Abstract
Background and Purpose
An imaging biomarker of myelin integrity is an unmet need in multiple sclerosis (MS). Selective inversion recovery (SIR) quantitative magnetization transfer imaging (qMT) provides assays of myelin content in the human brain. We previously translated the SIR method to 7T and incorporated a rapid turbo field echo (TFE) readout for whole-brain imaging within clinically acceptable scan times. We herein provide histological validation and test in vivo feasibility and applicability of the SIR-TFE protocol in MS.
Methods
Clinical (T1- and T2-weighted) and SIR-TFE MRI scans were performed at 7T in a post mortem MS brain and MRI data were acquired in 10 MS patients and 14 heathy volunteers to test in vivo. The following parameters were estimated from SIR data: the macromolecular-to-free water pool-size-ratio (PSR), spin-lattice relaxation rate of water (R1f), and the MT exchange rate (kmf). Differences in SIR parameters across tissue types e.g., white matter lesions (WM-Ls) and normal appearing WM (NAWM) in patients, and normal white matter (NWM) in heathy volunteers were evaluated. Associations between SIR parameters and disability scores were assessed.
Results
For post mortem scans, correspondence was observed between WM-Ls and NAWM from histology and PSR/R1f values. In vivo differences were detected for PSR, R1f, and kmf between WM-Ls and NWM (p≤0.041). Associations were seen between WM-Ls/NAWM PSR and disability scores (r≤−0.671, p≤0.048).
Conclusions
SIR-qMT at 7T provides sensitive, quantitative measures of myelin integrity for clinical and research applications.
Keywords: Myelin, magnetic resonance imaging, multiple sclerosis, quantitative magnetization transfer imaging
INTRODUCTION
There is an urgent need to validate a novel magnetic resonance imaging (MRI) biomarker that can provide an accurate in vivo quantification of myelin integrity in patients with multiple sclerosis (MS).1 This biomarker would serve as an imaging surrogate of neurodegeneration and repair and would allow for (1) an objective study of the mechanisms of disease evolution, (2) an improved understanding of the patient disability substrate, and (3) a more accurate assessment of treatment response in future clinical trials.
Magnetization transfer (MT) imaging was pursued herein because previous studies indicated that MT methods offer a non-invasive means of assaying myelination changes in MS.2–5 MT imaging assays myelination by probing interactions between protons on mobile water molecules,6 from which the conventional MRI signal is derived, and protons associated with immobile macromolecules (primarily lipids in myelin).7–9 The latter protons do not directly contribute to the observed MRI signal in conventional sequences because of their short transverse relaxation times. The MT ratio (MTR) is a semi-quantitative measure of the MT effect that can be estimated from an MT-weighted and reference image.10,11 Studies indicate that MTRs change with myelin and axonal content12,13 indicative of the degree of neurodegeneration but also water, ultimately reflecting inflammation and edema.14 Post mortem, MTR values related to myelin/axons quantities in normal appearing white matter (NAWM) regions close to WM lesions (WM-Ls) and to the content of activated microglia in NAWM areas distant from WM-Ls.15 In animal models of MS, MTRs were found to be associated to the degree of macrophage infiltration.16
Together, the findings support the sensitivity to myelin of MTR values but raise concerns regarding their pathological specify. Aside from being non pathologically specific, MTR is also reflective of differences in non-biological parameters17,18 (i.e., scanner hardware, radio frequency power, and sequence timings) and tissue relaxation times, which can also be affected by inflammation and edema.
Quantitative MT (qMT) methods attempt to remove these confounding influences by fitting a series of MT-weighted images with a two-pool model of the MT effect. This process yields tissue-specific indices, including the macromolecular to free water pool-size-ratio (PSR), the rate of MT exchange from the free (f) to macromolecular (m) pool (kmf), and the spin-lattice relaxation rate of free water (R1f). PSR and R1f are two complementary measures in that they are sensitive to the relative quantity of macromolecules, which is primarily reflective of total myelin content in WM.19–22 R1f, the reciprocal of which corresponds to the T1 typically reported in literature, is additionally sensitive to iron content and, therefore, may be a less specific assay of myelination.23 The relationship between the rate of MT exchange (kmf) and underlying tissue composition is less clear; however, previous work has suggested that kmf may reflect changes within the myelin lipid structure.24
The two primary impediments to the adoption of many qMT imaging methods in clinical and pre-clinical studies are: (1) the complicated acquisition/analysis associated with the method (e.g., independent measures of the spin-lattice relaxation time, T1, along with transmit/main magnetic field variations are typically required) and (2) the resulting long acquisition times. We previously demonstrated that a novel qMT method known as selective inversion recovery (SIR) can partially address this limitation by providing qMT measures in human brain at 3.0 Tesla (3T) without the need for time-consuming measurements of the T1.25 Subsequently, we translated the SIR method to 7T and incorporated efficient turbo field-echo readout (SIR-TFE) to provide whole-brain qMT maps within clinically acceptable scan times.23 In controls, the resulting PSR values were reproducible and consistent with known brain regional variations in myelin content. In this proof of concept study we build upon this previous work to: (1) provide initial histological validation of our novel SIR-TFE protocol at 7T in MS brains post mortem and (2) test feasibility and applicability of this method in a small cohort of MS patients in vivo.
METHODS
This is a collaborative project between the Neurology and Radiology Departments at the University of Vanderbilt Medical Center and the Brain Research Center of the University of Vienna.
