Abstract
An abasic (AP) site is a ubiquitous DNA lesion that is produced via several cellular processes. Although AP sites are cytotoxic and mutagenic, cells are protected from them by different DNA damage tolerance and repair pathways, including base excision repair (BER). AP lesions are alkali-labile but the half-life for strand scission is several weeks in free DNA at around neutral pH. AP lifetime is reduced ~100-fold in nucleosome core particles (NCPs) because the histone proteins promote strand scission. The reactivity of other DNA lesions to BER enzymes and exogenous reagents is highly dependent upon rotational positioning within the NCP. We examined strand scission at AP sites as a function of rotational position over approximately one helical turn of DNA. The rate constant for strand scission at AP varies ~4-fold, a much smaller range in reactivity than observed for processes that involve reaction with diffusible reagents in solution. In addition, the change in rate constant does not exhibit an obvious pattern with respect to rotational position. The small dependence of reactivity on rotational position is attributed to interactions with histone proteins. A molecular model based upon NCP X-ray crystal structures indicates that histone protein tails access AP sites via the major or minor groove, and are therefore not limited to regions where one particular groove is exposed to solvent. Determining the roles of individual proteins is difficult due to the unstructured nature of the histone tails and the chemical mechanism, which involves reversible Schiff base formation, followed by irreversible elimination.
Graphical abstract
INTRODUCTION
An abasic site (AP) is the most ubiquitous DNA lesion. AP sites arise from a variety of processes, including spontaneous depurination, which is accelerated upon DNA alkylation. AP sites are also formed as intermediates during base excision repair (BER) of damaged nucleotides. Under typical conditions, ~10,000 AP sites are produced per cell/day.1, 2 It is well established that AP sites adversely affect replication and transcription.3 More recently, chemical studies in the test tube have identified additional possible cellular consequences of AP sites. For instance, AP sites produce DNA interstrand cross-links, a very deleterious form of DNA damage, with suitably positioned purines.4–9 In addition, histone proteins in nucleosome core particles (NCPs) accelerate DNA cleavage at AP sites and various oxidized abasic sites by as much as 1,500-fold, and their half-lives are as short as 10 min.10–15 Although the lifetimes of oxidized abasic sites in cells are unknown, estimates of AP site lifetimes range from an hour to more than one day.16, 17 Since oxidized abasic sites are repaired less efficiently than AP sites in the test tube, the lifetimes of some lesions in NCPs are sufficiently short that the histone promoted chemistry may be competitive with BER.18, 19 We wish to report the rotational effects within NCPs on AP reactivity.
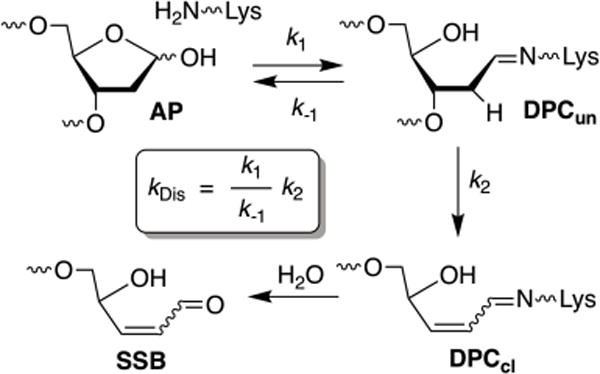
DNA is readily cleaved at AP sites upon alkaline treatment (e.g. 0.1 M NaOH/H2O). However, the half-life of this alkaline labile lesion in duplex DNA is ~1,000 h (several weeks) in phosphate buffered saline at 37 °C.10, 11 AP half-life in an identical sequence is reduced ~100-fold when present within a NCP. The reaction mechanism of this process was examined most thoroughly for an AP site at position 89 (superhelical location (SHL) 1.5, Figure 1A). The DNA is kinked at this site, and is a hot spot for DNA damaging molecules.20, 21 A variety of kinetics experiments support a mechanism in which AP cleavage proceeds through transient DNA-protein cross-links containing uncleaved DNA (DPCun) from which β-elimination occurs (DPCcl) (Scheme 1).11, 22 Site specific mutagenesis experiments revealed that the lysine-rich histone H4 tail is responsible for the bulk of the increased AP89 reactivity within the NCP. Despite lacking carboxylic-containing amino acids which catalyze the β-elimination, the histone protein acts much like a lyase enzyme. The lysine residues are responsible for Schiff-base formation and for inducing β-elimination.
Figure 1.
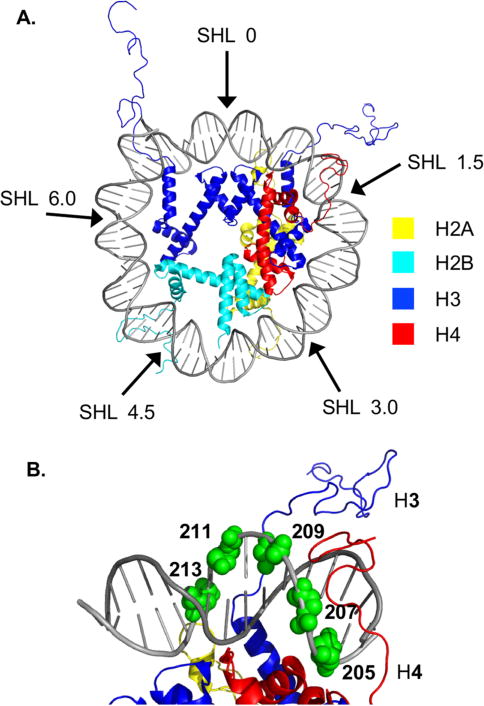
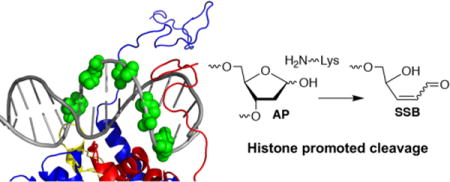
Nucleosome core particle structure. (A.) Overview of NCP structure with one DNA gyre hidden to enhance visibility of DNA-octamer. (B.) Zoomed in region between SHL 0 and 1.5. Positions (205, 207, 209, 211, 213) at which AP sites are introduced are indicated in green spheres. (Structure taken from PDB: 1kx5.)
