Abstract
Vocal folds are connective tissues housed in the larynx, which can be subjected to various injuries and traumatic stimuli that lead to aberrant tissue structural alterations and fibrotic-induced biomechanical stiffening observed in patients with voice disorders. Much effort has been devoted to generate soft biomaterials that are injectable directly to sites of injury. To date, materials applied toward these applications have been largely focused on natural extracellular matrix (ECM)-derived materials such as collagen, fibrin or HA; these approaches have suffered from the fact that materials are not sufficiently robust mechanically nor offer sufficient flexibility to modulate material properties for targeted injection. We have recently developed multiple resilin-inspired elastomeric hydrogels that possess similar mechanical properties as those reported for vocal fold tissues, and that also show promising in vitro cytocompatibility and in vivo biocompatibility. Here we report studies that test the delivery of resilin-based hydrogels via injection to the subcutaneous tissue in a wild-type mice model; histological and genetic expression outcomes were monitored. The rapid kinetics of crosslinking enabled facile injection and ensured the rapid transition of the viscous resilin precursor solution to a solid-like hydrogel in the subcutaneous space in vivo; the materials exhibited storage shear moduli in the range of 1000~2000Pa when characterized via oscillatory rheology. Histological staining and gene expression profiles suggested minimal inflammatory profiles three weeks after injection, thereby demonstrating the potential suitability for site-specific in vivo injection of these elastomeric materials.
Keywords: resilin, injectable hydrogels, viscoelastic properties, biocompatibility, vocal folds, tissue engineering
INTRODUCTION
Vocal fold scarring resulting from voice abuse or surgery causes significant changes in the biological environment and viscoelastic mechanics of vocal fold tissues, and consequently hampers the self-sustained oscillations of the vocal folds that are essential to voice production.(1),(2) Impaired voice production holds significant implications for individual health and wellness, social and occupational function, and societal productivity with up to 9% of Americans affected yearly.(2)–(4) Treatment of voice disorders or scarring, which can be caused by extracellular matrix (ECM) injury or loss, has been limited due to the fact that current surgical operations fail to adequately address the ECM biomechanical properties; in addition, most currently employed biomaterials are unable to mimic the complex ECM compositions or fulfill the stringent mechanical requirements of native vocal fold tissues.(5)–(7)
Regenerative medicine-based biomimetic tissue engineering strategies that focus on the function of biomaterials to actively stimulate tissue healing and regeneration may be beneficial in treating vocal fold scarring and other ECM-based defects of the lamina propria, particularly for the superficial and intermediate layers.(8) Hydrogel injection, in particular, is an applicable tissue engineering approach predominately reported for potential treatment of vocal fold scars.(9)–(11) Delivery of the hydrogel by injection minimizes the degree of mucosal invasion compared to surgical implantation and scar lysis procedures. This procedure can be performed as an in-office treatment with local anesthetic, which increases the accessibility of surgical treatments to the vocal fold lamina propria for a greater number of patients while mitigating the risk associated with general anesthesia. Furthermore, in situ hydrogel cross-linking allows polymer solutions to conform to the complex vocal fold dimensions upon delivery and then to form solid hydrogels in vivo with improved adherence to the surrounding tissue. Local surface micro-roughness can further secure the hydrogel to strengthen the interface native tissue, minimizing biomechanical interruption during subsequent vocal vibration.
Previous investigations using injectable materials for vocal fold augmentation and repair have relied largely on chemically crosslinked hyaluronic acid (HA)-based materials due to the fact that HA is a major component of the ECM of the superficial lamina propria (SLP) and because of its contribution in modulating the viscoelastic properties of vocal fold tissue.(9),(12) Clinical investigations of vocal fold treatments that employ HA-based biomaterials have yielded overall improvements in glottal closure and phonation quality when injected into the thyroarytenoid muscle; unfortunately a HA product specifically for the lamina propria treatment is not yet available. Although the mechanical properties of biomaterials can be easily tailored to match those required for vocal fold tissue engineering, there is limited information available regarding transient mechanical properties upon application and release of strain and limited ability, to date, to independently control mechanical features and biological functions. Thus, novel injectable biomaterials that maintain mechanical integrity during vibration under high frequencies, exhibit dynamic mechanical response, and show superior elastomeric features present valuable alternatives in material selection, particularly for materials whose composition and biological function can be tuned.
The protein resilin offers unique and unprecedented advantages in engineering new elastomeric and highly mechanically active biomaterials that can be tailored for vocal fold tissue repair.(8),(13)–(16) Resilin, an insect structural protein, exhibits rubber-like elasticity featured by low storage modulus, reversible extensibility with superior resilience, and efficient energy storage and release, all of which support its critical roles in insect actions such as flight and jumping.(17)–(19) Natural resilin also exhibits an amazing life-long fatigue resistance, in select cases surviving up to 400 million repeated contraction/extension cycles at frequencies ranging from 200 to 4000Hz.(20)–(22) Recently, multiple versions of recombinant resilins based upon consensus repetitive amino acid sequences derived either from Drosophila melanogaster (GGRPSDSYGAPGGGN) or the mosquito analogue Anopheles gambiae (AQTPSSQYGAP) have been developed,(14),(15),(23),(24) thus opening routes to the fabrication of biologically active and mechanically robust hydrogels with desired high-frequency responsiveness.
Given the potential utility of resilin-based materials in biomedical applications, there have been reports of resilin-like polypeptides (RLPs) that have been engineered with peptide mimics of BMP-2 (bone morphogenetic protein 2) to accelerate osteogenic differentiation for bone tissue engineering(24) and with a VEGF (vascular endothelial growth factor)-mimetic QK peptide to promote endothelial differentiation of human mesenchymal stem cells (MSC) for vascular tissue engineering.(25) Resilin-like polypeptides have also been designed to incorporate other repeating sequences to synthesize alternating diblock, triblock or multi-block elastomeric proteins where resilin domains were intercepted by other domains to simulate the passive elasticity mechanical properties of muscle protein titin,(23) induce assembly,(26)–(28) as well as capture the biophysical and biochemical characteristics of native ECM microenvironments.(29) More recently, resilin has been combined with other natural materials or synthetic polymers to fabricate biocomposites with enhanced material complexity that encompass a wide spectrum of physically and chemically controllable properties such as pH- and thermo-responsiveness(30),(31) and liquid-liquid phase separation and formation of microstructured domains.(32),(33)
Our previous success in generating RLPs has demonstrated that these polypeptides can be easily and quickly cross-linked (<10 mins) to form solid hydrogels via multiple chemical methods(32),(34),(35) that afford tunable elastic shear and Young’s moduli. The storage moduli of the RLP hydrogels are comparable to those measured for vocal fold tissues (1–20kPa (G’), 10–50kPa (E’)), with excellent extensibility (up to 300%) and superior resilience (>95%), indicating their flexibility and utility to efficiently transmit dynamic mechanical forces.(34)–(37) The similarities in properties between recombinant resilin and the vocal fold tissue (soft, low modulus, high toughness, high resilience and similar mechanical moduli at elevated frequencies (1000–2000Pa))(34),(37),(38) suggest that RLPs hold considerable promise for capturing the mechanical properties of the vocal fold tissues, and we and others have demonstrated their flexibility for modulating biological functions, cellular interactions and growth factor sequestration and release.(25),(29),(35),(39) These design handles, largely absent in other materials applied for these applications,(35) suggest exciting new avenues for treating vocal fold disorders.(8),(16) Indeed, initial subcutaneous implantation of preformed RLP hydrogels in a rat model resulted in minimal acute inflammation.(39) In this study, we further evaluate the feasibility of in vivo injection of chemically crosslinked RLP-based hydrogels and investigate the subsequent biocompatibility of these hydrogels in the subcutaneous tissues in a wild-type mice model. Histological outcomes were used to quantify any inflammatory responses, while gene expression analysis was employed to monitor ECM production and oscillatory rheology used to confirm the mechanical properties of the materials employed during injection.
