f6. [DOI] [PubMed] [Google Scholar]
- 45.Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW. Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol. 2015;309:H1904–1914. doi: 10.1152/ajpheart.00308.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faloia E, Gatti C, Camilloni MA, Mariniello B, Sardu C, Garrapa GG, Mantero F, Giacchetti G. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J Endocrinol Invest. 2002;25:309–314. doi: 10.1007/BF03344010. [DOI] [PubMed] [Google Scholar]
- 47.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 48.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger rna. Hypertension. 1988;11:591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- 50.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 51.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in a-zip/f-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez JG, O’Malley EJ, Ho WSV. Pro-contractile effects of perivascular fat in health and disease. Br J Pharmacol. 2017;174:3482–3495. doi: 10.1111/bph.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Cao ZF, Stoffel E, Cohen P. Role of perivascular adipose tissue in vascular physiology and pathology. Hypertension. 2017;69:770–777. doi: 10.1161/HYPERTENSIONAHA.116.08451. [DOI] [PubMed] [Google Scholar]
- 54.Rudic RD, Fulton DJ. Pressed for time: The circadian clock and hypertension. J Appl Physiol. 2009;107:1328–1338. doi: 10.1152/japplphysiol.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda N, Maemura K. Circadian clock and vascular disease. Hypertension Res. 2010;33:645–651. doi: 10.1038/hr.2010.68. [DOI] [PubMed] [Google Scholar]
- 56.Brown TM, McLachlan E, Piggins HD. Angiotensin ii regulates the activity of mouse suprachiasmatic nuclei neurons. Neuroscience. 2008;154:839–847. doi: 10.1016/j.neuroscience.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 57.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin ii induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 58.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 59.Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R244–251. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller JE. Circadian variation and triggering of acute coronary events. Am Heart J. 1999;137:S1–S8. doi: 10.1016/s0002-8703(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 61.Lemmer B, Mattes A, Bohm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension. 1993;22:97–101. doi: 10.1161/01.hyp.22.1.97. [DOI] [PubMed] [Google Scholar]
- 62.Langheinrich M, Lee MA, Bohm M, Pinto YM, Ganten D, Paul M. The hypertensive ren-2 transgenic rat tgr (mren2)27 in hypertension research. Characteristics and functional aspects. Am J Hypertens. 1996;9:506–512. doi: 10.1016/0895-7061(95)00400-9. [DOI] [PubMed] [Google Scholar]
- 63.Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin ii receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monosikova J, Herichova I, Mravec B, Kiss A, Zeman M. Effect of upregulated renin-angiotensin system on per2 and bmal1 gene expression in brain structures involved in blood pressure control in tgr(mren-2)27 rats. Brain Res. 2007;1180:29–38. doi: 10.1016/j.brainres.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 65.Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: Antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004;26:177–189. doi: 10.1081/ceh-120028556. [DOI] [PubMed] [Google Scholar]
- 66.Ruger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of obesity on perivascular adipose tissue vasorelaxant function: Nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res. 2015;52:299–305. doi: 10.1159/000443885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez-Santos C, Gomez-Abellan P, Madrid JA, Hernandez-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engin A. Circadian rhythms in diet-induced obesity. Adv Exp Med Biol. 2017;960:19–52. doi: 10.1007/978-3-319-48382-5_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