The study has a post mortem and an in vivo component. The post mortem study was approved by the local institutional review board in Vienna. No institutional review board approval was required at Vanderbilt University Medical Center, where post mortem scanning was performed. The donor consented to the study prior to death and the study was conducted in accordance with HIPAA compliance. The Rocky Mountain MS Center Brain Bank (Englewood, CO, USA) donated the tissue samples to the Neuroimmunology Division at Vanderbilt University Medical Center. The in vivo study, which was entirely done at the Vanderbilt site, was approved by the local institutional review board and a signed consent was obtained prior to all examinations.
Post mortem study
The samples used for this study derive from a 43 year-old female MS patient who was known to be not affected by any other neurological condition apart from MS. About 18 hours after death brain tissue was fixed in paraformaldehyde. The whole brain was first fixed and then cut in ~1-cm thick slices. By the time of the MRI scan, the brain parts have been in fixation for several years. Details on tissue collection and preparation for the MRI have been previously described.26,27 In brief, samples consisted of a single 1-cm thick coronal slice that included 1 hemisphere as well as several other temporal and frontal lobes WM samples. Each slice was then fixed in 4% paraformaldehyde until imaged. For imaging brain samples were placed into a cylindrical, custom-fabricated tissue container and imaged in 4% paraformaldehyde fluid in the container. The base ring of the container was manufactured from 140 mm inner diameter clear polyvinylchloride pipe of 22 mm height. The detachable endplates were manufactured from polycarbonate and were bolted to the pipe using anodized aluminum screws.
MRI acquisition protocol
All scans were acquired using a whole-body 7T Achieva MRI scanner (Philips Healthcare, Cleveland, OH) equipped with a volume transmit and 32-channel receive head coil (NOVA Medical, Wilmington, MA).
The post mortem protocol relevant for this study included a (1) multi-slice T2-weighted (T2-w) spin-echo gradient-echo (GRASE) imaging for evaluation of WM lesion burden and anatomical co-localization of lesions26 and (2) SIR-TFE sequence for qMT. The following parameters were employed for the GRASE sequence: 40 contiguous slices of 0.7 mm3 isotropic resolution, field-of-view (FOV) = 18 × 24 cm2, repetition time (TR) = 23 s, turbo SE (TSE) factor = 4, sensitivity encoding parallel imaging acceleration factor (SENSE) = 2 (Right-Left), flip angle = 90°, echo time (TE)/echo planar image (EPI) factor = 50/3. The SIR-TFE sequence used for qMT has been described previously.23,25 Briefly, the sequence employs a composite inversion pulse that is optimized to be insensitive to both the main magnet field (B0) and to the transmit radiofrequency field (B1+) variations experienced at 7.0 T. The inversion pulse is followed by variable duration inversion recovery period to sample a biexponential recovery that arises due to MT and a center-out TFE readout for efficiency. Data were acquired in post mortem brains using the following parameters: inversion times (TI) = 6–2000 ms (16 logarithmically space values), pre-delay time (TD) = 1.0 s, SENSE factor = 1, TFE pulse interval/TE/flip angle = 5.6 ms/2.6 ms/15°, echoes per shot = 71, resolution = 0.7×0.7×0.7 mm3, FOV = 150×150×28 mm3, number of signal averages = 1, and total acquisition time ≈ 60 min.
Image analysis
The qMT parameter maps, e.g. PSR (%), R1f, and kmf (seconds−1 [s-1]) were estimated by fitting SIR data to a two-pool model of the MT effect using our previously published method.21 The resulting parameter maps were transformed into the T2-w GRASE image space via the FSL software package (https://fsl.fmrib.ox.ac.uk/fsl/). WM-Ls and NAWM regions of interest (ROIs) were identified and delineated on anatomical T2-w GRASE using the graphic tools available in the MIPAV software package (https://mipav.cit.nih.gov/). To avoid partial volume effects, care was taken to ensure each ROI was (1) larger than 10 voxels, (2) positioned away from boundary regions (e.g., at the interface GM/WM or normal/disease tissue), and (3) that the distance to any boundary was greater than 10 voxels. The mean of each qMT parameter within each ROI was tabulated.
Histology
After scanning, formalin-fixed brain tissue was dehydrated and embedded in paraffin. Samples were cut with a microtome to have a thickness of 7–10 μm and mounted on glass slides with appropriate size. For basic evaluation of general pathology and demyelination, hematoxylin and eosin, and Luxol fast blue (LFB) - periodic acid-Schiff (PAS) myelin stains were performed. Additionally, immunohistochemistry against the major myelin protein proteolipid protein (PLP) was performed as described.28 The primary antibody against PLP was applied twice, that is, a first time overnight at 4°C and a second time for 1 hour at room temperature after 5 rinses with wash buffer. Immunohistochemical staining for PLP was done for detailed evaluation of demyelination, remyelination, and primary Wallerian degeneration with secondary myelin reduction due to axonal loss (PLP staining not shown, but used for pathological characterization). For the direct comparison with MRI, a color-coded map, indicating areas of MS-related pathology on the LFB-PAS-stained slice, was generated using Adobe Photoshop® CS4. Green indicates WM areas of complete demyelination without demyelinating activity (complete loss of myelin in both LFB-PAS and PLP stains, no PLP-positive myelin degradation products in macrophages). Yellow indicates remyelinated WM areas (myelin reduction visible in LFB-PAS staining but normal staining intensity in PLP staining). Orange indicates deep GM areas showing complete demyelination without demyelinating activity (complete loss of myelin in both LFB-PAS and PLP stains). Red areas indicate Wallerian degeneration with secondary myelin reduction (myelin reduction visible in both LFB-PAS and PLP stains).
