Abstract
Fetal Alcohol Spectrum Disorder (FASD) is one of the leading causes of mental health issues worldwide. Analysis of zebrafish exposed to alcohol during embryonic development confirmed that even low concentrations of alcohol for a short period of time may have lasting behavioral consequences at the adult or old age. The mechanism of this alteration has not been studied. Here, we immersed zebrafish embryos into 1% alcohol solution (vol/vol%) at 24 hours post-fertilization (hpf) for 2 hours, and analyze potential changes using immunohistochemistry. We measured the number of BDNF (brain derived neurotrophic factor) and NCAM (neuronal cell adhesion molecule) positive neurons and the intensity of synaptophysin staining in eight brain regions: lateral zone of the dorsal telencephalic area, medial zone of the dorsal telencephalic area, dorsal nucleus of the ventral telencephalic area, ventral nucleus of the ventral telencephalic area, parvocellular preoptic nucleus, ventral habenular nucleus, corpus cerebella and inferior reticular formation. We found embryonic alcohol exposure to significantly reduce the number of BDNF and NCAM positive cells in all brain areas studied as compared to control. We also found alcohol to significantly reduce the intensity of synaptophysin staining in all brain areas except the cerebellum and preoptic area. These neuroanatomical changes correlated with previously demonstrated reduction of social behavior in embryonic alcohol exposed zebrafish, raising the possibility of a causal link. Given the evolutionary conservation across fish and mammals, we emphasize the implication of our current study for human health: even small amount of alcohol consumption may be unsafe during pregnancy.
Keywords: Fetal alcohol spectrum disorder (FASD), prenatal, teratogen, neuronal protein, brain plasticity
Graphical Abstract
Introduction
Brain development involves a variety of processes that support cellular proliferation, apoptotic cell death, cellular differentiation, cell migration and the formation of synapses. Exposure to alcohol at different stages of pregnancy may disrupt several of these processes. Fetal alcohol spectrum disorder (FASD) represents a cluster of diseases with differing symptom sets and severity, all resulting from the developing fetus being exposed to alcohol in utero (see review by Riley et al., 2011). Although its specific mechanisms and mode of action are unclear, the particular effects of alcohol are known to depend upon the developmental stage at which alcohol reached the embryo, the concentration and duration of exposure to this substance, as well as the genotype and health status of the mother and the fetus (Riley & McGee, 2005; Ramsay, 2010). FASD may include both physical and behavioral abnormalities, such as anatomical malformations, problems with learning and memory, difficulties with comprehension or with following directions, reduced ability to control emotions, impaired communication, abnormalities with a variety of social behaviors, and issues even with daily life skills, for example, eating and bathing (see review by O’Connor & Paley, 2009). FASD is the leading cause of preventable developmental disability in the world. That is, the global prevalence of FASD among children and youth is approximately 1% of the general population (Lange et al., 2017; Popova et al., 2016). Efficient treatment for FASD does not exist, despite that embryonic alcohol exposure exerts life-long suffering for the patient and his/her family. Thus, FASD represents a clear and urgent unmet medical need.
Animal models have been developed in the hope that they will speed up discovery of the mechanisms of FASD and in turn will lead to the development of treatment for FASD patients (Meyer et al., 1990; Becker et al., 1996; Fernandes & Gerlai, 2009; Kelly et al., 2009; McClure et al., 2011; Church et al., 2012). The zebrafish is one of these animal models proposed for the analysis of FASD (Fernandes & Gerlai, 2009) as it offers some advantages. For example, zebrafish eggs are externally fertilized and develop outside of the mother, which allows one to precisely control the timing and dose of alcohol exposure during embryonic development (Tanguay & Reimers, 2008). Furthermore, ontogenesis is fast (it completes in 5 days), and the development of the zebrafish brain has been well mapped in terms of anatomical changes, patterns of expression of numerous genes and important markers aiding structural and functional characterization of developmental processes (Wullimann et al., 1996).
Previously, exposure to low concentrations of alcohol (up to 1%, vol/vol bath) employed for a short period of time (2 hours) during embryonic development was shown not to induce observable, gross, physical abnormalities, but to lead to significant reduction in the response of the exposed fish to social stimuli (Fernandes & Gerlai, 2009; Fernandes et al., 2015a, Buske & Gerlai, 2011). This impaired shoaling response was found to be accompanied by reduced levels of neurotransmitters dopamine, serotonin and their metabolites in the adult fish brain (Buske & Gerlai, 2011; Mahabir et al., 2014; Fernandes et al., 2015b). In addition, short duration exposure to low levels of alcohol during embryonic development was also found to lead to impaired learning in zebrafish (Fernandes et al., 2014). Other mechanisms potentially underlying the effects of embryonic alcohol exposure on shoaling in zebrafish are not known.
In the present study, we examine whether exposure of zebrafish embryos to a low concentration of alcohol (1% vol/vol bath immersion) for a short period of time (2 hours) results in alteration in the expression of three neuronal proteins important for zebrafish brain development and plasticity: brain derived neurotrophic factor (BDNF), neuronal cell adhesion molecule (NCAM) and synaptophysin, a synaptic protein. The developmental stage at which alcohol is administered is identical to what was employed before, and the concentration we chose corresponds to the highest dose that was previously found to induce robust behavioral impairment of social behavioral responses, and abolishment of dopaminergic responses to social cues in zebrafish without gross anatomical alterations (Fernandes & Gerlai, 2009; Buske & Gerlai, 2011; Fernandes et al., 2015b). Coincidentally, this external bath concentration and exposure regimen was found to lead to an alcohol dose inside the immersed zebrafish egg that corresponds to blood alcohol levels slightly under the considered legal limit for driving in North America (Mahabir et al., 2014).
We chose BDNF as one of the molecules of focus in this study because it plays a vital role during brain development as well as in the adult brain. It is a nerve growth factor that is also involved in the survival of a variety of neurons in mammals such as the dorsal root ganglion cells, hippocampal and cortical neurons (Liu et al., 2016; Lipsky & Marini, 2007). BDNF is known to modulate the function of both excitatory (glutamatergic) (Martin & Finsterwald, 2011) as well as inhibitory (GABAergic) synapses (Wardle & Poo, 2003) and is also known to play roles in the establishment of hippocampal long-term potentiation (LTP), i.e. in synaptic plasticity (Bekinschtein et al., 2008; Xu et al., 2000). BDNF also plays a significant role in neurogenesis (Bath et al., 2012; Egelandet al., 2015), another process which it mediates via activity-dependent synaptic plasticity learning and memory (Lu, 2003; Yamada et al., 2002). We also chose to measure the expression of NCAM, a neuronal cell surface glycoprotein, because in mammals it is known to mediate cell signaling, guidance, cellular differentiation, cell adhesion, neurite outgrowth, axon guidance, neural cell migration, myelination and synapse formation, crucial processes during brain development (Hildebrandt et al., 2007). Furthermore, in the adult mammalian brain, NCAM mediates neuronal plasticity as well as nerve regeneration (Rønn et al., 1998; Rønn et al., 2000a) via intracellular signaling through Src non-receptor protein tyrosine kinases (Maness et al., 1996). Last, we chose synaptophysin, a synaptic vesicle membrane protein which constitutes about 7–10% of the total synaptic vesicle proteins, because it is involved in fundamental processes of neurotransmitter packaging and neurotransmitter vesicle functions (Leube, 1995; Leube et al., 1989; Kwon & Chapman, 2011; Faludi & Mirnics, 2011; Eastwood et al., 2000), and its manipulation has been shown to alter complex learning and memory processes in mice (Schmitt et al., 2009).
Using immunohistochemistry, we examined the expression of these neuronal proteins in eight brain areas of the adult zebrafish: lateral zone of the dorsal telencephalic area (Dl), medial zone of the dorsal telencephalic area (Dm), dorsal nucleus of the ventral telencephalic area (Vd), ventral nucleus of the ventral telencephalic area (Vv), parvocellular preoptic nucleus (PPa), ventral habenular nucleus (Hav), corpus cerebelli (CCe) and inferior reticular formation (IRF).