Scheme 1.
Experiments with histone H4 variants also indicated that even the most distal of the lysine residues within the tail interact with electrophilic DNA at position 89.11, 12, 14, 23 However, the proximity of AP89 to the location from which the flexible protein tail protrudes through the DNA provides greater interaction between the lysine residues and DNA lesion. We speculated that DNA-protein interactions would vary when the abasic sites were generated at positions that were further removed from the point at which the histone H4 tail exits from the octameric core (Figure 1A). Positioning AP sites closer towards the dyad axis would require the H4 tail to extend, but would also increase the proximity to the lysine rich amino terminal tails of other histone proteins, particularly H3. Furthermore, variable positioning of the abasic site along the helix alters its rotational orientation with respect to the octameric core. Rotational positioning within a nucleosome core particle affects DNA reactivity with freely diffusible hydroxyl radical.24, 25 The reaction of damaged DNA with repair enzymes varies with respect to rotational positioning, although not always in a manner that one would predict based upon the orientation of the DNA groove with respect to the octameric histone core.26–29 Perhaps most relevant to the current investigation, the inherent reactivity of damaged DNA, specifically the rate of deamination in 5-methylcytosine (mC) within cyclobutane pyrimidine photodimers formed with thymidine (TmC), varies by more than 35-fold depending upon rotational positioning.30, 31 We examined the variation of AP reactivity within a NCP as a function of rotational positioning. The dependence of AP reactivity in NCPs as a function of rotational position is much smaller than that of other processes. This is attributed to a fundamental difference in reactivity. AP cleavage in a NCP is dependent upon its interaction with one or more histone proteins and not a diffusible species in solution.
MATERIALS AND METHODS
General Methods
Oligonucleotides were synthesized on an Applied Biosystems Incorporated 394 oligonucleotide synthesizer. Oligonucleotide synthesis reagents were purchased from Glen Research (Sterling, VA). T4 polynucleotide kinase (T4 PNK), T4 DNA ligase, uracil DNA glycosylase (UDG), proteinase K and DNase I were purchased from New England Biolabs (NEB). Nuclease P1 (from Peniciliumcitrinum) was obtained from Sigma and dissolved in water (1 U/μL). Amicon® Ultra Centrifugal Filters were purchased from Millipore. Salmon sperm DNA (10 mg/mL) was purchased from Invitrogen. The 145 bp 601 DNA containing AP precursor or internally labeled 5′-32P-AP was prepared as previously described.32 Expression and purification of all core histone proteins, as well as refolding and purification of the histone octamer, were carried out as previously described.33 DNA photolysis was carried out in a Rayonet photoreactor (RPR-100) equipped with 16 lamps with a maximum output at 350 nm. All experiments with NCPs were conducted in clear siliconized tubes (Bio Plas Incorporated). Quantification of radiolabeled oligonucleotides was carried out using a Molecular Dynamics Phosphorimager equipped with ImageQuant TL software.
General procedure for DNase I footprinting of nucleosome core particles
Reconstituted nucleosome core particles were concentrated to ~30 μL using a 10 K Amicon® filter (Millipore). The concentrated NCPs (10 μL, ~300,000 cpm) were treated with various amounts of DNase I (0.2 U, 0.5 U or 1.0 U) in a 15-μL reaction containing 1 × DNase buffer (10 mM Tris-HCl, 7.5 mM MgCl2, 0.5 mM CaCl2, pH 7.6) for 5 min at room temperature and naked DNA (~300,000 cpm) was treated with DNase I (0.05 U or 0.1 U) in a 20-μL reaction for 30 s at room temperature. The reactions were quenched by adding 2 μL DNase quench buffer (500 mM EDTA, 50 μg/mL salmon sperm DNA) and the DNA was purified by 6% native PAGE (acrylamide/bisacrylamide, 59:1, 10 × 8 × 0.15 cm). Intact nucleosome core particles were excised from the native PAGE gel and eluted overnight in 500 μL elution buffer containing 0.1% SDS and 3.2 U proteinase K at room temperature. The solution (~300 μL) was decanted from the gel segment and mixed with 1 μg Salmon sperm DNA. The DNA was precipitated by adding 3 M NaOAc (30 μL, pH 5.4) and cold ethanol (900 μL). The DNA was analyzed by 10% denaturing PAGE (acrylamide/bisacrylamide; 19:1, 7 M urea, 40 × 32 × 0.04 cm).
General procedure for determining the reactivity of AP in nucleosome core particles
NCPs containing AP precursor were photolyzed (350 nm) for 15 min at room temperature, and immediately incubated at 37 °C. Aliquots (~10,000 cpm) were removed at appropriate times and frozen at −80 °C. All aliquots were then treated with fresh NaBH4 (0.1 M) at 4 °C for 1 h and then room temperature for 2 h. Half of the reduced samples were analyzed by 10% SDS PAGE (20 × 16 × 0.1 cm, acrylamide/bisacrylamide 29:1, 5% stacking layer) to determine the DNA-protein cross-links (DPCs) and single-strand breaks (SSBs). The other half of the samples were treated with proteinase K (1.6 U) for 30 min at room temperature prior to analyzing by 10% denaturing PAGE (20 × 16 × 0.1 cm, acrylamide/bisacrylamide 19:1) to determine the total DNA strand cleavage. To determine the photolysis efficiency, two aliquots were removed immediately after the photolysis, treated with NaOH (0.1 M) for 30 min at 37 °C, neutralized by HCl and analyzed by 10% denaturing PAGE. For reactions carried out in the presence of NaBH3CN (10 mM), fresh reductant was added just before the photolysis. The data collected from time course experiments were normalized based on the NCP reconstitution and photolysis efficiency:
DNAcl and DNAun are the cleaved and uncleaved DNA respectively. The rate constant for AP reaction was obtained by fitting the disappearance of DNAun to a first order reaction.
