Abstract
Somatic KRAS mutations are the most common oncogenic variants in lung cancer and are associated with poor prognosis. Using a Kras–induced lung cancer mouse model, CC-LR, we previously showed a role for inflammation in lung tumorigenesis through activation of the NF-κB pathway, along with induction of interleukin 6 (IL6) and an IL17-producing CD4+ T-helper cell response. IL22 is an effector molecule secreted by CD4+ and γδ T cells that we previously found to be expressed in CC-LR mice. IL22 mostly signals through the STAT3 pathway and is thought to act exclusively on non-hematopoietic cells with basal IL22 receptor (IL22R) expression on epithelial cells. Here, we found that higher expression of IL22R1 in patients with KRAS mutant lung adenocarcinoma was an independent indicator of poor recurrence-free survival. We then showed that genetic ablation of Il22 in CC-LR mice (CC-LR/IL22KO mice) caused a significant reduction in tumor number and size. This was accompanied by significantly lower tumor cell proliferation, angiogenesis, and STAT3 activation. Il22 ablation was also associated with significant reduction in lung-infiltrating inflammatory cells and expression of pro-tumor inflammatory cytokines. Conversely, this was accompanied with increased antitumor Th1 and cytotoxic CD8+ T cell responses, while suppressing the pro-tumor immunosuppressive T regulatory cell response. In CC-LR/IL22KO mice, we found significantly reduced expression of core stemness genes and the number of prototypical SPC+CCSP+ stem cells. Thus, we conclude that IL22 promotes Kras mutant lung tumorigenesis by driving a pro-tumor inflammatory microenvironment with proliferative, angiogenic, and stemness contextual cues in epithelial/tumor cells.
Keywords: Lung Cancer, K-ras, IL22, STAT3, Stemness
Introduction
Tumor-promoting inflammation is a cancer hallmark which depends on cytokines and their downstream pathways (1,2). The roles of interleukin 22 (IL22) and IL22-expressing cells have been reported in a variety of human diseases (3,4), yet, our knowledge of their functions in cancer, particularly in lung oncogenesis, is lacking. IL22 is a unique member of the IL10 family of cytokines, and the functional outcome of its expression depends on the presence and relative amounts of other cytokines (5,6). IL22 seems to act exclusively on non-hematopoietic cells expressing a heterodimer transmembrane complex of IL22R1 and IL10R2 (7). IL22R1 is almost entirely expressed on cells of non-hematopoietic origin. The main signaling pathway downstream of IL22R1 is the STAT3 cascade, which mediates most IL22-induced effects (7). IL22 is known to be produced mostly by CD4+ T helper (Th) lymphocytes (Th17, Th22, and Th1), natural killer T cells (NKTs), innate lymphoid cells (ILCs), γδ T cells, and less commonly CD8+ lymphocytes (6–8).
Previous studies have demonstrated high IL22 and IL22+ cells in primary lung tumors and sera of patients with lung cancer (9,10). It is also known that high expression of IL22R1 in non-small cell lung cancer (NSCLC) is an independent indicator of poor overall survival (11). These suggest a role for IL22 signaling in the pathogenesis of lung cancer. KRAS mutations are the most common oncogenic mutation in NSCLCs and are associated with chemoresistance and poor prognosis (12). Our group and others have shown that activation of oncogenic K-ras in the lung (mouse and human) is associated with intrinsic inflammatory responses characterized by activation of the NF-κB pathway, the release of IL6, IL17A, and IL22, and subsequent activation of the transcription factor STAT3 (13–17). Therefore, we sought to evaluate the impact of IL22 in Kras mutant lung tumorigenesis. We found that IL22 has an essential role in the promotion of Kras mutant lung cancer through induction of a pro-tumor lung inflammatory microenvironment and protection of stemness characteristics in lung epithelial cells.
Methods and Materials
Human Data
IL22R1 mRNA expression in association with clinical outcome was interrogated in the Profiling of Resistance patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) expression dataset (18) comprised of 150 surgically resected lung adenocarcinomas (KRAS wild-type: n=111; KRAS mutant: n=39) who did not receive neoadjuvant therapy. PROSPECT patient clinicopathologic information has been reported previously by us (14). IL22R1 mRNA expression from these patients was log base2 transformed and median expression was computed in the R statistical language and environment. Patients were then dichotomized based on median IL22R1 mRNA expression and the different patient subgroups were then analyzed for differences in overall or recurrence-free survival.
Mouse Models
CCSPCre/LSL-K-rasG12D mice (CC-LR) were generated by crossing 6-week old mice harboring the LSL-K-rasG12D allele (LR mice; kindly provided by Dr. Tylor Jacks, Koch Institute, MIT) with mice containing Cre recombinase inserted into the Club cell secretory protein (CCSP) locus (CCSPCre mice) as we previously described (17). Il22 knockout (IL22 KO) and Stat3 conditional knockout (Stat3f/f) mice were kindly provided by Dr. Wenjun Ouyang (Genentech, South San Francisco, CA) and Dr. John DiGiovanni (the University of Texas at Austin, Austin, TX). CC-LR mice were crossed to IL22 KO and Stat3f/f mice separately in order to develop CC-LR/IL22KO and LR/STAT3Δ/Δ mice, respectively. All mice were housed under specific pathogen-free conditions and handled in accordance with the guidelines of Institutional Animal Care and Use Committee of MD Anderson Cancer Center.
Lung tissue and bronchoalveolar lavage fluid collection
Mice were anesthetized by intraperitoneal (IP) injection of avertin (Sigma), and their tracheas were cannulated with a blind needle of the appropriate size and sutured into place at the age of 14 weeks. In all mice (n=10 per group per time point), lung surface tumor numbers were counted if visible. In some mice (n=4 per group per time point), the lungs were perfused with PBS through the right heart atrium, inflated with 10% buffered formalin (Sigma), removed, and processed (as described below) for histological analysis. In other mice, bronchoalveolar lavage fluid (BALF) was obtained by sequentially installing and collecting two aliquots of 1 mL PBS through a tracheostomy cannula. Total leukocyte count was determined using a hemocytometer, and differential cell populations were determined by cytocentrifugation of 300 µl of BALF followed by Wright-Giemsa staining. The remaining BALF samples and collected whole lung tissues from mice were stored at −80°C for RNA and protein-based analysis.