In rats, the ventral tegmental area (VTA) and nucleus accumbens (nAC) are involved in dopamine-signaling dependent functions associated with reward, pleasure, cognition, drug addiction and in general, motivation. In the zebrafish brain, evolutionarily, functionally and anatomically homologous areas to these mammalian regions are the diencephalic posterior tuberculum and ventral telencephalic area (Vd and Vv respectively) (Rink & Wullimann, 2001; 2002). The ventral telencephalic (Vv) area represents the subpallium (Wullimann & Rink, 2002). The dorsal nucleus of the ventral telecephalic area (Vd) is considered to be part of the striatum and contains dopaminergic cells and fibers (Rink & Wulliman, 2001), while the ventral nucleus of the ventral telencepalic area (Vv) is considered to be part of the septum (Wullimann & Rink, 2002). In mammals, the hippocampus is primarily responsible for relational learning, the cerebellum is involved in motor function and simple associative learning, and the amygdala is involved in fear and other emotion related behavioral phenomena (Eichenbaum et al., 1994). The corresponding homologous brain areas in zebrafish are the lateral zone of the dorsal telencephalic area (Dl, equivalent to the hippocampus), medial zone of the dorsal telencephalic area (Dm, equivalent to the amygdala) and cerebellum (Mueller et al., 2011; Braford, 1995; Castro et al., 2006; Northcutt, 2006; Portavella et al., 2002; 2003; Rodriguez et al., 2005; Ganz et al., 2015). The dorsal telencephalic area in the zebrafish brain represents the pallium (Ganz et al., 2015). The parvocellular preoptic nucleus (PPa) is involved in zebrafish agonistic behavior (Larson et al., 2006) and is suggested to be a homolog of the supraoptic nucleus in mammals (Herget et al., 2014). Furthermore, serotonin has been shown to be the neurotransmitter linked to aggression, and in the mammalian brain, the serotonergic center is the lateral habenula, which is comparable to ventral habenula in zebrafish (Popova, 2008; Amo et al., 2010). Serotonergic cell populations have also been shown to emerge in the reticular formation area of the hindbrain (Panula et al., 2010) as well as it contains dopaminergic and noradrenergic neuron populations (Ma, 1997).
Thus, analysis of the three chosen neuronal markers in these eight zebrafish brain areas should provide us with a reasonable overview of potential neuroanatomical and functional changes that embryonic alcohol exposure may have induced. Although detailed analysis of the relevance of these brain areas, and molecular targets, for particular aspects of zebrafish behavior is often lacking, we hope that our pioneering study will allow the establishment of working hypotheses as to how exposure to alcohol during embryonic development leads to lasting functional changes in the vertebrate brain. We hypothesize that a single, short, low concentration alcohol exposure during development will result in a change in expressions of BDNF, NCAM, and synaptophysin in adult zebrafish brains. A change in these neuronal proteins could offer a plausible explanation for the altered brain functions (learning deficit, and impaired social behavioral responses) in adult life that has been reported in zebrafish as well as in the milder forms of FASD in humans. Changes in these molecular targets could explain the previously reported reduction of levels of dopamine, serotonin and their metabolites in the zebrafish brain. Furthermore, analysis of these molecular targets may lead to identification of biomarkers that could be used as diagnostic tools for FASD.
Materials and Methods
Housing and alcohol treatment
Adult zebrafish (Danio rerio) of the AB strain were bred in-house (University of Toronto Mississauga Vivarium, Mississauga, ON, Canada) to obtain fertilized eggs. All experiments described below were approved by the University of Toronto Animal Care Committee, and were conducted in accordance with the guidelines of the Canadian Council for Animal Care (CCAC). The eggs were kept in system water, reverse osmosis filtered and sterilized water supplemented with 60 mg/l Instant Ocean Sea Salt (Big Al’s Pet Store, Mississauga, ON, Canada). At 24 hours post-fertilization (hpf) each group of eggs was immersed in either 0% or 1% (vol/vol) alcohol solution for 2 hrs. Subsequently, the eggs were washed with system water three times. The concentration, and the timing of exposure, employed in the current study was based upon prior studies that showed administration of 1% alcohol during this stage of development to result in robust and significant behavioral changes in adult zebrafish without any observable deleterious morphological effects or negative influence on growth rate and mortality (Fernandes & Gerlai, 2009; Buske & Gerlai, 2011). It is also notable that immersion in the 1% alcohol solution was shown to result in alcohol concentration inside the egg that was about 1/14th of the bath concentration, i.e. 0.07% (Mahabir et al., 2014). The legal limit for driving in most parts of North America is between 0.05 and 0.08%, thus the 0.07% alcohol concentration inside the zebrafish egg is comparable to what one may expect to reach the human fetus when a pregnant woman consumes moderate to low amounts of alcohol.
Eggs hatched at approximately 3 days post-fertilization (dpf). At 5 dpf (free swimming stage) the fish were placed in nursery racks where they were initially fed Larval Artificial Plankton 100 (dried fish formula with particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA) and subsequently freshly hatched brine shrimp nauplii (Artemia salina). At 3 weeks post-fertilization the developing fish were fed a 1:1 mixture of flake food (Tetramin Tropical fish flake food, Tetra Co, Melle, Germany) and powdered spirulina algae (Jehmco Inc., Lambertville, NJ, USA), which continued to adulthood.
Tissue fixation cryoprotection, and tissue processing
Adult zebrafish from control and alcohol exposed groups were quickly decapitated and the heads immediately placed in 4% paraformaldehyde (in PBS) for 24 h at 4°C. Heads were subsequently sequentially placed in 10% (2 h), 20% (4 h), and 30% (24 h) sucrose at 4°C in order to cryoprotect the tissue before sectioning.
The entire zebrafish head was sectioned at 30 microns using a Microm HM 520 Cryostat after mounting with HistoPrep frozen tissue embedding media (Cat. No. SH75-125D; Fisher Scientific) at −25°C. Tissue sections were transferred onto Fisherbrand Superfrost Plus Microscope slides and were dried for an additional hour. Slides were subsequently stored at −80°C until immunohistochemistry was performed. Slides were dried at 45°C on a slide warmer for 1h before staining. Background autofluorescence was reduced by incubating with 1 mg/ml sodium borohydride in ice-cold sPBS for 30 min, and rinsed with sPBS three times. Following sodium borohydride treatment, sections were incubated in 5% normal goat serum in sPBS containing 0.3% Triton X-100 for 20 min to block non-specific binding. Sections were then incubated in the primary antibodies: anti-BDNF (rabbit polyclonal, Chemicon, Temecula, CA, Cat# AB1779SP); anti-NCAM (Sigma Chemicals, St. Louis, MO) (Cat# ABIN335373, AB_10766836); anti-synaptophysin (Sigma Chemicals, St. Louis, MO) (Cat# ABIN335305, AB_10768391) (1:200 dilution using 5% normal goat serum in sPBS) overnight at 4°C. The slides were then washed with sPBS four times (5 min each), and incubated with the secondary anti-rabbit antibody coupled to FITC at 1:200 dilution using 5% goat serum in sPBS for BDNF, or anti-mouse antibody coupled to TRITC at 1:200 dilution using 5% goat serum in sPBS for NCAM and synaptophysin for 2 h at room temperature in the dark. The sections were then washed four times (5 min each) with sPBS and counterstained with 0.5 μg/mL DAPI in sPBS for 10 min in the dark and subsequently washed with sPBS four times. Slides were then mounted using Shandon PermaFluor Mountant with Fisherbrand coverslips. Sections were dried overnight in the dark and subsequently stored at 4°C. For our negative control experiment, we examined non-specific binding by the secondary antibody by substituting the primary antibody with 5% normal goat serum in sPBS.
Image analysis
Immunoreactive cells were imaged using an immunofluorescence microscope (Olympus BX60), and quantified using Image Pro Plus (Media Cybernetics, Inc.) as described before for rat brain (Chatterjee et al, 2007). Appropriate excitation filters for FITC, TRITC and DAPI were used to obtain images at 400x magnification. Different brain regions were identified on the basis of neuroanatomical landmarks, and pictures were taken from identical sites. Schematic diagrams of the sites where photomicrographs were taken are shown in figures 2, 4 and 6. For analysis, pictures were taken of a particular brain area from 4 consecutive sections. Pictures were taken at 400x magnification for counting using a fixed exposure period for each type of stain (100 ms for DAPI and 6 s for BDNF, NCAM and synaptophysin). The exposure time chosen was based upon the most optimal signal to noise ratio. Number of immunoreactive cells (for BDNF and NCAM positive cells) were quantified using Image-Pro Plus (Media Cybernetics, Inc.) using an automated count with a minimum cut-off area size of 200 pixels. For synaptophysin immunoreactivity, we have counted the pixels (as a measure of synaptophysin immunoreactivity) using Image-Pro Plus (Media Cybernetics, Inc.) using an automated pixel count. Background parameters for all pictures were set to identical settings using the digital control of the Image-Pro Plus software (Media Cybernetics, Inc.). In addition, the size of the area within which quantification of positively stained structures was performed was standardized for all three neuronal markers analyzed, in order to allow us direct comparison of quantified results across the selected brain regions. The sample size (n) of control and treated fish was 6, and the number sections analyzed per area of brain was 4.
