Abstract
The indirect pathway striatal medium spiny projection neurons (iMSNs) are critical to motor and cognitive brain functions. These neurons express a high level of cAMP-increasing adenosine A2a receptors (A2aRs). However, the potential effects of cAMP production on iMSN spiking activity have not been established, and recording identified iMSNs in freely moving animals is challenging. Here we show that in the transgenic mice expressing cAMP-producing G protein Gs-coupled designer receptor exclusively activated by designer drug (Gs-DREADD) in iMSNs, the baseline spike firing in MSNs is normal, indicating DREADD expression does not affect the normal physiology of these neurons. Intraperitoneal (IP) injection of the DREADD agonist clozapine-N-oxide (CNO; 2.5 mg/kg) increased the spike firing in 50% of the recorded MSNs. However, CNO did not affect MSN firing in Gs-DREADD negative mice. We also found that CNO injection inhibited the spike firing of globus pallidus external segment (GPe) neurons in Gs-DREADD positive mice, further indicating CNO excitation of iMSNs. Temporally coincident with these effects on spiking firing in the indirect pathway, CNO injection selectively inhibited locomotion in D2 Gs-DREADD mice. Taken together, our results strongly suggest that cAMP production in iMSNs can increase iMSN spiking activity and cause motor inhibition, thus addressing a long-standing question about the cellular functions of the cAMP-producing A2aRs in iMSNs.
Keywords: Adenosine receptor, basal ganglia, cAMP (cyclic adenosine monophosphate), chemogenetics, dopamine receptor, Parkinson’s disease, striatal medium spiny neuron, tetrode spike recording
Graphical abstract
In this study, we used chemogenetic techniques to mimic adenosine A2a receptor activation and to increase cAMP production selectively in indirect pathway medium spiny neurons (iMSNs), while monitoring their spike firing in freely moving mice. Our results show that cAMP-producing Gs-DREADD activation by intraperitoneal injection of clozapine N-oxide excites iMSNs, causing an inhibition of globus pallidus external segment (GPe) neuron spike firing and an excitation of a subgroup of neurons in the subthalamic nucleus (STN). These effects lead to an inhibition of motor activity. Our results are consistent with iMSN activation and provide important insights about the function of the cAMP signaling mechanism in iMSNs.
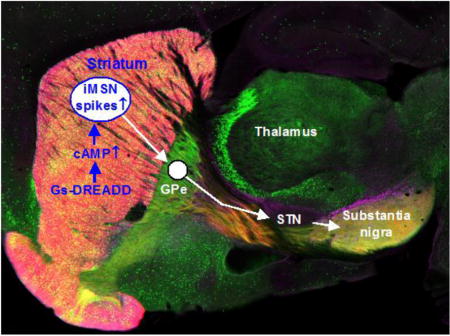
Introduction
The striatum profoundly affects motor and nonmotor behaviors as demonstrated by neurological psychiatric disorders originated in or involving the striatum (Obeso et al. 2014, Jahanshahi et al. 2015, Haber 2016, Simpson & Kellendonk 2017). Medium spiny neurons (MSNs) comprise ≥90% of the neuronal population in the striatum and serve as the output neurons for the striatum (Gerfen & Bolam 2017). In both rodents and primates including humans, one group of MSNs heavily express cAMP-increasing dopamine (DA) D1 receptors (D1Rs) and project to and inhibit the high frequency firing GABAergic neurons in the globus pallidus internal segment (GPi) and the substantia nigra pars reticulata (SNr), the output nuclei of the basal ganglia, forming the direct pathway, and these MSNs are referred to as D1-MSNs or dMSNs (Gerfen & Bolam 2017). The other group of MSNs heavily express cAMP-decreasing D2Rs and cAMP-increasing adenosine A2a receptors (A2aRs), and project to and inhibit the high frequency firing GABAergic neurons in the globus pallidus external segment (GPe), forming the indirect pathway, and these MSNs are often referred to as D2-MSNs or iMSNs (Gerfen & Bolam 2017, Schiffmann et al. 2007). The GPe GABAergic neurons project to and inhibit the autonomous firing of the glutamatergic neurons in the subthalamic nucleus (STN) that in turn excites SNr GABA projection neurons. The functional importance of iMSNs is clearly seen in Huntington’s disease in which iMSNs are preferentially lost, leading to or critically contributing to the profound motor and other behavioral symptoms (Mitchell et al. 1999, Glass et al. 2000, Walker 2007, Obeso et al. 2014).
To our knowledge, despite the heavy expression of A2aRs, the potential effects of the cAMP-increasing A2aR activation on iMSN spiking activity have not been established either in vitro brain slices or in intact and freely moving animals. The only published study in the literature reports that in a rat brain slice preparation, A2aR agonism alone had no effect on MSN membrane potential or spike firing (Azdad et al. 2009). Furthermore, spike recording of identified iMSNs in freely moving animals remains challenging because dMSNs and iMSNs intermingle with each other and are anatomically similar in the striatum, except their different projection targets; the spike waveform, amplitude and frequency of dMSNs and iMSNs are similar or identical. Consequently, there are only a few studies that recorded identified iMSNs in awake and freely moving animals, and these studies used optogenetic techniques to stimulate and identify iMSNs (Freeze et al. 2013, Lemos et al. 2016, Coffey et al. 2017), but optogenetic stimulation is invasive with the optical fiber (together with other technical difficulties). DA receptor-based pharmacological identification of dMSNs and iMSNs may not be reliable because the effects of DA activity on MSN membrane potential are mild, in the face of the stronger glutamatergic excitatory inputs from the cerebral cortex and thalamus; indeed, contradicting D1 agonistic effects and contradicting D2 agonistic effects on MSN firing have been reported (Inase et al. 1997, Kravitz et al. 2010), although good D1 and D2 receptor-based pharmacological identification of dMSNs and iMSNs has also been reported (Owesson-White et al. 2016).
To determine the potential effects of A2aR activation-induced intracellular cAMP increase on iMSN spiking activity, we have used chemogenetic or DREADD (designer receptor exclusively activated by designer drug) technology techniques to mimic A2aR activation and to increase cAMP production selectively in iMSNs while monitoring their spike firing in freely moving animals. Our results show clearly that cAMP-producing Gs-DREADD activation by intraperitoneal (IP) injection of CNO can reliably excite D2-MSNs both in the dorsal and ventral striatum providing evidence about the cellular effects of cAMP increase in iMSNs.
Materials and methodsAnimals
The chemogenetics-enabled mouse is the adenosine A2aR promotor-driven, D2-MSN or iMSN-specific, adora2A-rM3Ds-mCherry transgenic mouse created by Farrell et al. (2013). The breeder mice were purchased from The Jackson Laboratory (Bar Harbor, Maine), [B6.Cg-Tg (Adora2a-Chrm3*,-mCherry) AD6Blr/J; stock # 017863, RRID: IMSR_JAX:017863,] (Farrell et al. 2013). These mice had a normal growth, body weight and reproduction. Mice were genotyped using a PCR-based genotyping protocol and primers described by the Jackson Laboratory. The primers were the following: Transgene Forward: TCT TGT GAG GAA AGG GCT GC; Transgene Reverse: CAC ACA GTC AAT GGA GAG CCA; the transgene PCR product appears at 525 bp; Internal genomic DNA positive Control Forward: CTA GGC CAC AGA ATT GAA AGA TCT; Internal Positive Control Reverse: GTA GGT GGA AAT TCT AGC ATC ATC C; the internal genomic DNA positive control PCR product appears at 324 bp (Fig.S 1a). These mice are referred to as D2 Gs-DREADD in this paper because the DREADD is expressed in the D2R-expressing iMSNs that project to the GPe, and D1R-expressing dMSNs do not express this DREADD, clearly indicated by the mCherry reporter gene (Fig. S1b-e) (Farrell et al. 2013). Equally important, biochemical assays have determined that CNO activation of this Gs-DREADD activates adenylyl cyclase-coupled Gs/olf and consequently stimulates intracellular cAMP production (Farrell et al. 2013).