MATERIALS AND METHODS
Materials
Chemically competent E. coli strain M15-[pREP4] cells (for recombinant DNA plasmid transformation) and Ni-NTA agarose resin (for polypeptide purification) were purchased from Qiagen (Valencia, CA). Amine-functionalized 4-arm PEG (20kDa) was purchased from Creative PEG Works (Winston Salem, NC). The trifunctional cross-linker tris(hydroxymethyl phosphine) (THP) was purchased from Strem Chemicals (Newburyport, MA). Phosphate-buffered saline (PBS) was purchased from Mediatect (Manassas, VA). High-capacity endotoxin removal resin was purchased from Pierce Biotechnology (Rockford, IL). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Waltham, MA) and were used as received unless otherwise noted. Water was deionized and filtered through a NANOpure Diamond water purification system (Dubuque, IO).
RLP expression and purification
The RLP construct was cloned, transformed, expressed and purified (via Ni-NTA affinity chromatography) as described in our previously reported protocols.(34),(35) Approximate 20–30mg of each RLP construct per liter of cell culture was obtained after dialysis at 4°C and lyophilization. Resilin-like polypeptides were further purified using a high-capacity endotoxin removal column (Pierce Biotechnology, Rockford IL) to minimize the endotoxin content in protein samples, which was followed by a final purification step via preparative HPLC on a Waters Symmetry C18 column under a linear gradient from 95:5 to 5:95 of water/acetonitrile (containing 0.1% of trifluoroacetic acid) at 5mL/min over 65 min. Collected RLP elution fractions were directly frozen and lyophilized before using for subcutaneous injection experiments in vivo.
Oscillatory shear rheological characterization of RLP hydrogels
Bulk oscillatory shear rheology experiments of RLP-based hydrogels were conducted at 37°C on a stress-controlled rheometer (AR-G2), TA Instruments, (New Castle, DE) with a 20 mm diameter cone-on-plate geometry, 1 degree cone angle and at a 25µm gap distance. Dynamic oscillatory time, frequency and strain sweeps were performed. Two amounts (4mg and 6mg) of RLP were dissolved in degassed, pH 7.4 PBS at a final concentration of 100 and 150mg/mL respectively. Stock solutions of both the RLP and the cross-linker THP (100mg/mL) were chilled on ice before mixing in order to slow the reaction speed, preventing cross-linking during handling. 1.3µL and 1.9µL THP stock solution was added to the 38.7µL and 38.1µL RLP stock solutions at a final solution volume of 40µL to yield 10wt% and 15wt% gels with a 1:1 cross-linking ratio that is indicated as the molar ratio of lysine residues to reactive hydroxymethylphosphine (HMP) groups (or a 3:1 ratio of lysines per THP). The 1:1 crosslinking ratio (Lys:HMP) was chosen based on our previous success in generating robust and rapidly crosslinked hydrogels under these conditions. In order to achieve rapid crosslinking and hydrogel formation in vivo, we maintained the 1:1 crosslinking ratio and varied the overall polypeptide concentration to study the impact of various THP concentrations on hydrogel performance in vivo (e.g., 10wt% and 15wt% RLP hydrogels). To ensure homogeneous mixing, the mixture was vortexed gently for 5 seconds after the addition of the THP, and then followed by careful pipetting onto the bottom plate of the rheometer for in situ rheological characterization. Strain sweep measurements were performed on samples from 0.01% strain to a maximum strain of 1000% to determine the limit of the linear viscoelastic regime (LVE). Rheological properties were examined by time sweep (t=0~120mins, ω=6.28rad/s) and frequency sweep experiments (ω = 0.1–100 rad/s) in the LVE at fixed strain amplitude of 1%. Experiments were repeated on 3 samples and representative data are presented.
In vivo subcutaneous injection of soluble components and hydrogel materials
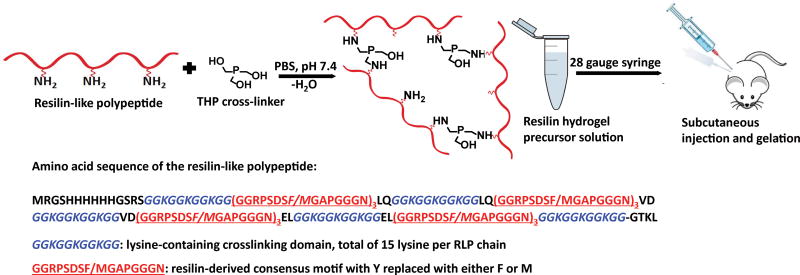
Injection of soluble components include RLP solutions at various concentrations (0.5wt%, 1wt%, 2.5wt%, 5wt%, 7.5wt% to 10wt%), 10wt% PEG solution, and solutions of the cross-linker THP (150µL, 0.42mg and 300µL, 0.84mg); each injection volume was 150µL or 300µL in PBS at room temperature. For injections of RLP hydrogels, 5µL or 7.5µL THP cross-linker (stock concentration at 100mg/mL) was added to 10wt% or 15wt% RLP solution (final polypeptide weight percentage, respectively) with the pH adjusted to 8 with 12.5µL or 18.75µL of 1M NaOH to reach a final injection hydrogel precursor solution of 150µL. For RLP+PEG solutions, 4µL THP cross-linker (stock concentration at 100mg/mL) was added to 10wt% RLP+PEG 50/50 (w/v) solution (final material weight percentage) with pH adjusted to 8 by addition of 6µL 1M NaOH to reach a final injection volume of the hydrogel precursor solution of 150µL. For PEG hydrogel injection, 15mg of 20kDa, 4-arm, amine-functionalized PEG was dissolved in pH 7.4 PBS followed by the addition of 2µL THP cross-linker (stock concentration at 100mg/mL) to achieve the final injection hydrogel precursor solution 150µL. The well-vortexed mixture was then transferred to 25-gauge, 1mL syringes and maintained at least for 10mins at room temperature prior to subcutaneous dorsal injection (one injection per mouse) of the wild-type BALB/C male mice.
Surgical procedures
A total of 123 BALB/C male mice were anesthetized with isoflurane (2% to 3% delivered at 0.8 to 1.5L/min). Dorsal injections (150µL or 300µL) were then made with 28-gauge needles subcutaneously above the tail. At day 5, a total of 62 mice were euthanized; the remaining mice (61) were euthanized at day 21. To euthanize, mice were placed in a non-precharged chamber and 100% CO2 was introduced at a rate of 10–20% of the chamber volume per minute, as regulated by the flowmeter attached to the CO2 canister. Death was confirmed by verifying cardiac and respiratory arrest. The backs of the mice were shaved over the injection site and tissue was harvested for histological and transcription expression. All animal experiments used in this study were performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison.
Histological analysis
Subcutaneous tissues extracted from 86 mice were processed for histological analysis. Tissues were fixed in 10% neutral buffered formalin overnight, dehydrated via an ethyl alcohol series, and embedded in paraffin. Five-micron sections were cut and stained with hematoxylin and eosin (H&E) for histological analysis. Representative high magnification images for each condition by day were captured with an Olympus DP25 digital camera mounted on an Olympus BX41 microscope and were scored by a blinded pathologist for evaluation of the biocompatibility of the injected hydrogels. Lower magnification H&E images for all material compositions are included in Supplementary Information.