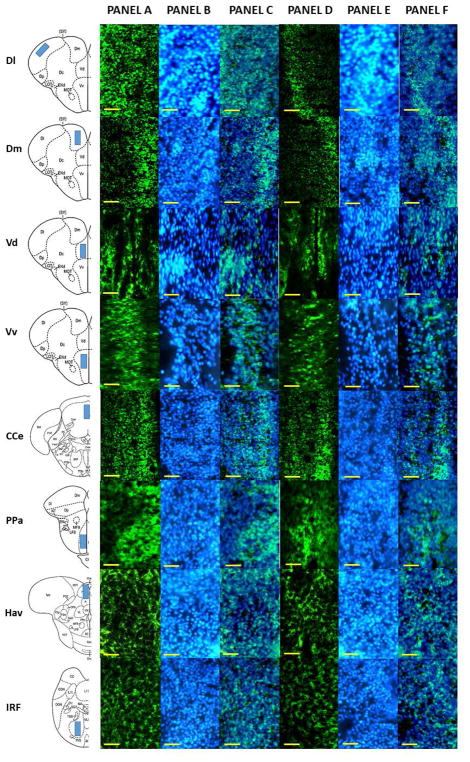
Figure 2.
Representative photomicrograph of different areas of adult zebrafish brain stained with anti-BDNF antibody. The first column of the figure displays a schematic diagram of the areas analyzed. Panel A depicts the anti-BDNF stained brain section of control fish (0% alcohol exposed) and Panel B depicts a DAPI stained brain section of the corresponding area. Panel C shows Panel A and B superimposed. Panel D depicts the anti-BDNF stained brain section of 1% alcohol exposed zebrafish, and Panel E depicts a DAPI stained brain section of the corresponding area. Panel F displays Panel D and E superimposed. The scale bar represents 10μm.
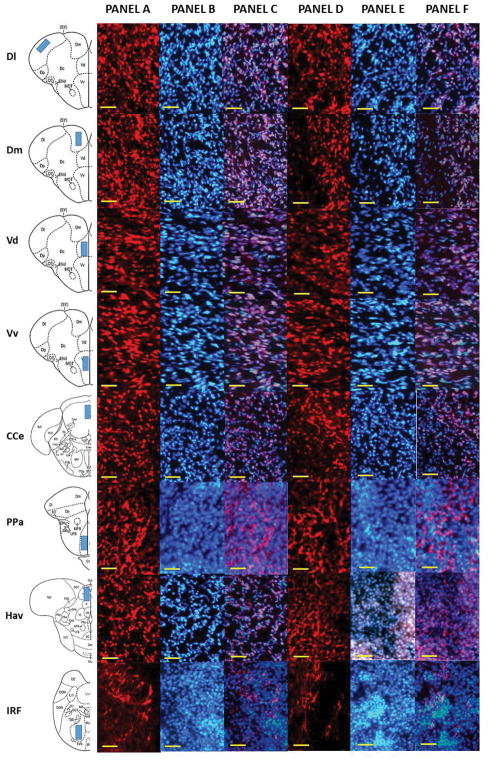
Figure 4.
Representative photomicrograph of different areas of adult zebrafish brain stained with anti-NCAM antibody. The first column of the figure displays a schematic diagram of the areas analyzed. Panel A depicts the anti-NCAM stained brain section of control fish (0% alcohol exposed), and Panel B depicts a DAPI stained brain section of the corresponding area. Panel C shows Panel A and B superimposed. Panel D depicts the anti-NCAM stained brain section of 1% alcohol exposed zebrafish, and Panel E depicts a DAPI stained brain section of the corresponding area. Panel F displays Panel D and E superimposed. The scale bar represents 10μm.
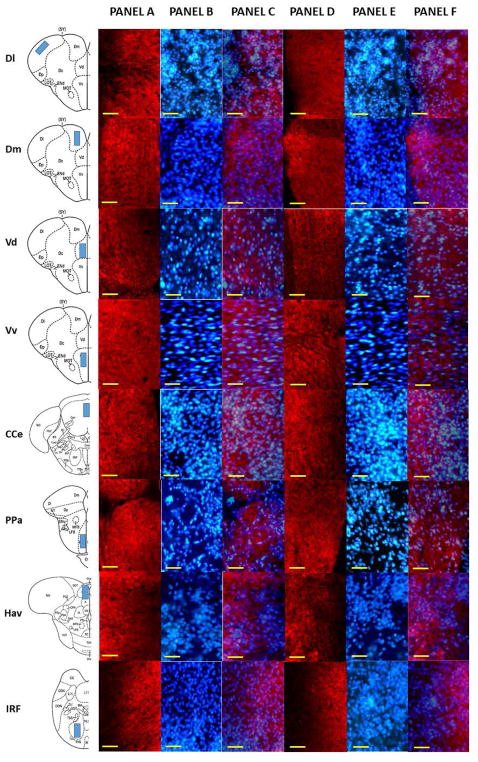
Figure 6.
Representative photomicrograph of different areas of adult zebrafish brain stained with anti-synaptophysin antibody. The first column of the figure displays a schematic diagram of the areas analyzed. Panel A depicts the anti-synaptophysin stained brain section of control fish (0% alcohol exposed), and Panel B depicts a DAPI stained brain section of the corresponding area. Panel C shows Panel A and B superimposed. Panel D depicts the anti-synaptophysin stained brain section of 1% alcohol exposed zebrafish, and Panel E depicts a DAPI stained brain section of the corresponding area. Panel F displays Panel D and E superimposed. The scale bar represents 10μm.
Western blot analysis
Zebrafish brains were dissected and stored at −80°C until use. For NCAM and synaptophysin western blots, brains were sonicated in 30μL of Tris-buffered saline (TBS). Protein concentration was determined using Bio-Rad protein assay dye reagent (Bio-Rad). 4X Laemmli buffer was added to each sample (3 volume sample lysis +1 volume sample buffer) and boiled for 2 min. For BDNF western blot, the tissue was prepared different to facilitate better dissolution of BDNF from tissue. For BDNF western blot, dissected tissues were homogenized in 10 volumes of 100mM phosphate buffer containing 1mM EDTA, 2M guanidine hydrochloride (pH7.2), and three protease inhibitors (10mM N-ethylmaleimide, 0.36mM pepstatin, and 1mM PMSF). Homogenates were subsequently sonicated and processed in Laemmli buffer as described above for NCAM and synaptophysin. Twenty micrograms of protein from each sample were loaded onto each lane of a 7.5% polyacrylamide gel (Bio-Rad) and separated by electrophoresis. Separated proteins were transferred to a nitrocellulose membrane using a Bio-Rad mini transblot apparatus. Membranes were incubated with 5% non-fat dry milk in TBS containing 0.1% Tween-20 for 2 h at room temperature to block nonspecific binding. The membrane was then incubated in the primary antibodies: anti-BDNF (rabbit polyclonal, Chemicon, Temecula, CA, Cat# AB1779SP); anti-NCAM (mouse monoclonal, Sigma Chemicals, St. Louis, MO, Cat# C-9672); anti-synaptophysin (mouse monoclonal, Sigma Chemicals, St. Louis, MO, Cat# S-5768) respectively (all primary antibodies were 1:200 dilution using 5% normal goat serum in sPBS) overnight at 4°C. The membranes were subsequently washed with TBS containing 0.1% Tween-20 four times (5 min each). The membranes were then incubated in a secondary anti-rabbit antibody coupled to HRP (1:10000 dilution for BDNF antibody) or in anti-mouse antibody coupled to HRP (1:10000 dilution for NCAM and synaptophysin antibody) for 1 h at room temperature and subsequently washed with TBS containing 0.1% Tween-20. Immunoreactivity was visualized using the enhanced chemiluminescence method according to the manufacturer’s instructions (GE Healthcare Bio-Sciences Corp.), exposed to X-Ray film for 30 sec and developed in a Kodak automated developer. Side by side we have blotted solubilized rat brain homogenates with the same anti-NCAM, anti-synaptophysin and anti- BDNF antibody to confirm the staining of specific proteins in the zebrafish brain.