Fourteen DREADD positive male mice and seven DREADD negative control mice (weighing 25-30 g) were individually housed after microdrive-tetrode assembly implantation in a room maintained under constant temperature and humidity conditions at the University of Tennessee Health Science Center (UTHSC) animal facility with a 12:12 h light–dark cycle (light on at 7:00 AM). Food and water were available ad libitum. All experiments were carried out with the approval of the Institutional Animal Care and Use Committee at UTHSC (animal protocol # 13-056.0). This study was not pre-registered and no randomization was performed. Animals were assigned into different groups based on genotype and no blinding was performed.
Surgical procedures and microdrive-tetrode assembly implantation
All surgeries were performed under aseptic conditions, prior to each surgery the mice were anesthetized with a mixture of ketamine 100 mg/Kg (Ketathesia HCl) and xylazine 10 mg/kg, (Henry Schein, Dublin, OH) administered intraperitoneally and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). This ketamine and xylazine anesthesia is a standard rodent procedure, specified in our institutional animal protocol (UTHSC animal protocol # 13-056.0) and based on the institutional animal care and use committee guidelines (IACUC). A subcutaneous (s.c.) injection of buprenorphine 0.1 mg/kg (Buprenex®, Henry Schein, Dublin, OH) was given prior to the scalp incision, in order to minimize animal suffering. After cleaning and making the skull flat, the craniotomy was made above each desired recording site, the size of the craniotomy was large enough to accommodate the tetrodes bundle. The dura mater was carefully removed, and then the tetrode bundle was lowered slowly above the striatum (in mm relative to bregma, AP: + 1.1, L: +1.25, n=5), GPe (AP: −0.46, L: +1.75, n=3) and STN (AP: −2.06, ML: +1.5, n=3) according to the stereotaxic coordinates of the mouse brain atlas (Paxinos & Franklin 2001).
Each tetrode was home-constructed from four nickel–chromium wires (diam. 12.7μm) twisted together (Sandvik AB, Hallstahammar, Sweden). The tetrodes were placed into 3 or 4 independently movable microdrive (Fig. S2a), which were also homemade. The microdrive-tetrode assembly (Fig. S2b) was anchored to the skull surface using four cranial screws and dental cement, the ground wire was attached to one of these screws. During the surgery, the body temperature was maintained at 37° using a heating pad. At the end of surgery, a s.c. injection of sterile 0.9% NaCl (1 ml) was administered to prevent dehydration and an antibiotic cream (Water-Jel Technologies, LLC, Carlstadt, NJ, USA) was applied to the surgical site. The mice were allowed to recover during at least one-week period after the implantation surgery, during which the tetrodes were gradually lowered to the target depth (2.5 mm for striatum, 3.5 mm for GPe and 4.4 mm for STN). Tetrodes impedances were 250-350 KΩ after gold plating with a non-cyanide Au solution (Neuralynx Inc., Bozeman, MT, USA). In order to minimize animal suffering, a s.c. injection of buprenorphine 0.1 mg/kg (Buprenex®, Henry Schein, Dublin, OH) was given each 12h during the first 3 days of postsurgical recovery.
In vivo extracellular electrophysiology in freely behaving mice
Data collection
Recordings were made in a plexiglas open field cage (23 × 23 ×20 cm) placed in a shielded and sound-attenuating recording chamber, the microdrive-tetrode assembly was connected to a commutator using a lightweight headstage cable allowing the mice to behave freely (Omniplex, Plexon Inc., Dallas, TX). The mice were allowed to explore the open field cage during ~ 20 min before starting recording. Extracellular spike activity was amplified, band-pass filtered at 100–8000 Hz, and sampled at 40 kHz using an Omniplex 16-channel acquisition system (Plexon Inc, Dallas, TX, USA). Spikes were detected online by threshold crossing and further analyzed offline. Basal firing of neurons was recorded for 60 min, then CNO was injected intraperiotoneally and the recorded neurons were followed for 60 min. After each recording session, the individual tetrodes were lowered in ~ 40 μm increments to avoid repeated sampling the same neurons through sessions. We took care during the spike sorting analysis to verify that a single neuron was not mistakenly included in the analysis twice.
Data analysis
Single units were sorted offline (Offline Sorter, Plexon) using cluster separation via principal component analysis. The quality of sorting was based on cluster separation and the presence of the refractory period. Spike firing parameters were calculated using Neuroexplorer (NeuroExplorer, Nex Technologies, Littleton, MA, USA) program and waveforms extraction using Matlab (MathWorks, Inc., Natick, MA). Following established methods (Raz et al. 1996, Berke et al. 2004, Berke 2008, Barnes et al. 2005, Mallet et al. 2005, Elias et al. 2007, Hernandez et al. 2013, Kubota et al. 2009, Miller et al. 2008, Steigerwald et al. 2008, Benhamou et al. 2012, Singh et al. 2016), we used the combined properties of spike waveform, rate, autocorrelogram and interspike interval (ISI) histogram to identify and classify single unit spikes in the striatum (Fig. S3a,b), GPe and STN.
The average firing rates before and during CNO were calculated as the average frequency of discharge during the 60 min period before CNO injection and during the 60 min period after CNO injection. Neurons were considered excited or inhibited by CNO based on their firing with reference to the basal firing rate. Using a published practice (Delaville et al. 2015, Benazzouz et al. 2000), a change by more than 20% was considered a significant increase or decrease in the firing rate.
Open field test
The effect of CNO on motor behavior was evaluated in D2 Gs-DREADD positive mice receiving either CNO or vehicle (pure water) injection (n=11) and D2 Gs-DREADD negative mice receiving CNO injection (n=5) using the open field test, each mouse was individually placed in the middle of the arena (47 × 37 × 20 cm). The total locomotor activity was recorded by beams breaks of the equipped 16 infrared beams. The distance traveled was analyzed using an automated tracking system (Ethovision, Noldus Information Technology, Wageningen, the Netherlands). The distance traveled was measured during 20 min before CNO or vehicle injection and 60 min after injection.
Clozapine N-Oxide
All the mice received an IP injection of CNO-2HCl (clozapine-N-oxide dihydrochloride, product # HB6149, Hello Bio Inc, Princeton, NJ, USA) dissolved in pure water at the dose of 2.5 mg/Kg.