General procedure for determining the protein(s) involved in cross-linking with AP
The NCPs containing internally labeled 5′-32P-AP were reconstituted at 4 °C. The NCPs (~1,000,000 cpm) were incubated at 37 °C for an appropriate length of time (AP205: 60 h; AP207: 12 h; AP209: 37 h; AP211: 37 h; AP213: 52 h) in the presence of NaBH3CN (10 mM) and then concentrated to ∼30 μL using a 10 K Amicon® filter (Millipore). The concentrated NCPs (10 μL, ~100,000 cpm) were treated with DNase I (2 U) in a 15 μL reaction containing 1 × DNase I reaction buffer (10 mM Tris-HCl, pH 7.6, 7.5 mM MgCl2, 0.5 mM CaCl2) for 10 min at 37 °C. The reaction was quenched by addition of a 10% SDS solution (final SDS amount: 0.5%). The mixture was then heated at 90 °C for 3 min, quickly put on ice, and diluted to 50 μL with nuclease P1 buffer (50 mM Bis-Tris propane, pH 6.0, 1 mM MgCl2, 0.1 mM ZnCl2). Nuclease P1 (1 U, 1 μL) was added and the solution was incubated at 37 °C for 2 h. Additional Nuclease P1 (1 U, 1 μL) was then added and the solution was incubated at 37 °C for an additional hour. The histone proteins with 32P-AP transferred were precipitated by adding cold acetone (400 μL), followed by a 2 h incubation at −20 °C. The proteins were pelleted by centrifugation (16,000 × g, 30 min, 4 °C). The supernatant was carefully removed so as to disturb the pellet, followed by slowly adding 100 μL cold acetone to the precipitate. The proteins were pelleted again by centrifugation (16000 × g, 5 min, 4 °C). After drying in a Speed Vac concentrator, the proteins were resuspended in SDS PAGE gel loading buffer (60 mM Tris, pH 6.8, 10% glycerol, 2% SDS, 0.1 mg/mL bromophenol blue), and a portion of the sample (~2000 cpm) was analyzed by 20% SDS PAGE (20 × 16 × 0.1 cm, acrylamide/bisacrylamide 29:1, 5% stacking layer). If a protein band was detected with the size similar to H2A and H2B, the protein samples were dissolved in a buffer containing 8 M urea, 10 mM DTT and 5% acetic acid and analyzed by 15% triton/acid/urea (TAU) PAGE (10 × 8 × 0.15 cm, acrylamide/bisacrylamide 59:1, 8 M urea, 5% acetic acid and 0.37% Triton X-100). The TAU PAGE gel was prepared and utilized as previously described.34
RESULTS AND DISCUSSION
Design and preparation of NCPs containing site-specific AP
NCPs comprised of the “601” strong positioning DNA were generated containing an abasic site in a single DNA strand that extended from position 205 towards the dyad axis (Figure 1B).35 AP205 was chosen because it is proximal to the superhelical location (SHL) 1.5, where the DNA is kinked and is a hot spot for DNA damaging agents.20, 36 The AP sites spanned almost a complete helical turn from position 205 through 213 (Figure 1B). The accessibility of the DNA to histone tails was expected to change as the AP lesion position varies from position 205 through 213. The N-terminal tail of histone H4 protrudes from the octameric core in the vicinity of AP205, but X-ray crystallography suggests that the unstructured N-terminal tail of histone H3 and C-terminal tail of H2A tail are closer to AP213.36
Time course experiments were carried out using NCPs containing AP sites generated from an alkaline stable, photolabile precursor (1, Scheme 2). AP sites were generated within the reconstituted NCPs immediately prior to use via a brief, 15 min photolysis at 350 nm. AP generation was carried out after NCP formation to prevent adventitious cleavage during the reconstitution process. Experiments to identify histone proteins responsible for cross-linking to AP sites required 32P-labeling of the AP 5′-hydroxyl. Because oligonucleotides containing a 5′-1 are not labeled by polynucleotide T4 kinase, these experiments were carried out using 2′-deoxyuridine (dU) at the 5′-terminus. dU was transformed into AP within 145 bp, internally 32P-labeled duplex DNA using UDG prior to reconstitution.10 The NCPs containing 32P-AP were reconstituted at 4 °C to avoid non-specific histone-DNA cross-link formation and adventitious AP cleavage.
Scheme 2.
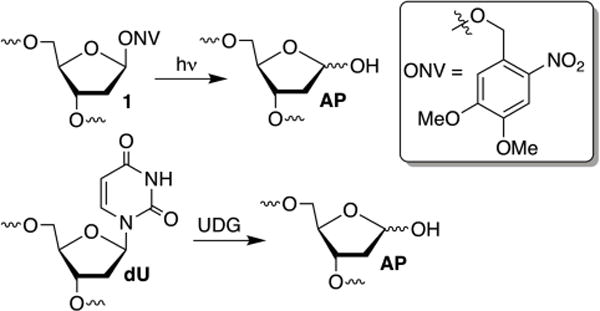
The oligonucleotides containing 1 were synthesized as previously described.10 Gel purified products were characterized by mass spectrometry. For kinetic experiments, with the exception of the 5′-terminal oligonucleotide, the synthetic oligonucleotides were 5′-phosphorylated, and ligated using T4 DNA ligase. Assembly of the 145 nt products was carried out on nanomole scale, and the products were purified by denaturing PAGE and stored at −80 °C. The 145mer was 5′-32P-labeled prior to hybridizing with its complement and reconstituted with purified histone octamer. Substrates for histone cross-linking experiments required internally 32P-labeled DNA. The 145nt material required was freshly prepared in a similar manner, with 5′-32P labeled dU containing oligonucleotides.
