Abstract
The purpose of this study is to determine neural, vascular, protein secretion, and cellular signaling changes with disease progression in lacrimal glands of the thrombospondin-1−/− (TSP-1−/−) mouse model of dry eye compared to C57BL/6 wild type (WT) mice. Neural innervation was reduced in TSP-1−/− lacrimal glands compared to WT controls, whereas the number of blood vessels was increased. Intracellular Ca2+ stores and the amount of lysosomes, mitochondria and secretory granules, but not the endoplasmic reticulum, were reduced in TSP-1−/− compared to WT acini at 12 weeks of age. Ex-vivo high KCl-evoked secretion was decreased in TSP-1−/− compared to WT lacrimal gland tissue pieces. The α1D-adrenergic agonist-stimulated response was increased in TSP-1−/− at 4 and 24 weeks but decreased at 12 weeks, and the ATP and MeSATP-stimulated peak [Ca2+]i responses were decreased at 24 weeks. These changes were observed prior to appearance of mononuclear infiltrates. We conclude that in the lacrimal gland the absence of TSP-1: injures peripheral nerves; blocks efferent nerve activation, decreases protein secretion, and, alters intracellular Ca2+ stores. Through these effects the absence of TSP-1 leads to disruption of ocular surface homeostasis and development of dry eye.
Introduction
Dry eye is a multifactorial disease, which affects over forty million Americans. Most of the currently available treatments and therapies for dry eye are restricted to palliative care and are limited in number.1–3 Previous literature has implicated lacrimal gland dysfunction as the primary cause of aqueous deficiency dry eye (ADDE).4–6 One form of ADDE is Sjogren’s syndrome, an autoimmune disease that occurs predominantly in females that represent 90% of Sjogren’s syndrome patients.7 The dysfunction in ADDE includes an alteration in protein, electrolyte and water secretion from lacrimal glands and has also been linked to ocular surface inflammation commonly diagnosed in dry eye patients.8 In this study we characterized lacrimal gland structural and functional changes in a novel mouse model of ADDE, the thrombospondin-1 null (TSP-1−/−) mouse, which previously was reported to mimic Sjögren’s-associated chronic dry eye progression as seen in humans with Sjögren’s-associated ADDE.9,10
Lacrimal gland secretion is regulated predominantly by a neural reflex in which stimuli on the ocular surface activate afferent sensory nerves. These activated nerves by a complex neural reflex stimulate both efferent parasympathetic and sympathetic nerves. The efferent nerves release their neurotransmitters acetylcholine, vasoactive intestinal peptide (VIP), and norepinephrine that interact with their receptors, muscarinic 3 acetylcholine receptors (M3AchR), VIP receptors (VPACR), and α1D adrenergic receptor (α1DAR), respectively on acinar and duct cells. Activation of these receptors stimulates a variety of calcium handling mechanisms and other signaling pathways to work in a tightly regulated fashion to produce lacrimal gland protein secretion.11 These proteins produced along with isotonic electrolyte and water secretion constitute the lacrimal gland primary fluid,12 that undergoes further modification by ductal cell secretion before being released onto the cornea and conjunctiva to help form the aqueous component of tears.13
The two major signaling pathways that regulate protein secretion by the lacrimal gland are the cholinergic (via muscarinic M3AchR) and adrenergic (via α1D-ADR) pathways.5 Acetylcholine activates M3AchRs, which are coupled to phospholipase Cβ (PLCβ). PLCβ activation produces the protein kinase C (PKC) activator diacylglycerol and inositol 1,3,5-trisphosphate (InsP3).14 InsP3 increases the intracellular [Ca2+] ([Ca2+]i) using thapsigargin sensitive intracellular Ca2+ stores that, along with the activation of PKCα, -δ, and -ε, stimulate the secretion of protein stored in preformed secretory granules.14,15 Stimulation of M3AchRs also activates extracellular-regulated kinase (ERK) 1/2 and phospholipase D, which attenuate secretion.16,17 Norepinephrine activates α1D-AR, which causes an increase in [Ca2+]i by a mechanism that is not yet determined but does not involve generation of InsP3 or use of thapsigargin-dependent cellular Ca2+ stores.18 α1D-ARs also transactivate the epidermal (EGF) receptor to increase ERK1/2 activity, which attenuates secretion.19,20 VIP interacts with VPAC1 to stimulate secretion by increasing cellular levels of cAMP and increasing [Ca2+]i.21 Increasing [Ca2+]i is a signaling mechanism in common to activation of these three receptors to stimulate protein exocytosis and secretion. Both increase in [Ca2+]i and protein secretion can serve as markers of lacrimal gland function. Dysfunction of these cellular signaling pathways potentially can disrupt secretory function of lacrimal gland and contribute to the development of dry eye disease.
Additionally, protein secretion into the tears by the lacrimal gland can also occur by a non-adrenergic non-cholinergic pathway (NANC). VIP, Substance P and purinergic receptors (P2X and P2Y) constitute some of the NANC candidates.22 In the lacrimal gland activation of P2X3 and P2X7 receptors increases [Ca2+]i and stimulates protein secretion.23 P2Y receptors play a role in the function of the myoepithelial cells that surround the acinar cells.24 Thus, abnormalities in protein secretion activated by stimulation of non-canonical receptors in the lacrimal gland could also be involved in dry eye disease progression.
TSP-1 is a matricellular protein that activates latent transforming growth factor25. TSP-1 regulates extracellular and intracellular signaling complexes as it interacts with cell surface receptors, growth factors, cytokines, and extracellular matrix proteins. This activates many functions within the cell including cell migration, proliferation, and cell death26. The use of TSP-1−/− mice as a model for ADDE was recently validated by Turpie et al. in male mice.10 In TSP-1−/− mice they detected progressively exacerbated infiltration of the lacrimal glands, with inflammatory infiltrates containing predominantly CD4 T cells, as typically reported in autoimmune Sjögren’s syndrome. Alterations in lacrimal gland protein secretion were observed as early as 8 weeks of age in TSP1−/− mice whereas mononuclear infiltrates were not seen in the lacrimal glands until these mice reached 24 weeks of age10. Therefore, unlike other mouse models where inflammation is indistinguishable as a cause or effect, our murine model exhibits destructive inflammatory responses preceded by distinct glandular dysfunction. We previously found that female TSP-1−/− mouse lacrimal glands also have minimal disease at 4 weeks of age, but extensive disease at 12 and 24 weeks of age.27 Inflammation in female mouse lacrimal glands is similar to that in males. In addition, female TSP1−/− mice have altered expression of the cytokines interleukin (IL)-1β, IL-6, Th17 and IFNγ, cellular morphology, progenitor cell markers and a decrease in cell proliferation.27
In the present study, we investigated potential aberrations in signaling pathways associated with lacrimal gland function at multiple levels in female TSP-1−/− compared to WT mice. We found that in the TSP−/− lacrimal gland peripheral nerves are damaged and efferent nerve activation is blocked leading to decreased protein secretion. Through these changes the absence of TSP-1 leads to disruption of ocular surface homeostasis and development of dry eye.
Results
Neural innervation (parasympathetic, sympathetic and sensory) is decreased in TSP-1 deficient lacrimal glands compared to WT glands
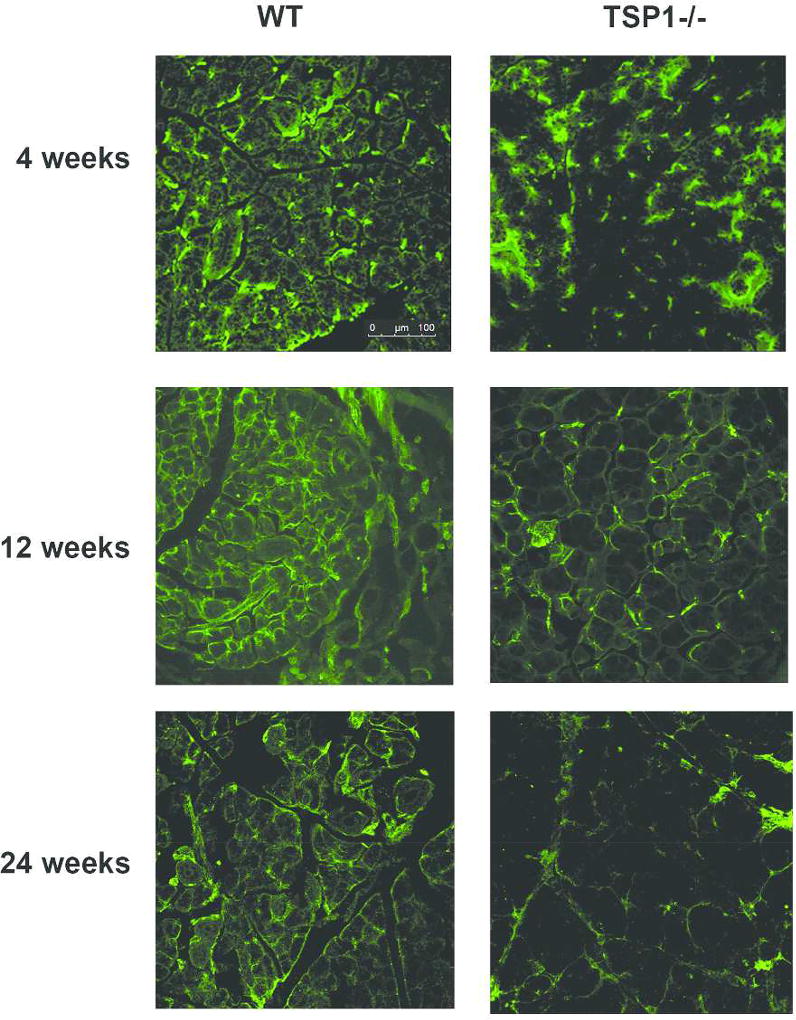
Lacrimal glands are innervated by sensory (Sub P and CGRP containing), parasympathetic (acetylcholine and VIP containing), and sympathetic (norepinephrine containing) nerves. To determine if the secretory defect detected in TSP-1−/− glands is related to their innervation, we used confocal microscopy to visualize innervation patterns in TSP-1 deficient lacrimal glands prior to (4 weeks), during (12 weeks) and after development of inflammation (24 weeks) compared to lacrimal glands from age-matched WT mice. The results obtained from analysis of TUJ-1 (a neural marker that indicates all nerve types) expression show substantially decreased innervation in 4, 12 and 24 week old TSP-1−/− mice when compared to age-matched WT acini (Fig. 1).