Histological procedures
After completion of experiment, mice were anesthetized with a mixture of ketamine/xylazine and an electrolytic lesion was applied by passing a current pulse of 30 μA for 3-4 seconds across each microwire to mark recordings sites. Twenty-four hours later, the mice were anesthetized with an overdose of ketamine/xylazine and intracardiacally perfused with phosphate-buffered saline (PBS) (Fisher Scientific, Pittsburgh, PA, USA) followed by 4% formalin (Fisher Scientific, Pittsburgh, PA, USA). Brains were removed and post-fixed overnight in 4% formalin. Coronal sections were then cut (100 μm) using a vibratome and examined on microscope. Only brains with clear tetrodes placements in the striatum, GPe and STN were used for data analysis.
Statistical analysis
An assessment of the normality of data was carried out by the Shapiro–Wilk test before the statistical tests. Statistical analyses were performed using Graph pad prism (Graph Pad Software, San Diego, CA, USA). Firing rates before and after CNO injection were compared using a paired t-test. Locomotor activity results were compared using a two-way ANOVA (treatment and time as factors) with Holm-Sidak post hoc analysis as a multiple comparison procedure. No sample calculation was performed to predetermine the sample size. No test for outliers was conducted on the data and no sample size differences between the beginning and end of the experiments. Data are presented as mean ± SEM. A p-value <0.05 was considered as significant.
RESULTS
Effect of CNO injection on the spontaneous firing of MSNs in D2 Gs-DREADD negative mice
In order to confirm that CNO’s potential effects on MSN spiking activity is specifically mediated by Gs-DREADD, we first examined the effects of IP injected CNO on MSN spiking activity in D2 Gs-DREADD negative mice (n=2). The CNO dose was 2.5 mg/kg because at this dose, CNO did not induce any overt behavioral effect in DREADD negative mice, but inhibited DREADD positive mouse locomotor activity (data presented in Fig. 7). We recorded single unit spikes in 19 MSNs in 2 freely moving DREADD negative mice. The mean firing rate of these neurons was 1.33 ± 0.17 Hz during the 1 hour before CNO injection and 1.38 ± 0.21 Hz during the 1 hour following CNO IP injection (paired t-test, p=0.53) (Fig. 1a–e and Fig. 2a,c); the paired scatter plot of before and after firing rates (Fig. 2a) and the scatter plot of firing rates after CNO normalized to the basal firing rates (Fig. 2c) show a total and homogenous lack of any CNO effect. Taken together, these results clearly indicate that CNO had no significant effect on the spike firing these MSNs neurons in DREADD negative mice.
Fig. 7.
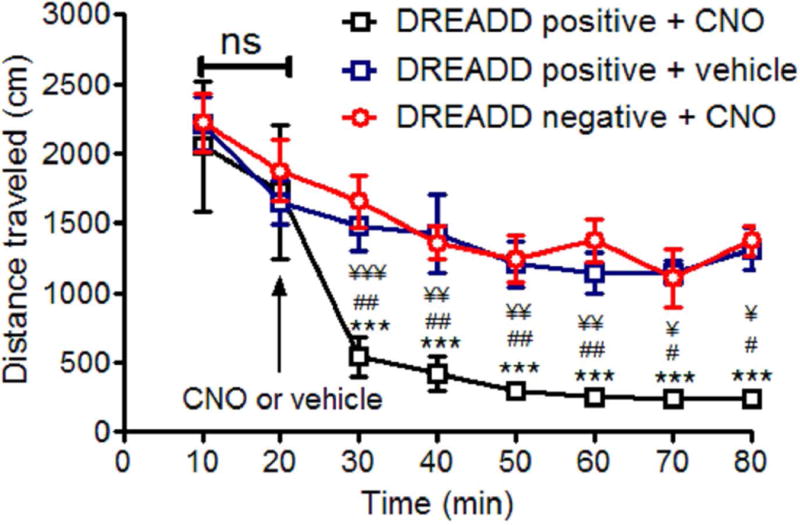
CNO inhibited locomotion in D2 Gs-DRERADD positive mice. Values are the mean ± SEM. The values of the three groups were compared using the two-way ANOVA followed by Holm-Sidak post hoc analysis as a multiple comparison procedure. ¥ ¥ ¥ p<0.001, ¥ ¥ p<0.01, ¥ p<0.05 compared with DREADD negative + CNO (n=5); ## p<0.01, # p<0.05 compared with DREADD positive + vehicle (n=5); ***p<0.001 compared with DREADD positive + CNO (n=6) at 20 min.
Fig. 1.
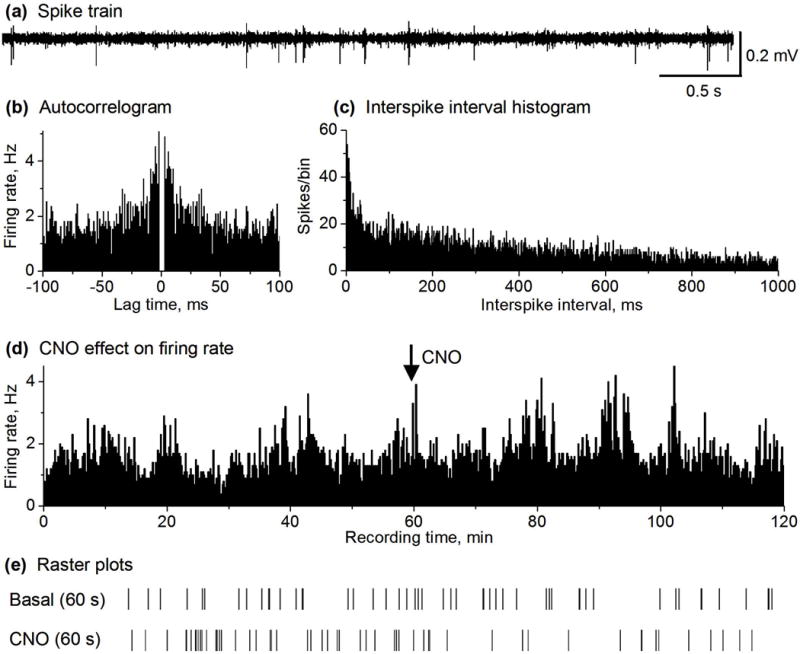
CNO did not affect MSN spike firing in D2 Gs-DREADD negative mice. (a) Representative example of spike train recorded in the striatum containing MSNs in a DREADD negative mouse. (b,c) autocorrelogram and ISI histogram (1 ms bin width) characterizing a MSN recorded in DREADD negative mouse. (d) Spike firing rate histogram before and after 2.5 mg/kg CNO injection showing no change in the MSN firing rate in DREADD negative mouse. Firing rates were calculated and plotted as the average frequency of discharge in 10-s bins. (e) Raster plots before and after CNO injection of an isolated MSN.
Fig. 2.