In vivo study
Ten patients with relapsing remitting MS and 14 healthy volunteers were consecutively enrolled. Each patient underwent a clinical exam assessing scores at the Expanded Disability Status Scale (EDSS) and a brain MRI within 1±2 weeks. Four patients were scanned twice within one month to assess inter-scan variations in qMT derived metrics. No intra-subject variability data were obtained in healthy volunteers since the robustness of qMT in this cohort of subjects has been already demonstrated by our team at both 3T and 7T scanners.23,25
In vivo MRI acquisition protocol
Each subject underwent the same 7T MRI protocol as that described for post mortem studies with modifications to account for differences in scan time constraints and relaxation times. The in vivo protocol included a (1) three-dimension (3D) 1 mm3 isotropic magnetization-prepared rapid gradient-echo (MPRAGE) sequence obtained before and 10 minutes after the injection of the contrast agent Gadolinium DTPA to identify active lesions, a (2) multi-slice T2-w GRASE (TR/TE = 17 sec/59 sec and resolution = 0.6 × 0.6 × 3 mm3), and (3) SIR-TFE for qMT. In vivo SIR-TFE data were acquired prior to contrast agent injection using a similar protocol to that described above for post mortem studies with modifications to account for differences in scan time constraints and relaxation times, including: TI = 6–8000 ms (14 logarithmically spaced values), TD = 2.5 s, SENSE factor = 4 (2 anterior–posterior, 2 superior–inferior), TFE pulse interval/TE/flip angle = 2.8 ms/1.4 ms/15°, echoes per shot = 53, resolution = 2×2×3 mm3, FOV = 212×212×75 mm3, number of signal averages = 1, and total acquisition time ≈ 16 min. PSR, kmf, the R1f were estimated from these data using the same approach described for post mortem data.
Image analysis
qMT imaging derived maps were registered on the T2-w GRASE sequence as done for post mortem scans. ROI labelling was also performed via in vivo T2-w GRASE images using the same method employed for post mortem images. PSR, kmf, the R1f were measured in each WM-L. Eight ROIs were placed in the NAWM and normal WM (NWM) of each patient’s and healthy volunteer’s scans, respectively. Care was taken to position ROIs in the same anatomical locations for both patents and healthy volunteers as to account for regional variations in myelin content. To further validate our manually defined ROIs, histogram analyses were performed, whereby NAWM masks were automatically generated using FSL’s FAST algorithm29 (after subtracting the manually defined WM-Ls ROIs above).
For inter-scan variability measurements, ROIs were traced on T2-w GRASE randomly obtained with the first or the second scan. qMT imaging derived map at each time point were then registered into the T2-w GRASE space selected for ROI labeling using the FSL software package as described above.
Statistical analysis
Data are expressed in mean ± standard deviation (SD) unless indicated otherwise. Differences in age and sex between patients and healthy volunteers were assessed using unpaired Student-t and a χ2 tests, respectively. In patients, differences in PSR, R1f, and kmf between WM-Ls and NAWM were computed using a paired t-test. Group (patients vs. healthy volunteers) comparisons between NAWM (patients) and NWM (healthy volunteers) and between NWM (healthy volunteers) and WM-Ls (patients) were obtained using a univariate analysis of covariance (ANCOVA), using age as controlling factor. This approach was undertaken to account for differences in age between patients and healthy volunteers. Given the explorative nature of the study, no correction for multiple comparisons was employed.
In patients, correlation analyses between SIR-qMT derived metrics and EDSS scores were obtained using a Spearman correlation analysis. Inter-scan reproducibility was demonstrated by providing percent (%) differences in group measurements between scans as well as coefficient of variations (COV) between scans. COV was computed as the ratio between SD (of differences)/mean (of differences) √2*100. The √2 accounts for the propagation in error for using a difference measurement to calculate the sample standard deviation.