RNA extraction and reverse transcription
Thirty-seven mice samples were used for transcript expression analysis. Tissues were excised and immersed in RNAlater and frozen at −80°C until processed. Total cellular RNA was isolated from subcutaneous tissues using an RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. A Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington DE) was used to determine RNA yield and purity. Gene expression was quantitated using the QuantiGene® 2.0 Plex Assay with magnetic plate washer (Panomics/Affymetrix Inc., Fremont, CA, USA) following manufacturer’s instructions for purified RNA. Four hundred nanogram RNA input samples were prepared in triplicate and randomly organized in two 96-well plates. Negative water controls were included in both plates. Genes measured included interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), transforming growth factor beta (TGF-β) and polymerase 2a. Samples first were lysed with 80µL lysis reagent followed by overnight incubation (54°C) with fluorescent microspheres (Luminex beads) and Oligonucleotide probe sets manufactured for hybridization of target genes. Samples were then incubated with DNA amplification molecules for signal amplification and washed in wash buffer according to manufacturer instructions. Luminescence signals were read using MAGPIX® Luminex flow cytometer instrument at recommended settings. Median fluorescence intensity (MFI) from Luminex beads correspond to the signal captured for each target gene. Bead counts of at least 50 were used for analysis. Data was presented in the form of MFI normalized to Polymerase 2a and averaged for each sample type. All MFI over 20,000 were considered nonlinear and excluded from analysis. MFI below the lower limit of quantitation for Polymerase 2a and TGF-β and below the limit of detection for IL-6 and TNF-α were also excluded from analysis.
Statistical analysis
Gene expression levels were compared among 7 groups: RLP+PEG hydrogel-treated (10wt%), PEG hydrogel-treated (10wt%), RLP solution-treated (different polypeptide concentrations), THP-treated (100µL and 300µL injection), saline-treated samples and no treatment groups. RLP hydrogel-treated groups were not included due to unsolved chronic inflammation at day 21 post injection and such material compositions will not be considered for future investigations. The transcript gene expression results were analyzed using two-way ANOVA with p values<0.05 were considered statistically significant.
RESULTS
Oscillatory rheological mechanical characterization of resilin hydrogels formation
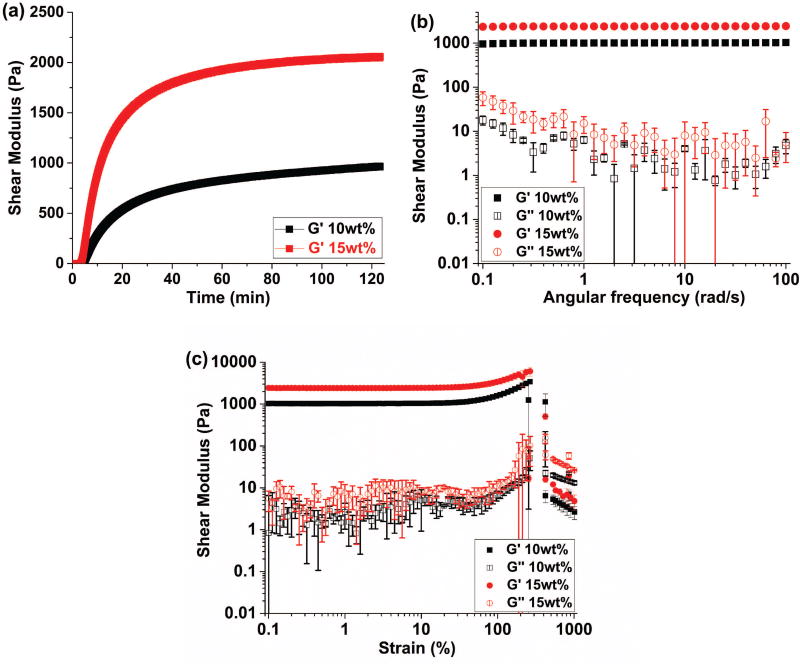
In situ oscillatory rheology results indicate the rapid increase of the shear storage modulus (G’) for both 10wt% and 15wt% RLP hydrogels within 10 mins (Fig. 1a) upon addition of THP crosslinker to resilin precursor solutions, confirming that the Mannich-type condensation reaction mediated rapid hydrogel formation. The storage moduli continued to increase over time and reached a plateau moduli around 1000Pa and 2000Pa for 10wt% and 15wt% RLP hydrogels, respectively. The stability of these hydrogels were further confirmed via frequency sweep measurements, where storage moduli (G’) were 100–200 fold greater than loss moduli (G”); the stability of the shear storage moduli over the full frequency range of the experiment (0.1–100 rad/s) (Fig. 1b) suggested the elastic-solid-like behavior of the covalently crosslinked RLP hydrogels as we have previously observed.(35),(36) Strain sweep measurements (Fig. 1c) of these chemically crosslinked hydrogels demonstrated high strain-to-break ratios with average values of 265% and 245% for 10wt% and 15wt% RLP hydrogels respectively, confirming the elasticity and extensibility of the RLP hydrogels employed in the in vivo studies. The RLP-PEG hydrogel composition was included as a potential candidate for in vivo vocal fold injection based upon our recent success in generating such biocomposite materials with intrinsic phase-separation behavior that is capable of promoting cell spreading and migration in 3D.(32) The bulk mechanical properties of THP-crosslinked RLP-PEG hydrogels has been characterized; the materials exhibit a shear storage modulus of approximately 1000Pa,(33) which is within the range of those for the RLP-based hydrogels reported here.
Figure 1.
Histology of in vivo injection of THP-crosslinked RLP hydrogels
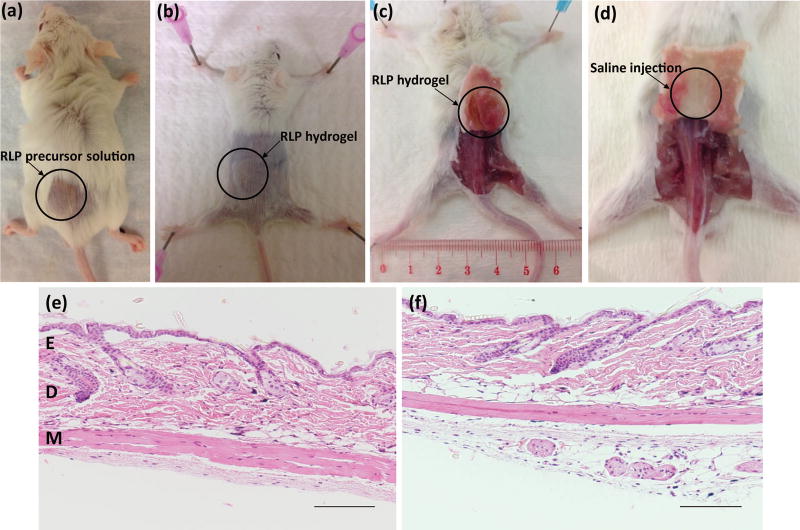
Standard wild-type BALB/C male mice were chosen for initial in vivo subcutaneous injection studies of the RLP hydrogels in order to test the biocompatibility of materials before moving to higher-level animal models (Scheme 1). Both 10wt% and 15wt% RLP-based solutions were delivered to the subcutaneous injection site through a 28 gauge needle (Fig. 2a) and formed stable solid hydrogels in vivo upon mixing with appropriate amount of the cross-linker THP. After sacrifice, backs of the mice were shaved over the injection site in order to better identify the positions of injected hydrogels (Fig. 2b) and tissue was harvested for histological assessment. RLP hydrogels were detectable in subcutaneous tissue after sacrifice, confirmed by the existence of solid material at subcutaneous tissue compared to the observed void space of the saline injections in the control mice samples (Fig. 2c–2d).
Scheme 1.
Figure 2.