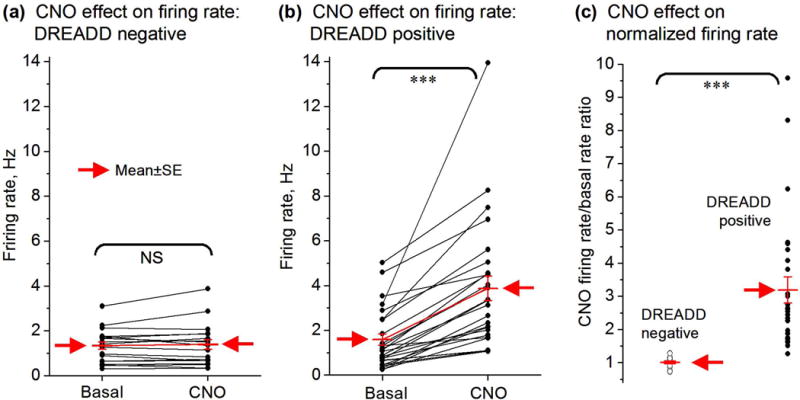
Summary data CNO effects on MSN firing rate in DREADD negative and positive mice. (a) Paired before-after scatter plot showing a total lack of 2.5 mg/kg CNO effect on the spike firing rate of all the 19 MSNs recorded in D2 Gs-DREADD negative mice (n=2). (b) Paired before-after scatter plot showing that CNO clearly increased the spike firing rate of the 27 of the 54 MSNs recorded in D2 Gs-DREADD positive mice (n=3). The other 27 MSNs showed no change and are described in Fig. S4. (c) Scatter plot of the ratio of CNO firing rate/basal firing rate (i.e. normalized firing rate) showing CNO effects on MSN firing in D2 Gs-DREADD negative and positive mice. ***, p<0.001, paired t-test.
Effect of CNO injection on the spontaneous spike firing of iMSNs in cAMP-producing D2 Gs-DREADD positive mice
Published data have established that CNO activation of Gs-DREADD stimulates cAMP production (Farrell et al. 2013), providing a method to increase intracellular cAMP. To determine the potential effects of cAMP-producing Gs-DREADD activation on MSN activity, we recorded single unit spikes in a total of 54 MSNs in freely moving D2 Gs-DREADD positive mice (n=3). In the basal condition (recorded for 1 hour), the firing rate of the 27 (out of a total 54) MSNs was 1.58 ± 0.25 Hz (Fig. 3a–e and Fig. 2b,c). In the 1 hour after IP injection of 2.5 mg/kg CNO, the firing rate of these 27 MSNs was 3.86 ± 0.54 Hz, or 144.3% above the basal firing rate (paired t-test, p<0.0001) (Fig. 3a–e and Fig. 2b,c), clearly indicating that CNO injection significantly increased the firing rate of these MSNs. Furthermore, no significant change was observed in the firing rate of the remaining 27 MSNs after CNO injection in these 3 same freely moving D2 Gs-DREADD positive mice (Fig. S4): the average firing rate was 1.82 ± 0.18 Hz during baseline and 1.87 ± 0.18 Hz (paired t-test, p= 0.18) during the 1 hour after CNO injection. Since Gs-DREADD is expressed only in D2-MSNs or iMSNs (Fig. S1; see also (Farrell et al. 2013)), these results suggest that CNO-activated MSNs were iMSNs whereas CNO-nonresponding MSNs might be dMSNs.
Fig. 3.
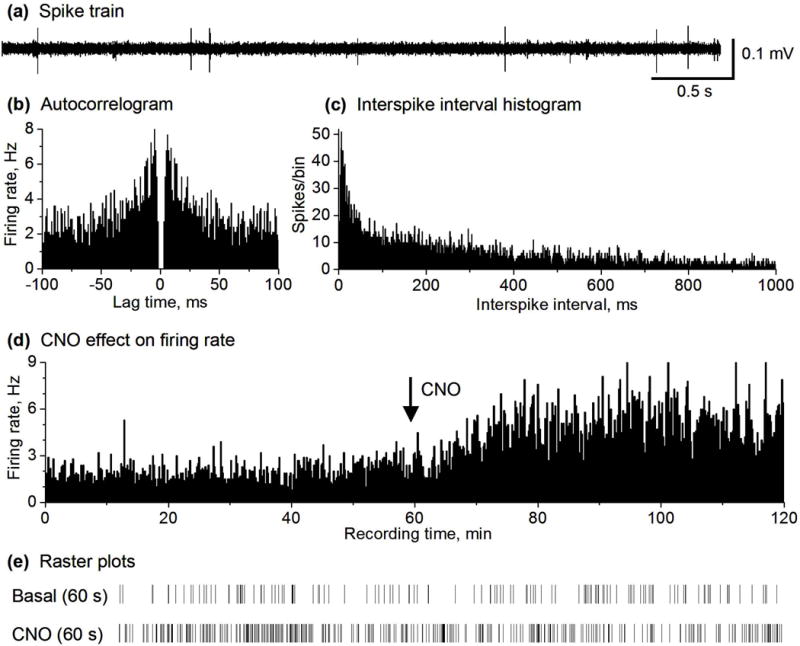
CNO increased the spike firing in 50% of the recorded MSNs in D2 Gs-DREADD positive mice. (a) Representative example of spike train recorded in the striatum containing MSNs in a DREADD positive mouse. (b,c) autocorrelogram and ISI histogram (1 ms bin width) characterizing a MSN recorded in DREADD positive mouse. (d) Spike firing rate histogram of a MSN recorded in DREADD positive mouse showing an increase in the firing rate after 2.5 mg/kg CNO injection. Firing rates were calculated and plotted as the average frequency of discharge in 10-s bins. (e) Raster plots showing the spikes in a representative 60 s before and after CNO injection of an isolated MSN. Pooled data are shown in Fig. 2b,c.
Effect of CNO injection on the spontaneous firing of GPe neurons in D2 Gs-DREADD positive mice
To confirm the reliability of our results on D2-MSNs described above, we next determined how IP injection of CNO affects the spike firing of GPe neurons. Based on the established anatomical and physiological facts that the GABAergic D2-MSNs innervate and inhibit GPe neurons (Gerfen & Bolam 2017, Wei et al. 2013, 2017), we predict that CNO activation of D2 Gs-DREADD will inhibit GPe neuron spiking firing. We recorded a total of 33 GPe neurons in D2 GS-DREADD positive mice (n=3). Before CNO injection the firing rate of 82% of GPe neurons (n=27/33) was 16.39 ± 2.46 Hz. CNO injection significantly decreased the firing rate of GPe neurons by 48% to 8.51 ± 1.61 Hz (paired t-test, p<0.0001, Fig. 4a–g). The firing rate of the remaining 6 (out of 33 GPe neurons, 18%) did not significantly change after 2.5 mg/kg CNO injection (16.69 ± 6.65 Hz versus 14.59 ± 5.43 Hz, paired t-test, p=0.17; Fig. S5 a-d).
Fig. 4.