Statistical analysis
SPSS (version 14.1) was used for data analysis. For each neuronal marker, a repeated measures Univariate Variance Analysis (ANOVA) was performed with two factors: Brain Area (the repeated measures within subject factor with 8 levels) and Embryonic Alcohol Treatment (the between subject treatment factor with 2 levels). Subsequently, as appropriate, a post hoc t-test with Holm-Bonferroni correction (to reduce type 1 error without inflating type 2 error) was performed for each brain area to compare the two treatment groups. The null hypothesis was rejected when its probability (p) was less than 0.05.
Results
Specificity of the BDNF, NCAM and synaptophysin antibody
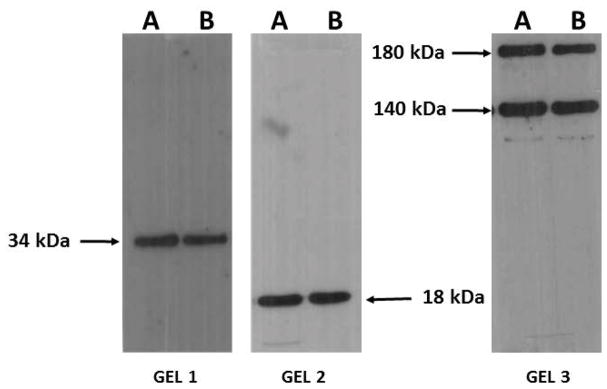
The antibodies employed in this study were developed using mammalian species. Although high nucleotide sequence homologies between zebrafish and human genes have been found and although similarly high amino-acid sequence homologies between zebrafish and human protein homologs have been well established, antibodies developed for particular mammalian molecular targets may not always work in zebrafish. To investigate whether our chosen antibodies have indeed specificity of binding for their intended targets we conducted western blot analyses. These analyses revealed that the anti-BDNF, anti-NCAM and anti-synaptophysin antibody used in this study detected identical bands in both rat and zebrafish brain homogenates. Anti-synaptophysin antibody recognized a band at 34 kDa and BDNF recognized a band at 18 kDa in both rat and zebrafish brain homogenates. The anti-NCAM antibody, as expected from product insert of the antibody (https://www.sigmaaldrich.com/catalog/product/sigma/c9672?lang=en®ion=CA), recognized two proteins, which corresponded to the molecular weight of 180 kDa and 140 kDa subunits of NCAM protein, both in rats and zebrafish brains. The results shown in Fig. 1 thus suggest that the antibodies used in this study specifically recognized the BDNF, NCAM and synaptophysin protein in the zebrafish brain, and were not binding other brain proteins.
Figure 1.
Western blot analysis of rat (A) and zebrafish (B) brain homogenates with anti-synaptophysin (gel 1), anti-BDNF(gel 2) and anti-NCAM(gel 3) antibodies used in the present study. Molecular weights of the proteins recognized by each antibody are indicated by arrows. Note the close correspondence of signals between rat and zebrafish brains.
Quantification of BDNF, NCAM and synaptophysin
Representative immunostained photomicrographs of different brain areas of zebrafish labeled with anti-BDNF, anti-NCAM, and anti-synaptophysin antibodies are shown in Fig. 2, 4, and 6 respectively. The schematic diagrams of the areas analyzed are also shown in these images. Panel “A” shows the representative immunostaining of control fish brain (brain of fish that received no alcohol during embryonic development). Panel “B” displays DAPI stains of the same area. Panel “C” displays the superimposed image of Panels “A” and “B”. Panel “D” showing the representative immunostaining in brains of embryonic alcohol treated fish (fish that received 1% alcohol during embryonic development). Panel “E” displays DAPI stains of the same area. Panel “F” displays the superimposed image of Panels “D” and E”. It is important to note that the pattern of DAPI stained nuclei were denser than immunostained cells, as DAPI stained all cells in any area, but immunostaining only stained a target-positive population, i.e. a sub-population, of neurons in that area. Furthermore, we found no significant differences in the total number of DAPI stained nuclei in each area studied between control and 1% alcohol treated fish (data not shown). Therefore, any difference observed in the immunostained cells between control and alcohol treated fish is likely not due to alcohol treated animals having a reduction in overall number of cells, but rather to changes specific to the immunostained target neuron population.
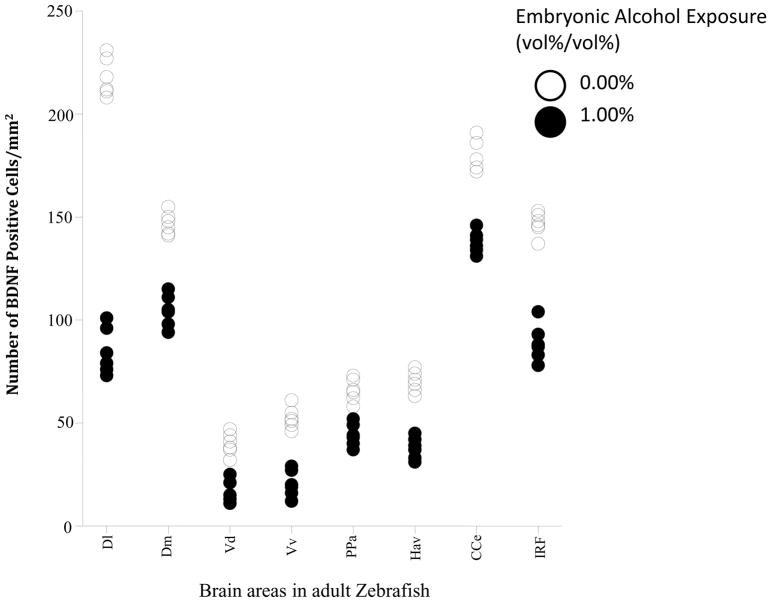
The number of BDNF positive cells quantified in different areas of the brains of control and 1% alcohol exposed zebrafish (expressed as number of positive cells/mm2area) is shown in Fig. 3. Repeated measures ANOVA detected a significant brain area effect (F(7, 70) = 819.72, p < 0.001) demonstrating that BDNF positive cells are not distributed evenly across the eight brain structures studied. It also found the effect of embryonic alcohol treatment to be significant (F(1, 10) = 667.81, p <0.001), confirming the lasting effect of early embryonic alcohol exposure on the brain in adult zebrafish. Last, it also detected a significant brain area x alcohol interaction (F(7, 70) = 96.43, p < 0.001) suggesting that the lasting effect of embryonic alcohol exposure was not equal in magnitude for all brain areas studied. Although the magnitude of alcohol effect varied across brain areas, post hoc two-sample t-tests revealed a significant reduction in the number of BDNF positive cells in fish exposed to alcohol compared to fish that received no alcohol in the lateral zone of the dorsal telencephalic area (D1) (t(10)= 22.23, p< 0.001), medial zone of the dorsal telencephalic area (Dm) (t(10)= 11.00, p< 0.001), dorsal nucleus of the ventral telencephalic area (Vd) (t(10)= 7.10, p< 0.001), ventral nucleus of the ventral telencephalic area (Vv) (t(10)= 9.39, p< 0.001), corpus cerebelli (CCe) (t(10)= 11.39, p< 0.001), parvocellular preoptic nucleus (PPa) (t(10)= 6.74, p< 0.001), ventral habenular nucleus (Hav) (t(10)=10.67, p< 0.001) and inferior reticular formation (IRF) (t(10)= 13.38, p< 0.001).
Figure 3.
Scatter-plot showing number of BDNF positive cells in different areas of the adult zebrafish brain. Note that alcohol (0%, control, white; or 1% alcohol black) was delivered during embryonic development and immunostaining to detect BDNF positive cells was conducted in adult zebrafish. Each point represents results from a single subject. The different brain areas analyzed were: lateral zone of the dorsal telencephalic area (Dl), medial zone of the dorsal telencephalic area (Dm), dorsal nucleus of the ventral telencephalic area (Vd), ventral nucleus of the ventral telencephalic area (Vv), parvocellular preoptic nucleus (PPa), ventral habenular nucleus (Hav), corpus cerebelli (CCe) and inferior reticular formation (IRF). n=6, and 4 sections were used per animal.