RESULTS
Post mortem study
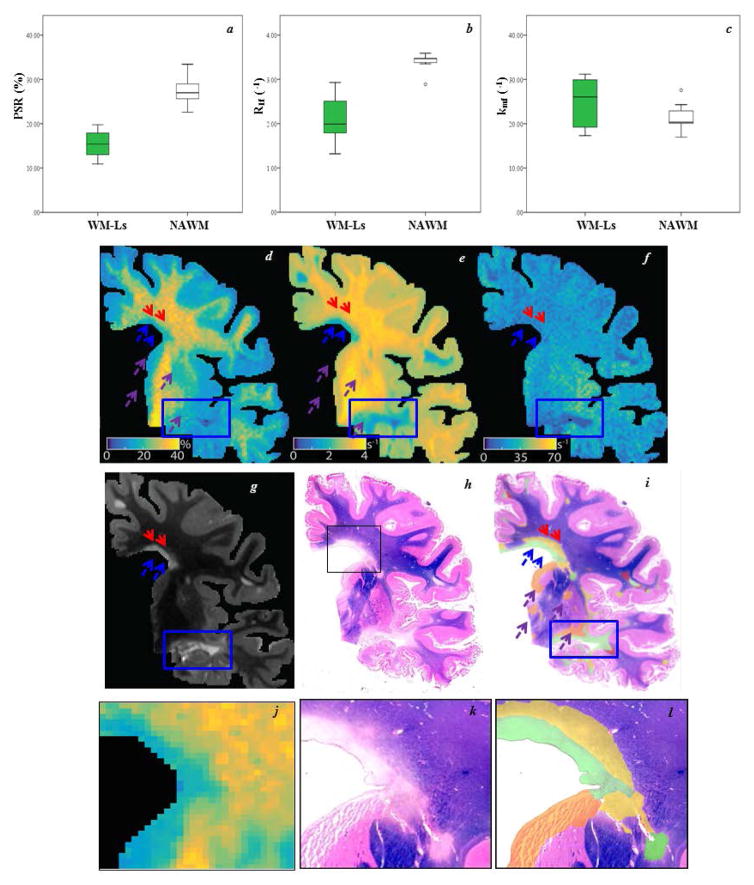
PSR, kmf, and R1f were measured in ROIs covering a total of 56.34 mm2 in 9 WM-Ls and 27.36 mm2 in 7 NAWM ROIs. The small sample size precluded formal quantitative analyses, but the data distributions are presented in Figure 1a–c. Side by side MRI-histology (Figure 1d–g) maps show the ability of T2-w GRASE MRI to detect WM-Ls, but not areas of remyelination, which were instead captured by PLP staining, PSR, and R1f maps. The blue rectangle captures an area of a demyelinated WM-L. This area, color-coded in green in the PLP staining (i), is entirely visible on the T2-w GRASE, (g), PSR (d) R1f (e), and kmf (f). In the same figure, blue and red arrows indicate a WM-L that has a (1) demyelinated component indicated by the blue arrows (g), color-coded in green in the hematoxylin and eosin staining map (i) and zoomed in j-l, and (2) a remyelinated component indicated by the red arrows and color-coded in yellow in the PLP map. One can appreciate lack of visibility of the remyelinated component on T2-w GRASE images and the sensitivity of PSR (d) and R1f (e) qMT to capture it. In the color-coded map, orange-coded (purple arrows in the qMT maps) represent areas of GM demyelination, which are again captured by PSR and R1f, but are not visualized on the T2-w GRASE and kmf maps. Dark pink areas represent those with axonal degeneration with secondary WM loss, which were not formally analyzed in this study due the small size of these regions.
Figure 1. Differences in free water pool-size-ratio (PSR), spin-lattice relaxation rate of water (R1f), and the MT exchange rate (kmf) between white matter lesions (WM-Ls) and normal appearing white matter (NAWM), post mortem.
Boxplots for each quantitative magnetization transfer imaging parameter (a=PSR in %, b=R1f and c=kmf in seconds−1 [s−1]), where green and white represent WM-Ls and NAWM regions of interest, respectively. Each box represents the 25th–75th percentile and the black line inside the boxes represents the median value. The vertical lines outside the box delineate the minimum and maximum values. Outliers (small circles, 10th – 90th percentile) are also represented. Side-by-side qMT parameter maps (d=PSR, e=R1f, and f=kmf), T2-weighted gradient echo spin echo (g), luxol fast blue-stained section (h) and proteolipid protein (PLP)-color-coded myelination maps (i). Corresponding zoomed insets (black box) are shown in the bottom row (j= PSR, k=LFP, and l=PLP). In the quantitative magnetization transfer imaging (qMT) maps, the blue/red arrows and rectangles indicate WM-Ls that have a demyelinated/remyelinated component, while the purple arrows indicate gray matter (GM) demyelination. In the PLP maps, green/yellow indicate demyelinated/remyelinated WM-Ls, orange areas indicate GM demyelination, and dark pink areas indicate Wallerian degeneration with secondary demyelination.
In vivo study
Tolerability of SIR-qMT scans and data reproducibility
Age was 45.2±12.4 in patients and 28.8±4.7 in healthy volunteers (p=0.004). The two groups were matched for sex (p=0.09). There were seven females and 3 males in the patients’ cohort and 4 females and 10 males in the healthy volunteers’ group. Table 1 describes the demographic, radiological, and clinical characteristics of the patient cohort.
TABLE 1.
Demographic, clinical and MRI characteristics of patients
| Patient | Age (years) | Sex | MS Years | EDSS | Therapy | WM-Ls volume (cm3) |
|---|---|---|---|---|---|---|
| 1* | 65 | Male | 36 | 2.5 | IFNβ-1a | 17.4 |
| 2* | 33 | Female | 5 | 0 | None | None ** |
| 3 | 59 | Female | 23 | 1.0 | Fingolimod | 2.3 |
| 4 | 61 | Female | 6 | 2.0 | IFNβ-1a | 20.9 |
| 5 | 48 | Female | 15 | 3.5 | IFNβ-1b | 2.7 |
| 6 | 45 | Male | 7 | 3.0 | IFNβ-1a | 0.6 |
| 7* | 46 | Female | 8 | 0 | IFNβ-1a | 0.18 |
| 8* | 24 | Female | 5 | 0 | Dimethyl Fumarate | 1.6 |
| 9 | 31 | Male | 3 | 1.0 | None | 2.2 |
| 10 | 43 | Female | 8 | 1.5 | IFNβ-1a | 0.04 |
EDSS= Expanded Disability Status Scale; IFNβ-1a=interferon beta 1a; MS=multiple sclerosis; WM-L=white matter lesion;
indicates patients who returned for a follow up scan to test inter-scan variability;
radiological presence of illness was in the spinal cord.