Immunohistochemistry/Immunostaining
Lung tissues were primarily embedded in paraffin, and then sectioned on glass slides, deparaffinized, and stained with hematoxylin and eosin (H&E). The H&E stained slides were examined by a pathologist blinded to genotype, and the lung proliferative lesions were evaluated by the recommendations of the Mouse Models of Human Cancer Consortium (19). Sectioned lungs on slides were also immunohistochemically stained and assessed for expression of Ki-67 (1:200; Abcam, Cat# Ab16667), ETS-related gene (ERG; 1:1000, Abcam, Cat# Ab92513), phosphorylated (p)STAT3 (Tyr705; 1:250; Cell Signaling Technology, Cat# 91125S), CCSP (1:2500, a kind gift from Dr. Barry Stripp), surfactant protein C (SPC; 1:1000; Millipore, Cat# AB3786), and aldehyde dehydrogenase (ALDH1; 1:500; Abcam, Cat# ab52492). Stained slides were imaged by DP Controller software (Olympus) using an upright microscope, and the number of labeled positive cells for each marker was quantitated as a fraction of total tumor nuclei per high-power field (40×) in 10 fields for 3 to 5 mice per group. Results were expressed as a percentage of positive cells±SEM.
Quantitative RT-PCR analysis (qPCR)
Total RNA was isolated from the whole lung according to the TRIzol reagent protocol (Invitrogen) and purified by E.Z.N.A. Total RNA Kit I (OMEGA). RT-PCR was performed on 1 µg of RNA using the qScript cDNA SuperMix (Quanta Biosciences). Quantitative RT-PCR was carried out according to a standard protocol using gene-specific primers in 3 replicates, as indicated in Supplementary Table S1. SYBR Green reactions were done using FastMix, Low ROX (Quanta Biosciences), and products were measured on an ABI Via 7 PCR System (Applied Biosystems). The expression of individual genes was calculated and normalized with the ΔΔCT method.
Western blot analysis
Mouse lung tissues were sonicated (30 seconds on, 2–3 minutes off, 30 seconds on) in lysis buffer, including RIPA buffer (Pierce Cat: 89901), protease inhibitor (Roche Cat: 05 056 489 001), and phosphatase inhibitor (Roche Cat: 04 906 837 001). After incubation for 20 minutes at 4°C with moderate shaking, samples were centrifuged at 14000 rpm for 10 minutes at 4°C, and supernatants were collected for further analysis. Protein concentration was measured using Bradford Assay (Bio-Rad Laboratories) and 40 µg/lane protein was loaded per well. Western blot (WB) analysis was performed according to standard procedures. Proteins were transferred onto polyvinylidene difluoride membranes, and an enhanced chemiluminescence system (GE Healthcare) was used for visualization. Anti-pSTAT3 (Tyr705; 1:2000; Cat# 9145), anti-pERK (1:2000; Cat# 4370), and anti-RasG12D (1:1000; Cat# 14429), all from Cell Signaling Technology, and anti–β-actin (1:20000; Sigma, Cat# A5441) were used. All antibodies were diluted in 1× tris-buffered saline with 5% Tween 20/ 5% bovine serum albumin.
Isolation of lung inflammatory cells and flow cytometry
Lungs of 14-week old mice were harvested after perfusion with PBS and were inflated with collagenase IV (100 µL) and DNase I (20 µL) (Thermo Fisher Scientific) in PBS for 25 minutes at 37°C, 5% CO2. Single-cell suspensions were prepared by mechanical dissociation of lung tissue through a 70 μm nylon mesh. Lung cells were suspended in PBS and layered on lymphocyte separation medium (Lonza). Cells were centrifuged at room temperature for 20 minutes at 900 × g, and mononuclear cells were harvested from the gradient interphase. Lymphocytes (2–5×105 cells) were re-stimulated with PMA (50 ng/mL; Sigma-Aldrich) and ionomycin (500 ng/mL; Sigma-Aldrich) for 5 hours in the presence of GolgiPlug (1:1000; BD Biosciences), followed by intracellular staining of IFNγ-PE (eBioscience, clone XMG1.2) with a BD CytoFix/CytoPerm intracellular staining kit (BD Bioscience) and analysis within a CD4+ and CD8+ T-cell gate (CD8–PerCP-Cy5.5, clone 53–6.7 and CD4-FITC, clone GK1.5; both from Biolegend). Intracellular staining for Foxp3 (Foxp3-APC; eBioscience, clone FJK-16s) and IL10 (IL10-PE; BD Biosciences, clone JES5-16E3) was performed by using a Foxp3 staining kit (eBioscience) and analyzed in CD4+ T-cell gate (CD4-PerCP; Biolegend, clone GK1.5). IL22 (clone 1H8PWSR, Thermo Fisher Scientific, Cat# 12-7221-80), Thy1 (clone 30-H12, eBioscience, Cat# 105327), CD4 (clone GK1.5, Biolegend, Cat# 100406), and γδTCR (clone UC7-13D5, Biolegend, Cat# 107504) antibodies were used to detect IL22-producing T cells. Cells were analyzed and gated as above and previously described (14) on a LSRII machine, and data were further analyzed by FlowJo V10 (FLOWJO, LLC).
NTHi lysate aerosol exposure
Mice were nebulized with an aerosol lysate (2.5 mg/mL in PBS) of a clinical isolate of non-typeable Haemophilus influenza (NTHi) strain 12 once a week starting at 6 weeks of age for 8 weeks for the induction of an inflammatory lung environment similar to chronic obstructive pulmonary disease (COPD), as we previously done (17). The aerosol delivery was done using an AeroMist CA-209 nebulizer (CIS-US) driven by 10 L/minute of room air supplemented with 5% CO2 for 20 minutes.
Statistical analysis
Data were analyzed by GraphPad prism 7 using two-tailed unpaired Student t tests and presented as mean±SEM with P<0.05 considered significant. Assessment of recurrence-free survival was performed using Cox proportional hazard regression analysis and the Kaplan–Meier method for estimation of survival probability.