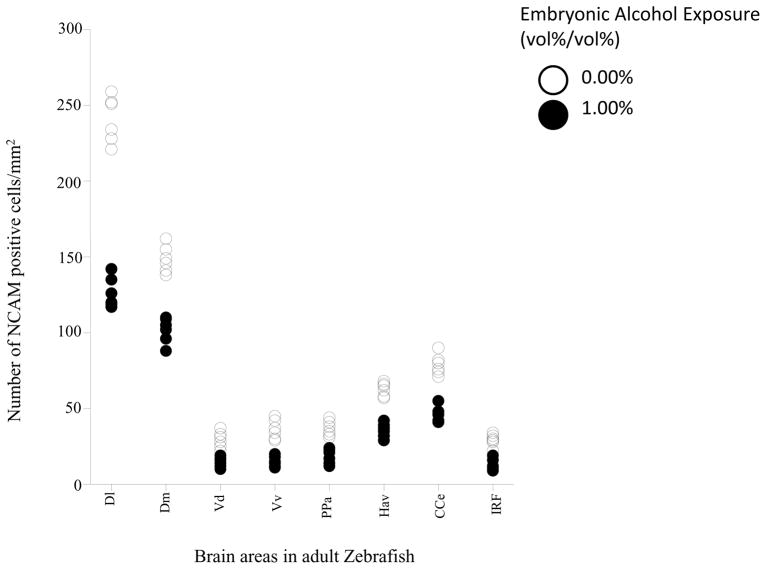
Figure 5 shows the number of NCAM positive neurons (expressed as number of positive cells/mm2area) quantified from 8 brain areas of adult zebrafish that were exposed either to embryonic alcohol or to fresh water (control). The figure demonstrates a robust reduction of the number of NCAM positive cells in all brain areas studied. It also suggests an unequal distribution of NCAM positive cells across the brain areas studied. Repeated measures ANOVA confirmed these observations and showed a significant brain area effect (F(7, 70) = 1004.98, p < 0.001) as well as a significant alcohol effect (F(1, 10) = 350.32, p < 0.001). In addition, the brain area x alcohol interaction term was also found to be highly significant (F(7, 70) = 78.55, p <0.001) demonstrating that although alcohol significantly reduced the number of NCAM positive neurons in the brain of adult fish, this reduction was brain area dependent. Post hoc two sample t-test comparisons of embryonic alcohol treated and control fish showed that the alcohol treatment significantly reduced the number of NCAM positive neurons in the lateral zone of the dorsal telencephalic area (D1) (t(10)= 15.35, p< 0.001), medial zone of the dorsal telencephalic area (Dm) (t(10)= 9.36, p< 0.001), dorsal nucleus of the ventral telencephalic area (Vd) (t(10)= 5.62, p< 0.001), ventral nucleus of the ventral telencephalic area (Vv) (t(10)= 7.10, p< 0.001), corpus cerebelli (CCe) (t(10)= 9.46, p< 0.001), parvocellular preoptic nucleus (PPa) (t(10)= 6.49, p< 0.001), ventral habenular nucleus (Hav) (t(10)=10.19, p< 0.001) and inferior reticular formation (IRF) (t(10)= 7.08, p< 0.001).
Figure 5.
Scatter-plot showing number of NCAM positive cells in different areas of the adult zebrafish brain. Note that alcohol (0%, control, white; or 1% alcohol black) was delivered during embryonic development and immunostaining to detect NCAM positive cells was conducted in adult zebrafish. Each point represents result from a single subject. The different brain areas analyzed were: lateral zone of the dorsal telencephalic area (Dl), medial zone of the dorsal telencephalic area (Dm), dorsal nucleus of the ventral telencephalic area (Vd), ventral nucleus of the ventral telencephalic area (Vv), parvocellular preoptic nucleus (PPa), ventral habenular nucleus (Hav), corpus cerebelli (CCe) and inferior reticular formation (IRF). n=6, and 4 sections were used per animal.
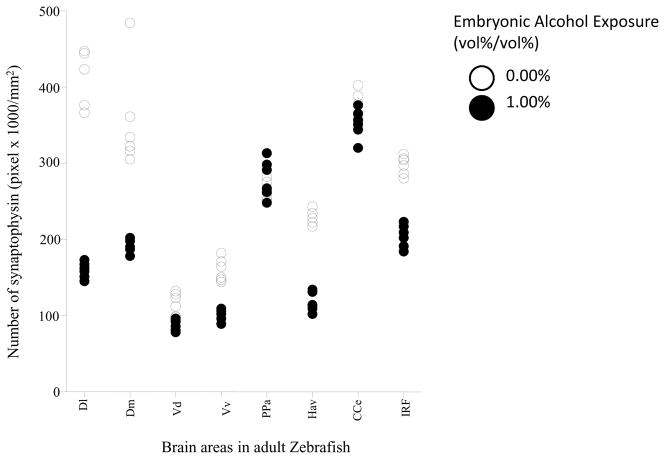
Figure 7 shows the intensity of synaptophysin immunostaining (expressed as number of pixels x 1000/mm2 area) in 8 brain areas of adult fish that were exposed either to alcohol or to freshwater during embryonic development. Similarly to BDNF and NCAM, embryonic alcohol exposure appears to have reduced the signal compared to control. Repeated measures ANOVA found the effects of brain area (F(7, 70) = 167.92, p < 0.001) and of alcohol treatment (F(1, 10) = 467.25, p < 0.001) significant. The interaction between these two factors was also significant (F(7, 70) = 38.82, p < 0.001), suggesting that although alcohol in general reduced the intensity of synaptophysin immunostaining, this effect was dependent upon the brain area in which it was quantified. Post hoc two-sample t-tests comparing alcohol and freshwater treated fish found a significant reduction in the intensity of synaptophysin in fish exposed to alcohol compared to fish that received no alcohol in the lateral zone of the dorsal telencephalic area (D1) (t(10)= 15.73, p< 0.001), medial zone of the dorsal telencephalic area (Dm) (t(10)= 5.95, p< 0.001), dorsal nucleus of the ventral telencephalic area (Vd) (t(10)= 4.12, p= 0.0122), ventral nucleus of the ventral telencephalic area (Vv) (t(10)= 8.73, p< 0.001), ventral habenular nucleus (Hav) (t(10)=17.10, p< 0.001) and inferior reticular formation (IRF) (t(10)= 11.34, p< 0.001). However, the alcohol effect was found non-significant for the corpus cerebelli (CCe) (t(10)= 2.19, p= 0.1075) and parvocellular preoptic nucleus (PPa) (t(10)= −1.33, p= 0.2253),
Figure 7.
Scatter-plot showing intensity (as measured by pixel count) of synaptophysin positive staining in different areas of the adult zebrafish brain. Note that alcohol (0%, control, white; or1% alcohol black) was delivered during embryonic development, and immunostaining to detect synaptophysin expression was conducted in adult zebrafish. Each point represents result from a single subject. The different brain areas analyzed were: lateral zone of the dorsal telencephalic area (Dl), medial zone of the dorsal telencephalic area (Dm), dorsal nucleus of the ventral telencephalic area (Vd), ventral nucleus of the ventral telencephalic area (Vv), parvocellular preoptic nucleus (PPa), ventral habenular nucleus (Hav), corpus cerebelli (CCe) and inferior reticular formation (IRF). n=6, and 4 sections were used per animal.