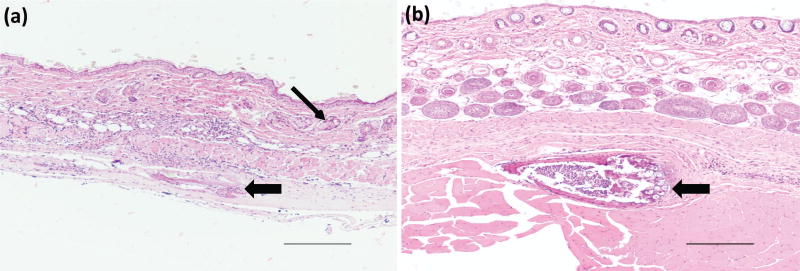
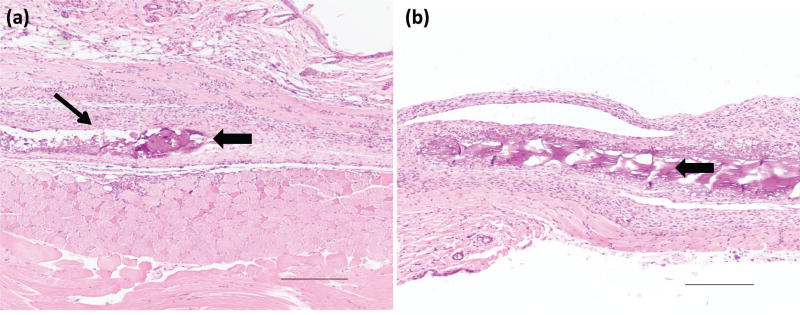
At days 5 and 21, H&E results for non-injection control mice (Fig. 2e) and saline injection (Fig. 2f) showed minimal infiltration of inflammatory cells in the epidermis, dermis, or deep muscle layers; tissues harvested from these samples were judged to be histologically normal. For THP-crosslinked RLP hydrogels, at day 5, in a 10wt% hydrogel (Fig. S1), focal parakeratosis and necrosis were identified in the epidermis, with acute inflammation extending below the muscle layer; in 15wt% gel (Fig. 3a and Fig. S2a) inflammatory cells were also observed in the tissue around the area of the hydrogels. The epidermis was thinned over the area of the hydrogel and adipocytes formed within the dermis. Acute inflammation and early wound repair characterized by neutrophils and fibroblasts were present, extending to the deep dermis and subcutaneous tissue. Inflammatory cells persisted at day 21, for both RLP hydrogels, in the dermis (Fig. 3b and Fig. S2b) there was increased collagen deposition. In some tissues, necrotic scabs formed around the hydrogels with formation of new epidermis noted under the area of the scab. The tissue surrounding the 10wt% RLP hydrogel contained an increase in the number of hair follicles in subcutaneous tissue, indicative of wound-healing.
Figure 3.
Histology of in vivo injection of THP-crosslinked RLP-PEG hydrogels
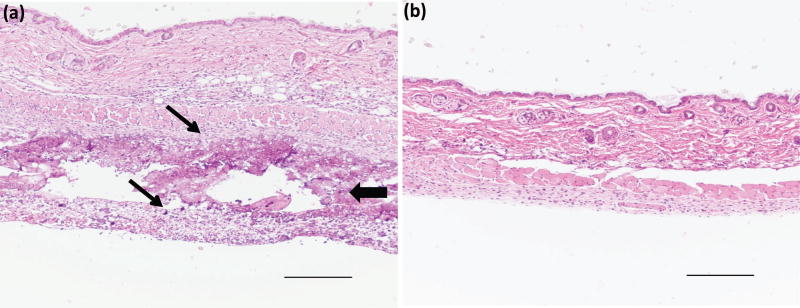
For the hydrogel samples that comprised both RLP and PEG, inflammation through the epidermis, dermis and subcutaneous tissues was observed at day 5 (Fig. 4a and Fig. S3a) indicated by a band of infiltrated macrophages and neutrophils in the dermis. Inflammation observed at day 5 was mostly resolved by day 21 (Fig. 4b and Fig. S3b), however, some evidence of acute and chronic inflammation persisted in the dermis characterized by neutrophils and lymphocytes surrounding the remaining RLP-PEG hydrogel.
Figure 4.
Histology of in vivo injection of THP-crosslinked PEG hydrogels
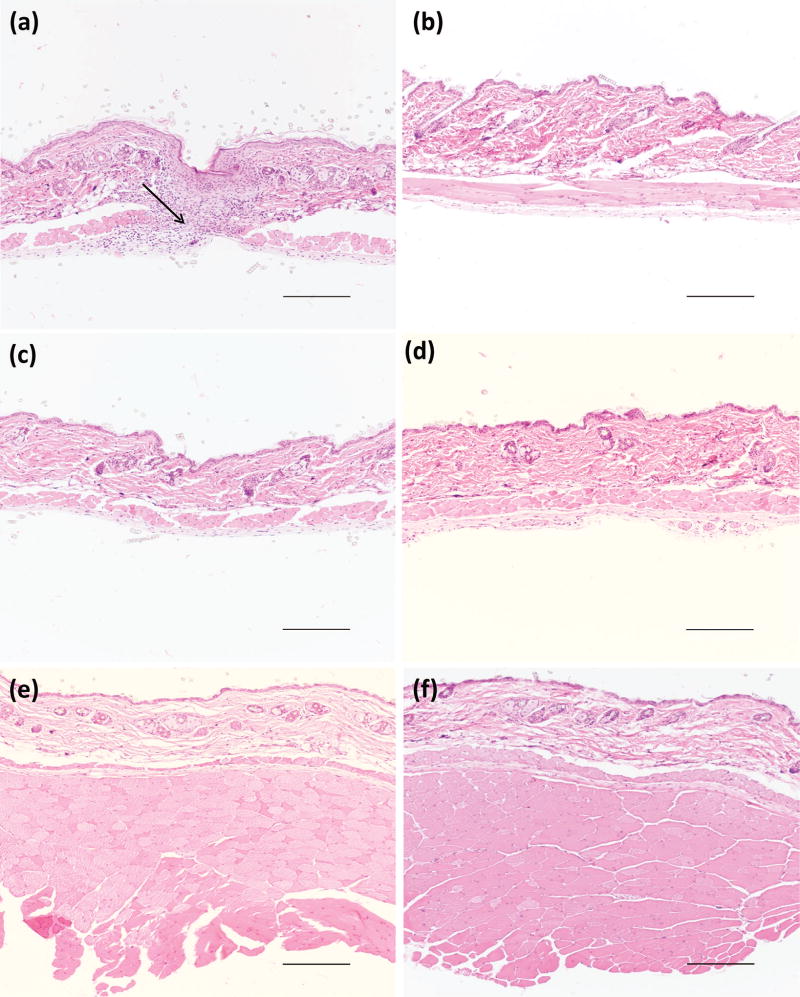
The epidermis and dermis appeared mostly intact in the tissues surrounding the PEG-only hydrogels. Acute inflammation was present between and extended below the muscle layers with a predominance of macrophages and fibroblasts with some residual foreign materials being noted at day 5 (Fig. 5a and Fig. S4a). The inflammatory response observed in the subcutaneous tissue at day 5 was mostly resolved by day 21 (Fig. 5b and Fig. S4b); the tissue appears to be histologically normal without observation of residual foreign material.
Figure 5.