Results
IL22+ cells and IL22 are present in Kras mutant lung cancer
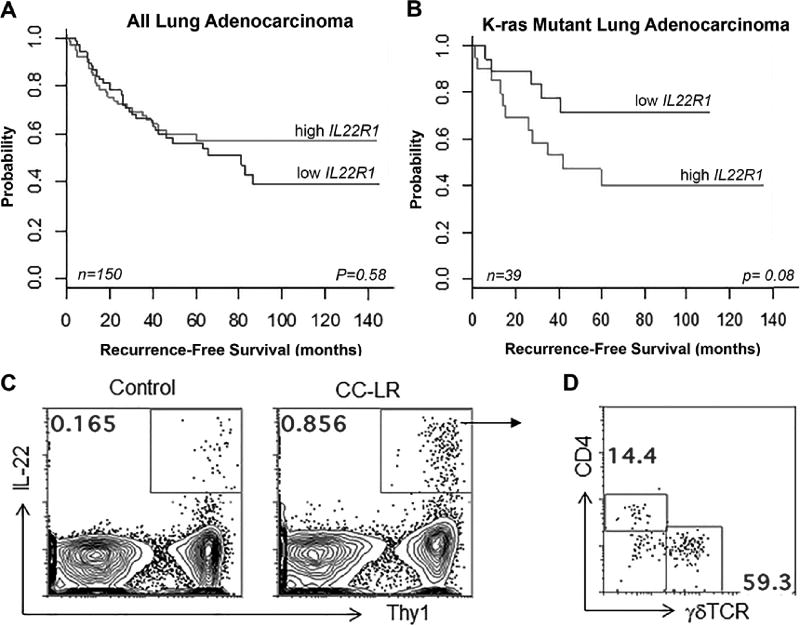
It has been shown that IL22 and IL22R1 are expressed in human lung cancer, and IL22R1 high expression correlates with poor prognosis (9,11). We investigated whether IL22R1 is associated with clinical outcome in KRAS mutant human lung adenocarcinomas. IL22R1 mRNA expression was not significantly associated with recurrence-free survival (RFS) when we assessed the entire set of lung adenocarcinomas (n=150), irrespective of mutation status (P=0.58) (Fig. 1A). Relatively higher (above the median) IL22R1 mRNA expression was associated with patterns of worse RFS in KRAS mutant lung adenocarcinomas (P=0.08), albeit not reaching statistical significance (Fig. 1B). These data suggest that IL22/IL22R1 pathway activation worsens clinical outcomes of patients with KRAS mutant lung adenocarcinomas.
Figure 1. IL22/IL22R1 signaling in KRAS mutant lung tumors.
(A) All (N=150), and (B) KRAS mutant adenocarcinomas (N=39) from the PROSPECT cohort were stratified based on IL22R1 median mRNA expression (high: gray, all patients: n = 75, KRAS mutant patients: n = 20; low: black, all patients: n = 75; KRAS mutant patients: n = 19) and were then analyzed for differences in recurrence-free survival using the Kaplan-Meier method for estimation of survival probability and P-values were obtained using the log-rank test in the R statistical language and environment. (C) Representative plots displaying frequencies of IL22+ cells in the lungs of 14 week–old control and CC-LR mice, and (D) representative distribution of CD4+ T cells and γδ T cells among IL22+ cells.
We previously demonstrated high expression of the IL22 protein in the bronchoalveolar lavage fluid (BALF) of a Kras mutant lung cancer mouse model, CC-LR (15). We further confirmed high expression of IL22 by qPCR analysis of whole lung homogenate, with IL22 expression upregulated in CC-LR mice compared to controls (LR mice) during tumor progression (Supplementary Fig. S1A). To determine the source of IL22 in the lung (IL22+ cells), we isolated mononuclear cells from lung tissues. Control lungs showed a very low frequency of IL22+ cells (Fig. 1C), whereas in CC-LR lungs, IL22-producing cells were increased and mostly confined to a Thy1+ fraction. Approximately half of the IL22-producing cells were γδ T cells, with many of the rest found to be TCRβ+ CD4+ T cells (Fig. 1D). Based on our findings, γδ T cells and CD4+ T cells appear to be the main sources of IL22 in Kras mutant lung cancer.
IL22 overexpression is required for Kras mutant lung tumorigenesis
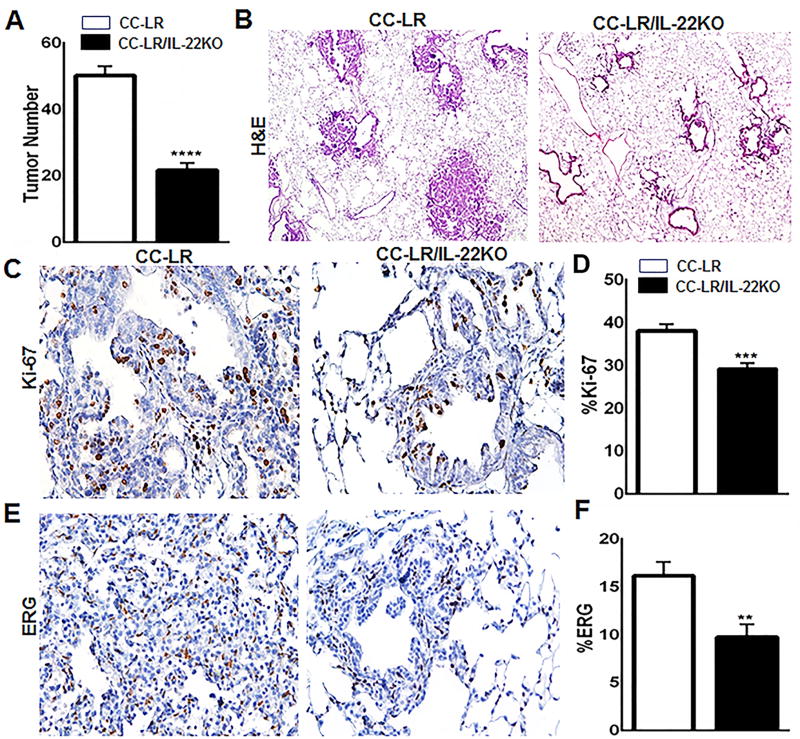
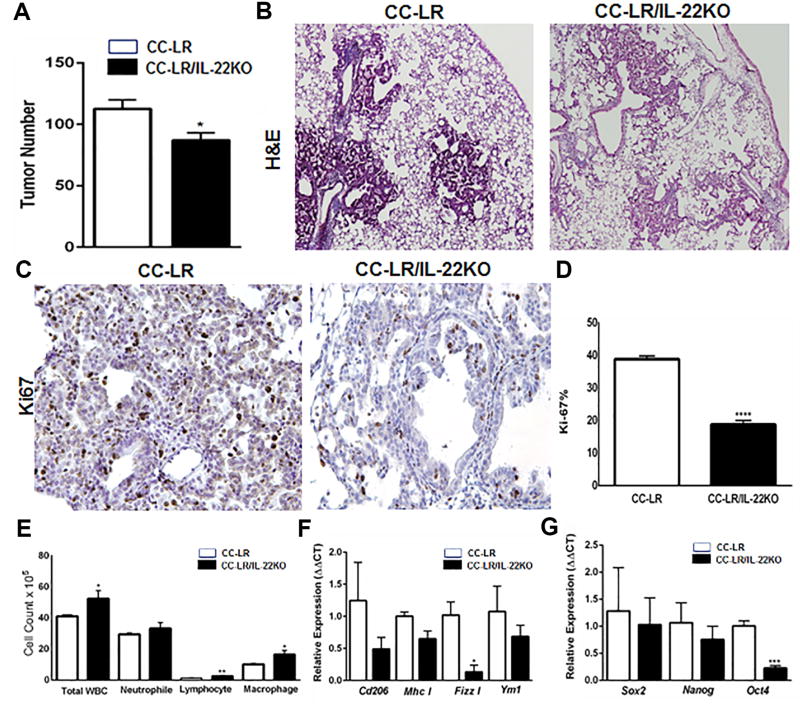
To better characterize the functional significance of IL22 overexpression in Kras mutant lung tumorigenesis, CC-LR mice were crossed with globally IL22 knocked-out (KO) mice (20). CC-LR/IL22KO mice showed a significant reduction (2.1-fold, ~54%) in the number of visible macroscopic lung tumors on the surface compared with CC-LR mice (Fig. 2A), indicating that IL22 promotes lung tumorigenesis. Tumor burden reduction was confirmed by analyzing expression of KrasG12D in the absence of IL22 (Supplementary