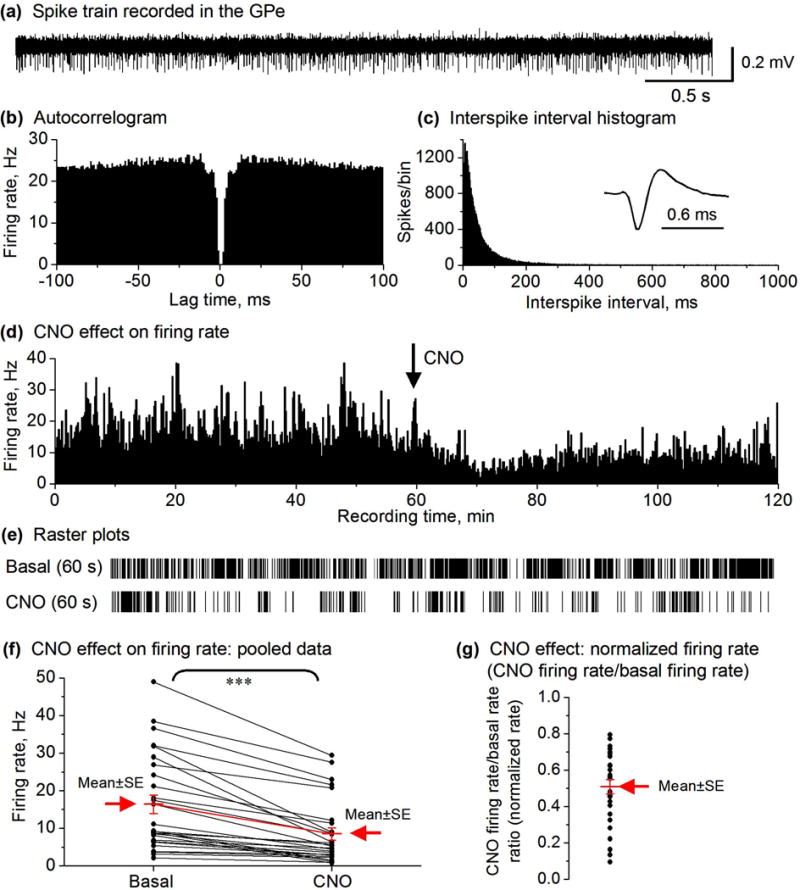
CNO IP injection decreased the spike firing in the majority of GPe neurons (27 out of total 33) in D2-MSN Gs-DREADD mice. (a) Representative example of spike train recorded in the GPe in a DREADD positive mouse. (b,c) autocorrelogram and ISI histogram (1 ms bin width) characterizing a GPe neuron recorded in DREADD positive mouse. (d) Spike firing rate histogram of a GPe neuron recorded in DREADD positive mouse showing a decrease in the firing rate after 2.5 mg/kg CNO injection. Firing rates were calculated and plotted as the average frequency of discharge in 10-s bins. (e) Raster plots showing the spikes in a representative 60 s before and after CNO injection of an isolated GPe neuron. (f) Paired before-after scatter plot showing that CNO decreased the firing rate of the 27 GPe neurons in D2 Gs-DREADD positive mice (n=3), *** p<0.001, paired t-test. (g) CNO reduced the normalized firing rate in GPe neurons. Each black dot represents the normalized firing rate of a single GPe neuron. The mean ± SE are shown in red and indicated by the arrow.
Effect of CNO injection on the spontaneous firing of STN neurons in D2 Gs-DREADD positive mice
Since GPe neurons inhibit STN neurons (Hallworth & Bevan 2005, Sano et al. 2013), we predicted that CNO-induced excitation and the consequent inhibition of GPe neurons would disinhibit STN neurons. We recorded a total of 40 STN neurons in D2 GS-DREADD positive mice (n=3). Upon CNO injection, in 42% of these STN neurons (n=17 out of 40), the firing rate increased from 5.23 ± 0.87 Hz to 11.74 ± 2.39 Hz (an increase by 124%, paired t-test, p= 0.0017; Figs. 5 and 6); 20% of STN neurons (n=8/40) showed a 27.2%, significant decrease in their firing rate (from 12.59 ± 5.30 Hz to 9.16 ± 4.35 Hz, paired t-test, p=0.01). The remaining STN neurons (n=15/40, 38%) showed no significant change in their firing rate after CNO injection (11.58 ± 3.19 Hz versus 10.82 ± 2.91 Hz, paired t-test, p=0.05) (Figs. 5 and 6).
Fig. 5.
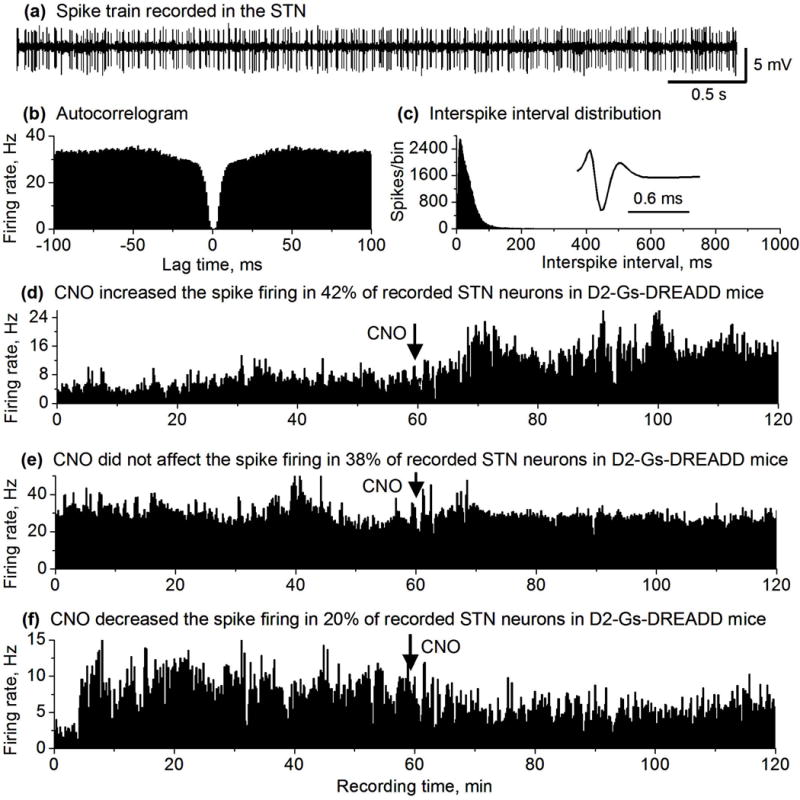
CNO effects on STN neuron spike firing in DREADD positive mice. (a) Representative example of spike train recorded in the STN in a DREADD positive mouse. (b,c) autocorrelogram and ISI histogram (1 ms bin width) characterizing an STN neuron recorded in DREADD positive mouse. (d,e,f) Spike firing rate histograms of 3 representative STN neurons recorded in DREADD positive mice showing an increase (d), no change (e) and a decrease (f) in the spike firing rate after 2.5 mg/kg CNO injection. Firing rates were calculated and plotted as the average frequency of discharge in 10-s bins.
Fig. 6.