Kinetic analysis of AP reactivity as a function of rotational position
The half-lives for AP sites at a small number of distinct regions within NCPs have been reported.10, 11, 22 AP reactivity varied by less than 4-fold within these regions, a small difference compared to the overall ~100-fold acceleration compared to reaction within free DNA. However, no information is available regarding the effect of rotational position on abasic site reactivity within nucleosomal DNA. AP reactivity was methodically examined over approximately one turn of the helix (Table 1, Figure 1B). Of the positions previously studied, AP205 is amongst the slowest to react.10, 11 The rate constant for its disappearance (kDis) measured here (Table 1) was within experimental error of that previously reported.11 As the AP site is moved along the DNA strand towards the dyad axis, the half-life for its disappearance initially decreases (AP207), but then reacts at approximately the same rate at positions 209 and 211 as does AP205. AP213 reacts most slowly, but its half-life is only slightly more than 4-fold longer than the most reactive position (AP207).
Table 1.
Kinetics of AP disappearance as a function of position and histone structure.
| WTa | H3 Del 1-37 | H4 Del 1-20 | ||||
|---|---|---|---|---|---|---|
| AP position | kDis × 10−6 (s−1) | t1/2 (h) | kDis × 10−6 (s−1) | t1/2 (h) | kDis × 10−6 (s−1) | t1/2 (h) |
| 205 | 6.3 ± 0.2 | 30.5 ± 1.1 | 15.5b | 12.4 | 1.8b | 104.2 |
| 207 | 13.1 ± 2.7 | 15.1 ± 3.0 | 14.5b | 13.3 | 1.3b | 149.4 |
| 209 | 7.2 ± 1.1 | 27.2 ± 3.9 | 6.3 ± 1.2c | 31.1 ± 5.9 | 1.2 ± 0.1c | 158.0 ± 6.6 |
| 211 | 6.0 ± 0.6 | 32.3 ± 3.3 | 120.3b | 1.6 | 7.6b | 25.4 |
| 213 | 3.0 ± 0.5 | 65.9 ± 10.3 | 3.8 ± 0.3c | 50.8 ± 3.7 | 2.1 ± 0.2c | 91.4 ± 8.2 |
Rate constants are the ave. ± std. dev. of 3 experiments, each consisting of 3 replicates.
Rate constants are from one experiment consisting of 3 replicates.
Rate constants are the ave. ± std. dev. of 2 experiments, each consisting of 3 replicates.
The range of AP reactivity over the span of approximately one turn of the helix is much smaller than the differences in rates of deamination of a 5-methylcytidine•thymidine cyclobutane pyrimidine photodimer (TmC).30 TmC deaminates more than 35 times faster when it is in a strand facing towards solvent (“out”) than when the strand is oriented towards the histone octamer. The authors point out that the large difference in reactivity is not simply due to variable accessibility to water (the nucleophile), because the C4-carbon that water attacks to induce deamination is not obstructed by the protein when the photodimer is in either rotational position. However, the rationale for why there is such a large difference in reactivity is uncertain.
In contrast to TmC deamination and base excision repair of damaged nucleotides, AP reactivity is significantly accelerated in NCPs compared to free DNA at every position. Furthermore, this is true for every type of abasic site reported on.10–14, 22 The fundamental difference between the other processes referred to above, is that abasic site reactivity within a NCP does not depend upon access to a diffusible agent, such as water or an enzyme. Instead, AP reactivity in NCPs is primarily dependent upon interactions with histone tails, and is in effect an intramolecular interaction. More specifically, cleavage at AP sites (Scheme 1) requires access to the anomeric carbon (Schiff base formation) and the C2-position (for elimination).
The DNA sequences employed in this work are based upon the strong positioning 601 DNA.35 The crystal structure (PDB: 3lz0) of the 601 NCP consists of an octameric core with structurally unsolved histone tails.37 This prevents us from closely examining their interactions with AP. Thus, a model NCP structure was created by overlaying the 601 DNA with the protein portion of a crystal structure composed of α-satellite DNA and histones with tails (PDB: 1kx5) (Figure 2).36 Inspection of this model from multiple perspectives reveals that the AP sites are accessible to the lysine-rich amino terminal histone tails from either the major or minor groove, but which groove and tail(s) depends upon the position (Figure 2). For instance, the model suggests that AP205 – AP209 (Figure 2A, 2B) are accessible to the histone H4 tail from the minor groove. AP209 also appears to be accessible to histone H3 from the minor groove, as does AP211. AP213 is the only position of those examined that appears accessible to the C-terminal tail of H2A, although the histone H3 tail is also well positioned in the minor groove (Figure 2C). Overall, each of the abasic lesions is accessible to one or more lysine rich histone tails. Experiments involving histone variants indicated that all but ~5-fold of the ~100-fold rate acceleration of AP89 reactivity in a NCP is attributable to the lysine rich amino terminal tails.22 If the observations in those experiments can be extrapolated to other positions and histone tails, it is not surprising that a relatively modest rotational dependence is observed for AP reactivity in NCPs, because at each position one or more tails are well positioned to react.
Figure 2.
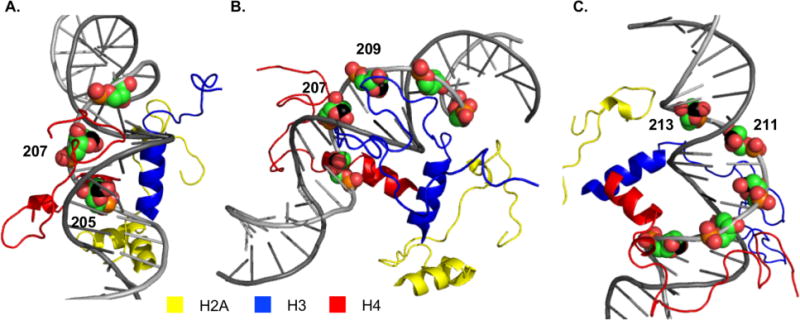
Nucleosome core particle model structures showing AP sites from different perspectives. (A.) Perspective showing access to AP205 and AP207. (B.) Perspective showing access to AP207 and AP209. (C.) Perspective showing access to AP211 and AP213. The anomeric carbon of AP sites is shown in black. (Model created by combining PDB: 1kx5, 3lz0. See text for explanation.)