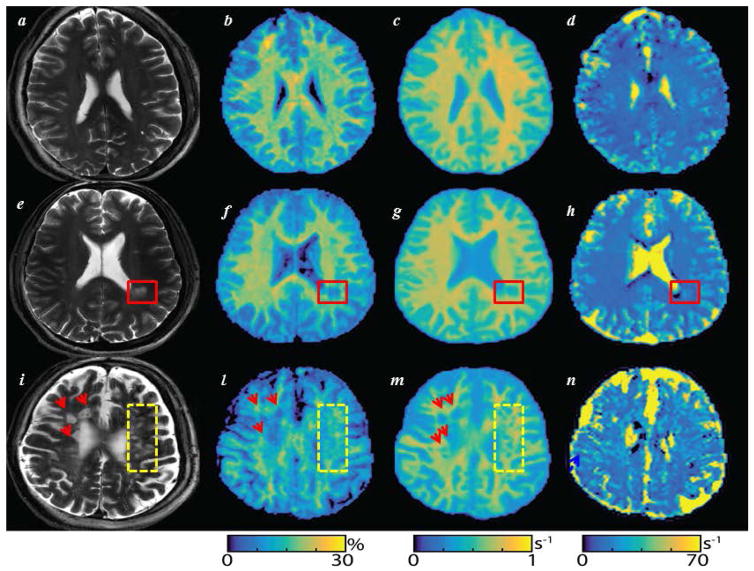
SIR-qMT was well tolerated by all study subjects. Figure 2 shows MRIs of three representative patients, where one can see the correspondence between WM-Ls and changes in SIR-qMT derived maps. Averaged relative differences across all ROIs between the two measurements were 0.5%, 0.05%, and 4.0% for PSR, R1f, and kmf values, respectively. These differences translated into COVs of 5.6%, 1.4%, and 11.4% for PSR, R1f, and kmf values, respectively.
Figure 2. Pool-size-ratio (PSR), spin-lattice relaxation rate of water (R1f), and the MT exchange rate (kmf) maps, in vivo.
Side-by-side T2-weighted gradient echo spine echo (T2-w GRASE), (a–e–i), PSR (in %, b–f–l), R1f (in second−1 [s−1], c–g–m), kmf (in s−1, d–h–n) maps. The first row displays a T2-w GRASE and selective inversion recovery quantitative magnetization transfer imaging derived PSR (b), R1f (c), and kmf (d) maps of a healthy volunteer. Data are derived from a 31year-old healthy gentleman. One can see the net distinction between white matter/grey matter signal on the PSR (b) and R1f (c) maps, but not on the kmf (d). Rows 2 and 3 show the same maps in multiple sclerosis (MS) patient #1 (e–h) and #8 (i–n) in Table 1. A clearly demarcated white matter lesion (WM-L) is seen on T2-w GRASE image of MS patient #1 (red rectangle in figures e–h). Patient #8 instead shows several diffuse WM-Ls on T2-w GRASE image (i) indicated by red arrows and yellow rectangles. These WM-Ls displayed several degrees of intensity on T2-w GRASE images which were reflected by differences in visibility on PSR (l) and kmf (m) maps. The elevated kmf near tissue boundaries (e.g., near the ventricles or large lesions) is related to partial volume averaging as previously described23 and contributed to the large observed variability for this index.
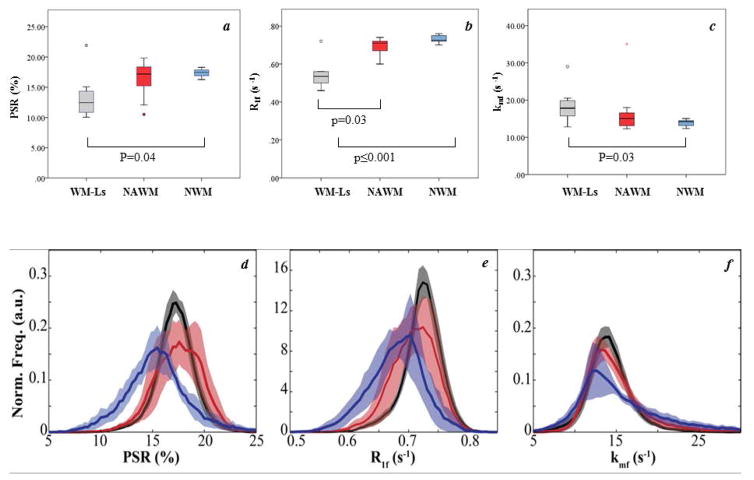
Group differences in SIR-qMT derived metrics
Figure 3a–c displays PSR, R1f, and kmf values in all ROIs. Differences in R1f (p=0.003) were observed when comparing NAWM vs. WM-Ls in patients, a trend toward a difference was observed for PSR (p=0.10), and no difference was detected for kmf values (p=0.66). After correcting for age, differences in all derived metrics were seen between WM-Ls (in patients) and NWM in healthy volunteers in R1f values (p≤0.0001), PSR (p=0.04), and kmf (p=0.03). Conversely, there was no difference in any of the SIR-qMT derived measures between NAWM (in patients) and NWM (in healthy volunteers) with p values of 0.76 for R1f, 0.14 for PSR and 0.47 for kmf.