Fig. 1. Innervation Is Decreased with Age in TSP-1−/− Compared to WT Mice.
Lacrimal gland of 4, 12, and 24 week old female WT (left) and TSP-1−/− (right) mice were fixed, sectioned, and stained with anti-Tuj1 antibody (green) that indicates sympathetic, parasympathetic, and sensory nerves. Representative micrographs taken with confocal microscopy are shown from three independent animals.
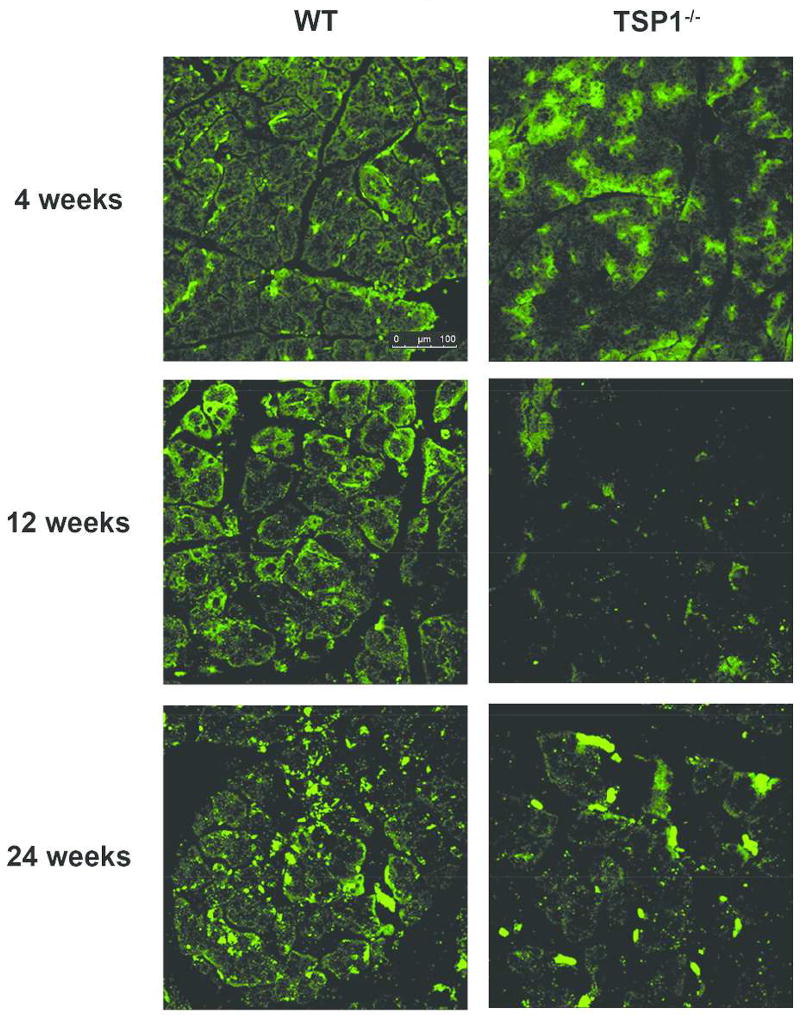
To specifically determine the effect of TSP-1 deficiency on the extent of parasympathetic innervation, we used anti-VIP antibodies in the following set of experiments. As shown in Fig. 2, a decrease in parasympathetic nerves was detected in 4, 12, and 24 week-old TSP-1−/− lacrimal acini when compared to WT controls. As parasympathetic nerves are the major nerves in the lacrimal gland, it is not surprising that a similar change was detected in the staining by both anti- TUJ-1 and VIP antibodies.
Fig. 2. Parasympathetic Innervation is Decreased with Age in Lacrimal Glands in TSP-1−/− Compared to WT Mice.
Lacrimal gland of 4, 12, and 24 week old female WT (left) and TSP-1−/− (right) mice were fixed, sectioned, and stained with anti-VIP antibody (green) that indicates parasympathetic nerves. Micrographs taken with confocal microscopy are representative of three independent animals.
These findings suggest that TSP-1 is important for nerve development or maintenance and the disruption of neural connections from an early age prior to development of inflammation might be a contributing factor in the development of dry eye in TSP-1 deficient mice.
Increased blood vessels are detected in TSP-1 deficient lacrimal glands compared to WT glands
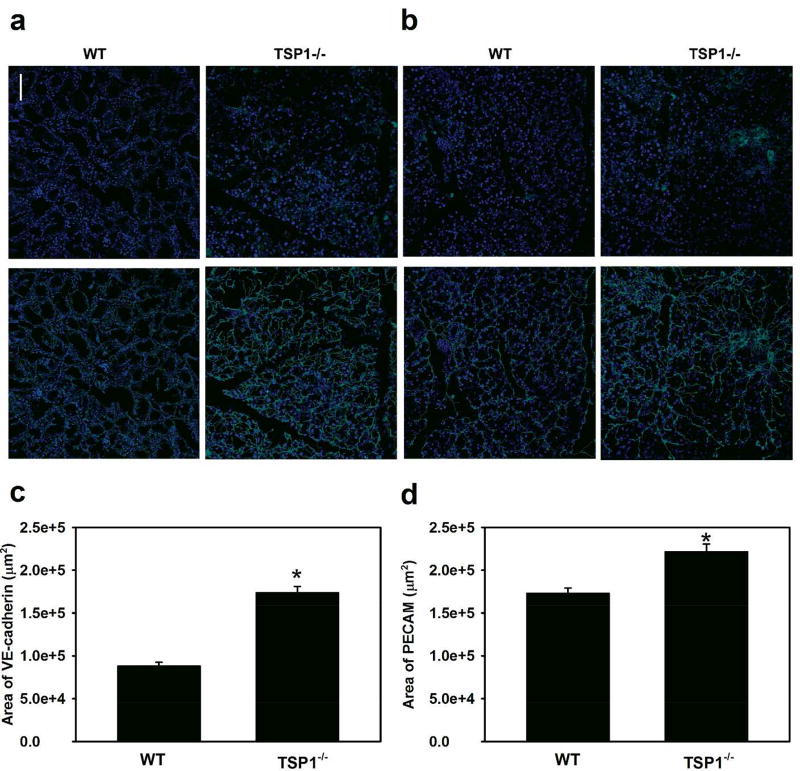
As lacrimal gland morphological changes were seen in 12 week-old TSP1−/− mice27, the total area of blood vessels in lacrimal glands from 12 week old mice was determined. Blood vessels were detected with anti-VE-cadherin (Fig. 3a) or anti-PECAM (Fig. 3b) antibody. When VE-cadherin was used the amount of blood vessels was 174,159 ± 6,062 µm2 in TSP-1−/− mouse glands, a value significantly (p=0.00004) increased from that in WT glands, which was 88,386 ± 3,444 µm2 (Fig. 3c). Similarly when PECAM was used the amount of blood vessels was 221,985 ± 8,001 µm2 in TSP-1−/− mouse glands, a value significantly (p=0.014) increased from that in WT glands, which was 173,452 ± 5,287 µm2 (Fig. 3d). These results support detection of significantly increased blood vessels in TSP-1 deficient lacrimal glands, which is in accordance with the antiangiogenic activity of TSP-1 previously described in the literature and serves as a positive control.28
Fig. 3. Area of VE-Cadherin and PECAM Is Decreased in Lacrimal Glands in TSP-1−/− Compared to WT Mice.