Fig. S1B). Histopathologic analysis of the lungs from CC-LR/IL22KO mice revealed fewer and smaller lesions with a lower percentage of adenoma/adenocarcinoma lesions, and most were early-stage hyperplastic lesions (Fig. 2B). To assess whether IL22 increases the tumor burden by promoting tumor cell proliferation and angiogenesis, we performed immunohistochemical examination of Ki-67 and ERG, respectively. CC-LR/IL22KO mice showed a significant reduction in Ki-67 (Fig. 2C and D) and ERG (Fig. 2E and F) expression in lung tumor tissues compared with CC-LR mice, suggesting that IL22 promotes tumor cell proliferation and angiogenesis in Kras mutant lung cancer.
Figure 2. Targeting IL22 reduces lung tumor burden, tumor cell proliferation, and tumor angiogenesis.
(A) Lung surface tumor numbers and (B) histopathologic appearance of the H&E stained lung sections in CC-LR mice vs CC-LR/IL22KO mice at 14 weeks of age (4× magnification, N=10–15 per group). (C) Representative photomicrographs and (D) quantitative analysis of immunohistochemically stained lung tumor cells for Ki-67 in lung tumors of CC-LR mice vs CC-LR/IL22KO mice at 14 weeks of age (20× magnification, N=3 per group). (E) Representative photomicrographs and (F) quantitative analysis of lung tumors immunohistochemically stained for ERG in CC-LR mice vs CC-LR/IL22KO mice at 14 weeks of age (20× magnification, N=3 per group). Data represent mean±SEM; unpaired t-test, **p<0.005, ***p<0.001, ****p<0.0001.
IL22 regulates the lung tumor microenvironment
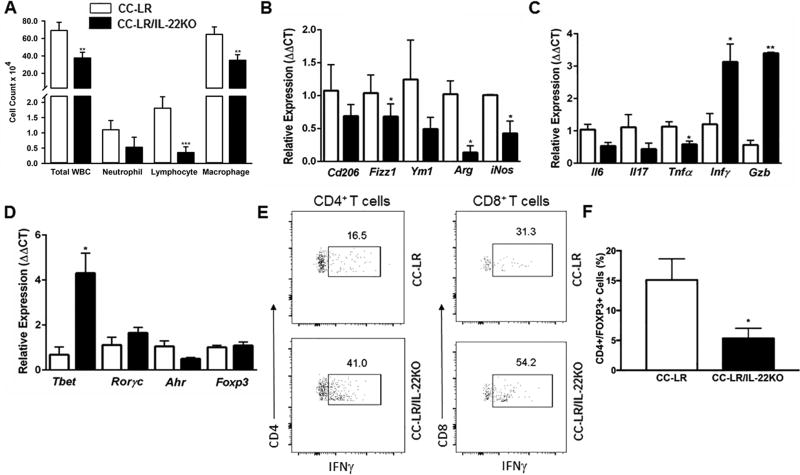
Uncontrolled expression of IL22 could amplify inflammation through induction of various inflammatory mediators alone or by synergizing with other cytokines in the lung tumor microenvironment (7,21). We, therefore, examined the effect of IL22 on airway inflammation in Kras mutant lung cancer. Analysis of the BALF from 14 week–old tumor-bearing CC-LR/IL22KO mice showed a significant reduction in total white blood cell (WBC) and macrophage numbers compared to the CC-LR mice. The number of infiltrating lymphocytes and neutrophils were also slightly reduced (Fig. 3A).
Figure 3. Targeting IL22 changes the lung microenvironment in Kras-induced lung tumors.
(A) Total inflammatory cell and lineage-specific leukocyte numbers from the BALF of CC-LR (N=10) and CC-LR/IL22KO (N=6) mice at the age of 14 weeks. (B) Relative expression of type 2 macrophage mRNA signature in whole lungs from CC-LR (N=4) and CC-LR/IL22KO (N=5) mice at the age of 14 weeks. (C) Relative expression of Il6, Il17, Tnfa, Ifng, and Gzmb mRNA in whole lungs of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks. (D) Relative mRNA expression of T cell-specific transcription factors in whole lungs of CC-LR (N=4) and CC-LR/IL22KO (N=5) mice at the age of 14 weeks. (E) Representative flow cytometry analysis of IFNγ-expressing CD4+ and CD8+ T cells in whole lungs of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks (N= 6 per group). (F) Percentage of CD4+Foxp3+ T regulatory cells in whole lungs of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks (N= 6 per group). Data represent mean±SEM; unpaired t-test, data representative of 2 independent experiments, *p<0.05, **p<0.005, ***p<0.001.