Figure 3. Differences in free water pool-size-ratio (PSR), spin-lattice relaxation rate of water (R1f), and the MT exchange rate (kmf) between white matter lesions (WM-Ls), normal appearing white matter (NAWM) and normal white matter (NWM), in vivo.
Boxplots for each quantitative magnetization transfer imaging (qMT) parameter (a=PSR in %, b=R1f and c=kmf in seconds−1 [s−1]), where gray, red, and blue represent WM-Ls, NAWM, and NWM, respectively. Significant differences are detailed in figures. Each box represents the 25th– 75th percentile and the black line inside the boxes represents the median value. The vertical lines outside the box delineate the minimum and maximum values. Outliers (small circles, 10th – 90th percentile) and extreme outliers (stars, 5th – 95th percentile) are also represented. NAWM histograms for each qMT parameter (d=PSR, e=R1f and f=kmf). All masks were generated automatically via FSL’s FAST algorithm29 after subtracting manually defined WM-L regions. The dark line represents the mean values and the shaded region the mean ± standard error. Note the overlap between PSR for healthy controls (black line) and patients with negligible disability (red line, patients with expanded disability status scale [EDSS] score ≤ 1), and the trend of decreasing PSR in NAWM with increasing disability (blue line, patients with EDSS >1). For R1f, a similar trend of decreasing values with increasing disability was observed, while kmf values were similar across all cohorts.
Histograms analyses were also performed for NWM and NAWM values, the latter grouped into patients with EDSS ≤1.0 (n=5) or >1.0 (n=5). For all qMT parameters, one can see some degree of overlap in controls and patients with EDSS ≤1.0, which can be quantified by the peak histogram values of the control (PSR: 17.4±0.4%, R1f: 0.73±0.01 s−1, kmf: 13.8±1.3 s−1) and EDSS ≤1.0 cohorts (PSR: 17.9±1.7%, R1f: 0.71±0.03 s−1, kmf: 13.5±1.0 s−1). For the cohort with EDSS > 1, reductions in the peak histogram values for PSR and R1f were observed, while kmf exhibited overlap with controls (PSR:15.6±1.2%, R1f: 0.67±0.03 s−1, kmf: 13.1±1.1 s−1). All WM-Ls identified on T2-w GRASE images were hypo- intense on the T1-w MPRAGE images; therefore, no formal analyses of black holes were performed.
Associations between SIR-qMT derived metrics and EDSS scores
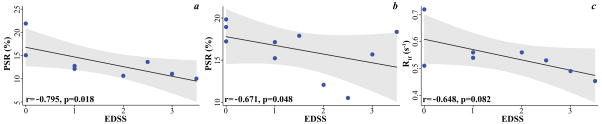
Spearman rank correlation anlayses showed significant associations between EDSS and PSR of WM-Ls (r=−0.795, p=0.018, Figure 4a) and NAWM (r=−0.671, p=0.048, Figure 4b), as well as between EDSS and WM-Ls R1f (r=−0.648, p=0.082, Figure 4c).
Figure 4. Associations between clinical and imaging metrics, in vivo.
Individual points plots showing the significant associations between white matter lesions pool-size-ratio (PSR, in %) (a) and spin-lattice relaxation rate of water (in seconds−1 [s−1]b) as well as normal appearing white matter PSR (c) and expanded disability status scale (EDSS) scores in patients. The gray shadows represent the 95% confidence interval of the regression.
DISCUSSION
In the present study, we demonstrate that SIR-qMT derived measures represent a sensitive biomarker of myelin integrity in MS patients. In the notion that a valid surrogate marker needs to be validated by histopathology, reflect clinical outcome, and dynamically change over time, our work represents a first step toward the validation of SIR-qMT derived measures as a biomarker of myelin integrity in MS patients.
Post mortem validation data
Post mortem data, although derived from a single sample, provided demonstration of the ability of PSR and R1f metrics to capture differences in myelin content. Remyelinated areas, known to have different quantity and thickness of myelin than WM-Ls and NAWM, were clearly discernible on PSR maps. Our histology findings on PSR are in accordance with other previous histology-based studies on both animal models of demyelination22 and humans with MS.19 For example, Janve and collaborators22 measured PSR in the brain WM of mice with type III oligodendrogliopathy. The authors demonstrated a net separation in PSR values between demyelinated plaques and NAWM as well as a strong linear correlation between PSR and myelin intensity in WM-Ls. Similarly Schmierer and collaborators19 found differences in the fraction of macromolecular protons between WM-Ls and NAWM post mortem. Given the larger sample size, the authors were able of adding the demonstration that the fraction of macromolecular protons also differed between remyelinated plaques and NAWM, but not between chronic demyelinated and remyelinated plaques.