Female WT and TSP-1−/− mouse lacrimal glands were removed at 12 weeks of age, fixed, and stained with VE-cadherin (a) and PECAM (b) antibodies to study blood vessels. Representative micrographs are shown in a and b. Quantification of positive staining was performed and the total area was calculated. Data are mean ± SEM from four independent animals and shown in c and d. * indicates a significant difference from WT..
Neural stimulation of protein secretion is decreased in TSP-1 deficient lacrimal glands compared to WT glands
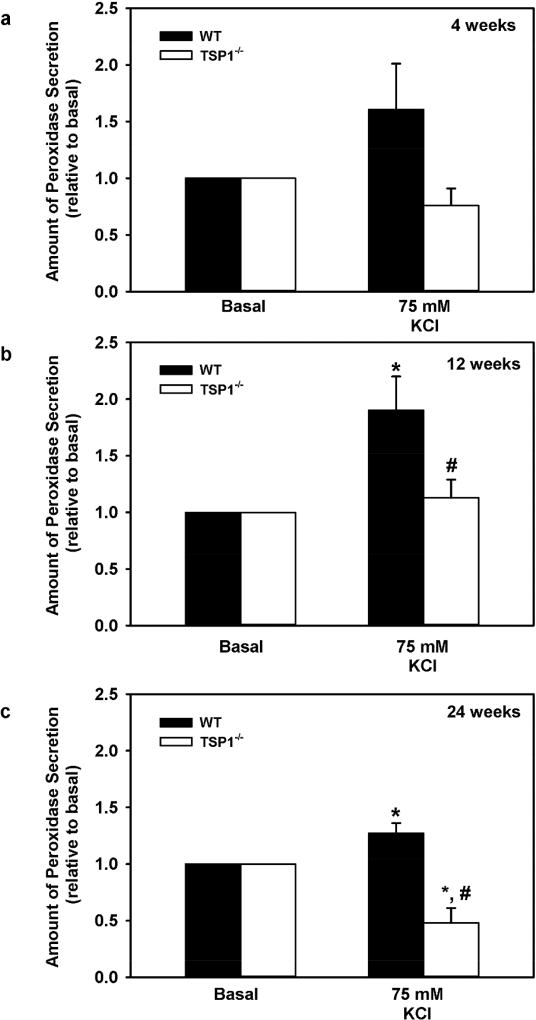
Upon depolarization, nerve endings release neurotransmitters which bind to the membrane surface receptors located on lacrimal gland acinar cells,5 leading to initiation of signaling cascades resulting in a rise in [Ca2+]i and protein secretion. We next determined if disrupted neural connections in TSP-1 deficient lacrimal glands lead to abnormalities in their protein secretion in an ex-vivo experiment. To depolarize nerves we incubated lacrimal gland tissue pieces that contained functioning efferent nerve endings in buffer containing 4mM basal KCl or 75 mM high KCl, and measured protein secretion (Fig. 4 a–c). As expected high KCl-stimulated secretion was significantly increased over basal KCl in WT lacrimal gland tissues derived from both 12 (Fig. 4b) and 24 (Fig. 4c) week old mice. However, similar stimulation in lacrimal gland tissues derived from age-matched TSP-1−/− mice was significantly reduced 83.9% (p=0.026) and 294.2% (p=0.0037) as compared to WT lacrimal gland tissue. In addition, at 24 weeks, high KCl-stimulated secretion was significantly reduced from secretion with basal KCl (p=0.007). Thus, in TSP-1−/− lacrimal glands a decrease in nerves or their function corresponds to a decrease in neural-stimulated protein secretion.
Fig. 4. High KCl Induced Secretion is Decreased with Age in in Lacrimal Glands in TSP-1−/− Compared to WT Mice.
Lacrimal gland pieces were stimulated with normal 4 mM KCl (basal) and high 75 mM KCl and protein secretion was measured in 4 (a), 12 (b), and 24 (c) week old mice. Data are mean ± SEM from at least three independent animals for each condition. * indicates statistically significant different from basal; #indicates a significant difference from WT.
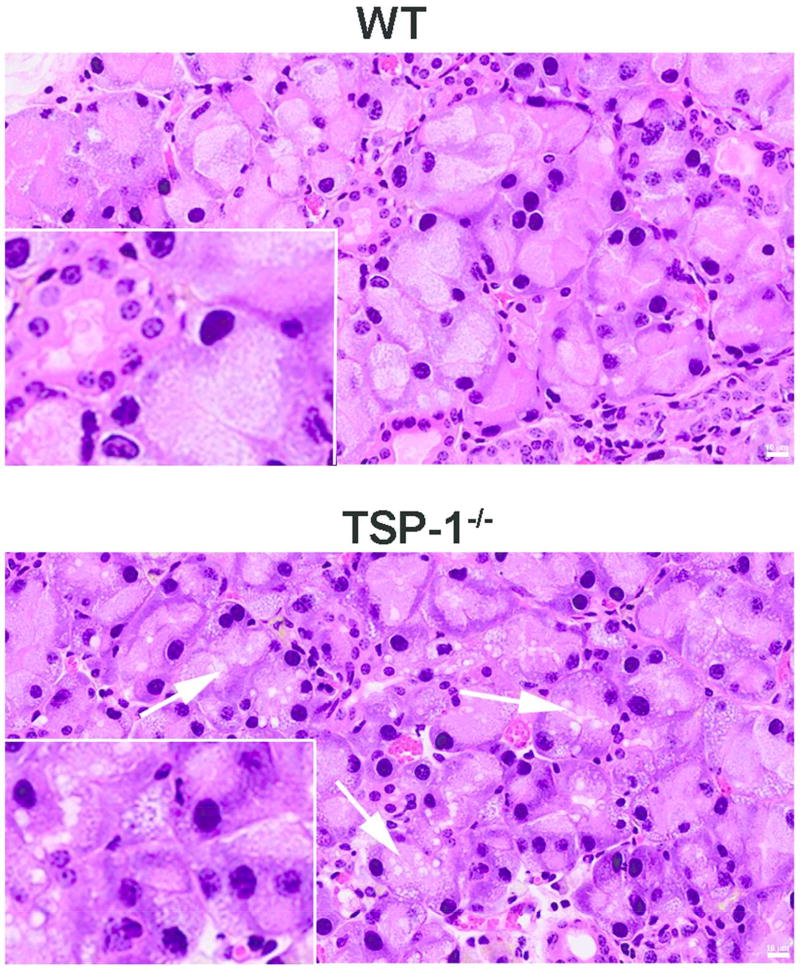
TSP-1 deficiency alters structure of lacrimal gland acinar cells
Consistent with their abnormal innervation which was observed at 12 weeks of age, we also detected altered morphology of lacrimal gland acini in TSP-1 deficient lacrimal gland. Lacrimal gland sections derived from 12 week-old mice were stained with hematoxylin and eosin (H&E) and examined under higher power magnification. As shown in Fig. 5, TSP-1−/− acini look visibly altered in that they appear coarser and contain more vacuoles compared to WT acini. This result suggests that an absence of TSP-1 could affect the expression of intracellular organelles thereby manifesting a distinct cellular morphology in TSP-1−/− mice.
Fig. 5. Structural Changes of Lacrimal Glands in TSP-1−/− Compared to WT Mice.
Lacrimal glands from 12 week-old WT and TSP-1−/− mice were isolated, fixed, and stained with hematoxylin/eosin. High power magnification shows altered acinar cell morphology in TSP-1−/− mice. Arrows indicate vacuoles inside of cytoplasm of TSP-1−/− acinar cells. The scale bar is 10 microns. Micrographs are representative of 3 independent animals.