Histology of in vivo injection of RLP and PEG solutions at various concentrations
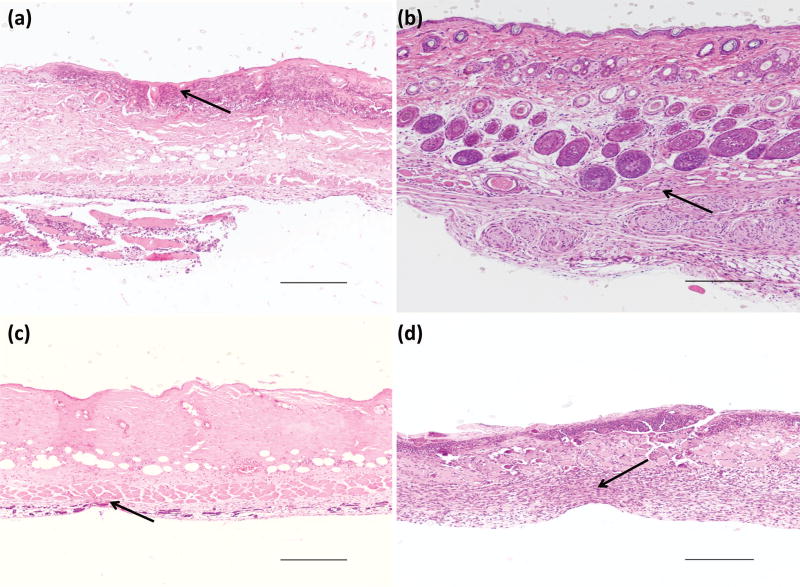
Soluble RLP solution injections were completed with various protein concentrations, ranging from 0.5wt%, 1wt%, 2.5wt%, 5wt%, 7.5wt% to 10wt% (Fig. 6, Fig. S5 and S6) in order to determine the critical RLP concentration threshold with respect to inflammatory induction, only representative images are included. The epidermis, dermis and muscle layers appeared intact for any of the above concentrations, and no visual observation of skin ulceration or scab formation for both days 5 and 21 was noted. For 0.5, 1, 2.5 (Fig. 6a and 6b) and 5wt% RLP (Fig. 6c and 6d) solution, there were minimal inflammatory cells in the epidermis, dermis or muscle at day 5 and day 21, and these tissues appeared histologically normal compared to saline injections. For 7.5 and 10wt% RLP solutions (Fig. 6e and 6f), there was mild chronic inflammation characterized by the infiltration of macrophages between the muscle layers at day 5; scant evidence of the mild chronic inflammation persisted in the subcutaneous tissue by day 21. Soluble PEG solution at 10wt% with an injection volume of 150µl was also conducted. A localized area of inflammation in the epidermis was found and was indicative of this skin sample being from the injection site. Overall the tissue appeared to be histologically normal at both days 5 and 21 time points (images not included).
Figure 6.
Histology of in vivo injection of THP cross-linker solutions
In vivo injection of soluble cross-linker THP was characterized at two different injection volumes: 150µl THP (Fig. 7a and 7b, Fig. S7a and S7b) (0.42mg, 2.8mg/mL in PBS, the amount of THP needed to crosslink 10wt% 15mg RLP for a 150µl total hydrogel volume) and 300µl THP (Fig. 7c and 7d, Fig. S7c and S7d) (0.84mg, 2.8mg/mL in PBS, the amount of THP needed to crosslink 10wt% 30mg RLP for 300µl total hydrogel volume). At day 5, ulceration of the epidermis was evident in both cases with surrounding acanthosis and hyperkeratosis. The inflammatory response was characterized by the infiltration of both neutrophils and lymphocytes in epidermis and dermis. Necrotic tissue extended to the muscle layer with focal dystrophic calcification being noted within degenerating muscle fibers. At day 21, in the case of 150µl THP injection, ulceration was mostly resolved, chronic inflammation was characterized by fibroblasts/macrophages throughout the epidermis; for 300µl THP injections, the epidermis was still ulcerated with acanthosis and hyperkeratosis around the perimeter of the ulcer and a dense mixed inflammatory infiltration with necrosis and calcification being present, extending down to the deep dermis and subcutaneous muscle layer with possible residue scarring surrounding the injection site being characterized by loss of hair follicles.
Figure 7.
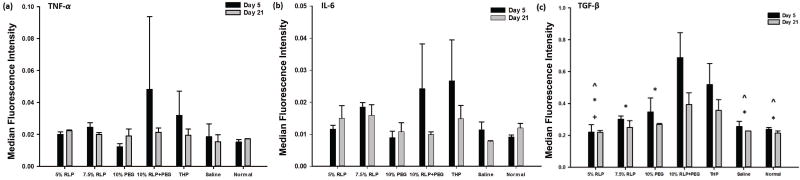
Transcript expression
Gene expression for mice whose subcutaneous tissues were injected with different materials were compared across treatment groups at two different time points (days 5 and 21). Relative expression levels of TNF-α, IL-6 and TGF-β were plotted and are shown in Fig. 8. There are no significant differences among treatment groups for TNF-α (Fig. 8a, p=0.4809) or IL-6 (Fig. 8b, p=0.0838). There are no significant differences in mean MFI between day 5 and day 21 for each group for TNF-α (p=0.3927) or IL-6 (p=0.1388). There are no interaction effects in mean MFI between days and groups for TNF-α (p=0.6545), IL-6 (p=0.1506) or TGF-β (p=0.0701). Although we do not have definitive information on the source of higher variability of expression level of TNF-α and IL-6 (compare to other formulations), it is possible that there were differences in the injections and/or the THP concentrations in these samples that might contribute to this variability particularly in the markers of inflammation. Gene expression levels of TGF-β exhibited significant differences among days (p<0.0088) and hydrogel groups (p<0.0002, Fig. 8c). At day 5 there was significantly greater expression of TGF-β (Fig. 8c, p=0.0088) than at day 21 for all groups. The 10wt% RLP+PEG hydrogel and THP injection groups showed significantly higher level of TGF-β expression than 5wt% RLP, saline and no injection at both day 5 and 21 (5wt% RLP, 7.5wt% RLP, 10wt% PEG, saline and no injection). Significant differences between 5wt% RLP and 10% PEG were measured for each time point and lastly 7.5% RLP and 10% PEG were significantly less than 10% RLP+PEG but not THP.
Figure 8.
DISCUSSION
Vocal fold scarring is characterized by induced vocal fibrosis and increased tissue viscosity and stiffness.(40)–(42) The concomitant effects of these pathophysiological changes directly alter patient vocal quality as well as create detrimental dysphonia due to the loss of normal vibratory functions.(1) Therefore, current surgical treatments such as lysis/excision and microflaps are not ideal as these techniques are likely to disrupt native ECM biomechanics of healthy surrounding VF tissues and cause secondary injuries leading to scar formation during surgical intervention.(2),(5),(43) In recent years, regenerative medicine strategies have introduced injectable hydrogels with tailored material functionalities to repair vocal fold injuries, a methodology with great applicability as it can minimize the invasiveness of the procedure and overcome aforementioned limitations of traditional surgical techniques.(12) Motivated by the recent success of fabricating synthetic resilin-based nanoparticles,(44) fibers,(26),(45) microstructures,(32),(33) films and hydrogels(14),(16),(23),(46) with tunable biofunctionality, we have demonstrated the advantages of combining the mechanical properties of natural resilin with independent control of biological features to engineer elastomeric and degradable hydrogels that promote cell adhesion, spreading and proliferation. Additional promising results indicate that the RLPs are well-tolerated in vitro and in vivo; minimal inflammatory responses are elicited by surgical implantation of preformed RLP hydrogels.(39) In this study, we aim to evaluate protocols for in vivo injection of chemically crosslinked RLP-hydrogels. We investigate their biocompatibility in the subcutaneous tissue using a murine model via assessment of histological outcomes and quantification of corresponding expression of inflammatory markers and vocal fold ECM.
The zero-length tri-functional THP cross-linker was employed to react with lysine residues from RLP to form solid hydrogels, due to its robust chemical reaction over a wide range of solution conditions (e.g., temperature, pH, ionic strength).(34),(47) The THP-based Mannich-type condensation reaction facilitated rapid gelation, which allows for facile in vivo injection. Rheological characterization of RLP hydrogel crosslinking indicated the formation of soft but mechanically stable hydrogels with tunable shear moduli between 1000 and 2000Pa via modulation of polypeptide concentrations; the resulting shear moduli are within the bulk range of those measured on porcine and human vocal fold tissues.(37) Such rapid in situ gelation kinetics validate the feasibility and applicability of RLP hydrogel formation in vivo, which is confirmed in the present studies by the maintenance of solid RLP-based materials in the subcutaneous tissues of mice (Fig. 2a–d).