Identification of protein(s) responsible for Schiff base formation
To test the hypothesis that AP interactions with histone tails correlate with their reactivity in NCPs, we first determined which proteins form transient DPCs, presumably via Schiff base formation with lysine residues. This was accomplished using NCPs in which the 5′-phosphate of the AP site is 32P-labeled (Scheme 3).10 The DPCs were trapped by sodium cyanoborohydride. Following DNA digestion, the modified proteins were separated by SDS PAGE (or TAU PAGE if H2A and/or H2B were tagged). The proteins were identified using purified histones as standards, and the relative amounts of transient DPCs formed with each histone were determined via 32P quantification. Histone-AP cross-linking is dominated by H4 at positions 205-209, and DPCs involving histone H4 are the only cross-links detected at AP207 (Table 2). Cross-linking at AP211 is still distributed between histone H3 and H4, but the preference (~3:1) is shifted toward the former. A more drastic change is observed at AP213 where more than 40% of the DPCs involve H2A.
Scheme 3.
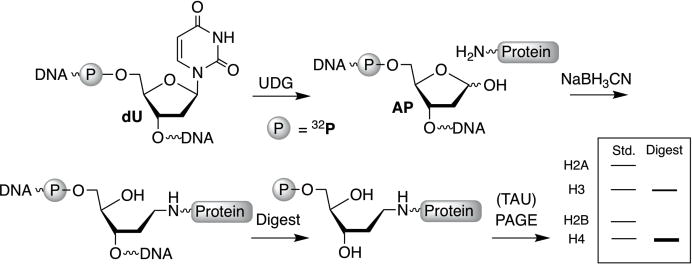
Table 2.
Distribution of DNA-protein cross-links as a function of AP position.a
| AP Position | H2A (%) | H2B (%) | H3 (%) | H4 (%) |
|---|---|---|---|---|
| 205 | 0 | 0 | 22.7 ± 3.4 | 77.3 ± 3.4 |
| 207 | 0 | 0 | 0 | 100 |
| 209 | 0 | 0 | 27.5 ± 3.0 | 72.5 ± 3.0 |
| 211 | 0 | 0 | 76.0 ± 3.2 | 24.0 ± 3.2 |
| 213 | 41.7 ± 0.8 | 0 | 35.2 ± 1.8 | 23.1 ± 1.2 |
Values are the ave. ± std. dev. of 3 experiments.
The distribution of proteins responsible for transient DPC formation is largely consistent with expectations based upon the proximity between the histone tails and AP predicted from the model (Figure 2). Histone H4 dominates reaction at AP205 – AP209, while reactivity transitions more towards histone H3 at AP211. Histone H2A reaction with AP213 is also consistent with the anticipated proximity of its carboxy tail to this position. The most surprising observation is formation of a minor amount of AP213 cross-linking with histone H4. However, this observation is not unreasonable, as extension of the unstructured tail places it within reach of the abasic site.
The effects of histone tail deletions on AP reactivity
Previous studies established a general mechanism (Scheme 1) in which reversible DPCun formation is followed by a rate limiting, irreversible elimination step to form a DPC containing cleaved DNA (DPCcl). DPCcl is ultimately converted to single-strand breaks upon Schiff base hydrolysis. The data used to determine rate constants for AP disappearance (kDis, Table 1) were obtained by denaturing PAGE, following protein digestion. Hence, irreversible elimination is required for detecting AP disappearance. Consequently, the distribution of proteins responsible for DPC formation (Table 2) need not correlate with AP half-life in the NCP, since DPCun are not accounted for in this analysis. Hypothetically, slow elimination from a Schiff base (DPCun) involving one protein relative to a DPCun involving a different histone protein that traps the abasic site less efficiently could result in less significant contribution from the former to kDis. We also cannot rule out Schiff base formation involving one histone protein and a second protein promoting elimination.
Additional insight into which protein(s) were responsible for strand scission at the AP sites was gleaned from utilizing histone variants in which the amino terminal tails were deleted (Table 1, Scheme 4). Deleting the 20 N-terminal amino acids from histone H4 resulted in a significant increase in lifetimes of AP sites at positions 205, 207, and 209. In contrast, AP207 and AP209 reactivity was unchanged in NCPs lacking the 37 N-terminal amino acids of histone H3 even though histone H3 contributes to transient DPC formation at AP209. Initially surprising was the more than 2-fold increase in AP205 reactivity upon histone H3 tail deletion. Although we have no proof, this observation could be an example of what was described in the previous paragraph. One contribution to this observation is that elimination (k2, Scheme 1) following Schiff base formation (k1, Scheme 1) between AP205 and histone H3 is very slow, making this an unproductive event. Hence, by removing the H3 tail, the concentration of histone H4-AP205 Schiff base(s), which may undergo elimination more rapidly (Scheme 1) could increase, resulting in a higher overall reaction rate.
Scheme 4.