Discussion
Previously, low doses of ethanol administered for a short period of time during the embryonic development of zebrafish were shown to induce lasting changes in the behavior. These alterations included reduction or abolishment of response to computer generated social stimuli (Fernandes & Gerlai, 2009; Fernandes et al., 2015a; 2015b), and impaired shoaling with live conspecifics (Buske & Gerlai, 2011), as well as impaired associative learning (Fernandes et al., 2014). The mechanisms underlying these embryonic alcohol exposure-induced behavioral changes are largely unknown, although some neurotransmitter systems (particularly the dopaminergic system) have already been implicated (Buske & Gerlai, 2011; Fernandes et al., 2015b; Mahabir et al., 2013). Social cognition as well as learning and memory are supported by a complex array of molecular mechanisms (Bicks et al., 2015; Mayford et al., 2012), and alcohol is known to interact with many of them (Wozniak et al., 2004). Due to the fact that alcohol interacts directly and indirectly with a large number of molecular targets and thus influences numerous biochemical pathways, its effects on embryonic development are also expected to be rather complex, developmental-stage and alcohol-concentration dependent, analysis of which necessitates comprehensive systematic time-course studies. However, comprehensive analysis of embryonic alcohol induced mechanistic changes would be a substantial undertaking. Instead of such a comprehensive analysis, we decided to focus on a particular embryonic stage, the 24th hpf time point of development that has been shown to be particularly sensitive to alcohol effect in the context of social behavior; a single dose (1% external bath concentration) of alcohol that has been shown to abolish responses to social stimuli; and only three neuronal markers that are fundamentally important in many processes underling cognitive and including social behavioral functions. The three chosen targets, BDNF, NCAM and synaptophysin, are also known to play crucial roles during brain development, and are likely to be engaged by embryonic alcohol exposure.
Using an immunohistochemical approach, we investigated potential effects of embryonic alcohol exposure on the expression of these neuronal markers quantified in the fully developed adult zebrafish. We found a robust reduction in the number of BDNF and NCAM positive cells and also the strength of staining for synaptophysin in the brains of adult zebrafish that received embryonic exposure to alcohol. This is notable, because the changes were observed in fully mature adult zebrafish, thus demonstrating the long-lasting consequences of early developmental exposure to alcohol. It is also important to note that, although we found the alcohol effect significant in most brain regions, the magnitude of this effect depended upon the particular brain region studied. In other words, the impact of embryonic alcohol treatment varied according to brain region. How these immunohistochemical/molecular changes may contribute to alteration of behavior is not known at this point.
The BDNF gene is well conserved across species, from fish to mammals (Heinrich & Pagtakhan, 2004; Aid et al., 2007; Pruunsild et al., 2007), suggesting that this protein has a strong functional significance as well as translational relevance. Many neurodevelopmental diseases characterized by deficits with synaptic plasticity have been associated with abnormalities in the expression of BDNF (Alberch et al., 2004; 2006; Arancio & Chao, 2007). BDNF plays a vital role during the development of the brain (Cohen-Cory et al., 2010). For example, BDNF is involved in dendritic development, synaptic connectivity and growth and branching of axon terminals (Zweifel et al., 2005; Ming et al., 2001;). In adulthood, BDNF plays a pivotal role in the molecular mechanisms of synaptic plasticity, and many studies demonstrate the involvement of BDNF in learning and memory, neurogenesis, synaptic morphological rearrangements and plasticity (Hall et al., 2000; Mu et al., 1999; Cunha et al., 2010; Waterhouse et al., 2012; Bath et al., 2012 and Vilar & Mira, 2016; An et al., 2008; Tongiorgi et al., 2006). These studies indicate the importance of BDNF both in the adult and in the developing brain, and how alteration of this protein can impact the neuronal circuit.
BDNF is also known to modulate monoaminergic synaptic transmission, for example, antidepressants primarily acting on the serotonergic and noradrenergic systems increase BDNF mRNA and protein expression (Lee & Kim, 2010; Binder & Scharfman, 2004). Importantly, BDNF expression has been demonstrated in numerous parts of the zebrafish brain including those studied here (Cacialli et al., 2016). We speculate that the reduction of BDNF expressions we observed in brain areas where dopaminergic neurons reside (Vd, D1, Dm) and in areas where serotonergic neurons are found (Hav and IRF) may have contributed to the impaired shoaling and learning performance reported previously for zebrafish exposed to alcohol during embryonic development (Fernandes & Gerlai, 2009, 2014). It is possible that abnormal functioning of the dopaminergic system may have diminished the motivation to join a shoal, and alteration of the serotoninergic system may have modified fear or aggression related phenotypes, which could have contributed to modification of cognitive processes involved in shoaling and learning.
NCAM is closely linked to cellular processes involving neural development and plasticity (see Hinsby et al., 2004 for review) and learning and memory (Bailey et al., 1992; Mayford et al., 1992; Schuster et al., 1996). Polysialylated NCAM (PSA-NCAM), or embryonic NCAM (eNCAM), plays a role in nervous system development and plasticity (Rutishauser & Landmesser, 1996;; Durbec & Cremer, 2001; Kleene & Schachner, 2004). During development, PSA-NCAM is expressed with axon outgrowth, branching and contact formation in the cortocospinal and the retinotectal systems as well as in the formation of the neuromuscular junction (Daston et al., 1996; Fraser et al., 1984; Yin et al., 1995; Williams et al., 1996; Landmesser et al., 1990). In the adult brain, the degree of polysialylation of NCAM (PSA-NCAM) is low and most NCAM molecules play a role in stabilizing structures. In vitro studies indicate the role of NCAM in long term potentiation (LTP) (Luthi et al., 1994; Rønn et al., 2000a; Muller et al., 1996) as well as neurite extension (Hildebrandt et al., 2007). The mechanisms underlying NCAM’s role in synaptic plasticity, regeneration, or adult learning and memory is still poorly understood.
The expression of NCAM was also affected in all of the brain regions we studied. NCAM has been found to play a role in the serotonergic system and in the development and survival of dopaminergic neurons (Stork et al., 2000; Kohl et al., 2013; Ghitza et al., 2010; Xiao et al., 2009). Based on these studies, the reduced NCAM expression in the brain of embryonic alcohol exposed zebrafish may explain the lower serotonin and dopamine levels as well as the reduced levels of metabolites of these neurotransmitters previously reported as resulting from embryonic alcohol exposure in zebrafish (Buske & Gerlai, 2011). Thus, both BDNF and NCAM may underlie the previously observed behavioral abnormalities of embryonic alcohol exposed fish, including impaired social behavior and learning performance.
Synaptophysin is exclusively localized to synaptic vesicles and is widely used as a marker for pre-synaptic terminals. It is one of the most frequently used protein markers of synapse formation and synaptic plasticity in the brain (Counts et al., 2006; Reddy et al., 2005; Knaus et al., 1986). Synaptophysin also plays a vital role in learning and memory (Sze et al., 1997; Frick & Fernandez, 2003). A study by Sun et al. (2007) indicated that synaptophysin may be involved in plasticity-related changes after injury of hippocampal neurons and in age-related cognitive impairment (Smith et al., 2000). Recent studies also revealed increased synaptophysin in hippocampal neurons in aged brains, indicating the possibility of compensation for the cognitive decline associated with aging (Grilloa et al., 2013). Though synaptophysin is one of the major synaptic vesicle proteins, its involvement in the regulation of neurotransmission is unknown (McMahon et al., 1996).
In our study, the expression of synaptophysin was significantly reduced by embryonic alcohol treatment in the D1, Dm, Vd, Hav, Vv and IRF regions of zebrafish exposed to embryonic alcohol treatment as compared to fish that received no alcohol. Notably, we found no such alcohol effect on the cerebellum and preoptic area. These results suggest a decrease in synaptic connections, which likely include dopaminergic and serotoninergic synapses, in D1, Dm, Vd, Vv, Hav and IRF, but no synaptic level alterations in the cerebellum and preoptic regions. This finding is intriguing as embryonic alcohol treatment has been found to decrease serotonin and dopamine levels in a dose dependent manner (Mahabir et al., 2014) as well as to impair behaviors that may be mediated or influenced by these neurotransmitter systems (reward, learning, social interactions) in adult zebrafish (Fernandes & Gerlai, 2009, 2014).
Very few studies reported on the effects of prenatal ethanol on BDNF (Caldwell et al., 2008; Feng et al., 2005; Maier et al., 1999), NCAM and synaptophysin (Medina, 2011; Klintsova et al., 2013; Kumar et al., 2013) in the vertebrate brain, and in all of these studies and others mentioned in this paper, the concentration of alcohol employed during development was high and the duration of exposure was long, thus these studies may only have translational relevance for the most extreme cases of FASD. Our current study is the first to show decreased expressions of BDNF, NCAM, and synaptophysin in the brain of adult zebrafish due to a single, short and low dose alcohol exposure during development. Prior published results (e.g. Fernandes et al., 2015b; Mahabir et al., 2013; Buske & Gerlai, 2011) and our own unpublished findings suggest that the alcohol exposure regimen and timing employed in the current study particularly affects the dopaminergic and serotoninergic neurotransmitter systems leaving other neurotransmitter systems, e.g. the GABAergic and glutamatergic, relatively intact at least in the context of neurochemical levels analyzed using HPLC. Thus, our current study together with the previous findings offer working hypotheses about the mechanisms underlying the altered brain functions, e.g. learning deficit and impaired social behavioral responses, detected previously in fully mature or old zebrafish, and, as such, opens new research lines into the analysis of the effects of embryonic alcohol exposure in vertebrates.