These quantitative changes in lung microenvironment were associated with changes in phenotypes of infiltrating leukocytes. Expression of immunosuppressive Fizz1, Arg, and iNos was significantly reduced in the absence of IL22 in CC-LR mice, indicating a reduction in immunosuppressive inflammatory responses (Fig. 3B). We detected a moderate decrease in expression of Il6 and Il17 mRNA in CC-LR/IL22KO mice, suggesting a reduction in IL6-driven Th17 differentiation and infiltration. We also found an increase in expression of Ifng and granzyme B (Gzmb), suggestive of enhanced Th1 differentiation and CD8+ cytotoxic T-cell activation (Fig. 3C). We did not find any significant change in Rorc mRNA expression between CC-LR and CC-LR/IL22KO mice, whereas Tbet expression significantly increased in CC-LR/IL22KO mice (Fig. 3D), indicating induction of a Th1 response. Flow cytometric analysis of whole lungs from 14 week–old mice also confirmed induction of an IFNγ–producing Th1/CD8+ T-cell population, along with a reduction in the T regulatory cell population in CC-LR/IL22KO mice, despite no changes in the total numbers of CD4+ or CD8+ T cells (Fig. 3E and F, Supplementary Fig.S1C and D).
IL22 activates STAT3 and MAPK pathways in Kras mutant lung cancer
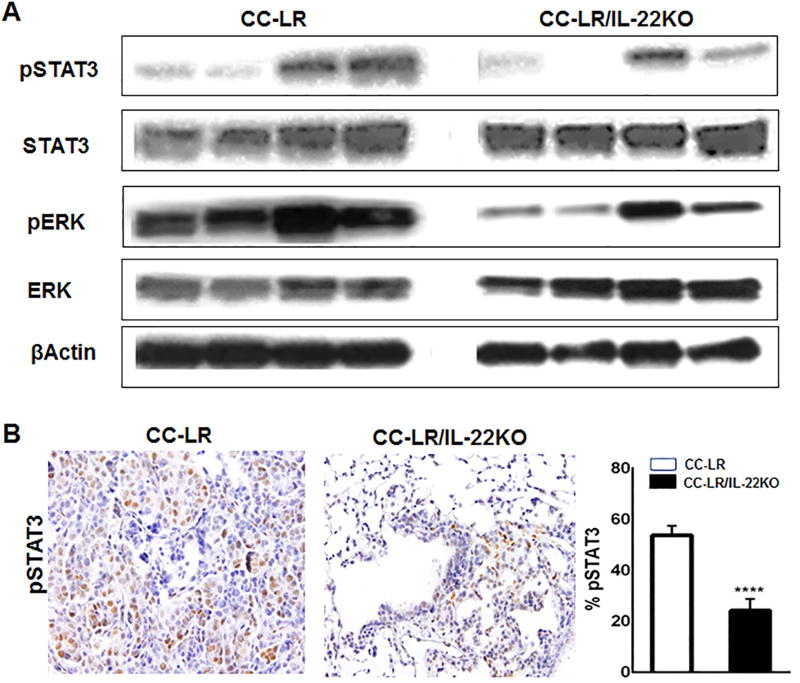
The IL22-IL22R1/IL10R2 complex activates the JAK/STAT pathway, leading to phosphorylation of mainly STAT3 proteins at the Tyr705 residue (5,13,22,23). Besides STAT3 signaling, activation of mitogen-activated protein kinase (MAPK) has been also noted after IL22 activation (5,23). In CC-LR/IL22KO mice, we observed a significant reduction in tyrosine-phosphorylation of STAT3 and ERK signaling pathways (Fig. 4A and B). These data suggest that IL22 might induce proliferation and angiogenesis in Kras mutant lung cancer through activation of STAT3 and MAPK signaling pathways.
Figure 4. IL22 activates STAT3 and ERK pathways in Kras mutant lung tumors.
(A) Western blot of pSTAT3 and pERK protein expression in whole lung lysates from CC-LR and CC-LR/IL-22KO mice at the age of 14 weeks. (B) Representative photomicrograph (40× magnification) and quantitative analysis of immunohistochemically stained slides for pSTAT3 in lung tissue of CC-LR and CC-LR/IL22KO mice at 14 weeks of age (N=3 per group). Data represent mean±SEM; unpaired t-test, ****p<0.0001.
IL22 induces stemness properties in Kras mutant lung cancer
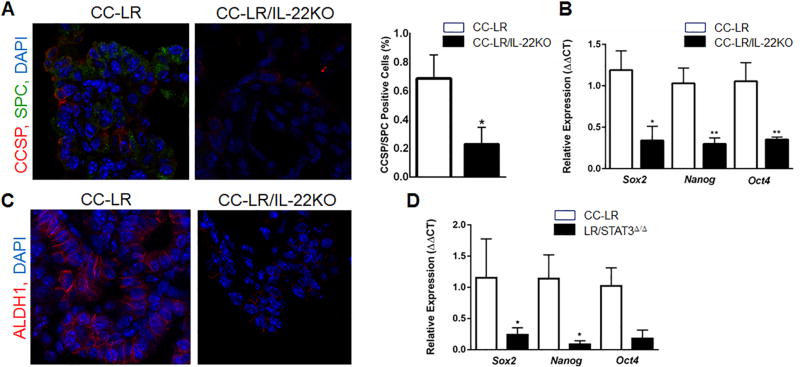
IL22 is required for epithelial barrier integrity and functional repair (7,24,25). This, coupled with the reduction in lung tumors in CCLR/IL22KO mice, suggests that IL22 might be involved in the initiating steps of Kras mutant lung tumorigenesis. Therefore, we assessed whether IL22 deficiency affects cancer stem cells (CSCs) in Kras mutant lung cancer. CSCs represent a minor population of undifferentiated cells, which are responsible for initiation, maintenance, and recurrence of cancer (26). In the lung, stem-like cells are mostly found at the bronchoalveolar duct junction (BADJ) and express the Club cell secretory protein (CCSP) and surfactant protein C (SPC) markers (27). Quantitative analysis of lung tissue sections showed a significant reduction in percentage and distribution of these double positive cells in the absence of IL22 compared with CC-LR mice (Fig. 5A). We noted that a significant fraction of CCSP+SPC+ double positive cells were located within tumors and not within the airway, the BADJ, or the lung surface.