Upon histological evaluation, it was observed that injections of 10wt% and 15wt% RLP hydrogels (Fig. 3 and Fig. S1) resulted in typical foreign body acute inflammatory responses at day 5 with chronic inflammation at day 21 observed from H&E staining. 10wt% RLP+PEG (Fig. 4) and 10wt% PEG (Fig. 5) hydrogels also showed acute inflammation, at the injection sites. However, these early inflammatory responses observed at day 5 were regularly resolved by day 21. The recovery of the tissue at the later time points is consistent with examples from Shim et al, in which the biocompatibility of pH- and temperature-sensitive block copolymer PEG hydrogels was evaluated following dorsal subcutaneous injection of rats. It was found that after one and two weeks, inflammation was present surrounding the hydrogels; the injected areas began to show recovery following injection of the gel with decreased inflammation and subsequent proliferation of new tissue 4 weeks post injection.(48)
The observed differences in inflammatory response between injections of RLP-only hydrogels and RLP+PEG or PEG only gels are likely due to the variation in the amount of THP in the samples. In addition, we have also observed that the gelation kinetics are consistently faster in the case of forming RLP+PEG and PEG hydrogels compared to RLP-only hydrogels, which is likely to yield a lower amount of un-reacted THP cross-linkers in these compositions, another possible explanation why RLP+PEG and PEG gels are better tolerated in vivo. While the inclusion of PEG as a matrix component may modulate the overall in vivo biocompatibility of the RLP+PEG material, the main impact is likely to the significant reduction in the amount of THP required for hydrogel formation of RLP+PEG hydrogels compared to the amount needed to crosslink RLP-only hydrogels. The poor histological outcomes for the THP solution injections (Fig. 7) clearly indicated the incompatibility of this crosslinker for subcutaneous injection in these mice models. The larger quantity of toxic THP required in crosslinking RLP hydrogels amplified the early acute inflammatory response and developed into unresolved chronic inflammation which was observed at day 21.(49) This chronic inflammation was further confirmed by gene expression analysis of tissue that received soluble THP injections at the same concentration and same volume as that of RLP-only hydrogel injections. This observation pinpoints deleterious effects of soluble THP and THP-crosslinked biomaterials for this application, and highlights the RLP+PEG material as a better potential candidate for future vocal fold engineering. The RLP+PEG hydrogel effectively combines the biocompatibility and chemical versatility of PEG with intrinsic elastomeric mechanical features derived from native resilin protein, which carries the potential for enhancing in vivo outcomes when incorporated into vocal fold (currently under investigation).
In contrast to injections of crosslinked hydrogels, all injections of RLP and PEG solutions (Fig. 6, Fig. S5 and S6) at various material concentrations showed minimal or no inflammatory reactions at days 5 and 21, which confirmed the biocompatibility of the soluble RLP and PEG. These differences between hydrogel and material solution injections are almost certainly due to the presence of THP; any THP that is present at the hydrogel-tissue interface or THP that remains unreacted during hydrogel formation in vivo is capable of diffusing out of the hydrogels and could react with amines in proteins in the surrounding tissue and thus cause chronic inflammation.(49) In the presence of injected RLP-only hydrogels (without any biological motifs included or cells encapsulated), minimized tissue remodeling encourages the persistence of such non-attenuated inflammation and potentially leads to chronic inflammation characterized by the formation of fibrotic or necrotic tissues. In validation of this hypothesis, H&E images confirmed that injection of solutions of the THP cross-linker induced the most severe acute inflammation with extended chronic inflammation, characterized by persistent ulceration in the epidermis and dermis layers as well as necrosis and calcification in the deep muscle layer at day 21. Studies on elastin-like polypeptides (ELPs) first reported that a THP cross-linker could be used for in vitro procedures to maintain viability of mouse fibroblast cells in 3D culture.(47) Similarly, Li et al. also employed THP cross-linkers to form solid, elastomeric RLP hydrogels that were able to encapsulate hMSCs and maintain cell viability over two weeks in culture.(35) However, in both of these examples, cell encapsulated hydrogels were washed vigorously immediately after cross-linking, a process not feasible for in vivo injection. Kim et al., in contrast, suggested that resilin crosslinked with THP resulted in significantly fewer live cells in 2D cytotoxicity assays as compared to transglutaminase-based enzymatic cross-linking, indicating that THP-based Mannich-type condensation reaction may be toxic to cells.(49) Recent advances in studying in vivo performance of biomaterials identified crucial chemical modifications and material geometry features (e.g., size and shape) that can substantially reduce the inflammatory response and enhance their performance in rodents and non-human primates.(50)–(52) Such knowledge on bulk material properties will guide our future material design and choice of hydrogel crosslinking chemistries to modulate in vivo mechanical stability, surface roughness and protein adsorption. Additionally, multiple biological domains (e.g., cell-adhesive, matrix degradation and growth factor sequestration) and relevant cell types (e.g., human vocal fold fibroblasts and hMSCs) will be readily incorporated in RLP hydrogels in order to promote the integration of injected cell-gel matrix and native tissue towards tissue remodeling for applications in vocal fold tissue engineering.
Pro-inflammatory cytokines, a family of important mediators that regulate cross-talk among multiple cell types, play pivotal roles in directing various physiological and pathophysiological processes and thus are highly involved in the early stages of wound healing, inflammatory reactions and foreign body responses.(53)–(55) Off-balance and/or dysregulated acute and chronic phases of inflammation mediated by these cytokines can cause abnormal wound healing and damage host tissue. To further evaluate the in vivo response of injected RLP components, we thus chose to examine transcript expression of inflammatory markers such as IL-6, TNF-α and TGF-β as these signaling molecules not only are known to be associated with inflammatory response to foreign materials but also are involved in playing significant roles during the healing process.(12),(56),(57) TNF-α and IL-6 are both pro-inflammatory cytokines predominately secreted by invading neutrophils, monocytes/macrophages and T-lymphocytes after injury. These cells play important roles in mediating the progression of inflammatory events, fibroblast recruitment for fibrotic collagen deposition and foreign body reaction at the site of implantation/injection of biomaterials.(56),(58),(59) Previous work clarified that these cytokines were involved in early stages of wound-healing and inflammation in a mouse skin model with IL-6-deficient BALB/c knockout mice exhibiting significant decrease in gene expression level of multiple key molecules (TNF-α and TGF-β) and reduction of wound area characterized with delayed attenuated leukocyte infiltration, re-epithelialization and angiogenesis compared to wide type (WT) mice.(59) Similarly, subcutaneous implantation of silk-based hydrogels demonstrated up-regulation of IL-6 at early time-point followed by a down-regulation after 3 weeks in a mouse model.(60)
TGF-β, on the other hand, is a multifunctional, stimuli modulatory growth factor involved in the promotion of cell proliferation, recruitment, differentiation, migration and ECM accumulation and deposition. Up-regulation of TGF-β plays an important role in regulating tissue inflammation and regeneration after injury, which is also essential for signaling during the proliferation stage of wound-healing.(61)–(63) Rousseau and colleagues, for example, reported a bimodal distribution of TGF-β mRNA expression level with a prominent peak by day 7 but stable expression at day 28 using an injured male Sprague-Dawley rat vocal fold model.(61) In our studies, despite the differences observed between samples in the histological analysis, there were no significant differences in expression of IL-6 and TNF-α between any of the soluble or hydrogel injections (Fig. 8a–b) as compared to each other or to saline controls. All groups exhibited a very low level expression of TNF-α and IL-6 at both time points, indicating that the hydrogel and soluble injections were not causing up-regulated gene expression of the inflammatory cytokines due to either the injection procedures or the composition of the materials at the time points evaluated. Interestingly, the 10wt% RLP+PEG hydrogel and THP solution injections exhibited up-regulated TGF-β expression (Fig. 8c) corresponding to histological findings of increased and extended fibrotic inflammatory response after injection in these two groups. This almost certainly is a result of the inclusion of THP in these two material compositions, resulting in the profibrotic response with up-regulated production of TGF-β as compared to the non-THP soluble injections and PEG hydrogel injection in which a lower amount of THP was required for hydrogel formation. In comparison, Thibeault and coworkers investigated short-term macrophage inflammatory response with adipose tissue-derived stromal cells (ASC) embedded in a hyaluronan scaffold injected into injured pig vocal folds where they profiled expression of multiple inflammatory genes and observed higher secretion of both TNF-α and TGF-β compared to others (e.g., IL-4, IL-10, IL-12 and IFN) at both day 3 and 7. In our case, the overall effect of down-regulation of TGF-β by day 21 compared to day 5 across groups suggests a regenerative and remodeling transition from an early pro-inflammatory stage (where multiple cell types are recruited to invade into injection sites/wound bed to induce the immune reaction) to a later stage with a resolved foreign body response characterized by new tissue remodeling.(3),(12) Examination of the impact of RLP-derived hydrogels on vocal fold tissue regeneration, particularly in the context of related gene expression profiles and ECM deposition, requires further in vivo studies. Future investigations will require new non-toxic, biocompatible crosslinking chemistry for in vivo hydrogel formation with the inclusion of multiple cell-instructive biological motifs and relevant cell types present in these injected hydrogel matrices. Even in light of the required modifications to the RLP formulations, our recent development of RLP+PEG biocomposites that support in vitro 3D cell spreading and proliferation,(32),(33) coupled with our preliminary in vivo results here, suggest the potential of RLP-containing materials as injectable cell-gel constructs for engineering vocal fold ECM. Such chemical crosslinking strategies and other RLP-containing materials with enhanced cytocompatibility (e.g., RLP+PEG, RLP+hyaluronan) are currently under development. Further studies might also examine tissue response at later time points to explore ECM expression associated with acute and chronic inflammation, long-term cell retention and function, hydrogel degradation and biocompatibility in physiologically healthy and/or injured vocal fold tissues with large animal models (e.g., rabbit and pig).