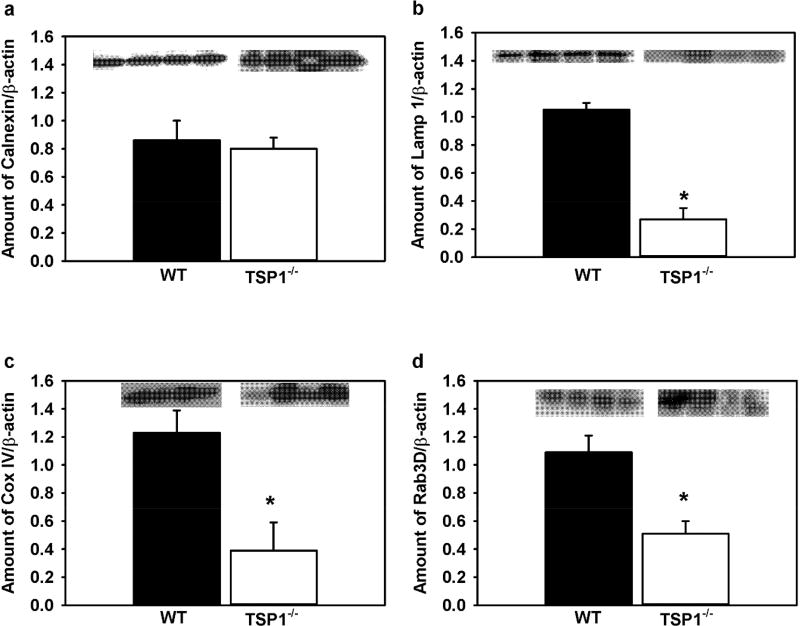
To assess quantitative changes in intracellular organelles including endoplasmic reticulum (ER), lysosomes, mitochondria, and secretory granules in TSP-1−/− compared to WT lacrimal gland acini from 12 week-old mice, we performed western blot analysis (Fig. 6). The amount of ER, critical in protein synthesis and in regulation of the [Ca2+]i, was determined using anti-calnexin antibody (Fig. 6a). However, the amount of these organelles was not significantly different in TSP-1−/− compared to WT lacrimal glands. Lysosomes are responsible for degradation and recycling of proteins to maintain cell homeostasis. Lysosomes can play a role in regulation of [Ca2+]I as they can take up Ca2+ released from the ER through multiple Ca2+ channels.29 The amount of Lamp1 protein, a lysosomal marker, was significantly decreased by 74.3% in TSP-1−/− compared to WT lacrimal glands (Fig. 6b). Mitochondria play a role in regulating the basal [Ca2+]i, in cell oxidative stress, and in cell death. The amount of mitochondria, determined with anti-cox IV antibodies, was significantly decreased by 68.3% in TSP-1−/− compared to WT lacrimal glands (Fig. 6c). Finally, lacrimal gland protein secretion is dependent on protein exocytosis. Rab3D marks secretory vesicles that fuse to the apical membrane and release their cargo (secretory proteins) into the lacrimal gland duct system. The amount of Rab3D protein was significantly decreased 53.2% in TSP-1−/− compared to WT lacrimal glands (Fig. 6d).
Fig. 6. Amount of Organelle-Related Protein Is Decreased in Lacrimal Glands in TSP-1−/− Compared to WT Mice.
The amount of calnexin (a), Lamp 1 (b), Cox IV (c), Rab3D (d), and β-actin was measured by western blot in lacrimal gland samples from 12 week-old WT and TSP-1−/− mice. Western blots are shown as insets where each lane is a separate animal. The amount of each organelle-related protein was standardized to β-actin (not shown). Data are mean ± SEM from four independent animals * indicates significance from WT expression.
Our results show comparable expression of ER and a significant reduction in markers for expression of lysosomes, mitochondria, and secretory vesicles in TSP-1−/− compared to WT lacrimal glands. These data suggest a reduction of select intracellular organelles in TSP-1−/− lacrimal gland acini and might contribute towards an altered morphology and function of TSP-1−/− lacrimal glands.
Intracellular Ca2+ signaling is altered in TSP-1−/− compared to WT lacrimal gland acini
The reduction in organelles associated with protein secretion in TSP-1 deficient acini prompted us to assess Ca2+i signaling that is integral part of lacrimal gland protein and fluid secretion. Any, aberrations in Ca2+ handling mechanisms might directly affect lacrimal gland protein secretion. Therefore, we first determined the concentration dependency of the rise in [Ca2+]i evoked by both parasympathetic and sympathetic agonists, major stimuli of secretion, in lacrimal gland acini from 24 week old WT mice, an age in which the lymphocytic infiltration is extensive. Additionally, we also determined Ca2+ increase evoked by NANC candidates: ATP, an activator of purinergic (P) P2X receptors, and 2MeS-ATP, an activator of P2Y receptors.
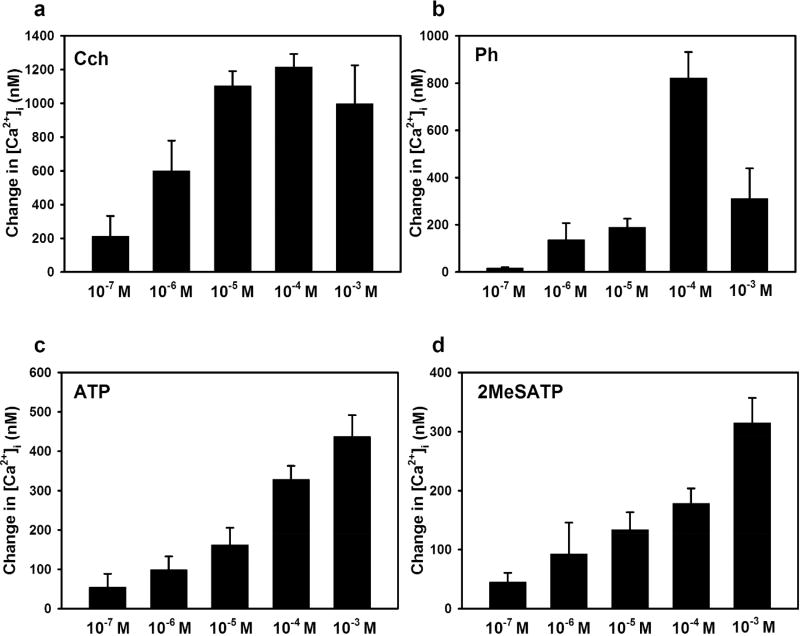
Carbachol, a M3AchR agonist, increased the [Ca2+]i in a concentration dependent manner with a maximum [Ca2+]i of 1216.05 ± 76.81 nM at 10−4 M (Fig. 7a). The α1D-adrenergic agonist phenylephrine also increased [Ca2+]i in a concentration dependent manner with a maximum increase of 712.27 ± 202.58 nM at 10−4 M (Fig. 7b). ATP and 2MeS-ATP each increased [Ca2+]i, but a maximum response was not obtained (Fig. 7c, d). The highest response was 437.02 ± 54.90 nM at 10−3 M ATP and 314.72 ± 42.33 nM at 10−3 M 2MeS-ATP. We used the concentration of agonist that gave the maximal or highest Ca2+ response for subsequent experiments.
Fig. 7. Concentration Dependency of the [Ca2+]i Response in WT Lacrimal Gland Acini.
Lacrimal gland acinar cells from 24 week old WT mice were treated with increasing doses of carbachol (Cch) (a), phenylephrine (Ph) (b), ATP (c), and 2MeSATP (d) and the change in peak [Ca2+]i was measured. Data are mean ± SEM from three independent animals.
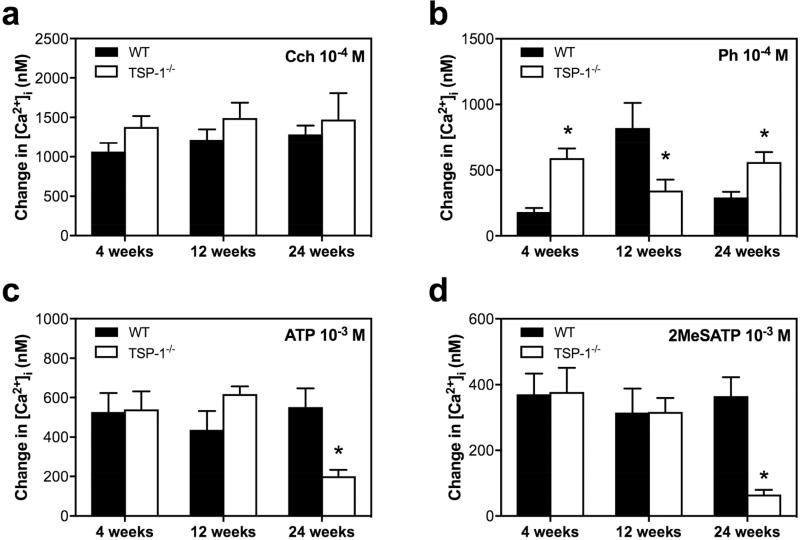
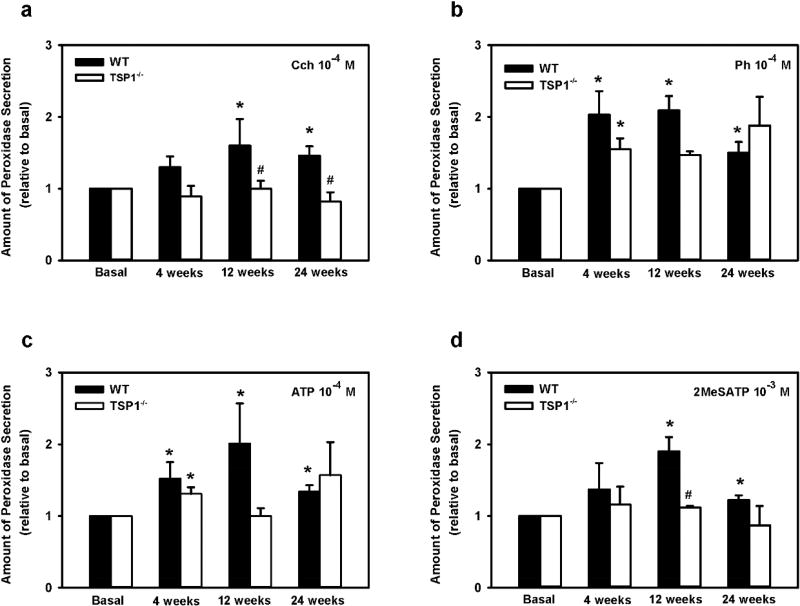
We next compared the peak agonist-stimulated rise in [Ca2+]i in TSP-1−/− versus WT lacrimal gland acini at 4, 12, and 24 weeks of age (Fig. 8). When carbachol was used at 10−4 M the increase in [Ca2+]i was not different between TSP-1−/− and WT acini at any age measured (Fig. 8a). In contrast, we found that the phenylephrine (Ph)-stimulated increase in [Ca2+]i was affected in all the three age groups (Fig. 8b). In WT mice, a time-dependent increase in Ph-stimulated increase in [Ca2+]i was observed in WT mice, with maximum increase occurring at 12 weeks, likely due to maturation of the mice. In TSP1−/−, the increase in [Ca2+]i does not change with age. The α1D-adrenergic pathway-mediated increase in [Ca2+]i at 12 weeks was significantly diminished by 57.9% in TSP-1−/− lacrimal acini in contrast to the other two age groups (4 and 24 weeks), where the stimulated [Ca2+]i was significantly enhanced in TSP-1−/− by 223.4% and 91.9%, respectively, when compared to the [Ca2+]i responses in WT acini.