Figure 5. IL22 induces stemness property in Kras mutant lung tumor.
(A) Representative photomicrograph (40× magnification) and quantitative analysis of CCSP/SPC double positive cells (CCSP: red, SPC: green, DAPI: blue) in lung tumor tissue of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks (N=3 per group). (B) Relative mRNA expression of core stemness genes in whole lungs of CC-LR (N=4) and CC-LR/IL22KO (N=5) mice at the age of 14 weeks. (C) Representative photomicrograph (40× magnification) of ALDH1 expression in lung tumor tissue of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks. (D) Relative mRNA expression of core stemness genes in whole lungs of CC-LR and CC-LR mice with epithelial-specific deletion of STAT3 (LR/STAT3Δ/Δ) at the age of 14 weeks (N=3 per group). Data represent means±SEM; unpaired t-test, *p<0.05, **p<0.005.
We further found that genetic ablation of Il22 significantly reduced mRNA expression of core stem cell genes Oct4, Sox2, and Nanog (28) (Fig. 5B). Aldehyde dehydrogenase (ALDH1) activity has been found to correlate with stemness properties in lung cancer (29). By immunostaining, we found a reduction in ALDH1 expression in lung tissue of CCLR/IL22KO mice (Fig. 5C). These data suggest that IL22 also enhances stemness in lung epithelial cells during K-ras–induced lung tumorigenesis.
IL22 may regulate stemness properties via activation of the STAT3 pathway
A critical role for STAT3 signaling has been shown in tumor initiation and maintaining stemness properties of colon cancer cells (30). Here, we crossed CC-LR mice to STAT3 conditional KO (Stat3f/f) mice (31) to specifically target STAT3 in Kras mutant–expressing lung epithelial cells (LR/STAT3Δ/Δ mice). Q-PCR analysis showed a significant reduction in Sox2 and Nanog and a modest decrease in Oct4 expression in LR/STAT3Δ/Δ mice (Fig. 5D). This finding suggests that the proposed role of IL22 in the induction of lung cancer stemness is mediated through activation of the STAT3 pathway.
Overexpression of IL22 is not required for promotion of lung cancer by COPD
Smokers with chronic obstructive pulmonary disease (COPD), an inflammatory disease of the lung, have an increased risk of lung cancer (3 to 10 fold) compared to smokers without COPD (32,33). Our group previously showed that induction of COPD-type inflammation through exposure to a lysate of non-typeable Haemophilus influenza strain 12 (NTHi) expands Th17 cells and related cytokines, including IL22, and significantly promotes lung cancer in CC-LR mice (15,17). To address the role of IL22 in the promotion of Kras mutant lung cancer by COPD, we exposed CC-LR/IL22KO and CC-LR mice to aerosolized NTHi lysate once weekly for 8 weeks to induce COPD-type lung inflammation as we previously described (17). Lack of IL22 only resulted in a 1.4-fold (~31%) tumor reduction in the NTHi-exposed CC-LR/IL22KO mice compared with the NTHi-exposed CC-LR mice (Fig. 6A and B). This amount of reduction could be attributed to the baseline effect of IL22 deficiency, which we observed in the absence of COPD-type airway inflammation as described above (2.1-fold, ~54%). We also found a reduction in tumor cell proliferation (Fig. 6C and D). However, no significant changes in pSTAT3 expression were seen (Supplementary Fig. S2A). We found a significant increase in the number of lung-infiltrating macrophages and lymphocytes. This finding was associated with decreased expression of immunosuppressive markers, suggesting induction of an antitumor type 1 immune response (Fig. 6E and F). No changes in T cell–related immune markers were observed (Supplementary Fig. S2B). These data indicated that IL22 may not be essential for the promotion of Kras mutant lung cancer by COPD. In the CC-LR/IL22KO mice after chronic exposure to NTHi, we found a trend in reduction of Sox2 and Nanog and a significant decrease in Oct4 expression (Fig. 6G). These results further indicated that IL22 has baseline effects in promoting Kras mutant lung tumorigenesis through inducing stemness properties, even in the presence of COPD.
Figure 6. IL22 does not play an essential role in the promotion of Kras mutant lung cancer by COPD-type airway inflammation.
(A) Lung surface tumor numbers and (B) histopathologic appearance of the H&E stained lung sections in CC-LR mice vs. CC-LR/IL22KO mice at age of 14 weeks after 8 weekly NTHi exposures (4× magnification, N=10–15). (C) Representative photomicrographs and (D) quantitative analysis of immunohistochemically stained positive tumor cells for Ki-67 in lung tumors of CC-LR mice vs CC-LR/IL22KO mice at the age of 14 weeks after 8 weekly NTHi exposures (40× magnification, N=3 per group). (E) Total inflammatory cell and lineage-specific leukocyte numbers from the BALF of CC-LR (N=10) and CC-LR/IL22KO (N=7) mice at the age of 14 weeks after 8 weekly NTHi exposures. (F) Relative expression of type 2 macrophage mRNA signature in whole lungs from CC-LR (N=3) and CC-LR/IL22KO (N=5) mice at the age of 14 weeks after 8 weekly NTHi exposures. (G) Relative mRNA expression of core stemness genes in whole lungs of CC-LR and CC-LR/IL22KO mice at the age of 14 weeks after 8 weekly NTHi exposures. Data represent mean±SEM; unpaired t-test, *p<0.05, **p<0.005, ***p<0.001, ****p<0.0001.
Discussion
Most cancers, including lung cancer, are associated with inflammation (2). We previously showed a promoting role for inflammation through IL6 and IL17 in Kras mutant lung tumorigenesis (14,15). IL22 is an effector cytokine that plays a major role in the modulation of inflammatory responses in multiple tissues (7). However, its role in the development of lung cancer, particularly Kras mutant lung cancer, is not fully understood. Studies have found high expression of IL22 in primary tumor tissues and sera of NSCLC patients, as well as expression of the IL22R1 on lung cancer cell lines (9,10). Importantly, it is known that IL22R1 expression is regulated at lung barrier surfaces where epithelial cells play an active role in the initiation, regulation, and resolution of immune responses (7,21). In this study, we showed that activation of the Kras mutant gene in lung epithelial cells increased expression of IL22 in mouse lungs. We also showed that genetic targeting of Il22 significantly decreased tumor burden, tumor cell proliferation, and angiogenesis in Kras mutant lung cancer. This was accompanied by decreased levels of pro-tumor inflammatory cytokines and T regulatory cell responses, along with increased antitumor Th1 and CD8+ cytotoxic T-cell responses.