In a sense, the effect of deleting the histone H4 tail on AP211 reactivity complements observations regarding histone H3 tail deletions on AP207 and AP209 lifetime. Sodium cyanoborohydride trapping indicates that histone H4 is a minor contributor to DPC formation (~25%) at AP211. Deleting this tail had little effect on the half-life for AP211 disappearance (Table 1). In contrast, the 20-fold increase in AP211 reactivity upon histone H3 tail deletion could be due to the proximity of the protein’s amino terminus (Figure 2C), which is more nucleophilic than the e-amino groups of lysines. A similar effect was observed at AP89 when the histone H4 tail was deleted.11 In addition, the histone amino terminus also showed higher reactivity with 5-formyl-2′-deoxycytidine in NCPs.38 Furthermore, the amino terminus of the truncated H3 protein is a proline, which is particularly nucleophilic, and is the nucleophilic amino acid in the bifunctional BER protein, DNA formamidopyrimidine glycosylase.39
Finally, even though AP213 is the lesion furthest removed from where histone H4 protrudes from the octamer, deleting its tail reduces cleavage at that position by ~33%. The effect of the more remote histone H4 tail on AP213 is greater than the more proximal H3 tail, the removal of which actually results in a slight increase in reactivity. Experiments utilizing NCPs containing histone H2A variants were not carried out. Overall, the histone H4 tail contributes greater to cleavage at AP sites than does the H3 tail, despite both proteins contributing to Schiff base formation.
Time dependent AP reaction product distribution in NCPs as a function of rotational position
The minimum mechanism established for AP cleavage in NCPs is fit to a pre-equilibrium situation (Scheme 1).11 Hence, the rate at which DPCun products form (k1), as well as their reversion rates (k−1) and elimination to form DPCcl (k2) all effect kDis. We examined individual product formation as a function of time using SDS PAGE in the absence (Figure 3) or presence (Figure 4) of sodium cyanoborohydride to determine whether there was a trend in the relative rates of individual steps in the NCPs at positions 207-213. Reactivity at AP205 was previously described.11 Little if any DPCun is detected at any position in the absence of sodium cyanoborohydride (Figure 3A-D). The largest build-up of DPCun is observed at AP211, a position at which Schiff base formation is dominated by histone H3. At AP213, the slowest reacting position, is also distinctive in that significant quantities of either DPCcl or DPCun do not accumulate in the absence of reductant (Figure 3D). In addition, the amounts of DPCs formed at AP213 in the presence of sodium cyanoborohydride are considerably lower than at the other positions, and one still observes SSBs (Figure 4D). These observations suggest that DPCs at AP213 are short lived, but from the data we cannot determine whether this is due to slow formation (small k1) and/or rapid reversion (k−1). Furthermore, higher amounts of SSBs are observed at AP213 in the presence of sodium cyanoborohydride than at AP207 – AP211, indicating that the DPCcl at this position hydrolyze more rapidly (Figure 4D). The possibility that sodium cyanoborohydride reacts more slowly with DPCs formed at AP213 cannot be ruled out but is considered unlikely. Overall, the varying ratios of products as a function of time at the different positions indicate that the cleavage induced by the histones at AP sites is highly dependent upon position, which likely affects the microscopic rate constants (k1, k-1, and k2), as well as which protein(s) is responsible.
Figure 3.
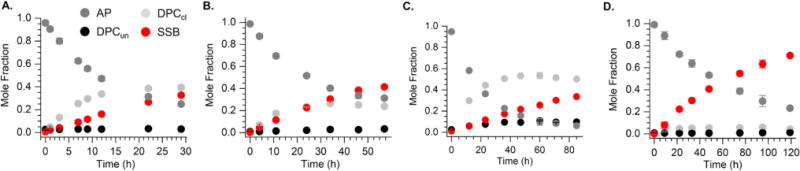
Time dependent product distribution from AP reactivity in NCPs in the absence of sodium cyanoborohydride. (A.) AP207 (B.) AP209 (C.) AP211 (D.) AP213.
Figure 4.
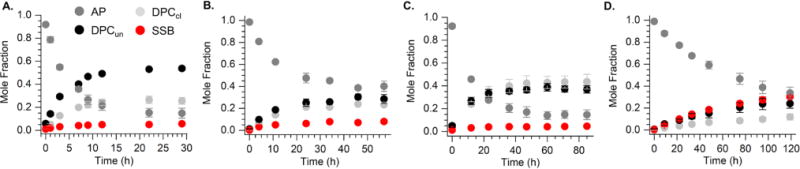
Time dependent product distribution from AP reactivity in NCPs in the presence of sodium cyanoborohydride. (A.) AP207 (B.) AP209 (C.) AP211 (D.) AP213.
CONCLUSIONS
Strand cleavage at abasic sites is accelerated within nucleosome core particles relative to free DNA. Over the span of one helical turn AP cleavage is more rapid in a NCP than in free DNA at each of 5 positions examined. This is in contrast to numerous reports on reactions of base excision repair enzymes with damaged DNA, cleavage of nucleosomal DNA by hydroxyl radical, and one other example of chemical reactivity of damaged DNA. In each of these instances, reactivity in NCPs waxes and wanes relative to that in free DNA as a function of rotational position. The fundamentally different rotational positioning effect on AP reactivity is ascribed to the fact that histone proteins themselves promote strand scission. That reactivity varies only ~4-fold over one helical turn is believed to be consistent with interaction between the unstructured histone protein tails and the AP sites. Unlike DNA lesions containing nucleobases, the reactive center of an abasic site is accessible via the major and minor grooves.
The histone tails play a major role in inducing cleavage of AP and various oxidized abasic sites in NCPs. Although previous studies concluded that the histone tails interacted predominantly with the linker DNA in nucleosomes, this did not affect abasic site reactivity.40 The reactivity of an oxidized abasic site was actually slightly faster at two positions within the core region of a nucleosome compared to a NCP.14 Extracting the roles of individual amino acids or even proteins responsible for cleavage at AP sites is difficult. While proximity certainly plays a role, the histone tails are capable of interacting with lesions that are one turn of the helix away, and maybe further. In addition, the heterogeneous structure of the histone tails indicates that they promote strand scission unequally, but their relative abilities are difficult to disentangle given the multiple variables; AP position, heterogeneous protein sequence, rate constants for Schiff base formation and reversion, as well as the rate constant for elimination. A variety of experiments could be carried out to improve our understanding of these reactions. For instance, molecular dynamics simulations could shed light on histone tail-AP site interactions as a function of position.