Fig. 8. Increase in [Ca2+]i Mediated by Carbachol, Phenylephrine, ATP, and 2MeSATP in TSP-1−/− compared to WT mice.
Lacrimal gland acini were isolated and the change in [Ca2+]i mediated by carbachol (Cch) 10−4 M (a), phenylephrine (Ph) 10−4 M (b), ATP 10−3 M (c) and 2MeSATP 10−3 M (d) was studied in 4, 12, and 24 week old WT and TSP-1−/− mice. * indicates significance from WT response at the same age. Data are mean ± SEM from three independent animals.
Finally, in mice at 4 and 12 weeks of age, both of the purinergic receptor (P2X and P2Y) mediated stimulation of the [Ca2+]i was unchanged, but at 24 weeks of age P2X and P2Y responses were both significantly diminished by 63.5% for ATP and 82.2% for 2MeSATP in TSP-1−/− compared to WT acini (Fig. 8c, d). Although our findings show different agonist specific alterations in the change in peak [Ca2+]i, α1D-adrenergic-, P2X-, and P2Y-agonist mediated changes in [Ca2+]i all were altered in TSP-1−/− compared to WT acini at the oldest age measured, indicating alterations in lacrimal glands of TSP1−/− mice and possible defective cellular Ca2+ handling with disease progression.
Protein secretion is affected in TSP-1−/− when compared to WT lacrimal gland acini
To determine if changes in peak [Ca2+]i correspond with protein secretion we compared ex-vivo stimulation of lacrimal glands with M3AchR, α1D-adrenergic, P2X, and P2Y agonists We measured protein secretion in TSP-1−/− versus WT lacrimal gland pieces at 4, 12 and 24 weeks of age (Fig. 9). We found that in WT mice, carbachol evoked secretion increased, though not significantly (p=0.07, power 0.36) at 4 weeks and was significantly increased at 12 and 24 weeks (p=0.05 and p= 0.03, respectively). There was no significant difference in TSP1−/− compared to WT mice, at 4 weeks (p=0.14, power 0.33), but was significantly diminished by 93.9% (p=0.05) and 139.0% (p=0.013) in 12 and 24 week old TSP-1−/− compared to WT pieces (Fig. 9a). In contrast to carbachol, phenylephrine- and ATP-stimulated secretion were not significantly different between the three ages in TSP-1−/− compared to WT lacrimal gland pieces with powers ranging from 0.07–0.4. (Fig. 9b, c). Maximum secretion in response to ATP and 2MeSATP was observed in 12 week old WT mice (Fig 9c,d). 2MeSATP stimulation of protein secretion was not significantly different between the two strains at 4 or 24 week old. However secretion was significantly diminished by 86.4% (p=0.002), in 12-week-old TSP-1−/− compared to WT pieces (Fig. 9d). Secretion from TSP1-1−/− mice was reduced compared to secretion from WT mice regardless of the stimuli..
Fig. 9. Lacrimal Gland Protein Secretion in TSP-1−/− Compared to WT Mice in Response to Stimuli.
Lacrimal gland pieces from 4, 12, and 24 week old WT and TSP1−/− mice were stimulated for 40 min with carbachol (Cch) (a), phenylephrine (Ph) (b), ATP (c), and 2MeSATP (d), and protein secretion was measured. Data are mean ± SEM from three independent animals.* indicates statistically significant different from basal; #indicates a significant difference from WT.
TSP-1−/− mouse lacrimal gland acini exhibit decreased intracellular Ca2+ stores compared to WT acini
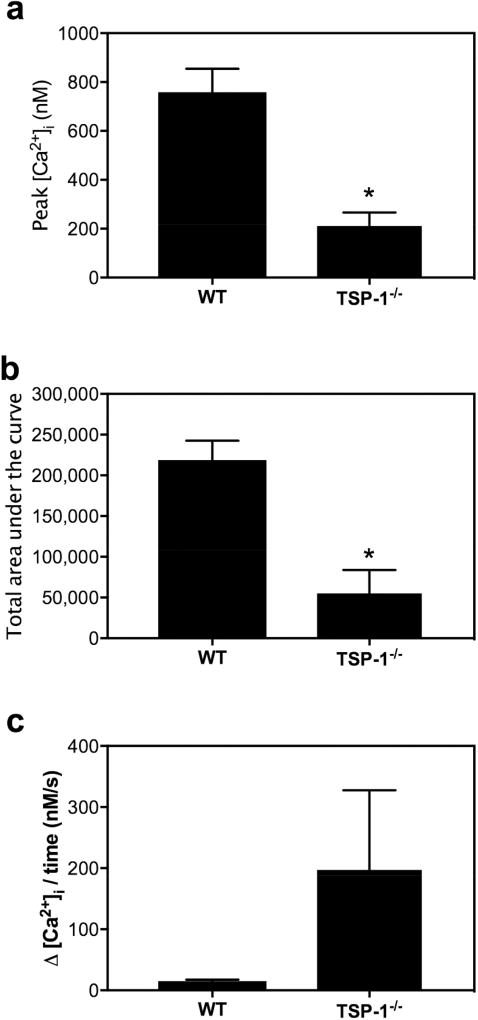
Protein secretion experiments demonstrated a significant decrease in M3AchR protein secretion in 12 and 24 week old TSP-1−/− compared to WT mouse lacrimal glands. As carbachol, the M3AchR agonist used in the present study, primarily evokes protein exocytosis via ER associated Ca2+ release stimulated by IP3R, we determined if thapsigargin-releasable intracellular Ca2+ stores, most likely located in the ER, were altered in TSP-1−/− compared to WT acini. The Ca2+ response to thapsigargin indicates the size of the intracellular Ca2+ store. When Ca2+ is introduced in the bathing solution, this results in refilling of ER stores. The rate of the Ca2+ change indicated the rate of influx of extracellular Ca2+. We detected a significant decrease in peak Ca2+ amplitude and area under the curve during thapsigargin exposure to lacrimal gland acini in 24-week-old TSP-1−/− compared to WT acini (Fig. 10). There was a 72.1% decrease in peak response (Fig. 10a) and a 74.8% decrease in area under the curve (Fig. 10b) in TSP-1−/− compared to WT acini. Thus, this finding can be interpreted as a reduction in ER store Ca2+ content in lacrimal acini of 24-week-old TSP-1−/− when compared to WT mouse acini. In contrast, the rate of refilling of ER stores by extracellular Ca2+ influx was unchanged between the two groups (Fig. 10c). Therefore, even though the store operated Ca2+ entry mechanism and ER as organelle remains unaltered at 24 weeks in TSP-1 deficient lacrimal gland acini compared to WT acini, the total Ca2+ content in the ER stores is drastically depleted and could account for the decrease in cholinergic agonist-stimulated protein secretion.
Fig. 10. Intracellular Ca2+ Stores are Decreased in TSP-1−/− Compared to WT Lacrimal Gland Acini.