Our results also open the possibility of identifying potential biomarkers that may be used for diagnosing FASD, a major limitation in the human clinic. In summary, given the translational relevance of the zebrafish (Gerlai, 2010), mechanistic understanding of the actions of alcohol in this species may have important clinical implications for FASD in humans, the ultimate goal of our study.
Acknowledgments
This work was supported by NIH grant (R01 AA14357-01A2) to RG.
Abbreviations
- BDNF
Brain derived neurotrophic factor
- CCe
Corpus cerebelli
- DAPI
4′,6-diamidino-2-phenylindole
- Dl
Lateral zone of the dorsal telencephalic area
- Dm
Medial zone of the dorsal telencephalic area
- dpf
days post-fertilization
- EDTA
Ethylenediaminetetraacetic acid
- eNCAM
embryonic Neuronal Cell Adhesion Molecule
- FASD
Fetal alcohol spectrum disorder
- FITC
Fluorescein isothiocyanate
- GABA
Gamma Amino Butyric Acid
- Hav
Ventral habenular nucleus
- hpf
hours post-fertilization
- HRP
Horse Radish Peroxidase
- HPLC
High Performance Liquid Chromatography
- IRF
Inferior reticular formation
- LTP
Long-term potentiation
- nAC
nucleus accumbens
- NCAM
Neuronal cell adhesion molecule
- PBS
Phosphate Buffered Saline
- PMSF
phenylmethylsulfonyl fluoride
- PPa
Parvocellular preoptic nucleus
- PSA-NCAM
Polysialylated Neuronal Cell Adhesion Molecule
- TBS
Tris Buffered Saline
- TRITC
Tetramethylrhodamine-isothiocyanate
- Vd
Dorsal nucleus of the ventral telencephalic area
- VTA
ventral tegmental area
- Vv
Ventral nucleus of the ventral telencephalic area
Footnotes
Competing Interests
No conflicts of interests.
Authors Contributions
All authors contributed to the conception and design of this study. Acquisition of the data was conducted by Samantha Mahabir, Diptendu Chatterjee, Dipashree Chatterjee and Keith Misquitta. Analysis and interpretation of data was carried out by Robert Gerlai and Samantha Mahabir. Drafting of the manuscript was done by Samantha Mahabir, Diptendu Chatterjee and Dipashree Chatterjee. Critical revisions were conducted by Robert Gerlai.
Data Accessibility
All data including original blots, unedited picture files, and counts are maintained at the University of Toronto Mississauga Vivarium, Mississauga, ON. All data can be accessed by contacting Samantha Mahabir at samantha.mahabir@mail.utoronto.ca.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–53. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberch J, Perez-Navarro E, Canals JM. Neurotrophic factors in Huntington’s disease. Prog Brain Res. 2004;146:195–229. doi: 10.1016/s0079-6123(03)46014-7. [DOI] [PubMed] [Google Scholar]
- Amo R, Aizawa H, Takahoko M, Koayashi M, Takahashi R, Aoki T, Okamoto H. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30:1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: An early step of learning- related synaptic growth in Aplysia. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Bath KG, Akins MR, Lee FS. BDNF control of adult SVZ neurogenesis. Dev Psychobiol. 2012;54:578–589. doi: 10.1002/dev.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. Thepolysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. PNAS. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived Neurotrophic Factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braford MR., Jr Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not. Brain Behav Evol. 1995;46:259–274. doi: 10.1159/000113278. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult Zebrafish. Neurotox Teratol. 2011;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacialli P, Gueguen MM, Coumailleau P, D’Angelo L, Kah O, Lucini C, Pellegrini E. BDNF expression in larval and adult zebrafish brain: distribution and cell identification. PLOS ONE. 2016;11:e0158057. doi: 10.1371/journal.pone.0158057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, Roehlk MJ, Alcon SN, Allan AM. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol Biochem Behav. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadon R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J Comp Neurol. 2006;494:435–459. doi: 10.1002/cne.20782. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–8. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Church MW, Hotra JW, Holmes PA, Anumba JI, Jackson DA, Adams BR. Auditory brainstem response (ABR) abnormalities across the life span of rats prenatally exposed to alcohol. Alcoholism: Clinic Exp Res. 2012;36:83–96. doi: 10.1111/j.1530-0277.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, kidane AH, Shirkey NJ, Marshak S. Brain-deprived neurotrophic factor and the development of structural connectivity. Dev Neurobiol. 2010;70:271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston MM, Bastmeyer M, Rutishauser U, O’Leary DDM. Spatially restricted increase in polysialic acid enchances corticospinal axon branching related to target recognition and innervations. J Neurosci. 1996;16:5488–5497. doi: 10.1523/JNEUROSCI.16-17-05488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec P, Cremer H. Revisiting the function of PSA- NCAM in the nervous system. Mol Neurobiol. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Cairns NJ, Harrison PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. Br J Psychiatry. 2000;176:236–42. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–518. [Google Scholar]
- Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nature Rev Neurosci. 2015;16:189–200. doi: 10.1038/nrn3855. [DOI] [PubMed] [Google Scholar]
- Faludi G, Mirnics K. Synaptic Changes in the Brain of Subjects with Schizophrenia. Int J Dev Neurosci. 2011;29:305–309. doi: 10.1016/j.ijdevneu.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng MJ, Yan SE, Yan QS. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring. Brain Res. 2005;1042:125–132. doi: 10.1016/j.brainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcoholism: Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Jia J, Gerlai R. The effect of the number and size of animated conspecific images on shoaling responses of zebrafish. Pharmacol Biochem Behav. 2015a;139:94–102. doi: 10.1016/j.pbb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Gerlai R. Embryonic alcohol exposure impairs the dopaminergic system and social behavioural responses in adult zebrafish. Int J Neuropsychopharmacol. 2015b;18:1–8. doi: 10.1093/ijnp/pyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Tran S, Abraham E, Gerlai R. Embryonic alcohol exposure impairs associative learning performance in adult zebrafish. Behav Brain Res. 2014;15:181–187. doi: 10.1016/j.bbr.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SE, Murray BA, Chuong CM, Edelman GM. Alteration of the retinotectal map in Xenopus by antibodies to neural cell adhesion molecules. Chem Senses. 1984;81:4222–4226. doi: 10.1073/pnas.81.13.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Ganz J, Kroehne V, Freudenreich D, Machate A, Geffarth M, Braasch I, Kaslin J, Brand M. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000 Research. 2015;3:308. doi: 10.12688/f1000research.5595.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. High-throughput Behavioral Screens: The First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav reviews. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilloa FW, Song S, Teles-Grilo Ruivoa LM, Huang L, Gao G, Knott GW, Maco B, Ferretti V, Thompson D, Little GE, Paola VD. Increased axonal bouton dynamics in the aging mouse cortex. PNAS. 2013;110:E1514–E1523. doi: 10.1073/pnas.1218731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J1, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Pagtakhan CJ. Both 5′ and 3′ flanks regulate Zebrafish brain-derived neurotrophic factor gene expression. BMC Neurosci. 2004;5:19. doi: 10.1186/1471-2202-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget U, Wolf A, Wullimann MF, Ryu S. Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic- hypothalamic area in zebrafish larvae. J Comp Neurol. 2014;522:1542–1564. doi: 10.1002/cne.23480. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Mühlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103:56–64. doi: 10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- Hinsby AM, Berezin V, Bock E. Molecular mechanisms of NCAM function. Frontiers Biosci. 2004;9:2227–2244. doi: 10.2741/1393. [DOI] [PubMed] [Google Scholar]
- Knaus P, Betz H, Rehm H. Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem. 1986;47:1302–1304. doi: 10.1111/j.1471-4159.1986.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hannigan JH. Animal models of fetal alcohol spectrum disorders: impact of the social environment. Dev Disabil Res Rev. 2009;15:200–8. doi: 10.1002/ddrr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Schachner M. Glycans and neural cell interactions. Nature Rev Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Hamilton GF, Boschen KE. Long-Term Consequences of Developmental Alcohol Exposure on Brain Structure and Function: Therapeutic Benefits of Physical Activity. Brain Sci. 2013;3:1–38. doi: 10.3390/brainsci3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl C, Riccio O, Grosse J, Zanoletti O, Fournier C, Klampfl SM, Schmidt MV, Sandi C. The interplay of conditional NCAM-knockout and chronic unpredictable stress leads to increased aggression in mice. Stress. 2013;16:647–654. doi: 10.3109/10253890.2013.840824. [DOI] [PubMed] [Google Scholar]