It has been shown that the effect of IL22 on cell proliferation and migration is mediated through activation of IL22R1/STAT3 and AKT signaling (7,13). We found that genetic targeting of IL22 significantly reduced phosphorylation of STAT3 and AKT signaling pathways, which could explain the observed reduction in tumor cell proliferation and angiogenesis. Interestingly, STAT3 and AKT are often activated in NSCLC (34), and we have previously found that increased STAT3 expression is significantly associated with poorer disease-free survival in patients with Kras mutant lung tumors (14). We and others have found a negative correlation between IL22R1 expression and overall and recurrence-free survival in patients with lung cancer. These findings suggest that IL22, through induction of aberrant STAT3 and probably AKT expression, promotes lung tumorigenesis and impacts clinical outcome among early-stage lung adenocarcinoma patients with KRAS mutations.
IL22 is released by several types of cells, including CD4+ T cells, NKT cells, γδ T lymphocytes, and ILC3s (7,35). In mice, IL22 release is preferentially associated with Th17 differentiation. However, in humans, only a limited number of Th17 cells co-release IL22 with IL17 (7). We found that IL22-producing cells were significantly increased, and IL22 was mainly produced by γδ T cells and CD4+ T cells in tumor-bearing Kras mutant lung tissues. Although IL22-producing ILC3s are reported in stage I/II NSCLC, where they are suggested to have antitumor activity and are associated with favorable prognosis (36), we did not see any contribution by these cells in producing IL22 in the lungs of mice with Kras mutant tumors. Metz et al. showed that IL22 induces the production of chemokines, including CCL2, by Kras mutant lung cancer cell lines (37). This could explain the observed reduction in the number of macrophages and expression of cytokines in our Kras mutant/IL22KO mouse.
IL22 is required for epithelial barrier integrity and has a protective role for the epithelium (38,39). It has also been shown that STAT3 activity is required for small intestine crypt stem cell survival (40). Recombinant IL22 could directly target intestinal stem cells (ISCs) and enhance proliferation of both mouse and human intestinal organoids by inducing STAT3 phosphorylation in Lrg5+ ISCs (41). In lung adenocarcinoma, OCT4 and SOX2 have increased expression and have been described to play a role in the maintenance of stemness and tumorigenicity (42–45). In the current study, we found a significant reduction in percentage and distribution of SPC/CCSP double positive stem cells and decreased expression of core stem cell genes in Kras mutant tumors in the absence of IL22, similar to what we demonstrated in mice with epithelial-specific oncogenic K-ras activation and STAT3 deletion. Thus, IL22 might be involved in the initiating steps of Kras mutant lung tumorigenesis by acting directly on epithelial stem cells through activating a STAT3 signaling cascade and inducing stemness. This is concordant with a previously described role for induction of colorectal cancer stemness by IL22-producing T cells through STAT3 activation (30,46). Expressions of ALDH1, OCT4, SOX2, and NANOG were also associated with poor patient survival through drug resistance, detoxification of chemotherapy agents, and induction of epithelial-mesenchymal transition in human lung adenocarcinoma (29,43,44,47,48), suggesting a role for IL22 in tumor progression and response to treatment.
The likelihood of developing lung cancer is greater in smokers with COPD compared to smokers with normal lung function (32,33). COPD is thought to be caused by the lung parenchymal response to inflammation from cigarette smoke and bacterial colonization of smoke-injured airways (49,50). We previously showed that COPD-like lung inflammation promotes lung cancer (17) and an essential role for Th17 cells in this process (15). It is known that IL17 and IL22 primarily act on the lung epithelium, inducing the production of antimicrobial proteins and neutrophil chemoattractants (51). Specifically, IL17 contributes to antibacterial immunity by stimulation of macrophages and DCs, while IL22 promotes epithelial proliferation and repair following injury. Previous studies showed that IL22 is expressed in the lung of stable COPD patients (52,53) and might protect against infection and exacerbation in COPD (54,55). In our studies, although we found that the IL22 response is amplified in mice with NTHi-induced COPD-type airway inflammation, it is not probably required for promotion of Kras mutant lung cancer by this type of inflammation, which could be attributed to the protective roles of IL22 in COPD as explained above. This negative finding may also be related to the significant presence and important role of IL17 in our COPD model and promotion of Kras mutant lung cancer as we previously described (15), which could compensate for the IL22 deficiency. However, a limitation to our COPD model is that it does not fully recapitulate all aspects of COPD (no emphysema and no mucous metaplasia), which could contribute to this negative result as well.
Taken together, we propose pharmacological targeting of IL22 as a potential therapeutic strategy in combination with conventional chemotherapy, immune checkpoint blockade, or other targeted therapies (e.g. MEK inhibition) for KRAS mutant lung cancer patients.
Supplementary Material
Acknowledgments
This study was supported a grant [RSG-11-115-01-CNE] from American Cancer Society, and a Start-Up fund from UT M. D. Anderson Cancer Center, both awarded to Seyed Javad Moghaddam, and by National Cancer Institute (NCI) grant R01CA205608 awarded to Humam Kadara, as well as by NIH grants R03CA219760 (NCI), R21AI120012 (NIAID) and R56AI125269 (NIAID) awarded to Roza Nurieva.
Footnotes
Authors’ contributions: N.K., and S.J.M. designed research; N.K., M.S.C., A.M.C., N.U., C.D.G., O.N., S.D., B.J.H., B.A.G., A.M.A., Y.Y., H.H.C., R.N., H.K., and S.J.M. performed research; S.E.E, S.H., J.C., and E.J.O. contributed new reagents/analytic tools; N.K., M.S.C., S.H.C, R.N., H.K., and S.J.M. analyzed data; N.K., H.K., E.J.O., and S.J.M. wrote the paper; and S.J.M. supervised and conceptualized the study.
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflict of interest.