The size of intracellular Ca2+ stores was measured with a 15 min thapsigargin stimulation (10−5 M) in the absence of extracellular Ca2+. The peak [Ca2+]i (a), the total area under the curve (b) were measured over the 15 min period, and the rate of refilling was measured with the addition of extracellular Ca2+ (c) and compared between 24 week-old WT and TSP-1−/− mice. Data are mean ± SEM from three independent animals. * indicates a significant difference from WT. At least three independent experiments were performed.
Discussion
Lacrimal gland dysfunction leading to dry eye has been widely associated with inflammation. Under specific pathophysiological settings, a number of studies have shown upregulation of proinflammatory cytokines, chemokines and metalloproteinases.6 This in turn leads to a rapid expansion of autoreactive T helper cells which then migrate to the lacrimal gland tissue and cause inflammation and tissue atrophy.31 In TSP-1−/− mouse lacrimal glands there is an age dependent influx of lymphocytes and pro-inflammatory cytokines that can alter lacrimal gland structure and function leading to decrease secretion and ocular surface disease.
The major mechanism by which lacrimal gland and tear production is increased is by activation of afferent sensory nerves in the ocular surface that by a reflex activate efferent nerves that release neurotransmitters. The released neurotransmitters interact with their receptors on lacrimal gland cells to stimulate secretion, both protein and electrolyte/water. A decrease in total nerves (measured by anti-TUJ-1 antibody) and in parasympathetic nerves (measured by anti-VIP antibody) occurred by the 4 week time point in TSP-1−/− compared to WT lacrimal glands and continued at 12 and 24 weeks of age, when there is also a decrease in protein secretion stimulated by activating the nerves. Thus neural regulation of protein secretion is drastically altered in TSP-1−/− compared to WT lacrimal glands especially at older ages.
Zoukhri and co-authors32 showed that high KCl stimulation of nerves in pieces of lacrimal gland releases the neurotransmitter acetylcholine from parasympathetic nerves of wild type mice. The parasympathomimetic/cholinergic agonist-stimulated signaling pathway is one of the major pathways for lacrimal gland and tear secretion. The decrease in parasympathomimetic agonist (carbachol)-stimulated protein secretion that occurred in mouse lacrimal glands at 12 and 24 weeks of age in the present study is consistent with a major effect of lack of TSP-1 on the parasympathetic agonist stimulated protein secretion at both the neural and the acinar cell level. These data suggest that TSP-1−/− compared to WT mouse lacrimal glands have a defect in both the nerve/neurotransmitter release mechanism as well as in the signaling pathways activated by parasympathomimetic agonists in acinar cells. Of interest is our finding that nerves and neurally-mediated protein secretion was decreased at 4 weeks of age, but exogenous agonist induced secretion was not affected until later in disease progression. This finding suggests that nerves are most sensitive to the loss of TSP-1 and that the neurotransmitter receptors and the signaling pathways they activate in the acinar cells are less sensitive and unchanged until the disease progresses. These results are different from those found in the lacrimal glands of MRL/Mp-Fas-lpr/lpr (MRL/lpr) mice, a murine model for Sjogren’s syndrome, where the efferent nerve density was not altered, but the high KCl stimulated acetylcholine release was impaired.33 In contrast to the TSP-1−/− model, denervation supersensitivity occurred in the MRL/lpr model, as the acinar cell responses to neurotransmitters were increased compared to control animals.34 Thus, a different process affects nerves, but not neurotransmitter activation of acinar cells in the TSP-1−/− compared to the MRL/lpr model. As IL-1β is elevated in both of these models, we propose that in the TSP-1−/− lacrimal gland one of the structural domains of TSP-1 rather than the increase in cytokines could be affecting M3AchR agonist stimulation of secretion.
Cholinergic agonist stimulated protein secretion is dependent upon an increase in the [Ca2+]i. An increase in [Ca2+]i also directly participates in exocytosis of secretory granules that release secretory proteins. Most of the canonical Ca2+ signaling pathways in lacrimal glands are IP3 mediated and utilize internal ER Ca2+ stores for increasing Ca2+ signals leading to lacrimal gland protein secretion. Although differences in ER expression could directly affect cellular Ca2+ handling, the amount of ER is not altered in TSP-1−/− compared to WT lacrimal glands. Mitochondria also play a vital role in calcium homeostasis by acting as a buffer for any spontaneous rises in [Ca2+]i that overwhelm the capacity of the ER stores.35 Mitochondria are also the cellular “powerhouse” and impart energy to carry out cellular functions. Additionally, Kawashima et al. demonstrated mitochondrial damage in the lacrimal glands of patients diagnosed with Sjogren’s syndrome.36 The down regulation of a mitochondrial marker (Cox IV) suggests that mitochondrial expression is affected in the TSP-1−/− mouse lacrimal glands thereby hampering Ca2+ buffering in these cells. Moreover, an effect of mitochondrial oxidative damage on secretory output can also not be ruled out. A change in the amount of secretory granules could affect protein secretion. Decreased expression of Rab3D suggests that this pool of secretory vesicles might not be available for stimulation of secretion. As protein secretion from lacrimal acini is dependent on the intracellular Ca2+ stimulus and availability of secretory vesicles, a decrease in secretory granules could directly affect exocytosis and protein secretion.
There are conflicting changes in the use of intracellular Ca2+ by cholinergic agonists to stimulate protein secretion in TSP-1−/− compared to WT lacrimal gland acini. Consistent with a decrease in the Ca2+-dependent signaling pathway used by cholinergic agonists in TSP-1−/− mice is a decrease in the thapsigargin-releasable intracellular Ca2+ stores, amount of mitochondria, amount secretory vesicles, and a decrease in protein secretion. Inconsistent with this signaling pathway in TSP-1−/− mouse lacrimal glands is no change in the carbachol-induced [Ca2+]i and in the amount of ER thought to be the site of the thapsigargin-releasable intracellular Ca2+ stores. To explain the lack of change in the carbachol-induced [Ca2+]i that we expected to lower with decreasing protein secretion is the possibility that there could be a decrease in Ca2+-independent signaling pathways used by cholinergic agonists such as the activation of the Ca2+-independent PKC isoform PKCε that is known to stimulate cholinergic agonist protein secretion.37 Another possibility is that there could be an increase in the inhibitory pathways activated by cholinergic agonists in the lacrimal gland including activation of phospholipase D and ERK1/2.20 A final possibility is that the rapid increase in [Ca2+]i that occurs for only minutes does not correlate with the longer term depletion of thapsigargin-releasable intracellular Ca2+ stores that occurs over 15 minutes or with secretion that is measured over 40 min. Thus, use of intracellular Ca2+ stores over the 40 min stimulation of secretion could deplete the decreased stores in the TSP-1−/−, but not in the normal stores of the WT mouse lacrimal gland. M3AchR agonist-stimulated secretion is dependent on intracellular Ca2+ and influx of extracellular Ca2+ when these are depleted for the 40 min stimulation of secretion.18,38,39 To explain the lack of change in the amount of ER with decreasing intracellular Ca2+ stores we suggest that that only a specialized portion of the ER could be involved in the intracellular Ca2+ stores or that the proteins that regulate the [Ca2+]i such as Ca2+ ATPase, STIM1, and Orai could be changed in TSP-1−/− compared to WT lacrimal gland acini. Supporting this latter possibility is that the type II and III repeats of TSP-1 bind about 30 Ca2+ and that TSP-1 is located inside the ER.40 Lack of TSP-1 inside the ER and a decrease in Ca2+ binding could account for the depletion of intracellular Ca2+ stores that we found in the TSP-1−/− mouse lacrimal glands and for the decrease in M3AchR stimulated protein secretion. Further studies on protein binding, direct/indirect associations and cellular location of TSP-1 need to be done to specifically determine the different roles of TSP-1 in modulation lacrimal gland function.