More than 35 years ago Hélene discovered that simple peptides (e.g. Lys-Trp-Lys) promote cleavage at abasic sites, predating even the discovery of lyase activity in base excision repair proteins.41–43 Many elegant studies followed in which chemists designed artificial nucleases that act on abasic sites.44, 45 At the time of these ground breaking studies, it was not yet known that histone proteins were capable of promoting these same reactions within their native environment, chromatin. One could envision engineering histone proteins with the intent of improving their lyase activity. Such experiments might help explain why the amino acid sequences of histone tails have evolved as they have.
Supplementary Material
Acknowledgments
We are grateful for financial support from the National Institute of General Medical Sciences (GM-063028). We thank Supratim Chakraborty for assistance with oligonucleotide synthesis.
Footnotes
Supporting Information. Mass spectra of oligonucleotides containing 1, DNAse I digests of NCPs, representative autoradiograms of time course and DPC trapping experiments Supporting Information is available free of charge on the ACS Publications website.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Walmacq C, Chong J, Kashlev M, Wang D. Structural basis of transcriptional stalling and bypass of abasic DNA lesion by rna polymerase ii. Proc Nat Acad Sci USA. 2018;115:E2538. doi: 10.1073/pnas.1722050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta S, Chowdhury G, Gates KS. Interstrand cross-links generated by abasic sites in duplex DNA. J Am Chem Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson KM, Price NE, Wang J, Fekry MI, Dutta S, Seiner DR, Wang Y, Gates KS. On the formation and properties of interstrand DNA‚äìdna cross-links forged by reaction of an abasic site with the opposing guanine residue of 5‚ä≤-cap sequences in duplex DNA. J Am Chem Soc. 2013;135:1015–1025. doi: 10.1021/ja308119q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price NE, Johnson KM, Wang J, Fekry MI, Wang Y, Gates KS. Interstrand DNA-DNA crosslink formation between adenine residues and abasic sites in duplex DNA. J Am Chem Soc. 2014;136:3483–3490. doi: 10.1021/ja410969x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalano MJ, Liu S, Andersen N, Yang Z, Johnson KM, Price NE, Wang Y, Gates KS. Chemical structure and properties of interstrand cross-links formed by reaction of guanine residues with abasic sites in duplex DNA. J Am Chem Soc. 2015;137:3933–3945. doi: 10.1021/jacs.5b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price NE, Catalano MJ, Liu S, Wang Y, Gates KS. Chemical and structural characterization of interstrand cross-links formed between abasic sites and adenine residues in duplex DNA. Nucleic Acids Res. 2015;43:3434–3441. doi: 10.1093/nar/gkv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z, Price NE, Johnson KM, Wang Y, Gates KS. Interstrand cross-links arising from strand breaks at true abasic sites in duplex DNA. Nucleic Acids Res. 2017;45:6275–6283. doi: 10.1093/nar/gkx394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sczepanski JT, Wong RS, McKnight JN, Bowman GD, Greenberg MM. Rapid DNA-protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc Natl Acad Sci U S A. 2010;107:22475–22480. doi: 10.1073/pnas.1012860108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou C, Sczepanski JT, Greenberg MM. Mechanistic studies on histone catalyzed cleavage of apyrimidinic/apurinic sites in nucleosome core particles. J Am Chem Soc. 2012;134:16734–16741. doi: 10.1021/ja306858m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C, Sczepanski JT, Greenberg MM. Histone modification via rapid cleavage of c4′-oxidized abasic sites in nucleosome core particles. J Am Chem Soc. 2013;135:5274–5277. doi: 10.1021/ja400915w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Greenberg MM. Histone-catalyzed cleavage of nucleosomal DNA containing 2-deoxyribonolactone. J Am Chem Soc. 2012;134:8090–8093. doi: 10.1021/ja302993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng L, Greenberg MM. Rapid histone-catalyzed DNA lesion excision and accompanying protein modification in nucleosomes and nucleosome core particles. J Am Chem Soc. 2015;137:11022–11031. doi: 10.1021/jacs.5b05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett RAO, Swerdlow PS, Povirk LF. Spontaneous cleavage of bleomycin-induced abasic sites in chromatin and their mutagenicity in mammalian shuttle vectors. Biochemistry. 1993;32:3188–3195. doi: 10.1021/bi00063a034. [DOI] [PubMed] [Google Scholar]
- 16.Atamna H, Cheung I, Ames BN. A method for detecting abasic sites in living cells: Age-dependent changes in base excision repair. Proc Nat Acad Sci USA. 2000;97:686–691. doi: 10.1073/pnas.97.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgakilas AG, Bennett PV, Wilson DM, Sutherland BM. Processing of bistranded abasic DNA clusters in γ-irradiated human hematopoietic cells. Nucleic Acids Res. 2004;32:5609–5620. doi: 10.1093/nar/gkh871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg MM, Weledji YN, Kim J, Bales BC. Repair of oxidized abasic sites by exonuclease iii, endonuclease iv, and endonuclease iii. Biochemistry. 2004;43:8178–8183. doi: 10.1021/bi0496236. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y-J, DeMottt MS, Hwang JT, Greenberg MM, Demple B. Action of human apurinic endonuclease (ape1) on c1′-oxidized deoxyribose damage in DNA. DNA Repair. 2003;2:175–185. doi: 10.1016/s1568-7864(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 20.Kuduvalli PN, Townsend CA, Tullius TD. Cleavage by calicheamicin g1 of DNA in a nucleosome formed on the 5s rna gene of xenopus borealis. Biochemistry. 1995;34:3899–3906. doi: 10.1021/bi00012a005. [DOI] [PubMed] [Google Scholar]