CONCLUSION
In summary, the present study demonstrates the feasibility of subcutaneous injection and chemical crosslinking of RLP-based elastomeric materials in vivo for further development in vocal fold tissue engineering. The Mannich-type condensation crosslinking reaction renders fast gelation kinetics to yield RLP-based materials with shear storage moduli approximate to those of native vocal fold tissues; the crosslinking rate supports the sol-gel transition post-injection. Histological staining and gene expression analysis suggest the biocompatibility of resilin-like polypeptide materials, although injection of THP-containing solutions or THP-crosslinked hydrogels elicits both acute and chronic inflammatory responses characterized by accumulation of macrophages and neutrophils at injection sites with up-regulation of pro-inflammatory cytokines. Such crosslinking reagents must be avoided in the engineering of future injectable RLP-based biomaterials for vocal fold treatment. RLPs combined with other synthetic polymers or natural polysaccharides, fabricated using cytocompatible chemistries, offer promising alternatives for increasing versatility and enhancing biocompatibility for future in vivo injection of materials into vocal fold tissues.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. David T. Yang, MD from University of Wisconsin, Madison, Department of Pathology for his help on histological analysis. Related work in the authors’ laboratories has been supported by the National Science Foundation (DMR 0239744), the University of Delaware Research Foundation, the National Center for Research Resources (NCRR), a component of the National Institutes of Health (P20-RR017716 and P20-RR015588), and the National Institute on Deafness and Other Communication Disorders (NIDCD RO1 DC4336 and RO1 DC011377). This publication was also supported by the Delaware COBRE program (NIGMS; 1 P30 GM110758-01) from the National Institute of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–120. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Gugatschka M, Ohno S, Saxena A, Hirano S. Regenerative medicine of the larynx. Where are we today? A review. J Voice. 2012;26:670.e7–e13. doi: 10.1016/j.jvoice.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Kutty JK, Webb K. Tissue Engineering Therapies for the Vocal Fold Lamina Propria. Tissue Eng Part B Rev. 2009;15:249–262. doi: 10.1089/ten.TEB.2008.0588. [DOI] [PubMed] [Google Scholar]
- 4.Benninger MS, Alessi D, Archer S, Bastian R, Ford C, Koufman J, Sataloff RT, Spiegel JR, Woo P. Vocal fold scarring: Current concepts and management. Otolaryngol Neck Surg. 1996;115:474–482. doi: 10.1177/019459989611500521. [DOI] [PubMed] [Google Scholar]
- 5.Isshiki N, Shoji K, Kojima H, Hirano S. Vocal Fold Atrophy and its Surgical Treatment. Ann Otol Rhinol Laryngol. 1996;105:182–188. doi: 10.1177/000348949610500303. [DOI] [PubMed] [Google Scholar]
- 6.Mallur PS, Rosen CA. Vocal Fold Injection: Review of Indications, Techniques, and Materials for Augmentation. Clin Exp Otorhinolaryngol. 2010;3:177–182. doi: 10.3342/ceo.2010.3.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr Opin Otolaryngol Head Neck Surg. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Stiadle JM, Lau HK, Zerdoum AB, Jia X, Thibeault SL, Kiick KL. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials. 2016;108:91–110. doi: 10.1016/j.biomaterials.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaston J, Thibeault SL. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter. 2013;3:e23799. doi: 10.4161/biom.23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdick JA, Prestwich GD. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016;40:35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett RS, Thibeault SL, Prestwich GD. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater. 2012;7:24103. doi: 10.1088/1748-6041/7/2/024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weis-Fogh T. A rubber-like protein in insect cuticles. J Exp Biol. 1960;37:889–907. [Google Scholar]
- 14.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 15.Qin GK, Hu X, Cebe P, Kaplan DL. Mechanism of resilin elasticity. Nat Commun. 2012;3:1003. doi: 10.1038/ncomms2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Kiick KL. Resilin-Based Materials for Biomedical Applications. ACS Macro Lett. 2013;2:635–640. doi: 10.1021/mz4002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas Gorb S, Wootton RJF, Haas F, Gorb S, Wootton RJ. Elastic joints in dermapteran hind wings: materials and wing folding. Arthropod Struct Dev. 2000;29:137–146. doi: 10.1016/s1467-8039(00)00025-6. [DOI] [PubMed] [Google Scholar]
- 18.Burrows M, Shaw SR, Sutton GP. Resilin and chitinous cuticle form a composite structure for energy storage in jumping by froghopper insects. Bmc Biol. 2008;6:41–57. doi: 10.1186/1741-7007-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JFV, Wegst UGK. Design and mechanical properties of insect cuticle. Arthropod Struct Dev. 2004;33:187–199. doi: 10.1016/j.asd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Bennet-Clark HC. The role of the timbal in cicada sound production. J Exp Biol. 1995;198:1001–1019. doi: 10.1242/jeb.198.4.1001. [DOI] [PubMed] [Google Scholar]
- 21.Bennet-Clark HC. Tymbal mechanics and the control of song frequency in the cicada Cyclochila australasiae. J Exp Biol. 1997;200:1681–1694. doi: 10.1242/jeb.200.11.1681. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca PJ, Bennet-Clark HC. Asymmetry of tymbal action and structure in a cicada: A possible role in the production of complex songs. J Exp Biol. 1998;201:717–730. doi: 10.1242/jeb.201.5.717. [DOI] [PubMed] [Google Scholar]
- 23.Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li HB. Designed biomaterials to mimic the mechanical properties of muscles. Nature. 2010;465:69–73. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Renner JN, Liu JC. Incorporating the BMP-2 peptide in genetically-engineered biomaterials accelerates osteogenic differentiation. Biomater Sci. 2014;2:1110–1119. doi: 10.1039/c3bm60333d. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Liu JC. Protein-engineered microenvironments can promote endothelial differentiation of human mesenchymal stem cells in the absence of exogenous growth factors. Biomater Sci. 2016;4:1761–1772. doi: 10.1039/c6bm00472e. [DOI] [PubMed] [Google Scholar]
- 26.Bracalello A, Santopietro V, Vassalli M, Marletta G, Del Gaudio R, Bochicchio B, Pepe A. Design and Production of a Chimeric Resilin-, Elastin-, and Collagen-Like Engineered Polypeptide. Biomacromolecules. 2011;12:2957–2965. doi: 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- 27.Weitzhandler I, Dzuricky M, Hoffmann I, Garcia Quiroz F, Gradzielski M, Chilkoti A. Micellar self-assembly of recombinant resilin-like/elastin-like block copolypeptides. Biomacromolecules. 2017;18:2419–2426. doi: 10.1021/acs.biomac.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S-C, Qian Z, Dan A-H, Hu X, Zhou M-L, Xia X. Rational Design and Hierarchical Assembly of a Genetically Engineered Resilin–Silk Copolymer Results in Stiff Hydrogels. ACS Biomater Sci Eng. 2017;3:1576–1585. doi: 10.1021/acsbiomaterials.7b00353. [DOI] [PubMed] [Google Scholar]