References
- 1.Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345(2):164–73. doi: 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. doi S1535-6108(12)00082-7 [pii];10.1016/j.ccr.2012.02.022 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev. 2014;13(6):615–20. doi: 10.1016/j.autrev.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13(1):21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 5.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23(3):159–63. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 6.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–32. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 8.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21(5):365–79. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Tufman A, Huber RM, Volk S, Aigner F, Edelmann M, Gamarra F, et al. Interleukin-22 is elevated in lavage from patients with lung cancer and other pulmonary diseases. BMC Cancer. 2016;16:409. doi: 10.1186/s12885-016-2471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14(20):6432–9. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 11.Guillon A, Gueugnon F, Mavridis K, Dalloneau E, Jouan Y, Diot P, et al. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur Respir J. 2016;47(4):1277–80. doi: 10.1183/13993003.01580-2015. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–80. doi: 10.1056/NEJMra0802714. doi 359/13/1367 [pii];10.1056/NEJMra0802714 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi Y, Cao J, Jin S, Lv L, Qi L, Liu F, et al. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem. 2016;415(1–2):1–11. doi: 10.1007/s11010-016-2663-8. [DOI] [PubMed] [Google Scholar]
- 14.Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-2840. doi 0008-5472.CAN-15-2840 [pii];10.1158/0008-5472.CAN-15-2840 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111(15):5664–9. doi: 10.1073/pnas.1319051111. doi 1319051111 [pii];10.1073/pnas.1319051111 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–12. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 17.Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L, et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40(4):443–53. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardnell RJ, Behrens C, Diao L, Fan Y, Tang X, Tong P, et al. An Integrated Molecular Analysis of Lung Adenocarcinomas Identifies Potential Therapeutic Targets among TTF1-Negative Tumors, Including DNA Repair Proteins and Nrf2. Clin Cancer Res. 2015;21(15):3480–91. doi: 10.1158/1078-0432.CCR-14-3286. doi 1078-0432.CCR-14-3286 [pii];10.1158/1078-0432.CCR-14-3286 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64(7):2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 21.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4(5):679–91. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277(37):33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 24.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol. 2004;31(2):220–6. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 25.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 27.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt R, Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012;13(10):251. doi: 10.1186/gb-2012-13-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei D, Peng JJ, Gao H, Zhang T, Tan Y, Hu YH. ALDH1 Expression and the Prognosis of Lung Cancer: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2015;24(8):780–8. doi: 10.1016/j.hlc.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40(5):772–84. doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18(17):4657–68. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191(3):285–91. doi: 10.1164/rccm.201407-1210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J Clin Oncol. 2014;5(4):660–6. doi: 10.5306/wjco.v5.i4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada D, Takigawa N, Kiura K. The Role of STAT3 in Non-Small Cell Lung Cancer. Cancers (Basel) 2014;6(2):708–22. doi: 10.3390/cancers6020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia L, Wu C. The biology and functions of Th22 cells. Adv Exp Med Biol. 2014;841:209–30. doi: 10.1007/978-94-017-9487-9_8. [DOI] [PubMed] [Google Scholar]
- 36.Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun. 2015;6:8280. doi: 10.1038/ncomms9280. [DOI] [PubMed] [Google Scholar]
- 37.Metz HE, Kargl J, Busch SE, Kim KH, Kurland BF, Abberbock SR, et al. Insulin receptor substrate-1 deficiency drives a proinflammatory phenotype in KRAS mutant lung adenocarcinoma. Proc Natl Acad Sci U S A. 2016;113(31):8795–800. doi: 10.1073/pnas.1601989113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44(3):369–76. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 40.Matthews JR, Sansom OJ, Clarke AR. Absolute requirement for STAT3 function in small-intestine crypt stem cell survival. Cell Death Differ. 2011;18(12):1934–43. doi: 10.1038/cdd.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528(7583):560–4. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest. 2011;91(12):1796–804. doi: 10.1038/labinvest.2011.140. [DOI] [PubMed] [Google Scholar]
- 43.Karoubi G, Gugger M, Schmid R, Dutly A. OCT4 expression in human non-small cell lung cancer: implications for therapeutic intervention. Interact Cardiovasc Thorac Surg. 2009;8(4):393–7. doi: 10.1510/icvts.2008.193995. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Yao Z, Wan Y, Lin D. Overexpression of OCT4 is associated with gefitinib resistance in non-small cell lung cancer. Oncotarget. 2016;7(47):77342–7. doi: 10.18632/oncotarget.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC, Chung CH, et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells. 2013;31(12):2607–19. doi: 10.1002/stem.1518. [DOI] [PubMed] [Google Scholar]
- 46.Sun D, Lin Y, Hong J, Chen H, Nagarsheth N, Peng D, et al. Th22 cells control colon tumorigenesis through STAT3 and Polycomb Repression complex 2 signaling. Oncoimmunology. 2016;5(8):e1082704. doi: 10.1080/2162402X.2015.1082704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park E, Park SY, Sun PL, Jin Y, Kim JE, Jheon S, et al. Prognostic significance of stem cell-related marker expression and its correlation with histologic subtypes in lung adenocarcinoma. Oncotarget. 2016;7(27):42502–12. doi: 10.18632/oncotarget.9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bora-Singhal N, Perumal D, Nguyen J, Chellappan S. Gli1-Mediated Regulation of Sox2 Facilitates Self-Renewal of Stem-Like Cells and Confers Resistance to EGFR Inhibitors in Non-Small Cell Lung Cancer. Neoplasia. 2015;17(7):538–51. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis. 2011;6:113–23. doi: 10.2147/COPD.S15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugade AA, Bogner PN, Thanavala Y. Murine model of chronic respiratory inflammation. Adv Exp Med Biol. 2011;780:125–41. doi: 10.1007/978-1-4419-5632-3_11. [DOI] [PubMed] [Google Scholar]
- 51.McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. 2014;260(1):129–44. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD. 2013;10(4):459–65. doi: 10.3109/15412555.2013.770456. [DOI] [PubMed] [Google Scholar]
- 53.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–24. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharan R, Perez-Cruz M, Kervoaze G, Gosset P, Weynants V, Godfroid F, et al. Interleukin-22 protects against non-typeable Haemophilus influenzae infection: alteration during chronic obstructive pulmonary disease. Mucosal Immunol. 2017;10(1):139–49. doi: 10.1038/mi.2016.40. [DOI] [PubMed] [Google Scholar]
- 55.Pichavant M, Sharan R, Le Rouzic O, Olivier C, Hennegrave F, Remy G, et al. IL-22 Defect During Streptococcus pneumoniae Infection Triggers Exacerbation of Chronic Obstructive Pulmonary Disease. EBioMedicine. 2015;2(11):1686–96. doi: 10.1016/j.ebiom.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.