The results of our study showed a distinct separation in PSR and R1f between WM-L and NAWM, thus encouraging further studies adding R1f as a sensitive marker of tissue injury. PSR and R1f are both predictive of myelin content;19–22 however, PSR may be more specific to myelin content because R1f is additionally sensitive to other potentially pathological factors present in MS, such as changes in iron content. In agreement with previous studies,22 kmf, was less sensitive and specific to pathology, which can partially be attributed to the difficulty of robustly estimating this parameter.30
In vivo preliminary experience on SIR-qMT in MS
Our in vivo findings on reproducibility of SIR-qMT derived metrics confirmed previously reported ones in our group on healthy brains imaged both at 3T and 7T.23,25 Inter-scan differences for PSR and R1f were <1%. Larger (<5%) differences were instead seen for kmf values, which is not surprising giving the reported difficulty in estimating the rate of MT exchange.30 Overall, these measures verify that SIR-qMT is highly reproducible between scans and, therefore, may be a viable approach for objectively quantifying changes over time when used for monitoring MS patients longitudinally. The latter is an important pre-requisite of an MRI biomarker.1
A net separation in all SIR-qMT related measures was observed in vivo when analyzing differences between NWM and WM-Ls. In measuring differences in SIR-qMT derived metrics between NAWM/WM-Ls and NAWM/NWM, only R1f differences in NAWM/NWM reached statistical significance. Our data, in conjunction with those derived from previous investigations on animal model of demyelination, yield the conclusion that the more distinct separation seen with R1f values may be due to the fact that this includes both relaxation and MT effects. Thus, R1f carries other biological (myelin content), anatomical (fiber orientation), and pathological (heme- and non-heme iron content and edema even in the absence of demyelination) information aside from the myelin quantity per se.30 Several explanations, not mutually exclusive, may instead subtend our findings on PSR and kmf. Firstly, the small sample size could have been underpowered to detect this difference. Secondly, one needs to take into account that 50% of our (small) patient population had a very small degree of brain disease and disability progression; thus, may not have had a high degree of pathology in NAWM, accounting for the spreading of the data towards the normal values. Lastly, one needs to consider that hyper-intense lesions on T2-w images encompass a heterogeneous degree of myelin quantity. While some of these areas may represent chronically demyelinated plaques with even some degree of axonal transection, others may instead represent lesions with some amount of repair and remyelination,31 resulting in similar myelin contents (and qMT-derived values) to minimally injured NAWM. This is further demonstrated in our histogram analyses in Figure 3, where we observed a large degree of overlap in PSR/R1f between healthy controls and patients with EDSS ≤ 1 along with a trend of increasing contrast for the cohort with EDSS > 1. Healthy volunteers had distinguishable peaks but measures overlapped predominantly with values quantified in patients with lower degree of disability. Although the small sample size precludes a quantitative analysis of this trend, this finding support the hypothesis that a larger sample size may capture statistically abnormal values of SIR-qMT derived metrics in the NAWM as well. In agreement, indeed, correlation analyses showed NAWM and WM-Ls PSR/R1f to be significantly (or nearly so) associated to the EDSS scores.
Study limitations, future directions and conclusions
Our work is not without limitations. First among these is the small sample size. While this is important and might have affected part of our results, we believe that given the stage of our analyses, aimed at proving feasibility and applicability, it was not a major drawback. Secondly, the high value of the specific absorption rate of radio-frequency energy arising from the series of RF refocusing pulses in turbo spin echo (TSE) MRI precluded acquiring T1-w/T2-w TSE images. On the basis of our previous work23 demonstrating equivalence of T2-w GRASE and T2-w TSE for clinical scans at 7T, T2-w GRASE sequence was acquired to evaluate lesion burden. However, lack of a T1-w SE precluded assessing differences in SIR-qMT derived metrics between T1-hypontense lesion and T2-hyperitense lesions. Third, we do not provide data regarding the relations between PSR/R1f and axonal count. This is an important piece of investigation that must be attempted in larger sample size to account for the well-known anatomical variations in axons counts. The small post mortem sample size also precluded obtaining any formal quantification between the myelin content and PSR measures.
Notwithstanding the above limitations which do not compromise the validity of the finindgs, our work represents an important proof of concept, feasibility study and first step toward the demonstration of SIR-qMT-based estimates of myelin content in MS patients. We deliver our methods at 7T where one can achieve higher spatial resolution, a crucial factor for studying disease pathology in MS. We are currently expanding both the post mortem and in vivo studies in a larger cohort of patients at both 3T and 7T to address all the recognized limitations, as well as provide additional insights into the specificity and sensitivity of SIR-qMT delivered metrics as biomarkers of myelin integrity for MS patients in both WM and GM structures.
Acknowledgments
ACKNOWLEDGMENTS and DISCLOSURES: We are grateful for the donation of brain specimens for scientific investigations and to each patient and healthy volunteer who kindly participated to our MRI study. We acknowledge the support of the National Multiple Sclerosis Society to the Rocky Mountain MS Center Tissue Bank for providing the specimen material.
We are indebted to the MRI technicians of the Vanderbilt University Institute of Imaging Science and Mr. David Pennell for his assistance with scanning the post mortem brains. The investigators gratefully acknowledge the help of Mr. Christopher J. Cannistraci and Ms. Kehaunani M. Hubbard for assistance with participants’ recruitment.