- Kumar A, La Voie HA, Di Pette DJ, Singh US. Ethanol Neurotoxicity in the Developing Cerebellum: Underlying Mechanisms and Implications. Brain Sci. 2013;3:941–963. doi: 10.3390/brainsci3020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847–854. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Dahm L, Tang JC, Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron. 1990;4:655–667. doi: 10.1016/0896-6273(90)90193-j. [DOI] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G. Global prevalence of Fetal alcohol Spectrum disorder among children and youth. JAMA Pediatr. 2017;171:948–956. doi: 10.1001/jamapediatrics.2017.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ET, O’Malley DM, Melloni RH., Jr Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006;167:94–102. doi: 10.1016/j.bbr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Leube RE. Thetopogenic fate of the polytopictransmembrane proteins, synaptophysinand connexin, is determined by their membrane-spanning domains. J Cell Sci. 1995;108:883–894. doi: 10.1242/jcs.108.3.883. [DOI] [PubMed] [Google Scholar]
- Leube RE, Wiedenmann B, Franke WW. Topogenesis and sorting of synaptophysin: synthesis of a synaptic vesicle protein from a gene transfected into nonneuroendocrine cells. Cell. 1989;59:433–446. doi: 10.1016/0092-8674(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psych Investi. 2010;7:231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–43. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu Z, Liu H, Li H, Pan X, Li Z. Brain-derived neurotrophic factor promotes vesicular glutamate transporter 3 expression and neurite outgrowth of dorsal root ganglion neurons through the activation of the transcription factors Etv4 and Etv5. Brain Res Bull. 2016;121:215–26. doi: 10.1016/j.brainresbull.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Ma PM. Catecholaminergic systems in the zebrafish. III. Organization and projection pattern of medullary dopaminergic and noradrenergic neurons. J Comp Neurol. 1997;381:411–427. [PubMed] [Google Scholar]
- Maier SE, Cramer JA, West JR, Sohrabji F. Alcohol exposure during the first two trimesters equivalent alters granule cell number and neurotrophin expression in the developing rat olfactory bulb. J Neurobiol. 1999;41:414–423. doi: 10.1002/(sici)1097-4695(19991115)41:3<414::aid-neu9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D, Buske B, Gerlai R. Maturation of shoaling in two zebrafish strains: A behavioural and neurochemical analysis. Behav Brain Res. 2013;247:1–8. doi: 10.1016/j.bbr.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D, Gerlai R. Strain dependent neurochemical changes induced by embryonic alcohol exposure in zebrafish. Neurotox Terato. 2014;41:1–7. doi: 10.1016/j.ntt.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness PF, Beggs HE, Klinz SG, Morse WR. Selective neural cell adhesion molecule signaling by Src family tyrosine kinases and tyrosine phosphatases. Perspect Dev Neurobiol. 1996;4:169–181. [PubMed] [Google Scholar]
- Martin JL, Finsterwald C. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun Integr Biol. 2011;4:14–16. doi: 10.4161/cib.4.1.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perpect Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure KD, French RL, Heberlein U. A Drosophila model for fetal alcohol syndrome disorders: role for the insulin pathway. Dis Model Mech. 2011;4:335–46. doi: 10.1242/dmm.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahaon HT, Bolshakov VY, Janz R, Hammer RE, Siegelbaum SA, Südhof TC. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc Natl Acad Sci USA. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE. Fetal Alcohol Spectrum Disorders and Abnormal Neuronal Plasticity. Neuroscientist. 2011;17:274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LS, Kotch LE, Riley EP. Alterations in gait following ethanol exposure during the brain growth spurts in rats. Alcoholism: Clin Exp Res. 1990;14:23–27. doi: 10.1111/j.1530-0277.1990.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Mueller T, Dong Z, Berberoglu MA, Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei) Brain Res. 2011;1381:95–105. doi: 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Li W, Yao Z, Zhou X. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections to the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–43. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15:225–34. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurbiol of Disease. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Popova NK. From gene to aggressive behavior: the role of brain serotonin. Neurosci Behav Physiol. 2008;38:471–475. doi: 10.1007/s11055-008-9004-7. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L, Rehm J. The Economic Burden of Fetal Alcohol Spectrum Disorder in Canada in 2013. Alcohol Alcohol. 2016;51:367–75. doi: 10.1093/alcalc/agv117. [DOI] [PubMed] [Google Scholar]
- Portavella M, Vargas JP, Torres B, Salas C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res Bull. 2002;57:397–399. doi: 10.1016/s0361-9230(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Portavella M, Salas C, Vargas JP, Papini MR. Involvement of the telencephalon in spaced-trial avoidance learning in the goldfish (Carassiusauratus) Physiol Behav. 2003;80:49–56. doi: 10.1016/s0031-9384(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmus T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genome. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay M. Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Med. 2010;2:27. doi: 10.1186/gm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell JW. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante AM, Warren KR. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychology rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behaviour. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Theteloestean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull. 2002;57:385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Duran E, Gomez A, Ocana FM, Alvarez E, Jimenez-Moya F, Broglio C, Salas C. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res Bull. 2005;66:365–370. doi: 10.1016/j.brainresbull.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Rønn LC, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol. 1998;33:853–64. doi: 10.1016/s0531-5565(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Rønn LC, Berezin V, Bock E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci. 2000;18:193–199. doi: 10.1016/s0736-5748(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Rønn LC, Bock E, Linnemann D, Jahnsen H. NCAM-antibodies modulate induction of long-term potentiation in rat hippocampal CA1. Brain Res. 2000a;677:145–151. doi: 10.1016/0006-8993(95)00147-i. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–7. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience. 2009;162:234–243. doi: 10.1016/j.neuroscience.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-Specific Alterations in Hippocampal Synaptophysin Immunoreactivity Predict Spatial Learning Impairment in Aged Rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O, Welzl H, Wolfer D, Schuster T, Mantei N, Stork S, Hoyer D, Lipp H, Obata K, Schachner M. Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. Eur J Neurosci. 2000;12:3291–3306. doi: 10.1046/j.1460-9568.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–44. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Reimers MJ. Analysis of ethanol developmental toxicity in zebrafish. Meth Mol Biol. 2008;1812:381–389. doi: 10.1007/978-1-59745-242-7_5. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Domenici L, Simonato M. What is the biological significance of BDNF mRNA targeting in the dendrites? Mol Neurobiol. 2006;33:17–32. doi: 10.1385/MN:33:1:017. [DOI] [PubMed] [Google Scholar]
- Vilar M, Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo M. Brain-Derived Neurotrophic Factor Modulation of GABAergic Synapses by Postsynaptic Regulation of Chloride Transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32:14318–30. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William DK, Gannon-Murakami L, Rougon G, Udin SB. Polysialylated neural cell adhesion molecule and plasticity of ipsilateral connections in Xenopustectum. Neuroscience. 1996;70:277–285. doi: 10.1016/0306-4522(95)00330-l. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hatman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Disease. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain: A topological atlas. Basal, Switzerland: Birkhäuser Verlag; 1996. [Google Scholar]
- Wullimann MF, Rink E. Theteleostean forebrain: a comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Res Bull. 2002;57:363–70. doi: 10.1016/s0361-9230(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Xiao MF, Xu JC, Tereshchenko Y, Novak D, Schachner M, Kleene R. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J Neurosci. 2009;29:14752–14763. doi: 10.1523/JNEUROSCI.4860-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Yin X, Watanabe M, Rutishauser U. Effect of polysialic acid on the behavior of retinal ganglion cell axons during growth into the optic tract and tectum. Development. 1995;121:3439–3446. doi: 10.1242/dev.121.10.3439. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophinsignaling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]