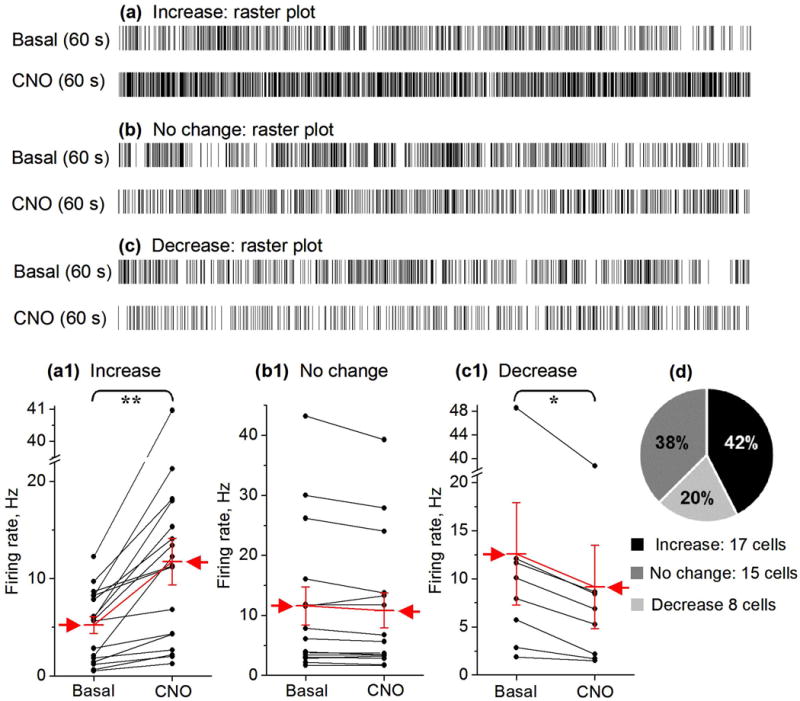
CNO effects on STN neuron firing rate in DREADD positive mice. (a, b, c) Examples of STN neuron spike response raster plots showing the spikes in a representative 60 s before and after 2.5 mg/kg CNO injection. (a1, b1, c1) Summary paired before-after scatter plots showing that CNO can increase, does not affect, or decrease the spike firing of STN neurons in DREADD positive mice (n=3). The mean ± SE is indicated by the red arrow.
** p<0.01, * p<0.05, paired t-test. (d) Circular plot representing the percentage of STN neurons showing an increase, a decrease or no change of their spike firing rate after CNO injection.
Effect of CNO on locomotion in D2 Gs-DREADD positive mice
Based on CNO’s cellular effects described in the preceding sections, we predicted that 2.5 mg/kg CNO IP injection can induce motor activity inhibition selectively in D2 Gs-DREADD positive mice. For this reason, we first monitored the locomotor activity in D2 Gs-DREADD positive mice before and after CNO or vehicle injection, and second in D2 Gs-DREADD negative mice before and after CNO injection. Before CNO or vehicle injections, the three groups traveled almost the same distance in the open filed during the first 20 min. After CNO or vehicle injections, while control mice; i.e. DREADD positive + vehicle and DREADD negative + CNO showed no significant change in the distance traveled between 20 min and 30 min (p=0.849 and p= 0.571, respectively), CNO IP injection significantly inhibited the locomotor activity, decreasing the distance traveled from 1727 ± 479.4 cm at 20 min to 541.5 ± 143.7 cm at 30 min (p<0.001, p<0.001 compared with DREADD negative +CNO at 30 min and p<0.01 compared with DREADD positive + vehicle at 30 min), (Fig. 7)). CNO inhibition of locomotor activity temporally coincided with CNO-induced D2-MSN excitation (compare with Fig. 3d).
Discussion
The main findings of this study are that in freely moving mice, non-invasive cAMP-producing chemogenetic activation can reliably excite iMSNs, triggering sustained increase in spike firing selectively in these neurons that in turn inhibits GPe neuron firing, leading to motor behavioral inhibition.
cAMP-producing Gs-DREADD activation excites iMSNs
It is established that A2aRs and dopamine D2Rs are highly expressed in iMSNs but not dMSNs, and A2aR activation stimulates adenylyl cyclase and cAMP production, whereas D2R activation inhibits adenylyl cyclase and cAMP production (Stoof & Kebabian 1981, Fuxe et al. 1998, Missale et al. 1998, Svenningsson et al. 1997, Svenningsson et al. 1998a, 1998b, Schiffmann et al. 2007, Beaulieu & Gainetdinov 2011, Herve 2011). However, to our knowledge, the potential effects of cAMP-producing A2aR activation on iMSN spiking activity have not been established either in vitro brain slices or in intact and freely moving animals. The only study (Azdad et al. 2009) that examined this question reported that co-application of an A2aR agonist and a D2R agonist increased NMDA type glutamate receptor activation-induced spike firing in mixed D1- and D2-MSNs; interpretation of these results may be confounded by the question of how A2aR activation (increasing cAMP) had no effect, but A2aR-D2R co-activation, i.e. increasing and decreasing cAMP in the same cell, increased MSN excitability.
In our present study, we used chemogenetic techniques to mimic A2aR activation and to increase cAMP production selectively in iMSNs (but not in dMSNs), while monitoring their spike firing in freely moving animals. Our results indicate that increasing cAMP is sufficient to increase iMSN excitability, thus providing important insights about the function of the cAMP signaling mechanism in iMSNs. Our results also indicate that A2aRs and D2Rs may exert their antagonistic effects on iMSN excitability by regulating cAMP production: When extracellular adenosine concentration rises, the excitability of iMSNs increases, and the excitability of iMSNs decreases when extracellular DA concentration rises, thus maintaining the excitability of these neurons at a level suitable for the behavioral requirement. This foundamental cellular neurobiological mechanism is supported by the fact that the A2aR antagonist istradefylline can improve motor function in parkinsonian patients in clinical trials (Kondo & Mizuno 2015, LeWitt et al. 2008, Mizuno et al. 2010, 2013).
In this study, we found that in 50% of the recorded MSNs in the dorsal striatum in D2 Gs-DREADD mice under a freely moving condition, IP injection of 2.5 mg/kg CNO increased the spike firing rate by ~ 144% from the baseline, and this effect coincided temporally with locomotor inhibition. The same CNO injection produced no effect on MSNs in D2 Gs negative mice (littermates of the D2 Gs-DREADD positive mice), indicating CNO effect is Gs-DREADD specific. We also found that the spike firing in 50% of the recorded MSNs in D2 Gs-DREADD mice was not affected. Since Gs-DREADD is expressed in only D2-MSNs and D2-MSNs constitute 50% of the MSNs, our results indicate that CNO excites D2-MSNs, while CNO’s lack of effect on MSNs suggest that these latter may be a D1-MSNs. We argue that this negative response-based association is reasonably reliable because of the consistency of the excitatory effect in D2-MSNs and there being only D2-MSNs and D1-MSNs; thus, if the MSN is not a D2 type, it is likely to be a D1 type. The moderate spike increase facilitates the tracking of the MSNs from baseline to CNO treatment. In contrast, a strong spike increase would complicate the tracking of the MSNs recording during baseline. Besides the consistency and moderation, the CNO-induced iMSN spiking increase is also sustained; this property facilitates testing behaviors that are commonly long-lasting.
Further, even if the recorded sustained excitation in MSNs is network-driven, this sustained excitation is in iMSNs only, because sustained network-driven excitation of dMSNs would have caused a sustained motor activation (but a sustained motor inhibition was observed), and a widespread excitation of MSNs (but a moderate excitation in only 50% of recorded MSNs was observed). Additionally, our ongoing brain slice-patch clamp experiments are showing that CNO induces prolonged, stable and sustained excitation in identified iMSNs in the absence of network excitation (with glutamate receptors being blocked). Certainly, future studies using combined intracellular recording and labeling can provide direct evidence for cell identity. The ion channel mechanism underlying CNO-induced excitation of D2-MSNs in D2 Gs DREADD mice is an ongoing project (Qian Wang and Fu-Ming Zhou) and will be published separately.