The lacrimal gland is also innervated by sympathetic nerves and activation of these nerves releases norepinephrine that interacts with α1D-adrenergic receptors to stimulate protein secretion. Although the sympathetic innervation to the WT mouse lacrimal gland is less dense than the parasympathetic one, sympathomimetic agonists such as the α1D-adrenergic agonist phenylephrine cause an effective if not a more effective increase in secretion than parasympathomimetic ones. In contrast, the increase in agonist-stimulated [Ca2+]i is less for phenylephrine than for carbachol. Furthermore, the changes in α1D-adrenergic agonist-induced increase in [Ca2+]i and protein secretion in TSP-1−/− compared to WT lacrimal glands were different when compared to those stimulated by M3AChR agonists. In WT mice, phenylephrine-induced [Ca2+]i was increased at 12 weeks of age compared to 4 weeks before declining at 24 weeks. This could be due to the normal maturation process. In TSP-1−/− compared to WT mouse glands phenylephrine-induced [Ca2+]i was increased at 4 weeks, decreased at 12 weeks, and increased at 24 weeks of age; whereas secretion was not altered at any age. This is in contrast to carbachol where the rise in [Ca2+]I was unchanged but protein secretion was decreased in TSP1−/− compared to WT mice. It is unclear why the stimulation of the [Ca2+]i by phenylephrine is inconsistent, as no other marker or function that we measured at the three different ages showed this pattern. Similarly to M3AChR agonist-, α1-adrenergic agonist-induced protein secretion did not follow the pattern of phenylephrine stimulated [Ca2+]i suggesting that phenylephrine-induced protein secretion was not dependent upon the rapid increases in [Ca2+]i stimulated by agonists. Unlike M3AchR agonists, α1D-adrenergic agonist-induced secretion is less dependent on intracellular Ca2+ and influx of extracellular Ca2+ when these are depleted for the 40 min stimulation of secretion.38,39,41,42 Furthermore, whereas M3AChR agonist-induced secretion was decreased in TSP1−/− compared to control lacrimal glands, α1D-adrenergic agonist-induced secretion was unchanged. As M3AChR agonists use different signaling pathways than α1-adrenergic agonists, it is not surprising that the secretory responses are opposite. α1D-Adrenergic agonists use eNOS and cGMP to stimulate secretion and transactivate the EGFR to activate ERK1/2 that attenuate secretion; whereas M3AchR agonists do not. Another possible reason for the difference in α1D-adrenergic agonist secretory response is that only M3AChR, not α1D-adrenergic agonists use the thapsigargin-dependent intracellular Ca2+ stores. The rapid increase in [Ca2+]I represents the release of Ca2+ from intracellular stores, that are decreased in TSP1−/− compare to WT. M3AChR agonist stimulated secretion is dependent upon these stores. The decrease in these stores in TSP1−/− compare to WT may not be extensive enough to alter the M3AChR agonist-induced intracellular Ca2+ response that occurs over seconds, but is enough to decrease secretion that is measured over 40 min. In contrast the α1D-adrenergic agonist secretory response is less dependent upon intracellular Ca2+ and would not be affected by the decrease in the Ca2+ store in TSP1−/− compared to WT mouse lacrimal gland acini.
Purinergic agonists such as ATP can be released from efferent sympathetic and parasympathetic nerves and in the lacrimal gland the signaling pathways activated by P2X agonists and their interaction with parasympathomimetic and sympathomimetic agonists have been delineated. We determined if the P2X agonist ATP and the P2Y agonist 2MeSATP stimulation of [Ca2+]i and protein secretion was altered in TSP-1−/− compared to WT lacrimal glands. There were no consistent changes in these parameters with age in TSP-1−/− compared to WT glands. Thus, release of ATP from nerves and purinergic receptor activation of acinar function probably does not play a role in dry eye that occurs in TSP-1−/− mice.
TSP-1−/− female mice have ADDE progression in a time dependent fashion allowing us to dissect out the anomalies, which contribute to glandular dysfunction. This study presents a comprehensive and detailed overview of the processes involved in such a scenario. Additional functional and expression studies are needed to delve into the mechanistic intricacies of such a phenotype. Moreover, gain of function or phenotypic rescue experiments will throw light into additional mechanisms that are not apparent. Recent literature on contribution of TSP-1 in ocular inflammation along with the phenotypic characteristics unraveled in this study makes the TSP-1 mouse model a very useful for study of ADDE at its early developmental stages. Additionally, this model also might serve be a useful and novel one for rapid screening of drugs to treat ADDE.
Materials and Methods
Animals
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee. TSP1−/− mice were originally obtained from Dr. J. Lawler (BIDMC, Harvard Medical School, Boston, MA) and bred at the Boston University Medical School Animal Facility. These mice were made on the C57BL/6J background. For that reason 4, 12, and 24 week old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor ME) and used as control. Only female mice were used in this study. For euthanasia, they were anesthetized for 5 minutes in CO2. Thereafter, both exorbital lacrimal glands were removed immediately.
Preparation of Lacrimal Gland Acini
Lacrimal glands were fragmented and digested with collagenase (100 U/mL, CLSIII; Worthington Biochemicals) in Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES) (119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose, at pH 7.45) plus 0.5% BSA for 30 minutes at 37°C. After incubation, digested tissue was filtered through a nylon mesh (150-µM pore size), and centrifuged at 50g for 2 minutes. The pellet was washed twice through KRB-HEPES containing 4% BSA. The dispersed acini recovered for 60 minutes at 37°C before use.
Immunohistochemistry
Lacrimal glands were extracted and fixed in 4% formaldehyde diluted in phosphate-buffered saline (PBS) for 2 days at 4°C. Then the glands were incubated in 30% sucrose for a day, embedded in OCT and sectioned (10 µm thick) using a cryostat. The sections were stained with hematoxylin/eosin (H&E) or with antibodies against TUJ-1 (1:500, BioLegend, San Diego, CA), VIP (1:100, Abcam, Cambridge, MA), VE-cadherin (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), or PECAM (1:100, BD Biosciences, San Jose, CA) overnight at 4°C in a humidified chamber. The secondary antibody conjugated to either Cy2 or Cy3 (1:300 dilution) was applied for 1 hour at room temperature (RT). Coverslips were mounted on slides, viewed by fluorescence microscopy (Eclipse E80i; Nikon, Tokyo, Japan), and micrographs were taken with a digital camera (Spot; Diagnostic Instruments, Inc., Sterling Heights, MI) or viewed by confocal microscopy (TCS-SP2, Leica Microsystems, Bannockburn, IL). Sections incubated in the presence of isotype controls served as the negative control. The length of blood vessels was measured using the Imaris program.
Measurement of Blood Vessel Area
Mouse lacrimal glands were labeled with VE-cadherin (labels endothelial cell junctions) or PECAM (labels vascular endothelial cells) and three-four z stacks per sample that were ~30 microns thick were collected from mouse lacrimal glands sections. Each z-stack was collected from different section. Then we used Imaris Image Analysis Software (Bitplane Zurich, Switzerland) to create 3D reconstructions from confocal Z series. To measure total blood vessels length in each z-stack the FilamentTracer module of Imaris software was used and filament tracing analyses were performed. This information was then used to quantify potential changes in vascular length in the TSP1−/− lacrimal glands. Two-Tailed t-test was used to determine whether the difference between TSP1−/− and WT blood vessels is statistically significant.
Western blotting analysis
Lacrimal glands were removed from mice and homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA) in the presence of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) to lyse the cells. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and processed for western blotting. The antibodies against Rab3D, calnexin, Lamp1, and CoxIV were used at 1:200 dilution (all from Santa Cruz Biotechnology). Immunoreactive bands were visualized by the enhanced chemiluminescence method. The films were analyzed with Image J software (http://rsbweb.nih.gov/ij/).
Measurement of [Ca2+]i
Lacrimal acini were incubated for 30 minutes at RT in the dark with KRB-HEPES containing 0.5% BSA, 0.5 µM fura-2/AM, 8 µM pluronic acid F127, and 250 µM sulfinpyrazone followed by washing in KRB-HEPES containing sulfinpyrazone. Lacrimal acini were stimulated with carbachol, phenylephrine, ATP, and 2MeSATP (all from Sigma-Aldrich). Calcium measurements were made with a ratio imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH) using excimer wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. Experiments were repeated in at least three animals. After the addition of agonists, data were collected in real time. Data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]I was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data are not shown, the plateau [Ca2+]i was affected similarly to the peak [Ca2+]i, except where indicated. To calculate intracellular Ca2+ stores, extracellular Ca2+ was removed. Then, cells were treated for 15 minutes with 10−5 M thapsigargin. Peak [Ca2+]i and total area under the curve were measured. After that, Ca2+ was reintroduced in the bath and the rate of refilling was analyzed.
Measurement of Peroxidase Secretion
Lacrimal pieces were incubated for 40 minutes in KRB-HEPES containing 4% BSA at 37°C in the presence of the agonists: carbachol, phenylephrine, ATP, and 2MeSATP. To terminate the incubation, pieces were pelleted by centrifugation, and the supernatant was collected. The pellet was homogenized in 10 mM Tris-HCl (pH 7.5). Peroxidase activity, an index of protein secretion, was measured in duplicate in both the supernatant and the pellet. Peroxidase was measured using Amplex Red reagent (Invitrogen), which, when oxidized by peroxidase in the presence of hydrogen peroxide, produces a highly fluorescent molecule. The amount of fluorescence in the supernatant and pellet was quantified using a fluorescence microplate reader (model FL600; BioTek) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Peroxidase was expressed as a percentage of peroxidase secreted into the media (supernatant) compared with total peroxidase present in the cells before stimulation (pellet plus supernatant). Data were expressed as fold increase over basal, which was set to 1.
Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM). Data were analyzed by Student's t-test. P< 0.05 was considered statistically significant.
Acknowledgments
This work was supported by NEI R01EY06177, NEI R01 EY026202, Fight for Sight Foundation Postdoctoral Award Grant and NIH P30 EY00379035.
Footnotes
Author Contributions
SB, RH, HM carried out the experiments and analyzed data. DD, SB designed the experiments. SB, DD, LGP drafted the manuscript, SM consulted and advised on mouse model.
Additional Information
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Coursey TG, de Paiva CS. Managing Sjogren's Syndrome and non-Sjogren Syndrome dry eye with anti-inflammatory therapy. Clinical Ophthalmology. 2014;8:1447–1458. doi: 10.2147/OPTH.S35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Deutsches Arzteblatt International. 2015;112:71–81. doi: 10.3238/arztebl.2015.0071. quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnati R, Laurie DE, Laurie GW. Lacritin and the tear proteome as natural replacement therapy for dry eye. Experimental Eye Research. 2013;117:39–52. doi: 10.1016/j.exer.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. The ocular surface. 2004;2:76–91. doi: 10.1016/s1542-0124(12)70146-5. [DOI] [PubMed] [Google Scholar]
- 5.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Progress in retinal and eye research. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. International Reviews of Immunology. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel R, Shahane A. The epidemiology of Sjogren's syndrome. Clinical Epidemiology. 2014;6:247–255. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pflugfelder SC, Stern ME. Mucosal environmental sensors in the pathogenesis of dry eye. Expert Review of Clinical Immunology. 2014;10:1137–1140. doi: 10.1586/1744666X.2014.944163. [DOI] [PubMed] [Google Scholar]
- 9.Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjogren's syndrome. PloS One. 2013;8:e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turpie B, et al. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. The American Journal of Pathology. 2009;175:1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You J, et al. Tear fluid protein biomarkers. Advances in Clinical Chemistry. 2013;62:151–196. doi: 10.1016/b978-0-12-800096-0.00004-4. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JH, Young JA, van Lennep EW. The ultrastructure of the duct system in the rat extraorbital lacrimal gland. Zeitschrift fur Zellforschung und Mikroskopische Anatomie. 1973;144:453–466. doi: 10.1007/BF00307373. [DOI] [PubMed] [Google Scholar]
- 13.Gilbard JP, Dartt DA. Changes in rabbit lacrimal gland fluid osmolarity with flow rate. Investigative Ophthalmology & Visual Science. 1982;23:804–806. [PubMed] [Google Scholar]
- 14.Dartt DA, et al. Lacrimal gland inositol trisphosphate isomer and inositol tetrakisphosphate production. The American Journal of Physiology. 1990;259:G274–281. doi: 10.1152/ajpgi.1990.259.2.G274. [DOI] [PubMed] [Google Scholar]
- 15.Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. The American Journal of Physiology. 1997;272:C263–269. doi: 10.1152/ajpcell.1997.272.1.C263. [DOI] [PubMed] [Google Scholar]
- 16.Hodges RR, Guilbert E, Shatos MA, Natarajan V, Dartt DA. Phospholipase D1, but not D2, regulates protein secretion via Rho/ROCK in a Ras/Raf-independent, MEK-dependent manner in rat lacrimal gland. Investigative Ophthalmology & Visual Science. 2011;52:2199–2210. doi: 10.1167/iovs.10-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoukhri D, Dartt DA. Cholinergic activation of phospholipase D in lacrimal gland acini is independent of protein kinase C and calcium. The American Journal of Physiology. 1995;268:C713–720. doi: 10.1152/ajpcell.1995.268.3.C713. [DOI] [PubMed] [Google Scholar]
- 18.Hodges RR, Dicker DM, Rose PE, Dartt DA. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. The American Journal of Physiology. 1992;262:G1087–1096. doi: 10.1152/ajpgi.1992.262.6.G1087. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, et al. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. American Journal of Physiology. Cell physiology. 2006;291:C946–956. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota I, et al. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. American Journal of Physiology. Cell physiology. 2003;284:C168–178. doi: 10.1152/ajpcell.00151.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Investigative Ophthalmology & Visual Science. 1997;38:610–619. [PubMed] [Google Scholar]
- 22.Venugopalan CS. Vasoactive intestinal peptide (VIP), a putative neurotransmitter of nonadrenergic, noncholinergic (NANC) inhibitory innervation and its relevance to therapy. Journal of Veterinary Pharmacology and Therapeutics. 1989;12:113–123. doi: 10.1111/j.1365-2885.1989.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 23.Hodges RR, Vrouvlianis J, Scott R, Dartt DA. Identification of P2X(3) and P2X(7) purinergic receptors activated by ATP in rat lacrimal gland. Investigative Ophthalmology & Visual Science. 2011;52:3254–3263. doi: 10.1167/iovs.10-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtomo K, et al. Increase of intracellular Ca2+ by purinergic receptors in cultured rat lacrimal gland myoepithelial cells. Investigative Ophthalmology & Visual Science. 2011;52:9503–9515. doi: 10.1167/iovs.11-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams JC, Lawler J. The thrombospondins. Cold Spring Harbor Perspectives in Biology. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Current Eye Research. 2014;39:759–774. doi: 10.3109/02713683.2013.877936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shatos MA, et al. Alteration in cellular turnover and progenitor cell population in lacrimal glands from thrombospondin 1−/− mice, a model of dry eye. Experimental Eye Research. 2016;153:27–41. doi: 10.1016/j.exer.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iruela-Arispe ML, Vazquez F, Ortega MA. Antiangiogenic domains shared by thrombospondins and metallospondins, a new family of angiogenic inhibitors. Annals of the New York Academy of Sciences. 1999;886:58–66. doi: 10.1111/j.1749-6632.1999.tb09400.x. [DOI] [PubMed] [Google Scholar]
- 29.Kania E, Roest G, Vervliet T, Parys JB, Bultynck G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Frontiers in Oncology. 2017;7:140. doi: 10.3389/fonc.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. The Journal of biological chemistry. 1991;266:21327–21330. [PubMed] [Google Scholar]
- 31.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Progress in retinal and eye research. 2012;31:271–285. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjogren's syndrome. Investigative ophthalmology & visual science. 2001;42:925–932. [PMC free article] [PubMed] [Google Scholar]
- 33.Zoukhri D, Hodges RR, Dartt DA. Lacrimal gland innervation is not altered with the onset and progression of disease in a murine model of Sjogren's syndrome. Clinical Immunology and Immunopathology. 1998;89:126–133. doi: 10.1006/clin.1998.4597. [DOI] [PubMed] [Google Scholar]
- 34.Zoukhri D, Hodges RR, Rawe IM, Dartt DA. Ca2+ signaling by cholinergic and alpha1-adrenergic agonists is up-regulated in lacrimal and submandibular glands in a murine model of Sjogren's syndrome. Clinical Immunology and Immunopathology. 1998;89:134–140. doi: 10.1006/clin.1998.4598. [DOI] [PubMed] [Google Scholar]
- 35.Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Archiv : European journal of physiology. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawashima M, et al. Comparison of telomere length and association with progenitor cell markers in lacrimal gland between Sjogren syndrome and non-Sjogren syndrome dry eye patients. Molecular vision. 2011;17:1397–1404. [PMC free article] [PubMed] [Google Scholar]
- 37.Zoukhri D, Hodges RR, Dicker DM, Dartt DA. Role of protein kinase C in cholinergic stimulation of lacrimal gland protein secretion. FEBS Letters. 1994;351:67–72. doi: 10.1016/0014-5793(94)00824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putney JW, Bird GS. Calcium signaling in lacrimal glands. Cell Calcium. 2014;55:290–296. doi: 10.1016/j.ceca.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dartt DA, Hodges RR, Zoukhri D. Signal transduction pathways activated by cholinergic and alpha 1-adrenergic agonists in the lacrimal gland. Advances in experimental medicine and biology. 1998;438:113–121. doi: 10.1007/978-1-4615-5359-5_15. [DOI] [PubMed] [Google Scholar]
- 40.Duquette M, et al. Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity. Matrix biology : journal of the International Society for Matrix Biology. 2014;37:15–24. doi: 10.1016/j.matbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dartt DA, Rose PE, Dicker DM, Ronco LV, Hodges RR. Alpha 1-adrenergic agonist-stimulated protein secretion in rat exorbital lacrimal gland acini. Experimental Eye Research. 1994;58:423–429. doi: 10.1006/exer.1994.1035. [DOI] [PubMed] [Google Scholar]
- 42.Hodges RR, Dicker DM, Rose PE, Dartt DA. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am J Physiol. 1992;262:G1087–1096. doi: 10.1152/ajpgi.1992.262.6.G1087. [DOI] [PubMed] [Google Scholar]