- 29.Lv S, Bu T, Kayser J, Bausch A, Li H. Towards constructing extracellular matrix-mimetic hydrogels: An elastic hydrogel constructed from tandem modular proteins containing tenascin FnIII domains. Acta Biomater. 2013;9:6481–6491. doi: 10.1016/j.actbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Whittaker JL, Dutta NK, Knott R, McPhee G, Voelcker NH, Elvin C, Hill A, Choudhury NR. Tunable Thermoresponsiveness of Resilin via Coassembly with Rigid Biopolymers. Langmuir. 2015;31:8882–8891. doi: 10.1021/acs.langmuir.5b01014. [DOI] [PubMed] [Google Scholar]
- 31.Richardson PM, McGuinness UM, Aguayo AJ, Fang W, Paananen A, Vitikainen M, Koskela S, Westerholm-Parvinen A, Joensuu JJ, Landowski CP, Penttilä M, Linder MB, Laaksonen P. Elastic and pH-Responsive Hybrid Interfaces Created with Engineered Resilin and Nanocellulose. Biomacromolecules. 2017;18:1866–1873. doi: 10.1021/acs.biomac.7b00294. [DOI] [PubMed] [Google Scholar]
- 32.McGann CL, Akins RE, Kiick KL. Resilin-PEG Hybrid Hydrogels Yield Degradable Elastomeric Scaffolds with Heterogeneous Microstructure. Biomacromolecules. 2016;17:128–140. doi: 10.1021/acs.biomac.5b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau HK, Li L, Jurusik AK, Sabanayagam CR, Kiick KL. Aqueous Liquid–Liquid Phase Separation of Resilin-Like Polypeptide/Polyethylene Glycol Solutions for the Formation of Microstructured Hydrogels. ACS Biomater Sci Eng. 2017;3:757–766. doi: 10.1021/acsbiomaterials.6b00076. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Teller S, Clifton RJ, Jia X, Kiick KL. Tunable Mechanical Stability and Deformation Response of a Resilin-Based Elastomer. Biomacromolecules. 2011;12:2302–2310. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Tong Z, Jia X, Kiick KL. Resilin-Like Polypeptide Hydrogels Engineered for Versatile Biological Functions. Soft Matter. 2013;9:665–673. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Kiick K. Transient Dynamic Mechanical Analysis of Resilin-based Elastomeric Hydrogels. Front Chem. 2014;2:20–32. doi: 10.3389/fchem.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teller SS, Farran AJE, Xiao L, Jiao T, Duncan RL, Clifton RJ, Jia X. High-Frequency Viscoelastic Shear Properties of Vocal Fold Tissues: Implications for Vocal Fold Tissue Engineering. Tissue Eng Part A. 2012;18:2008–2019. doi: 10.1089/ten.tea.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao T, Farran A, Jia X, Clifton RJ. High Frequency Measurements of Viscoelastic Properties of Hydrogels for Vocal Fold Regeneration. Exp Mech. 2009;49:235–246. doi: 10.1007/s11340-008-9126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Mahara A, Tong Z, Levenson EA, McGann CL, Jia X, Yamaoka T, Kiick KL. Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Adv Healthc Mater. 2016;5:266–275. doi: 10.1002/adhm.201500411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and Rheologic Characterization of Vocal Fold Scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 41.Bartlett RS, Gaston JD, Yen TY, Ye S, Kendziorski C, Thibeault SL. Biomechanical Screening of Cell Therapies for Vocal Fold Scar. Tissue Eng Part A. 2015;21:2437–2447. doi: 10.1089/ten.tea.2015.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graupp M, Bachna-Rotter S, Gerstenberger C, Friedrich G, Fröhlich-Sorger E, Kiesler K, Gugatschka M. The unsolved chapter of vocal fold scars and how tissue engineering could help us solve the problem. Eur Arch Oto-Rhino-Laryngology. 2016;273:2279–2284. doi: 10.1007/s00405-015-3668-8. [DOI] [PubMed] [Google Scholar]
- 43.Zeitels SM, Hillman RE, Mauri M, Desloge R, Doyle PB. Phonomicrosurgery in singers and performing artists: Treatment outcomes, management theories, and future directions. Ann Otol Rhinol Laryngol. 2002;111:21–40. doi: 10.1177/0003489402111s1203. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Luo T, Kiick KL. Temperature-Triggered Phase Separation of a Hydrophilic Resilin-Like Polypeptide. Macromol Rapid Commun. 2015;36:90–95. doi: 10.1002/marc.201400521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamburro AM, Panariello S, Santopietro V, Bracalello A, Bochicchio B, Pepe A. Molecular and Supramolecular Structural Studies on Significant Repetitive Sequences of Resilin. ChemBioChem. 2010;11:83–93. doi: 10.1002/cbic.200900460. [DOI] [PubMed] [Google Scholar]
- 46.Qin GK, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL. Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials. 2011;32:9231–9243. doi: 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl)phosphines in aqueous solution. Biomacromolecules. 2007;8:1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim WS, Kim J-H, Park H, Kim K, Chan Kwon I, Lee DS. Biodegradability and biocompatibility of a pH- and thermo-sensitive hydrogel formed from a sulfonamide-modified poly(ε-caprolactone-co-lactide)–poly(ethylene glycol)–poly(ε-caprolactone-co-lactide) block copolymer. Biomaterials. 2006;27:5178–5185. doi: 10.1016/j.biomaterials.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y, Gill EE, Liu JC. Enzymatic Cross-Linking of Resilin-Based Proteins for Vascular Tissue Engineering Applications. Biomacromolecules. 2016;17:2530–2539. doi: 10.1021/acs.biomac.6b00500. [DOI] [PubMed] [Google Scholar]
- 50.Grainger DW. All charged up about implanted biomaterials. Nat Biotech. 2013;31:507–509. doi: 10.1038/nbt.2600. [DOI] [PubMed] [Google Scholar]
- 51.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotech. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 56.Thibeault SL, Duflo S. Inflammatory cytokine responses to synthetic extracellular matrix injection to the vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2008;117:221–226. doi: 10.1177/000348940811700310. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–2948. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haines-Butterick LA, Salick DA, Pochan DJ, Schneider JP. In vitro assessment of the pro-inflammatory potential of beta-hairpin peptide hydrogels. Biomaterials. 2008;29:4164–4169. doi: 10.1016/j.biomaterials.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Z-Q, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Wise SG, Rnjak-Kovacina J, Kaplan DL, Bilek MMM, Weiss AS, Fei J, Bao S. Biocompatibility of silk-tropoelastin protein polymers. Biomaterials. 2014;35:5138–5147. doi: 10.1016/j.biomaterials.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 61.Ohno T, Hirano S, Rousseau B. Gene expression of transforming growth factor-β1 and hepatocyte growth factor during wound healing of injured rat vocal fold. Laryngoscope. 2009;119:806–810. doi: 10.1002/lary.20174. [DOI] [PubMed] [Google Scholar]
- 62.Gaston J, Quinchia Rios B, Bartlett R, Berchtold C, Thibeault SL. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS One. 2012;7:e30965. doi: 10.1371/journal.pone.0030965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopecki Z, Luchetti MM, Adams DH, Strudwick X, Mantamadiotis T, Stoppacciaro A, Gabrielli A, Ramsay RG, Cowin AJ. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J Pathol. 2007;211:351–361. doi: 10.1002/path.2113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.