A potential problem is that CNO penetrates the blood brain barrier poorly and binds to DREADDs with a μM affinity, whereas its back metabolite clozapine enters the brain tissue well and activates DREADD at a nM high affinity (Gomez et al. 2017, Raper et al. 2017). Our data show that CNO did not affect MSN firing in DREADD negative mice and that CNO inhibited locomotion in D2 Gs-DREADD positive mice but had no detectable effect on the locomotion in D2 Gs-DREADD negative mice; these data clearly indicate that CNO at 2.5 mg/kg is fully dependent on DREADDs and hence the IP CNO-induced effects are specifically mediated by DREADD, consistent with more recent findings (Mahler & Aston-Jones 2018).
Baseline D2-MSN firing is normal in D2 Gs-DREADD mice
In this study, in freely moving D2 Gs-DREADD positive mice, the baseline spontaneous average firing rate was around 1.5 Hz for activated D2-MSNs in the dorsal striatum; in freely moving D2 Gs-DREADD negative control mice, the baseline spontaneous average firing rate was 1.3 Hz for mixed D1- and D2-MSNs in the dorsal striatum, not statistically different from the firing rate in freely moving D2 Gs-DREADD positive mice. These baseline spontaneous average firing rates are consistent with those reported in the literature; for example, Jin and Costa (2010) reported that mixed MSN average firing rate was between 1 to 4 Hz in awake mice and Kim et al. (2014) reported that mixed MSN spontaneous firing rate was near 1 Hz in awake mice. The spontaneous average baseline MSN firing rate in awake rats is also around 1 Hz (Berke et al. 2004, Berke 2008, Coffey et al. 2016, Yael et al. 2013, Kish et al. 1999). Furthermore, mixed MSN baseline firing rate was reported to be about 1.6 Hz in monkeys and about 2 Hz in humans (Singh et al. 2016). Equally important, intracellular recording in awake rats indicates that mixed MSNs have a baseline firing rate of 2 Hz (Mahon et al. 2006). Thus, our recorded D2-MSNs in both D2 Gs-DREADD negative and positive mice are representative of the commonly recorded indirect pathway MSNs (also known as iMSNs or D2-MSNs).
Effects of D2-MSN Gs-DREADD activation on downstream nuclei in the indirect pathway
D2-MSNs are known to project to and inhibit GPe neurons (Gerfen & Bolam 2017, Kita & Kita 2011a, 2011b, Wei et al. 2013, 2017). Thus, Gs-DREADD activation in D2-MSNs should inhibit GPe neuron activity. Indeed, IP CNO injection and hence Gs-DREADD activation in D2-MSNs inhibited the spontaneously spike firing of the majority (82%) of the 33 recorded GPe neurons. This inhibition of GPe neuron firing temporally coincided with the CNO-induced excitation of D2-MSNs. Since Gs-DREADD is expressed in D2-MSNs but not in GPe neurons, it is reasonable to conclude that CNO-induced GPe neuron inhibition was mediated via the excitation of D2-MSNs expressing Gs-DREADD. This inhibition of GPe neuron firing is also a reflection of a global and consistent Gs-DREADD expression in D2-MSNs (Farrell et al. 2013), the Gs-DREADD excitation of D2-MSNs (our data in this study) and the anatomical fact that multiple D2-MSNs converge on a single GPe neurons (Gerfen & Bolam 2017, Oorschot 1996, Hardman et al. 2002). Of note, 18% or 6 out of 33 recorded GPe neurons did not significantly change their firing rate after CNO injection. We speculate that these GPe neurons may receive weak striatal inputs such that their activity is not detectably affected when striatopallidal neurons are only moderately excited by CNO.
We also monitored the spike firing of STN neurons during IP injection of CNO in D2 Gs- DREADD mice. The main response we observed is that CNO IP injection led to an increase in the spontaneous spike firing in 42% of our recorded STN neurons in the freely moving D2 Gs- DREADD mice. This response is fully consistent with the established anatomical fact that GABAergic GPe neurons project to and inhibit the glutamatergic STN neurons that in turn innervate and inhibit SNr GABA neurons; thus, GPe inhibition by CNO activation of Gs- DREADD-expressing D2-MSNs leads to STN neuron disinhibition and SNr GABA neuron excitation.
Additionally, we also observed that 38% of our recorded STN neurons did not change their spike firing, and the the remaining 20% of our recorded STN neurons modestly decreased their spike firing; the underlying mechanisms for this STN inhibition are not known, but it may be mediated via effects of non-canonical connections and effects through the cortico-STN pathway, i.e. a decreased glutamatergic drive to a subpolulation of STN neurons. A lack of response in some STN neurons may reflect the fact that the CNO activation of iMSNs is of moderate intensity and iMSNs do not directly synapse onto STN neurons.
We also need to note here that our data indicate that the overall effect of a global, moderate excitation (spike frequency doubling) of D2-MSNs on STN neurons is a modest excitation of STN neurons (all neurons polled, the spike frequency increased by 20%). This indicates that the normal low frequency baseline D2-MSN spontaneous spike firing probably has (via the GPe) little effect on STN neuron firing. This is understandable because otherwise, the basal spontaneous D2-MSN firing would excite STN neurons and inhibit behavior. We speculate that D2-MSNs can effectively disinhibit STN neurons only when D2-MSNs are phasically and strongly activated and a burst of high frequency spikes upon receiving cortical glutamatergic commands (Kravitz et al. 2010, Kita & Kita 2011b, Sano et al. 2013).
Conclusions
First, our data suggest that cAMP production can increase iMSN spike firing that can lead to behavioral inhibition (Fig. S6), addressing a long-standing question related to the neurophysiological functions of the intracellular cAMP. This conclusion is particularly important because iMSNs express high levels of cAMP-increasing A2aRs and cAMP-decreasing D2Rs, and may indicate a fundalmental mechanism that may be employed in other cell types such as dMSNs that express high levels of cAMP-increasing D1Rs. Second, our data strongly suggest that iMSNs can be reliably excited by IP injection of CNO in D2 Gs-DREADD transgenic mice.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01NS097671. Safa Bouabid was a recipient of the University of Tennessee Neuroscience Institute FY2017 postdoctoral fellowship. The authors also thank Shuhua Qi and Yuhan Wang for their technical help.
Abbreviations
- A2aR
adenosine A2a receptor
- ANOVA
analysis of variance
- Bp
base pair
- cAMP
cyclic adenosine monophosphate
- CNO
clozapine-N-oxide
- DA
dopamine
- D1R
dopamine D1 type receptor
- D2R
dopamine D2 type receptor
- DREADD
designer receptor exclusively activated by designer drug
- FSI
fast-spiking interneurons
- GABA
gamma amino butyric acid
- GPe
globus pallidus external segment
- GPi
globus pallidus internal segment
- Gs
GαS protein
- MSN
medium spiny neuron
- dMSN
direct pathway medium spiny neuron
- D1-MSN
dopamine D1 receptor-expressing MSN, same as dMSN
- iMSN
indirect pathway medium spiny neuron
- D2-MSN
dopamine D2 receptor-expressing MSN, same as iMSN
- ISI
interspike interval
- IP
intraperitoneal
- sc
subcutaneous
- NMDA
n-methyl-d-aspartate
- PCR
polymerase chain reaction
- RT
reticular thalamus
- RRID
Research Resource Identifier
- STN
subthalamic nucleus
- SNr
substantia nigra pars reticulata
- TAN
tonically active neurons
- ZI
zona incerta
Footnotes
DR. SAFA BOUABID (Orcid ID: 0000-0003-4184-3795)
PROF. FU-MING ZHOU (Orcid ID: 0000-0001-8313-3770)
Involves human subjects:
If yes: Informed consent & ethics approval achieved:
=> if yes, please ensure that the info “Informed consent was achieved for all subjects, and the experiments were approved by the local ethics committee.” is included in the Methods.