- 21.Davey G, Wu B, Dong Y, Surana U, Davey CA. DNA stretching in the nucleosome facilitates alkylation by an intercalating antitumor agent. Nucleic Acids Res. 2010;38:2081–2088. doi: 10.1093/nar/gkp1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sczepanski JT, Zhou C, Greenberg MM. Nucleosome core particle-catalyzed strand scission at abasic sites. Biochemistry. 2013;52:2157–2164. doi: 10.1021/bi3010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng L, Zhou C, Greenberg MM. Probing interactions between lysine residues in histone tails and nucleosomal DNA via product and kinetic analysis. ACS Chem Biol. 2015;10:622–630. doi: 10.1021/cb500737y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes JJ, Tullius TD, Wolffe AP. The structure of DNA in a nucleosome. Proc Natl Acad Sci U S A. 1990;87:7405–7409. doi: 10.1073/pnas.87.19.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes JJ, Bashkin J, Tullius TD, Wolffe AP. The histone core exerts a dominant constraint on the structure of DNA in a nucleosome. Biochemistry. 1991;30:8434–8440. doi: 10.1021/bi00098a022. [DOI] [PubMed] [Google Scholar]
- 26.Hinz JM, Rodriguez Y, Smerdon MJ. Rotational dynamics of DNA on the nucleosome surface markedly impact accessibility to a DNA repair enzyme. Proc Natl Acad Sci U S A. 2010;107:4646–4651. doi: 10.1073/pnas.0914443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Y, Stahley MR, Xu J, Friedman JI, Sun Y, McKnight JN, Gray JJ, Bowman GD, Stivers JT. Enzymatic excision of uracil residues in nucleosomes depends on the local DNA structure and dynamics. Biochemistry. 2012;51:6028–6038. doi: 10.1021/bi3006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilotti K, Tarantino ME, Delaney S. Human oxoguanine glycosylase 1 removes solution accessible 8-oxo-7,8-dihydroguanine lesions from globally substituted nucleosomes except in the dyad region. Biochemistry. 2018;57:1436–1439. doi: 10.1021/acs.biochem.7b01125. [DOI] [PubMed] [Google Scholar]
- 29.Cole HA, Tabor-Godwin JM, Hayes JJ. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J Biol Chem. 2010;285:2876–2885. doi: 10.1074/jbc.M109.073544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Q, Cannistraro VJ, Taylor JS. Rotational position of a 5-methylcytosine-containing cyclobutane pyrimidine dimer in a nucleosome greatly affects its deamination rate. J Biol Chem. 2011;286:6329–6335. doi: 10.1074/jbc.M110.183178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannistraro VJ, Pondugula S, Song Q, Taylor JS. Rapid deamination of cyclobutane pyrimidine dimer photoproducts at tcg sites in a translationally and rotationally positioned nucleosome in vivo. J Biol Chem. 2015;290:26597–26609. doi: 10.1074/jbc.M115.673301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou C, Sczepanski JT, Greenberg MM. Mechanistic studies on histone catalyzed cleavage of apyrimidinic/apurinic sites in nucleosome core particles. Journal of the American Chemical Society. 2012;134:16734–16741. doi: 10.1021/ja306858m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luger K, Rechsteiner TJ, Flaus AJ, Waye MMY, Richmond TJ. Characterization of nucleosome core particles containing histone proteins made in bacteria11edited by a. Klug. Journal of Molecular Biology. 1997;272:301–311. doi: 10.1006/jmbi.1997.1235. [DOI] [PubMed] [Google Scholar]
- 34.Lennox RW, Cohen LH. Analysis of histone subtypes and their modified forms by polyacrylamide gel electrophoresis. Meth Enzymol. 1989;170:532–549. doi: 10.1016/0076-6879(89)70063-x. [DOI] [PubMed] [Google Scholar]
- 35.Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- 36.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 37.Vasudevan D, Chua EYD, Davey CA. Crystal structures of nucleosome core particles containing the [`]601′ strong positioning sequence. J Mol Biol. 2010;403:1–10. doi: 10.1016/j.jmb.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Zhang Y, Bai J, Greenberg MM, Xi Z, Zhou C. 5-formylcytosine yields DNA-protein crosslinks in nucleosome core particles. J Am Chem Soc. 2017;139:10617–10620. doi: 10.1021/jacs.7b05495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zharkov DO, Rieger RA, Iden CR, Grollman AP. Nh2-terminal proline acts as a nucleophile in the glycosylase/aplyase reaction catalyzed by escherichia coli formamidopyrimidine-DNA glycosylase (fpg) protein. J Biol Chem. 1997;272:5335–5341. doi: 10.1074/jbc.272.8.5335. [DOI] [PubMed] [Google Scholar]
- 40.Angelov D, Vitolo JM, Mutskov V, Dimitrov S, Hayes JJ. Preferential interaction of the core histone tail domains with linker DNA. Proc Nat Acad Sci USA. 2001;98:6599–6604. doi: 10.1073/pnas.121171498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behmoaras T, Toulme JJ, Helene C. A tryptophan-containing peptide recognizes and cleaves DNA at apurinic sites. Nature. 1981;292:858–859. doi: 10.1038/292858a0. [DOI] [PubMed] [Google Scholar]
- 42.Behmoaras T, Toulme JJ, Helene C. Specific recognition of apurinic sites in DNA by a tryptophan-containing peptide. Proc Nat Acad Sci USA. 1981;78:926–930. doi: 10.1073/pnas.78.2.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailly V, Verly WG. Possible roles of beta-elimination and delta-elimination reactions in the repair of DNA containing ap (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988;253:553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fkyerat A, Demeunynck M, Constant JF, Michon P, Lhomme J. A new class of artificial nucleases that recognize and cleave apurinic sites in DNA with great selectivity and efficiency. J Am Chem Soc. 1993;115:9952–9959. [Google Scholar]
- 45.Lhomme J, Constant JF, Demeunynck M. Abasic DNA structure, reactivity, and recognition. Biopolymers. 1999;52:65–83. doi: 10.1002/1097-0282(1999)52:2<65::AID-BIP1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