This work was supported by the extramural program of the National Institutes of Health (NIBIB K01 EB009120, R01 EB000461, and K25 EB013659) and the Clinical and Translational Science Awards (UL1TR000445-06 from National Center for Advancing Translational Sciences/National Institutes of Health). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler. 2012;18:1534–40. doi: 10.1177/1352458512458169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalaa I, Grossman RI, Kolson DL, et al. Multiple sclerosis: magnetization transfer histogram analysis of segmented normal-appearing white matter. Radiology. 2000;216:351–5. doi: 10.1148/radiology.216.2.r00au16351. [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Rocca MA. Magnetization transfer magnetic resonance imaging in the assessment of neurological diseases. J Neuroimaging. 2004;14:303–13. doi: 10.1177/1051228404265708. [DOI] [PubMed] [Google Scholar]
- 4.Gass A, Barker GJ, Kidd D, et al. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol. 1994;36:62–7. doi: 10.1002/ana.410360113. [DOI] [PubMed] [Google Scholar]
- 5.Kalkers NF, Hintzen RQ, van Waesberghe JH, et al. Magnetization transfer histogram parameters reflect all dimensions of MS pathology, including atrophy. J Neurol Sci. 2001;184:155–62. doi: 10.1016/s0022-510x(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 6.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–44. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 7.Koenig S. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285–91. doi: 10.1002/mrm.1910200210. [DOI] [PubMed] [Google Scholar]
- 8.Kucharczyk W, Macdonal P, Stanisz G, Henkelman R. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology. 1994;192:521–9. doi: 10.1148/radiology.192.2.8029426. [DOI] [PubMed] [Google Scholar]
- 9.Schmierer K, Scaravilli F, Altmann D, Barker G, Miller D. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–15. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2017 doi: 10.1111/jon.12480. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Chou J, Lim SY, Tanasescu R, et al. Seven-tesla magnetization transfer imaging to detect multiple sclerosis white matter lesions. J Neuroimaging. 2017 doi: 10.1111/jon.12474. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Mottershead JP, Schmierer K, Clemence M, et al. High field MRI correlates of myelin content and axonal density in multiple sclerosis - a post-mortem study of the spinal cord. J Neurol. 2003;250:1293–301. doi: 10.1007/s00415-003-0192-3. [DOI] [PubMed] [Google Scholar]
- 13.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–54. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Vavasour IM, Laule C, Li DK, Traboulsee AL, MacKay AL. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J Magn Reson Imaging. 2011;33:713–8. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- 15.Moll NM, Rietsch AM, Thomas S, et al. Multiple sclerosis normal-appearing white matter: Pathology-imaging correlations. Ann Neurol. 2011;70:764–73. doi: 10.1002/ana.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blezer EL, Bauer J, Brok HP, Nicolay K, ‘t Hart BA. Quantitative MRI-pathology correlations of brain white matter lesions developing in a non-human primate model of multiple sclerosis. NMR Biomed. 2007;20:90–103. doi: 10.1002/nbm.1085. [DOI] [PubMed] [Google Scholar]
- 17.Berry I, Barker G, Barkhof F, et al. A multicenter measurement of magnetization transfer ratio in normal white matter. J Magn Reson Imaging. 1999;9:441–6. doi: 10.1002/(sici)1522-2586(199903)9:3<441::aid-jmri12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Henkelman R, Huang X, Xiang Q, Stanisz G, Swanson S, Bronskill M. Quantitative interpretation of magnetization transfer. Magn Reson Med. 1993;29:759–66. doi: 10.1002/mrm.1910290607. [DOI] [PubMed] [Google Scholar]
- 19.Schmierer K, Tozer D, Scaravilli F, et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging. 2007;26:41–51. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odrobina EE, Lam TY, Pun T, Midha R, Stanisz GJ. MR properties of excised neural tissue following experimentally induced demyelination. NMR Biomed. 2005;18:277–84. doi: 10.1002/nbm.951. [DOI] [PubMed] [Google Scholar]
- 21.Ou X, Sun S, Liang H, Song S, Gochberg D. The MT pool size ratio and the DTI radial diffusivity may reflect the myelination in shiverer and control mice. NMR Biomed. 2009;22:480–7. doi: 10.1002/nbm.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janve VA, Zu Z, Yao SY, et al. The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. Neuroimage. 2013;74:298–305. doi: 10.1016/j.neuroimage.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dortch RD, Moore J, Li K, et al. Quantitative magnetization transfer imaging of human brain at 7 T. Neuroimage. 2013;64:640–9. doi: 10.1016/j.neuroimage.2012.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SA, Golay X, Fatemi A, et al. Quantitative magnetization transfer characteristics of the human cervical spinal cord in vivo: application to adrenomyeloneuropathy. Magn Reson Med. 2009;61:22–7. doi: 10.1002/mrm.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dortch RD, Li K, Gochberg DF, et al. Quantitative magnetization transfer imaging in human brain at 3 T via selective inversion recovery. Magn Reson Med. 2011;66:1346–52. doi: 10.1002/mrm.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagnato F, Hametner S, Pennell D, et al. 7T MRI-Histologic Correlation Study of Low Specific Absorption Rate T2-weighted GRASE sequences in the detection of white matter involvement in multiple sclerosis. J Neuroimaging. 2015;25:370–8. doi: 10.1111/jon.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnato F, Yao B, Cantor F, et al. Multisequence-imaging protocols to detect cortical lesions of patients with multiple sclerosis: observations from a post-mortem 3 Tesla imaging study. J Neurol Sci. 2009;282:80–5. doi: 10.1016/j.jns.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602–15. doi: 10.1093/brain/awr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 30.Li K, Zu Z, Xu J, et al. Optimized Inversion Recovery Sequences for quantitative T1 and magnetization transfer imaging. Magn Reson Med. 2010;64:491–500. doi: 10.1002/mrm.22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkhof F, Bruck W, De Groot CJ, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol. 2003;60:1073–81. doi: 10.1001/archneur.60.8.1073. [DOI] [PubMed] [Google Scholar]