ARRIVE guidelines have been followed:
Yes
=> if No or if it is a Review or Editorial, skip complete sentence => if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.” unless it is a Review or Editorial
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
Open Science Badges
No, I am not interested to achieve Open Science Badge(s) => if yes, please see Comments from the Journal for further information => if no, no information need to be included in the manuscript
References
- Azdad K, Gall D, Woods AS, Ledent C, Ferre S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Benhamou L, Bronfeld M, Bar-Gad I, Cohen D. Globus Pallidus external segment neuron classification in freely moving rats: a comparison to primates. PloS one. 2012;7:e45421. doi: 10.1371/journal.pone.0045421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10075–10080. doi: 10.1523/JNEUROSCI.2192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Nader M, Bawa J, West MO. Homogeneous processing in the striatal direct and indirect pathways: single body part sensitive type IIb neurons may express either dopamine receptor D1 or D2. The European journal of neuroscience. 2017;46:2380–2391. doi: 10.1111/ejn.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Nader M, West MO. Single body parts are processed by individual neurons in the mouse dorsolateral striatum. Brain research. 2016;1636:200–207. doi: 10.1016/j.brainres.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaville C, McCoy AJ, Gerber CM, Cruz AV, Walters JR. Subthalamic nucleus activity in the awake hemiparkinsonian rat: relationships with motor and cognitive networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:6918–6930. doi: 10.1523/JNEUROSCI.0587-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2525–2538. doi: 10.1523/JNEUROSCI.4156-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, et al. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain research. Brain research reviews. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Bolam JP. The neuroanatomical organization of the basal ganglia. In: Steiner H, Tseng KY, editors. Handbook of Basal Ganglia Structure and Function. Vol. 24. Academic Press; 2017. pp. 3–30. [Google Scholar]
- Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science (New York, NY) 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. Corticostriatal circuitry. Dialogues in clinical neuroscience. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth NE, Bevan MD. Globus pallidus neurons dynamically regulate the activity pattern of subthalamic nucleus neurons through the frequency-dependent activation of postsynaptic GABAA and GABAB receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:6304–6315. doi: 10.1523/JNEUROSCI.0450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. The Journal of comparative neurology. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- Hernandez LF, Kubota Y, Hu D, Howe MW, Lemaire N, Graybiel AM. Selective effects of dopamine depletion and L-DOPA therapy on learning-related firing dynamics of striatal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:4782–4795. doi: 10.1523/JNEUROSCI.3746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Frontiers in neuroanatomy. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inase M, Li BM, Tanji J. Dopaminergic modulation of neuronal activity in the monkey putamen through D1 and D2 receptors during a delayed Go/Nogo task. Experimental brain research. 1997;117:207–218. doi: 10.1007/s002210050217. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nature reviews. Neuroscience. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Barter JW, Sukharnikova T, Yin HH. Striatal firing rate reflects head movement velocity. The European journal of neuroscience. 2014;40:3481–3490. doi: 10.1111/ejn.12722. [DOI] [PubMed] [Google Scholar]
- Kish LJ, Palmer MR, Gerhardt GA. Multiple single-unit recordings in the striatum of freely moving animals: effects of apomorphine and D-amphetamine in normal and unilateral 6-hydroxydopamine-lesioned rats. Brain research. 1999;833:58–70. doi: 10.1016/s0006-8993(99)01496-1. [DOI] [PubMed] [Google Scholar]
- Kita H, Kita T. Role of Striatum in the Pause and Burst Generation in the Globus Pallidus of 6-OHDA-Treated Rats. Frontiers in systems neuroscience. 2011a;5:42. doi: 10.3389/fnsys.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kita T. Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011b;31:10311–10322. doi: 10.1523/JNEUROSCI.0915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Mizuno Y. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clinical neuropharmacology. 2015;38:41–46. doi: 10.1097/WNF.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Liu J, Hu D, DeCoteau WE, Eden UT, Smith AC, Graybiel AM. Stable encoding of task structure coexists with flexible coding of task events in sensorimotor striatum. Journal of neurophysiology. 2009;102:2142–2160. doi: 10.1152/jn.00522.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, Alvarez VA. Enhanced GABA Transmission Drives Bradykinesia Following Loss of Dopamine D2 Receptor Signaling. Neuron. 2016;90:824–838. doi: 10.1016/j.neuron.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Annals of neurology. 2008;63:295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones G. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2018 doi: 10.1038/npp.2017.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington’s disease. Journal of neurophysiology. 2008;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Progress in neurobiology. 1999;59:691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Movement disorders: official journal of the Movement Disorder Society. 2010;25:1437–1443. doi: 10.1002/mds.23107. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2013;28:1138–1141. doi: 10.1002/mds.25418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet (London, England) 2014;384:523–531. doi: 10.1016/S0140-6736(13)62418-6. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. The Journal of comparative neurology. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Owesson-White C, Belle AM, Herr NR, Peele JL, Gowrishankar P, Carelli RM, Wightman RM. Cue-Evoked Dopamine Release Rapidly Modulates D2 Neurons in the Nucleus Accumbens During Motivated Behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:6011–6021. doi: 10.1523/JNEUROSCI.0393-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Vol. 1. Academic Press; 2001. [Google Scholar]
- Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A. Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS chemical neuroscience. 2017;8:1570–1576. doi: 10.1021/acschemneuro.7b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. Journal of neurophysiology. 1996;76:2083–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- Sano H, Chiken S, Hikida T, Kobayashi K, Nambu A. Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:7583–7594. doi: 10.1523/JNEUROSCI.4932-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Progress in neurobiology. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C. Insights About Striatal Circuit Function and Schizophrenia From a Mouse Model of Dopamine D2 Receptor Upregulation. Biological psychiatry. 2017;81:21–30. doi: 10.1016/j.biopsych.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Mewes K, Gross RE, DeLong MR, Obeso JA, Papa SM. Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:9629–9634. doi: 10.1073/pnas.1606792113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald F, Potter M, Herzog J, Pinsker M, Kopper F, Mehdorn H, Deuschl G, Volkmann J. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. Journal of neurophysiology. 2008;100:2515–2524. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Aubert I, Burbaud P, Fredholm BB, Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. The Journal of comparative neurology. 1998a;399:229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998b;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet (London, England) 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wei W, Ding S, Zhou FM. Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum. Journal of neurophysiology. 2017;117:987–999. doi: 10.1152/jn.00683.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Li L, Yu G, Ding S, Li C, Zhou FM. Supersensitive presynaptic dopamine D2 receptor inhibition of the striatopallidal projection in nigrostriatal dopamine-deficient mice. Journal of neurophysiology. 2013;110:2203–2216. doi: 10.1152/jn.00161.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yael D, Zeef DH, Sand D, Moran A, Katz DB, Cohen D, Temel Y, Bar-Gad I. Haloperidol-induced changes in neuronal activity in the striatum of the freely moving rat. Frontiers in systems neuroscience. 2013;7:110. doi: 10.3389/fnsys.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


